Abstract
Background/Aims:
Obstructive sleep apnea (OSA) is associated with an increased risk of obesity and non-alcoholic fatty liver disease (NAFLD), but it remains unclear whether the risk of NAFLD is independently related to OSA regardless of visceral obesity. Thus, the aim of the present study was to examine whether OSA alone or in combination with excessive daytime sleepiness (EDS) or short sleep duration was associated with NAFLD independent of visceral fat in Korean adults.
Methods:
A total of 621 participants were selected from the Korean Genome and Epidemiology Study (KoGES). The abdominal visceral fat area (VFA) and hepatic fat components of the participants were assessed using computed tomography scans and they were then categorized into four groups depending on the presence of OSA and EDS.
Results:
The proportions of NAFLD were 21.1%, 18.5%, 32.4%, and 46.7% in participants without OSA/EDS, with only EDS, with only OSA, and with both OSA and EDS, respectively. A combination of OSA and EDS increased the odds ratio (OR) for developing NAFLD (OR, 2.75; 95% confidence interval [CI], 1.21 to 6.28) compared to those without OSA/EDS, and this association remained significant (OR, 2.38; 95% CI, 1.01 to 5.59) even after adjusting for VFA. In short sleepers (< 5 hours) with OSA, the adjusted OR for NAFLD was 2.50 (95% CI, 1.08 to 5.75) compared to those sleeping longer than 5 hours without OSA.
Conclusions:
In the present study, OSA was closely associated with NAFLD in Korean adults. This association was particularly strong in those with EDS or short sleep duration regardless of VFA.
Keywords: Sleep apnea, obstructive; Fatty liver; Intra-abdominal fat
INTRODUCTION
Obstructive sleep apnea (OSA) is closely associated with all components of metabolic syndrome, including insulin resistance, dyslipidemia, and obesity [1,2]. In patients with metabolic syndrome, the prevalence of moderate to severe OSA is 50% to 60% [3] and the treatment of OSA with continuous positive airway pressure improves metabolic outcomes [4].
Non-alcoholic fatty liver disease (NAFLD) is regarded as the hepatic component of metabolic syndrome [5] and represents a wide spectrum of liver disorders from isolated steatosis to non-alcoholic steatohepatitis (NASH) and liver cirrhosis [6]. There is an increased prevalence of OSA in adults with NAFLD [7] and elevated serum aminotransferase levels are present in 20% to 50% of adults with OSA [8]. Furthermore, observational clinical studies have indicated that OSA is associated with the histological severity of NAFLD, particularly among patients with morbid obesity [9].
While an increasing amount of data indicates that OSA is related to NAFLD [8], the causal link between these two disorders has yet to be established. Because OSA and NAFLD have common underlying mechanisms, including insulin resistance and visceral obesity, the relationship between them can be coincidental rather than causal. On the other hand, an experimental study found that the chronic intermittent hypoxia associated with OSA triggers liver steatosis and inflammation in the absence of obesity [10], which suggests that OSA can cause NAFLD. Nonetheless, data linking OSA to NAFLD in humans are restricted to morbidly obese adults [9], and it remains unclear whether the association of OSA with NAFLD is dependent on visceral obesity.
Excessive daytime sleepiness (EDS) and sleep deprivation are major features of patients with OSA. Our research group has previously demonstrated that the combination of OSA with EDS or a short sleep time enhances the risk of glucose intolerance [11] and visceral obesity [12]. However, the joint effect of these factors on NAFLD has yet to be investigated. Therefore, the aim of the present study was to investigate whether OSA was associated with NAFLD independent of visceral fat in Korean adults. Additionally, the combined effects of OSA with EDS or short sleep duration on NAFLD were evaluated.
METHODS
Subjects
All participants in the present study were selected from the Ansan cohort of the Korean Genome and Epidemiology Study (KoGES), which is an ongoing population-based cohort study that began in 2001. The details of the study design and sampling method have been described previously [13]. The original cohort consisted of 5,020 participants (2,523 males and 2,497 females) from Ansan, South Korea who were 40 to 69 years of age. The participants in the KoGES have been biennially evaluated in terms of their demographic information, lifestyle factors, sleep-related factors, anthropometric and biochemical variables, and health statuses including medical illnesses and medications. All information was collected by trained interviewers. Of the 3,262 subjects who participated in the fifth biennial examination between March 2009 and February 2011, 851 who underwent polysomnography (PSG) tests and abdominal computed tomography (CT) scans were selected for the present study (Fig. 1). All PSG tests were conducted from September 2009 to November 2010 to randomly measure half of the KoGES participants that remained after excluding those refused the measurement.
Figure 1.
