Abstract
Background/Aims:
BK virus-associated nephropathy (BKVAN) is an important cause of allograft dysfunction in kidney transplant recipients. It has an unfavorable clinical course, and no definite treatment guidelines have yet been established. Here, we report our center’s experience with biopsy-proven BKVAN and investigate factors associated with its progression.
Methods:
From January 2004 to April 2013, 25 patients with BKVAN were diagnosed by biopsy at Seoul St. Mary’s Hospital. Of the 25 patients, 10 were deceaseddonor transplant recipients and 15 were living-donor transplant recipients. Three of the patients underwent retransplantation. The primary immunosuppressant used was tacrolimus in 17 patients and cyclosporine in eight patients.
Results:
BKVAN was observed at a mean duration of 22.8 ± 29.1 months after transplantation. The mean serum creatinine level at biopsy was 2.2 ± 0.7 mg/dL. BKVAN occurred with acute rejection in eight patients (28%). Immunosuppression modification was performed in 21 patients (84%). Additionally, leflunomide and intravenous immunoglobulin were administered to 13 patients (52%) and two (8%), respectively. Allograft loss occurred in five patients (27.8%) during the follow- up period at 0.7, 17.1, 21.8, 39.8, and 41.5 months after the BKVAN diagnosis. Advanced stages of BKVAN, increased creatinine levels, and accompanying acute rejection at the time of BKVAN diagnosis increased the risk of allograft failure.
Conclusions:
The clinical outcomes in patients with biopsy-proven BKVAN were unfavorable in the present study, especially in patients with advanced-stage BKVAN, poor renal function, and acute allograft rejection.
Keywords: BK virus, Kidney transplantation, Nephropathy
INTRODUCTION
BK virus (BKV) infection is one of the most important opportunistic infections in renal transplant recipients (RTRs) and can cause serious allograft dysfunction [1]. Primary BKV infection usually develops during childhood; the virus thereafter remains latent in the renal tubules and uroepithelial cells, which are the most important sites epidemiologically [2]. BKV is often activated in patients receiving immunosuppressants during the first year after kidney transplantation (KT) [3,4]. Its active replication has been identified to play a causative role in the development of BK virus-associated nephropathy (BKVAN) [5].
Since the first report of BKVAN in 1995, numerous studies have been conducted on BKV [6]. However, each showed diverse prevalences and outcomes of BKVAN [6,7]. Additionally, risk factors associated with the progression of this disease have not been well defined [7]. This may be due to differences in donor types among countries and, even more, the diversity in the immunosuppression and desensitization protocols used among centers [8]. The absence of established treatment and prevention guidelines for BKVAN may be another reason.
In this regard, it is important to investigate the prevalence and clinical outcomes of BKVAN at each center to establish BKVAN prevention or treatment guidelines and protocols specific to each center. Thus, we reviewed our center’s experience with biopsy-proven BKVAN and its clinical course and investigated risk factors associated with allograft outcome after BKVAN diagnosis.
METHODS
We conducted a retrospective single-center study of 25 RTRs with BKVAN, diagnosed by biopsy at Seoul St. Mary’s Hospital from 1 January 2004 to 15 March 2013. The patients’ demographic features and follow-up data were obtained from their hospital medical records. This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital (KC14RISI0220).
The immunosuppressive regimen and infection prophylaxis used in our center have been described previously [9]. All patients received induction therapy with basiliximab. The maintenance immunosuppressive regimen comprised prednisolone, mycophenolate mofetil, and a calcineurin inhibitor (cyclosporine A or tacrolimus). All patients received fluconazole and sulfamethoxazole/trimethoprim as prophylaxis against fungal and Pneumocystis jirovecii infection, respectively.
All RTRs with an unexplained elevation in their serum creatinine level of > 20% from the baseline value underwent an allograft kidney biopsy. A well-trained pathologist evaluated all the biopsy specimens using light microscopy and immunohistochemical staining with monoclonal antibodies directed against the SV40 large T antigen and electron microscopy to distinguish BKVAN from acute allograft rejection, according to the Banff criteria. Diagnosis of BKVAN was confirmed when all of the following criteria were met: (1) typical pathological findings of viral cytopathic effects on renal histological analysis with an inflammatory response suggestive of BKVAN, such as the presence of intranuclear viral inclusions; (2) immunohistochemical staining positive for SV40 large T antigen; and (3) detection of BKV replication in at least one laboratory test, including urine cytological examination, urine polymerase chain reaction (PCR) assay, or plasma PCR assay. The specimens diagnosed with BKVAN were also evaluated to confirm the BKVAN stage.