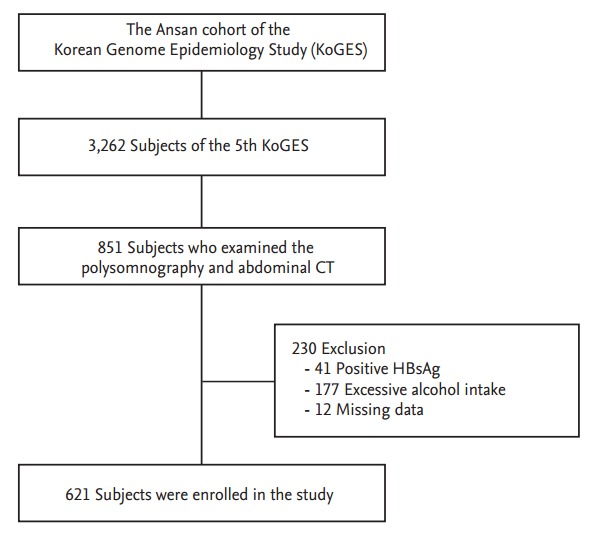
Flow diagram of the study protocol. CT, computed tomography; HBsAg, hepatitis B surface antigen.
To be eligible for the present study, the participants were required to fulfill the following criteria: no evidence of visceral hepatitis (hepatitis B surface antigen [+] or anti-hepatitis C virus antibody [+]), no history of current or past excessive alcohol drinking (an average of ≥ 140 g/week) [14], and no history of hemochromatosis, autoimmune hepatitis, cirrhosis, or other chronic liver diseases. After the exclusion of 230 subjects due to positive tests for serological markers of hepatitis B (n = 41), self-reported excessive alcohol intake (n = 177), or missing data (n = 12), a total of 621 individuals were enrolled in this study. Each participant signed an informed consent form, all procedures were performed in accordance with the principles of the Declaration of Helsinki of the World Medical Association, and the study protocol was approved by the Institutional Review Committee at Korea University Ansan Hospital.
Assessments
Anthropometric and laboratory measurements
All participants completed an interviewer-administered questionnaire and underwent a comprehensive physical examination. The lifestyle characteristics included smoking status and alcohol consumption, which were categorized as never, former, or current, and level of exercise, which was categorized as never, lightly (< 3 time/week and ≥ 30 min/session), or regularly (≥ 3 time/week and ≥ 30 min/session) during the previous month. The presences of chronic illnesses such as diabetes mellitus (DM), hypertension (HTN), and cardiovascular disease (CVD) were noted along with any prescribed medications. DM was defined according to the criteria of the American Diabetes Association using a 75 g oral glucose tolerance test (fasting plasma glucose [FPG] level ≥ 7.0 mmol/L or 2-hour plasma glucose level ≥ 11.1 mmol/L) or based on a medical history of anti-diabetic treatments [15]. HTN was defined as a systolic blood pressure (SBP) ≥ 140 mmHg, a diastolic blood pressure ≥ 90 mmHg, or based on medical history. Participants with a documented history or medical records of myocardial infarction, angina, heart failure, stroke, or peripheral artery disease were considered to have CVD. Waist circumference was measured to the nearest 0.5 cm along a horizontal plane at the level of the umbilicus at the end of a normal expiration.
All blood samples for the biochemical analyses were drawn after an overnight fast. Plasma glucose, serum triglyceride, high density lipoprotein cholesterol, and low density lipoprotein cholesterol levels were measured with an autoanalyzer (ADVIA 1650, Siemens, Tarrytown, NY, USA) and insulin levels were measured with an immunoradiometric assay kit (INS-IRMA Kit, BioSource, Nivelles, Belgium) using a Packard γ counter system. Insulin resistance was estimated with the homeostasis model assessment for insulin resistance (HOMA-IR) and was calculated as follows: fasting glucose (mmol/L) × fasting insulin (μU/mL) / 22.5 [16].
Sleep measurements
The overnight sleep assessments for each patient were performed at their home using a portable device (Embletta X-100, Embla Systems, San Carlos, CA, USA). Two trained sleep technologists visited the participants’ homes in the evening, applied the sensors, and instructed them how to turn the sensors on and off. The participants were also required to record the time that they turned the lights on and off and to report the times the following morning. The following recording channels were used: one for electroencephalography (C4–A1), one for electrooculography (right upper outer canthus/left lower outer canthus), one for chin electromyography, one for modified lead II electrocardiography, one for airflow from the nasal airflow pressure transducer, two for the respiratory efforts from the chest and abdominal respiratory inductance plethysmography, one for the pulse oximeter, and one for the position sensor. All data were scored by two well-trained technicians with ≥ 5 years of experience with PSG monitoring and scoring according to standard guidelines [17]. The internal consistency for scoring the apnea-hypopnea index (AHI) was high (Cronbach α = 0.996 and 1.00 for each rater) and the inter-rater reliability was strong (Cronbach’s α = 0.998). Although a validity study comparing the PSG recordings obtained at home with those obtained in the laboratory was not performed, the Sleep Heart Health Study clearly demonstrated that the median respiratory disturbance indices obtained in unattended home settings were similar to those obtained in attended laboratory settings with only a few differences of a small magnitude in some sleep parameters [18].
Obstructive apnea was defined as when airflow dropped by ≥ 90% of baseline with ongoing chest and abdominal movement, and hypopnea was defined as a reduction ≥ 30% in airflow associated with at least 4% oxygen desaturation; the duration threshold for both of these respiratory events was 10 seconds. The AHI was calculated and OSA was defined as an AHI ≥ 5.
All participants were asked to answer sleep-related questions based on their average sleep pattern over the past month. For example, the question “How many hours did you usually sleep per day during last month?” was used to designate self-reported sleep duration. Daytime sleepiness was assessed with the Epworth Sleepiness Scale (ESS) [19], which is a well-validated and frequently used subjective 8-item, self-administered questionnaire. The presence of EDS was defined when the ESS was 11 or above.
Visceral fat measurements
The abdominal adipose tissue area was quantified using single-slice CT scans obtained with a Brilliance 64 CT scanner (Philips, Cleveland, OH, USA) with a 120 kV exposure. A 5 mm CT slice scan was acquired at the L4 to L5 vertebral interspace and the images were converted into a format compatible with a commercial software package (EBW, Philips). The total area of intra-abdominal fat was delineated by manually tracing within the muscle wall and the visceral fat area (VFA) was defined as an area with an attenuation range between –190 and –30 Hounsfield units (HU). The subcutaneous abdominal fat area (SFA) was determined by subtracting the VFA from total abdominal fat area.
Definition of NAFLD
The hepatic attenuation was measured by randomly selecting three circular regions of interest (ROI; 1 cm2 each) on three different transverse sections: the T11 mid-body, T11 to T12 interspace, and T12 mid-body levels. To provide an internal control, the mean splenic attenuation was also calculated by averaging every two random ROI splenic attenuation measurement values on each of the three transverse sections used to evaluate hepatic attenuation. The liver attenuation index (LAI), which was derived from the difference between the mean hepatic and splenic attenuation, was used to diagnose NAFLD. A LAI of < 5 HU is a known predictor of > 5% steatosis [20] and the histological confirmation of NAFLD requires a minimum of 5% steatosis [21]. Therefore, the present study defined the presence of NAFLD as an LAI value < 5 HU.
Statistical analysis
The baseline characteristics of the groups were compared with a one-way analysis of variance for numerical variables and a chi-square test for categorical variables. Non-normally distributed variables, such as triglyceride levels and the HOMA-IR, are presented as the medians and interquartile ranges for each group and the differences were tested after a logarithmic transformation. Multivariate logistic regression analyses were conducted to identify the combined effects of OSA and EDS or short sleep duration; age, sex, exercise, alcohol, smoking, DM, HTN, CVD, and VFA were included as covariates. All statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) and a p < 0.05 was considered to indicate statistical significance.
RESULTS
Subject characteristics
Table 1 shows the baseline characteristics of the participants categorized according to the presence of OSA and EDS. The mean age of the entire population was 56.6 ± 6.9 years (range, 47 to 77) and the mean body mass index (BMI) was 24.7 ± 2.8 kg/m2 . Of the 621 participants included in the present study, 355 (57.2%) were males, 286 (46.1%) were diagnosed with OSA, and 57 (9.2%) reported EDS. Participants with OSA were older and had higher BMI, waist circumference, SBP, FPG, insulin, and HOMA-IR values than those without OSA, irrespective of EDS. The prevalence rates of DM, HTN, and CVD were higher in participants with OSA than in those without OSA. The group with OSA and EDS showed higher VFA and SFA values but a lower LAI than the other three groups. The prevalence rates for NAFLD were 21.1%, 18.5%, 32.4%, and 46.7% in participants without OSA/EDS, with only EDS, with only OSA, and with both OAS and EDS, respectively.
Table 1.