Statistical analysis
All statistical analyses were performed using SPSS version 19.0 (IBM Co., Armonk, NY, USA). Continuous variables are expressed as mean ± standard deviation, medians (minimum-to-maximum range), or counts with percentages and were compared using Student t test. For categorical variables, Pearson chi-square test and Fisher exact test were used. Allograft survival rates were calculated using the Kaplan-Meier method. All statistical tests were two-tailed, and results were considered significant at p values of <0.05.
RESULTS
Baseline characteristics
The baseline characteristics of the 25 patients are presented in Table 1. The mean age at BKVAN diagnosis was 39.4 ± 13.0 years, and 13 patients were male (52%). In total, 10 patients (40%) received kidneys from deceased donors, and three (12%) underwent second transplantations. Cyclosporine was used as the main immunosuppressant in eight patients (32%), and the remaining 17 patients (68%) received tacrolimus before BKVAN diagnosis.
Table 1.
Baseline characteristics (n = 25)
| Characteristic | Value |
|---|---|
| Age, yr | 39.4 ± 13.0 |
| Male sex | 13 (52) |
| Primary renal disease | |
| Chronic glomerulonephritis | 13 (56) |
| Diabetes mellitus | 4 (16) |
| Hypertension | 2 (8) |
| Unknown | 6 (24) |
| Deceased donor | 10 (40) |
| HLA mismatch number | 3.3 ± 2.0 |
| Second transplantation | 3 (12) |
| Immunosuppressant | |
| Cyclosporine | 8 (32) |
| Tacrolimus | 17 (68) |
Values are presented as mean ± SD or number (%).
HLA, human leukocyte antigen.
Laboratory and pathological findings associated with BKV infection
BKVAN was documented at 22.8 ± 29.1 months after transplantation, and the serum creatinine level at biopsy was 2.2 ± 0.7 mg/dL. In 18 patients, monitoring of BKV replication was performed using plasma BKV real-time PCR, and the replication levels exceeded 1.0 × 104 copies/mL in all patients (range, 1.6 × 104 to 5.0 × 107 ). The BKVAN stage distribution was as follows: stage A, nine patients (36%); stage B, 12 patients (48%); and stage C, four patients (16%). In six patients (24%), BKVAN presented with acute allograft rejection (acute T-cell-mediated rejection in five and acute antibody-mediated rejection in one).
Management and clinical outcomes of BKVAN
The follow-up duration after BKVAN diagnosis was 29.9 months (range, 0.7 to 90.1). The immunosuppression regimen was modified in 21 patients. Mycophenolate mofetil was discontinued in all patients, and the tacrolimus dose was reduced by 20% in nine patients. Leflunomide was used in 13 patients, and infused intravenous immunoglobulin (IVIG) was used for 5 days in four patients. Despite the change in immunosuppressant or treatment for BKVAN, the allograft function did not improve overall (Fig. 1A). In five patients, allograft rejection developed within 6 months of the BKVAN diagnosis. During the follow-up period, 20% (5/25) of the patients experienced allograft loss at 21.8 months (range, 0.7 to 41.5) from BKVAN diagnosis. The 5-year allograft survival rate from BKVAN diagnosis was 67% (Fig. 1B).
Figure 1.