Characteristics of the study participants
| Characteristic | OSA (–) |
OSA (+) |
p valuea | ||
|---|---|---|---|---|---|
| EDS (–) (n = 308) | EDS (+) (n = 27) | EDS (–) (n = 256) | EDS (+) (n = 30) | ||
| Age, yrb | 54.8 ± 6.1 | 54.3 ± 4.6 | 58.7 ± 7.3 | 57.4 ± 7.7 | < 0.001 |
| Male sexb | 157 (51.0) | 11 (40.7) | 175 (68.4) | 12 (40.0) | < 0.001 |
| Regular exercise | 113 (36.7) | 7 (25.9) | 92 (35.9) | 8 (26.7) | 0.510 |
| Smoking, current | 41 (13.3) | 1 (3.7) | 32 (12.5) | 1 (3.3) | 0.219 |
| Alcohol, currentb | 116 (37.7) | 10 (37.0) | 124 (48.4) | 12 (40.0) | 0.071 |
| Body mass indexb, kg/m2 | 24.2 ± 2.6 | 23.9 ± 2.1 | 25.4 ± 2.9 | 25.6 ± 2.7 | < 0.001 |
| Waist circumference, cmb | 78.9 ± 7.3 | 78.8 ± 4.8 | 84.3 ± 7.7 | 83.4 ± 8.1 | < 0.001 |
| SBP, mmHgb | 112.8 ± 14.8 | 109.7 ± 12.6 | 116.9 ± 15.0 | 118.1 ± 12.0 | 0.001 |
| DBP, mmHgb | 75.1 ± 9.3 | 75.1 ± 7.9 | 76.8 ± 9.4 | 77.9 ± 7.8 | 0.120 |
| FPG, mg/dLb | 98.3 ± 23.7 | 96.6 ± 16.3 | 101.9 ± 24.6 | 108.5 ± 48.0 | 0.084 |
| 2hPG, mg/dL | 131.0 ± 59.8 | 148.2 ± 50.6 | 133.1 ± 59.8 | 130.1 ± 64.1 | 0.571 |
| Insulin, μIU/mLb,c | 7.8 (6.3–9.9) | 7.5 (5.6–9.9) | 8.8 (7.0–11.9) | 8.7 (6.8–11.0) | < 0.001 |
| HOMA-IRb,c | 1.8 (1.4–2.4) | 1.8 (1.3–2.2) | 2.2 (1.6–3.0) | 2.0 (1.6–2.8) | < 0.001 |
| Triglyceride, mg/dLc | 122.5 (88.5–169) | 112 (82–220) | 128 (94–179.5) | 128.5 (82–174) | 0.317 |
| Total cholesterol, mg/dL | 199.5 ± 36.0 | 201.5 ± 46.7 | 199.2 ± 34.3 | 201.2 ± 43.4 | 0.983 |
| HDL-C, mg/dL | 43.3 ± 10.4 | 40.8 ± 7.9 | 42.4 ± 11.0 | 43.3 ± 11.9 | 0.579 |
| LDL-C, mg/dL | 129.0 ± 31.8 | 131.9 ± 37.3 | 126.8 ± 31.0 | 127.4 ± 38.9 | 0.782 |
| AST, IU/Lb,c | 23.0 (20.0–27.0) | 26.0 (21.0–25.0) | 25.0 (21.0–29.0) | 24.5 (18.0–28.0) | 0.031 |
| ALT, IU/Lb,c | 21.0 (16.0–28.0) | 25.0 (17.0-44.0) | 23.0 (18.0–33.0) | 22.5 (17.0–31.0) | 0.009 |
| VFAb, cm2 | 71.8 ± 34.6 | 78.2 ± 27.9 | 92.5 ± 40.1 | 92.9 ± 32.1 | < 0.001 |
| SFAb, cm2 | 179.3 ± 67.0 | 188.3 ± 54.3 | 191.0 ± 74.6 | 217.0 ± 68.4 | 0.018 |
| Liver attenuation indexb | 11.3 (6.2–15.1) | 10.1 (6.0–13.4) | 8.9 (2.6–13.5) | 5.8 (–3.6 to 12.0) | < 0.001 |
| Diabetes mellitusb | 62 (20.2) | 8 (29.6) | 90 (35.3) | 15 (50.0) | < 0.001 |
| Hypertensionb | 77 (25.0) | 4 (14.8) | 105 (41.0) | 12 (40.0) | < 0.001 |
| Cardiovascular diseaseb | 15 (4.9) | 1 (3.7) | 24 (9.4) | 3 (10.0) | 0.153 |
| NAFLDb | 65 (21.1) | 5 (18.5) | 83 (32.4) | 14 (46.7) | 0.001 |
Values are presented as mean ± SD, number (%), or median (interquartile range).
OSA, obstructive sleep apnea; EDS, excessive daytime sleepiness; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; 2hPG, 2-hour plasma glucose; HOMA-IR, homeostasis model assessment of insulin resistance; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; VFA, visceral fat area; SFA, subcutaneous fat area; NAFLD, non-alcoholic fatty liver disease.
Differences among the four groups.
p < 0.05 between groups with and without OSA regardless of EDS.
Statistical significance was estimated after logarithmic transformation.
ORs for NAFLD according to OSA with or without EDS
Multivariate logistic regression analyses were conducted to evaluate the association of OSA with NAFLD. After adjusting for age, sex, smoking, alcohol, exercise, DM, HTN, and CVD, the odds ratio (OR) for NAFLD was 1.62 (95% confidence interval [CI], 1.08 to 2.42) in subjects with OSA compared to those without OSA (Table 2). However, this association was not significant after further adjustment for VFA or BMI.
Table 2.
Associations between obstructive sleep apnea and the risk of non-alcoholic fatty liver disease
| Variable | OSA (–) (n = 335) | OSA (+) (n = 286) |
|---|---|---|
| Unadjusted prevalence, % | 20.8 | 33.8 |
| Crude | 1.00 (reference) | 1.94 (1.35–2.78) |
| Model | ||
| 1a | 1.00 (reference) | 1.78 (1.22–2.59) |
| 2b | 1.00 (reference) | 1.62 (1.08–2.42) |
| 3c | 1.00 (reference) | 1.26 (0.83–1.92) |
| 4d | 1.00 (reference) | 1.16 (0.76–1.79) |
Values are presented as odds ratio (95% confidence interval).
OSA, obstructive sleep apnea.
Model 1 adjusted for age and sex.
Model 2 adjusted for age, sex, exercise, alcohol, smoking, diabetes mellitus (DM), hypertension (HTN), and cardiovascular disease (CVD).
Model 3 adjusted for age, sex, exercise, alcohol, smoking, DM, HTN, CVD, and visceral fat area.
Model 4 adjusted for age, sex, exercise, alcohol, smoking, DM, HTN, CVD, and body mass index.
The OR for NAFLD according to OSA with or without EDS was also determined in the present study. A combination of OSA with EDS increased the OR for NAFLD (2.75; 95% CI, 1.21 to 6.28) compared to those without OSA/EDS (Table 3). This association remained significant (OR, 2.38; 95% CI, 1.01 to 5.59) after further adjustment for VFA, but not for BMI.
Table 3.
Odds ratios for non-alcoholic fatty liver disease according to OSA in combination with or without EDS
| Variable | OSA (–) |
OSA (+) |
||
|---|---|---|---|---|
| EDS (–) (n = 308) | EDS (+) (n = 27) | EDS (–) (n = 256) | EDS (+) (n = 30) | |
| Unadjusted prevalence, % | 21.1 | 18.5 | 32.4 | 46.7 |
| Crude | 1.00 (reference) | 0.85 (0.31–2.33) | 1.79 (1.23–2.62) | 3.27 (1.52–7.05) |
| Model | ||||
| 1a | 1.00 (reference) | 0.91 (0.33–2.51) | 1.60 (1.08–2.38) | 3.55 (1.62–7.75) |
| 2b | 1.00 (reference) | 0.68 (0.23–2.01) | 1.45 (0.95–2.21) | 2.75 (1.21–6.28) |
| 3c | 1.00 (reference) | 0.58 (0.19–1.80) | 1.08 (0.69–1.70) | 2.38 (1.01–5.59) |
| 4d | 1.00 (reference) | 0.72 (0.23–2.29) | 1.03 (0.65–1.62) | 2.15 (0.89–5.21) |
Values are presented as odds ratio (95% confidence interval).
OSA, obstructive sleep apnea; EDS, excessive daytime sleepiness.
Model 1 adjusted for age and sex.
Model 2 adjusted for age, sex, exercise, alcohol, smoking, diabetes mellitus (DM), hypertension (HTN), and cardiovascular disease (CVD).
Model 3 adjusted for age, sex, exercise, alcohol, smoking, DM, HTN, CVD, and visceral fat area.
Model 4 adjusted for age, sex, exercise, alcohol, smoking, DM, HTN, CVD, and body mass index.
ORs for NAFLD according to OSA and duration of sleep
The effects of OSA in combination with short sleep duration on NAFLD are presented in Table 4. The prevalence of NAFLD did not differ significantly between the short (< 5 hours) and long (≥ 5 hours) sleep duration groups (26.2% vs. 33.3%, respectively; p = 0.224). However, after adjusting for confounding variables, the short sleep duration group with OSA had an increased OR for NAFLD (OR, 3.69; 95% CI, 1.67 to 8.12) compared to the long sleep duration group without OSA. Even after further adjustments for either VFA or BMI, the ORs for NAFLD were 2.50 (95% CI, 1.08 to 5.75) and 2.95 (95% CI, 1.24 to 7.03), respectively, in short sleepers with OSA compared to those sleeping longer than 5 hours without OSA.
Table 4.
Odds ratios for non-alcoholic fatty liver disease according to OSA and duration of sleep
| Variable | Sleep duration ≥ 5 hours |
Sleep duration < 5 hours |
||
|---|---|---|---|---|
| OSA (–) (n = 307) | OSA (+) (n = 251) | OSA (–) (n = 28) | OSA (+) (n = 35) | |
| Unadjusted prevalence, % | 21.8 | 31.5 | 10.7 | 51.4 |
| Crude | 1.00 (reference) | 1.64 (1.12–2.39) | 0.43 (0.13–1.47) | 3.79 (1.85–7.76) |
| Model | ||||
| 1a | 1.00 (reference) | 1.44 (0.97–2.14) | 0.46 (0.13–1.59) | 4.34 (2.08–9.04) |
| 2b | 1.00 (reference) | 1.34 (0.88–2.05) | 0.49 (0.14–1.74) | 3.69 (1.67–8.12) |
| 3c | 1.00 (reference) | 1.05 (0.67–1.64) | 0.38 (0.10–1.42) | 2.50 (1.08–5.75) |
| 4d | 1.00 (reference) | 0.93 (0.59–1.47) | 0.38 (0.10–1.43) | 2.95 (1.24–7.03) |
Values are presented as odds ratio (95% confidence interval).
OSA, obstructive sleep apnea.