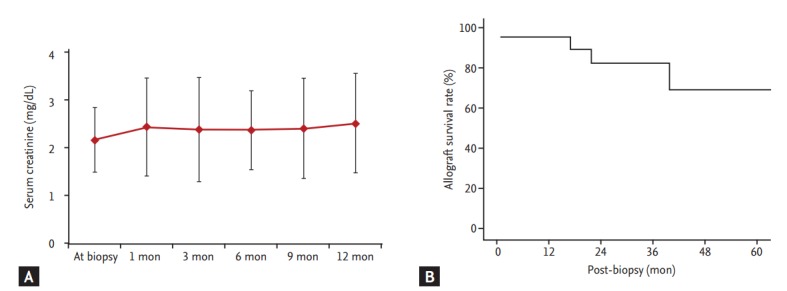
Changes in allograft function and allograft survival rate after BK virus-associated nephropathy (BKVAN) diagnosis. (A) Allograft function deteriorated after BKVAN diagnosis. (B) The 5-year allograft survival rate from biopsy was 67% using Kaplan-Meier analysis.
Risk factors associated with allograft failure after BKVAN diagnosis
The comparison between patients with and without graft failure is presented in Table 2. The duration from KT to BKVAN diagnosis was significantly longer in patients with than without graft failure (p < 0.01). Allograft biopsy findings showed that compared with patients without allograft failure, those with allograft failure had a more advanced stage of BKVAN and showed a significant decrease in allograft function, expressed as Modification of Diet in Renal Disease-estimated glomerular filtration rate (p < 0.01). Additionally, the proportion of combined allograft rejection showed a higher tendency in patients with allograft failure (p = 0.06). Treatment with IVIG or leflunomide or reduction of the immunosuppressant dose did not affect the clinical outcome of BKVAN.
Table 2.
Comparison of clinical parameters between patients with and without graft failure
| Parameter | Graft failure |
p value | |
|---|---|---|---|
| Yes (n = 5) | No (n = 20) | ||
| Age at diagnosis, yr | 35.9 ± 11.4 | 40.3 ± 13.5 | 0.52 |
| KT-biopsy, mon | 52.9 ± 42.4 | 15.3 ± 19.9 | < 0.01 |
| Male sex | 1 (20.0) | 12 (60.0) | 0.14 |
| Deceased donor | 3 (60.0) | 7 (35.0) | 0.34 |
| Retransplantation | 1 (20.0) | 2 (10.0) | 0.50 |
| Immunosuppressant | |||
| Cyclosporine | 2 (40.0) | 6 (30.0) | 0.60 |
| Tacrolimus | 3 (60.0) | 14 (70.0) | 0.75 |
| History of AR | 1 (20.0) | 3 (15.0) | 0.62 |
| Combined AR | 3 (60.0) | 3 (15.0) | 0.06 |
| sCr at biopsy, mg/dL | 3.0 ± 1.0 | 2.0 ± 0.4 | < 0.01 |
| eGFR at biopsy | 21.2 ± 7.9 | 35.8 ± 8.2 | < 0.01 |
| Treatment for BKVAN | |||
| IVIG | 1 (20.0) | 3 (15.0) | 0.67 |
| Leflunomide | 1 (20.0) | 12 (60.0) | 0.14 |
| IS reduction | 3 (60.0) | 18 (90.0) | 0.17 |
Values are presented as mean ± SD or number (%).
KT, kidney transplantation; AR, acute rejection; sCr, serum creatinine; eGFR, estimated glomerular filtration rate; BKVAN, BK virus-associated nephropathy; IVIG, intravenous immunoglobulin; IS, immunosuppressant.
Clinical outcomes based on risk factors
We analyzed clinical outcomes based on BKVAN stage, combined allograft rejection, and allograft function. The allograft failure rate was significantly higher and the allograft survival rate was significantly lower in patients with stage C BKVAN than in those with stage A or B (p = 0.045) (Fig. 2). Moreover, the allograft failure rate was higher (p = 0.05) and the allograft survival rate was significantly lower in patients presenting with acute rejection than in those without acute rejection (Fig. 3A and 3B). In addition, patients with combined allograft rejection showed a significantly higher rate of allograft rejection within 6 months from the diagnosis of BKVAN (p < 0.01) (Fig. 3C). Patients with decreased allograft function of < 30 mL/min/1.73 m2 showed a higher tendency for allograft failure (p = 0.12) and a lower tendency for allograft survival (p = 0.08) (Fig. 4).
Figure 2.