Model 1 adjusted for age and sex.
Model 2 adjusted for age, sex, exercise, alcohol, smoking, diabetes mellitus (DM), hypertension (HTN), and cardiovascular disease (CVD).
Model 3 adjusted for age, sex, exercise, alcohol, smoking, DM, HTN, CVD, and visceral fat area.
Model 4 adjusted for age, sex, exercise, alcohol, smoking, DM, HTN, CVD, and body mass index.
DISCUSSION
The present study investigated the relationship between NAFLD and OSA with or without hypersomnolence in non-morbidly obese subjects. Participants with OSA had a higher risk for NAFLD than those without OSA, even after adjusting for potential confounding factors. OSA combined with EDS or short sleep duration was associated with an increased risk of NAFLD regardless of visceral fat level.
OSA is thought to be a potential contributing factor in the exacerbation of NAFLD but whether OSA promotes liver injury independently of coexisting comorbidities is still unclear. Some studies have reported an independent relationship between OSA and NAFLD [22,23], while others have suggested that the association between OSA and NASH is coincidental [24,25]. Although experimental evidence indicates that chronic intermittent hypoxia may trigger liver injury [10], few studies have investigated the relationship between OSA and NAFLD in non-morbidly obese patients. In the present study, the mean BMI of the participants was 24.7 kg/m2 and 95.8% of the participants had a BMI less than 30 kg/m2 . Therefore, it is meaningful that these data demonstrated an association between OSA and NAFLD independent of the visceral fat level in relatively lean individuals.
Short sleep duration and poor sleep quality are significantly associated with an increased risk of NAFLD [26], but the combined effects of OSA with EDS or short sleep duration on NAFLD have yet to be investigated extensively. In the present study, although the relationship of OSA with NAFLD was not significant after adjusting for visceral fat or BMI, OSA participants with EDS or short sleep duration had an increased risk of NAFLD independent of visceral fat level. These findings suggest that their combination had a synergistic effect on NAFLD. In animal models, intermittent hypoxemia results in disturbed sleep architecture or damage to the neuronal structures that promote wakefulness [27]. Furthermore, a greater degree of hypoxemia predicts both sleepiness and insulin resistance [28,29]. Such findings suggest that the effects of hypoxemia on liver metabolism are likely to be more evident in individuals with comorbid OSA and EDS.
However, the mechanisms underlying the independent association between OSA and NAFLD regardless of visceral obesity remain unclear. In the absence of the accumulation of visceral fat, the intermittent hypoxia associated with OSA can increase lipogenesis and inhibit fat oxidation, which, in turn, would promote fat accumulation and the development of NAFLD [30]. Furthermore, chronic intermittent hypoxia increases sympathetic activity and promotes oxidative stress, the production of proinflammatory cytokines, endothelial dysfunction, and metabolic dysregulation [31]. Studies of lean mice have confirmed the role of chronic intermittent hypoxia in the progression of a fatty liver [10].
Although NAFLD is closely associated with visceral obesity, it often manifests independently of visceral fat-derived insulin resistance. The patatin-like phospholipase domain containing 3 (PNPLA3) genotype is considered to be an independent risk factor for NAFLD without obesity via the abolishment of triglyceride hydrolysis [32]. Additionally, patients with severe lipodystrophy develop severe hepatic steatosis and hepatic insulin resistance in the absence of visceral obesity [33], and reductions in visceral fat do not improve hepatic insulin resistance in obese individuals [34]. In conjunction with findings such as these, the present results suggest that visceral fat might not always be necessary to explain the relationship between NAFLD and OSA. Previous studies have shown that the association between NAFLD and metabolic disease is stronger in the non-obese than in the obese population [35,36]. Therefore, NAFLD may have the additional metabolic risk compared to visceral fat, particularly in non-obese individuals.
In the present study, there was a diminished association between OSA and NAFLD after adjusting for BMI. On the other hand, it has been shown that the amount of visceral fat, but not BMI, has a high degree of correlation with measures of OSA severity in obese populations [37]. It is possible that this discrepancy may be due to ethnic differences in body composition. For example, Asians generally have a lower BMI but a higher degree of visceral fat than Western populations [38]. Despite significantly lower rates of obesity compared to other ethnic groups, Asians continue to demonstrate a surprisingly high prevalence diabetes, metabolic syndrome and NAFLD [39,40]. Additionally, Asian patients with OSA were reported to have greater disease severity but to be less obese and have greater craniofacial restriction [41]. Because BMI, body fat distribution, and craniofacial morphology differ among ethnicities, it is unclear whether our findings would be consistent in non-Asian populations. Future studies are warranted in other ethnicities.
The strengths of the present work lie in the fact that it was a population-based study and its systematic evaluation of the participants using both PSG tests and CT scans to ensure the precise measurements of OSA and visceral fat. Nevertheless, the present study has several limitations. First, the causal relationship between OSA and NAFLD could not be determined due to the cross-sectional observational design of the study. Large prospective studies are needed to reveal the underlying mechanisms that link these diseases. Second, the present study based the diagnosis of NAFLD on CT scans despite the fact that a liver biopsy is the gold standard for the quantification of intrahepatic lipid content. However, due to its invasive nature this technique would not have been useful in the large community-based study.
In conclusion, the present findings demonstrated that OSA was independently associated with NAFLD in Korean adults and that this association was particularly strong in participants with EDS or short sleep duration, regardless of visceral fat level. Future studies should assess whether this is a universal relationship that exists in different populations, ethnicities, and obesity statuses.
KEY MESSAGE
1. Obstructive sleep apnea was independently associated with non-alcoholic fatty liver disease in non-morbidly obese subjects.
2. The association between obstructive sleep apnea and non-alcoholic fatty liver disease was particularly strong in subjects with excessive daytime sleepiness or short sleep duration, regardless of visceral fat level.
Acknowledgments
This research was supported by a fund (2009-E71002-00, 2010-E71001-00) from the Korea Centers for Disease Control and Prevention.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Bonsignore MR, Esquinas C, Barcelo A, et al. Metabolic syndrome, insulin resistance and sleepiness in real-life obstructive sleep apnoea. Eur Respir J. 2012;39:1136–1143. doi: 10.1183/09031936.00151110. [DOI] [PubMed] [Google Scholar]
- 2.Kim NH. Obstructive sleep apnea and abnormal glucose metabolism. Diabetes Metab J. 2012;36:268–272. doi: 10.4093/dmj.2012.36.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drager LF, Lopes HF, Maki-Nunes C, et al. The impact of obstructive sleep apnea on metabolic and inflammatory markers in consecutive patients with metabolic syndrome. PLoS One. 2010;5: doi: 10.1371/journal.pone.0012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest. 2008;134:686–692. doi: 10.1378/chest.08-0556. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed MH, Byrne CD. Current treatment of non-alcoholic fatty liver disease. Diabetes Obes Metab. 2009;11:188–195. doi: 10.1111/j.1463-1326.2008.00926.x. [DOI] [PubMed] [Google Scholar]
- 6.Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 7.Singh H, Pollock R, Uhanova J, Kryger M, Hawkins K, Minuk GY. Symptoms of obstructive sleep apnea in patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2005;50:2338–2343. doi: 10.1007/s10620-005-3058-y. [DOI] [PubMed] [Google Scholar]
- 8.Sookoian S, Pirola CJ. Obstructive sleep apnea is associated with fatty liver and abnormal liver enzymes: a meta-analysis. Obes Surg. 2013;23:1815–1825. doi: 10.1007/s11695-013-0981-4. [DOI] [PubMed] [Google Scholar]
- 9.Aron-Wisnewsky J, Minville C, Tordjman J, et al. Chronic intermittent hypoxia is a major trigger for non-alcoholic fatty liver disease in morbid obese. J Hepatol. 2012;56:225–233. doi: 10.1016/j.jhep.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 10.Savransky V, Nanayakkara A, Vivero A, et al. Chronic intermittent hypoxia predisposes to liver injury. Hepatology. 2007;45:1007–1013. doi: 10.1002/hep.21593. [DOI] [PubMed] [Google Scholar]
- 11.Kim NH, Cho NH, Yun CH, et al. Association of obstructive sleep apnea and glucose metabolism in subjects with or without obesity. Diabetes Care. 2013;36:3909–3915. doi: 10.2337/dc13-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim NH, Lee SK, Eun CR, et al. Short sleep duration combined with obstructive sleep apnea is associated with visceral obesity in Korean adults. Sleep. 2013;36:723–729. doi: 10.5665/sleep.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin C, Abbott RD, Lee H, Kim J, Kimm K. Prevalence and correlates of orthostatic hypotension in middle-aged men and women in Korea: the Korean Health and Genome Study. J Hum Hypertens. 2004;18:717–723. doi: 10.1038/sj.jhh.1001732. [DOI] [PubMed] [Google Scholar]
- 14.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 15.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.Iber C, Ancoli-Israel S, Chesson A, Jr, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification. Darien: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 18.Iber C, Redline S, Kaplan Gilpin AM, et al. Polysomnography performed in the unattended home versus the attended laboratory setting: Sleep Heart Health Study methodology. Sleep. 2004;27:536–540. doi: 10.1093/sleep/27.3.536. [DOI] [PubMed] [Google Scholar]
- 19.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 20.Limanond P, Raman SS, Lassman C, et al. Macrovesicular hepatic steatosis in living related liver donors: correlation between CT and histologic findings. Radiology. 2004;230:276–280. doi: 10.1148/radiol.2301021176. [DOI] [PubMed] [Google Scholar]