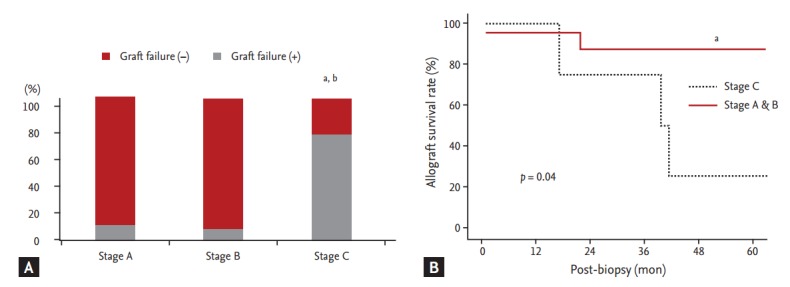
Impact of BK virus-associated nephropathy (BKVAN) stage on allograft outcome after BKVAN diagnosis. (A) Comparison of the development of allograft failure based on BKVAN stages. (B) Comparison of allograft survival rates between patients with stage A and B and patients with stage C using Kaplan-Meier analysis. The allograft failure rate was significantly higher and allograft survival rate was significantly lower in patients with stage C BKVAN than in patients with stage A and B. ap < 0.05 vs. stage A. bp < 0.05 vs. stage B.
Figure 3.
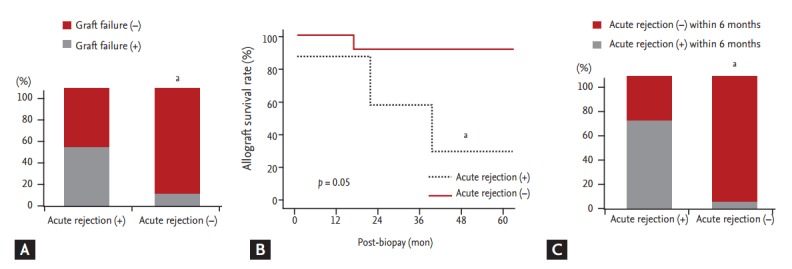
Impact of combined acute rejection on allograft outcome after BK virus-associated nephropathy (BKVAN) diagnosis. (A) Comparison of the development of allograft failure based on combined acute rejection. (B) Comparison of allograft survival rates between patients with and without combined acute rejection using Kaplan-Meier analysis. (C) Comparison of acute rejection rates within 6 months from BKVAN diagnosis. Patients with combined acute rejection had a higher risk of allograft failure, lower allograft survival rates, and higher acute rejection rates within 6 months from BKVAN diagnosis than did those without combined acute rejection. ap < 0.05 vs. acute rejection (+).
Figure 4.
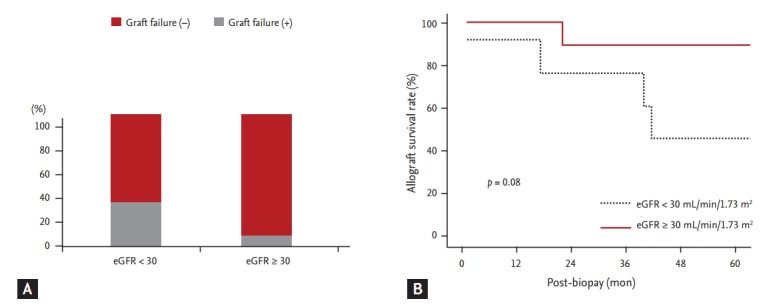
Impact of allograft function on clinical outcomes after BK virus-associated nephropathy (BKVAN) diagnosis. (A) Comparison of the development of allograft failure based on estimated glomerular filtration rate (eGFR). (B) Comparison of allograft survival rates between patients with an eGFR of ≥ 30 mL/min/1.73 m2 and those with an eGFR of < 30 mL/min/1.73 m2 using Kaplan-Meier analysis. Patients with an eGFR of < 30 mL/min/1.73 m2 had a higher risk of allograft failure and lower allograft survival rates than did those with an eGFR of < 30 mL/min/1.73 m2.
DISCUSSION
Because immunosuppressive regimens and treatment strategies for BKVAN are inconsistent among transplant centers, the incidence and outcomes are also variable among centers. In this regard, each center should compile their own data on BKVAN outcomes. We retrospectively reviewed the clinical course of BKVAN at our center. Our results indicated that RTRs with BKVAN had unfavorable clinical outcomes. Additionally, allograft function did not improve even after administration of antiviral agents such as leflunomide or IVIG and reduction of the immunosuppressant dose. The allograft loss rate was 20% in our center, which is similar to that reported previously [10-12].