- 21.Hubscher SG. Histological assessment of non-alcoholic fatty liver disease. Histopathology. 2006;49:450–465. doi: 10.1111/j.1365-2559.2006.02416.x. [DOI] [PubMed] [Google Scholar]
- 22.Minville C, Hilleret MN, Tamisier R, et al. Nonalcoholic fatty liver disease, nocturnal hypoxia, and endothelial function in patients with sleep apnea. Chest. 2014;145:525–533. doi: 10.1378/chest.13-0938. [DOI] [PubMed] [Google Scholar]
- 23.Musso G, Cassader M, Olivetti C, Rosina F, Carbone G, Gambino R. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease: a systematic review and meta-analysis. Obes Rev. 2013;14:417–431. doi: 10.1111/obr.12020. [DOI] [PubMed] [Google Scholar]
- 24.Daltro C, Cotrim HP, Alves E, et al. Nonalcoholic fatty liver disease associated with obstructive sleep apnea: just a coincidence? Obes Surg. 2010;20:1536–1543. doi: 10.1007/s11695-010-0212-1. [DOI] [PubMed] [Google Scholar]
- 25.Jouet P, Sabate JM, Maillard D, et al. Relationship between obstructive sleep apnea and liver abnormalities in morbidly obese patients: a prospective study. Obes Surg. 2007;17:478–485. doi: 10.1007/s11695-007-9085-3. [DOI] [PubMed] [Google Scholar]
- 26.Kim CW, Yun KE, Jung HS, et al. Sleep duration and quality in relation to non-alcoholic fatty liver disease in middle-aged workers and their spouses. J Hepatol. 2013;59:351–357. doi: 10.1016/j.jhep.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 27.Polotsky VY, Rubin AE, Balbir A, et al. Intermittent hypoxia causes REM sleep deficits and decreases EEG delta power in NREM sleep in the C57BL/6J mouse. Sleep Med. 2006;7:7–16. doi: 10.1016/j.sleep.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Punjabi NM, Shahar E, Redline S, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 29.Uysal A, Liendo C, McCarty DE, et al. Nocturnal hypoxemia biomarker predicts sleepiness in patients with severe obstructive sleep apnea. Sleep Breath. 2014;18:77–84. doi: 10.1007/s11325-013-0851-2. [DOI] [PubMed] [Google Scholar]
- 30.Piguet AC, Stroka D, Zimmermann A, Dufour JF. Hypoxia aggravates non-alcoholic steatohepatitis in mice lacking hepatocellular PTEN. Clin Sci (Lond) 2009;118:401–410. doi: 10.1042/CS20090313. [DOI] [PubMed] [Google Scholar]
- 31.Musso G, Olivetti C, Cassader M, Gambino R. Obstructive sleep apnea-hypopnea syndrome and nonalcoholic fatty liver disease: emerging evidence and mechanisms. Semin Liver Dis. 2012;32:49–64. doi: 10.1055/s-0032-1306426. [DOI] [PubMed] [Google Scholar]
- 32.Romeo S, Huang-Doran I, Baroni MG, Kotronen A. Unravelling the pathogenesis of fatty liver disease: patatin-like phospholipase domain-containing 3 protein. Curr Opin Lipidol. 2010;21:247–252. doi: 10.1097/mol.0b013e328338ca61. [DOI] [PubMed] [Google Scholar]
- 33.Petersen KF, Oral EA, Dufour S, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fabbrini E, Tamboli RA, Magkos F, et al. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology. 2010;139:448–455. doi: 10.1053/j.gastro.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwon YM, Oh SW, Hwang SS, Lee C, Kwon H, Chung GE. Association of nonalcoholic fatty liver disease with components of metabolic syndrome according to body mass index in Korean adults. Am J Gastroenterol. 2012;107:1852–1858. doi: 10.1038/ajg.2012.314. [DOI] [PubMed] [Google Scholar]
- 36.Kim HJ, Kim HJ, Lee KE, et al. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med. 2004;164:2169–2175. doi: 10.1001/archinte.164.19.2169. [DOI] [PubMed] [Google Scholar]
- 37.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–1158. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 38.Park YW, Allison DB, Heymsfield SB, Gallagher D. Larger amounts of visceral adipose tissue in Asian Americans. Obes Res. 2001;9:381–387. doi: 10.1038/oby.2001.49. [DOI] [PubMed] [Google Scholar]
- 39.Farrell GC, Wong VW, Chitturi S. NAFLD in Asia: as common and important as in the West. Nat Rev Gastroenterol Hepatol. 2013;10:307–318. doi: 10.1038/nrgastro.2013.34. [DOI] [PubMed] [Google Scholar]
- 40.Palaniappan LP, Wong EC, Shin JJ, Fortmann SP, Lauderdale DS. Asian Americans have greater prevalence of metabolic syndrome despite lower body mass index. Int J Obes (Lond) 2011;35:393–400. doi: 10.1038/ijo.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutherland K, Lee RW, Cistulli PA. Obesity and craniofacial structure as risk factors for obstructive sleep apnoea: impact of ethnicity. Respirology. 2012;17:213–222. doi: 10.1111/j.1440-1843.2011.02082.x. [DOI] [PubMed] [Google Scholar]