First, we investigated the timing of the BKVAN diagnosis and the BKVAN stages. Usually, BKV replication is initiated within 1 year from KT. However, the mean duration between KT and BKVAN was 22.8 months in the present study, and most patients were diagnosed ≥ 1 year after KT. In addition, the serum creatinine level was already above normal in most patients, and 16 patients (64%) showed stage B or C BKVAN. Considering the prototypical course of BKV infection, in which viremia can be detected before BKVAN development, these findings suggest that most cases were diagnosed after a long period from the start of BKV replication [13,14]. Indeed, all 18 patients who underwent BKV replication analysis using plasma BKV real-time PCR at the time of allograft biopsy showed significant BKV replication, with the BKV DNA copy number exceeding 1.0 × 104 /mL [2,15,16].
Second, we investigated the clinical outcomes after the BKVAN diagnosis and analyzed the effect of the use of therapeutic agents such as leflunomide and IVIG and reduction of the immunosuppressant dose. Various therapeutic agents, such as cidofovir, leflunomide, IVIG, and levofloxacin, have been used for BKVAN treatment to date, but none is clearly superior to the others [17,18]. In this study, we used leflunomide or IVIG for BKVAN treatment. It seemed that leflunomide may help to prevent allograft failure, but no significant effect was observed, perhaps because of the limited number of cases. Another possible reason is that other factors, such as chronic changes in allograft tissue and BKVAN stages, may have induced bias in the analysis of allograft outcome.
We reduced the immunosuppressant dose in most patients; however, even this showed no significant effect on BKVAN progression (Fig. 1). Furthermore, we observed five cases of allograft rejection after reducing the immunosuppressant dose. This finding is contrary to our previous results, showing that no rejection developed after immunosuppressant dose reduction during BK viremia [9]. The underlying reasons are unclear, but activation of the innate immune system by virus invasion of allograft tissue may activate adaptive immune cells, which can result in allograft rejection [19,20]. These findings suggest that after BKV infection of allograft tissue, reducing the immunosuppressant dose may have no beneficial effect on BKVAN progression and may increase the risk of allograft rejection, in contrast to dose reduction during viremia or viruria.
Next, we investigated risk factors associated with the development of allograft failure. In this study, allograft failure developed mostly in patients with advanced-stage BKVAN, consistent with previous studies reporting that the allograft failure rate reached 100.0% in patients with stage C BKVAN but was only 12.9% in those with stage A [10-12,21-23]. In patients with stage C BKVAN, histological examination of allograft biopsy specimens revealed extensive BKV replication, cell necrosis of the tubules and collecting ducts, and varying degrees of interstitial inflammation [6,24]. Additionally, allograft function decreased significantly in those patients, and the deteriorated allograft function did not recover even when BK viremia was successfully cleared [25].
Another risk factor for allograft failure was combined acute rejection of allograft tissue. Among the six patients with combined BKVAN and acute rejection, three (50%) experienced allograft loss, which was significantly higher than the allograft loss rate in patients without acute rejection (15%). The difficulty in the treatment of combined BKVAN and acute rejection is that we could provide neither sufficient antirejection therapy nor effective treatment for BKVAN because the two conditions require opposite treatment strategies. In those six patients, we first used steroid pulse therapy and later reduced the immunosuppressant dose. However, four of the six showed recurrent rejection within 6 months from BKVAN diagnosis, which suggests that rejection may not be treated sufficiently by steroid pulse therapy and that subsequent immunosuppression reduction may facilitate the recurrence of acute rejection in those cases. These findings suggest that steroid pulse therapy with immunosuppressant dose reduction is ineffective for not only BKVAN treatment, but also combined acute rejection treatment.
In terms of treatment for BKVAN with acute rejection, a two-step approach of antirejection treatment followed by a reduction in the immunosuppressant dose is supported by several studies reporting stabilization or improvement of allograft function. However, the prognosis differs from one center to another: some reports showed a worse prognosis of BKVAN combined with acute rejection than of BKVAN alone [4], as in our report, whereas Kim et al. [26] recently reported an allograft loss rate of BKVAN with acute rejection (20%) similar to that of BKVAN alone after anti-BKV therapy (22%). Further study is needed to determine the appropriate treatment option and prognosis for patients with BKVAN who develop acute rejection. Other demographic factors, including the donor type, retransplantation history, and type of immunosuppressant used, showed no significant correlation with allograft loss in this study.
A limitation of this study is the small number of patients. Thus, we did not perform multivariate analyses to investigate risk factors for allograft failure in patients with BKVAN. Second, we could not compare the therapeutic effects of each treatment method because the treatment protocol was not standardized.
In conclusion, the allograft loss rate was 25% among patients with BKVAN at our center. The allograft failure risk increased in patients with advanced-stage BKVAN, poor renal function, or BKVAN combined with acute allograft rejection at the time of BKVAN diagnosis. In addition, no therapy has proven effective in preventing allograft loss in patients with biopsy-proven BKVAN. Thus, early detection of BKV replication before BKVAN development is important, and when BKVAN is suspected, a prompt biopsy is needed for early diagnosis.
KEY MESSAGE
1. The risk for allograft failure increased in patients with advanced-stage BK virus-associated nephropathy (BKVAN), poor renal function, and BKVAN combined with acute allograft rejection at the time of diagnosis of BKVAN.
2. Early detection of BK virus replication before the development of BKVAN and, when suspected, a prompt biopsy for early diagnosis are important.
Acknowledgments
This study was supported by a grant (HI13C1232) from the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Hirsch HH. Polyomavirus BK nephropathy: a (re-)emerging complication in renal transplantation. Am J Transplant. 2002;2:25–30. doi: 10.1034/j.1600-6143.2002.020106.x. [DOI] [PubMed] [Google Scholar]
- 2.Costa C, Bergallo M, Astegiano S, et al. Monitoring of BK virus replication in the first year following renal transplantation. Nephrol Dial Transplant. 2008;23:3333–3336. doi: 10.1093/ndt/gfn289. [DOI] [PubMed] [Google Scholar]
- 3.Nickeleit V, Singh HK, Mihatsch MJ. Polyomavirus nephropathy: morphology, pathophysiology, and clinical management. Curr Opin Nephrol Hypertens. 2003;12:599–605. doi: 10.1097/00041552-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch HH, Brennan DC, Drachenberg CB, et al. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation. 2005;79:1277–1286. doi: 10.1097/01.tp.0000156165.83160.09. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch HH, Steiger J. Polyomavirus BK. Lancet Infect Dis. 2003;3:611–623. doi: 10.1016/s1473-3099(03)00770-9. [DOI] [PubMed] [Google Scholar]
- 6.Purighalla R, Shapiro R, McCauley J, Randhawa P. BK virus infection in a kidney allograft diagnosed by needle biopsy. Am J Kidney Dis. 1995;26:671–673. doi: 10.1016/0272-6386(95)90608-8. [DOI] [PubMed] [Google Scholar]
- 7.Lee SO. Common infections in solid organ transplant recipients. Korean J Med. 2013;84:145–157. [Google Scholar]
- 8.Chung BH, Jung MH, Bae SH, et al. Changing donor source pattern for kidney transplantation over 40 years: a single-center experience. Korean J Intern Med. 2010;25:288–293. doi: 10.3904/kjim.2010.25.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung BH, Hong YA, Kim HG, et al. Clinical usefulness of BK virus plasma quantitative PCR to prevent BK virus associated nephropathy. Transpl Int. 2012;25:687–695. doi: 10.1111/j.1432-2277.2012.01480.x. [DOI] [PubMed] [Google Scholar]
- 10.Hurault de Ligny B, Etienne I, et al. Polyomavirus-induced acute tubulo-interstitial nephritis in renal allograft recipients. Transplant Proc. 2000;32:2760–2761. doi: 10.1016/s0041-1345(00)01869-8. [DOI] [PubMed] [Google Scholar]
- 11.Ahuja M, Cohen EP, Dayer AM, et al. Polyoma virus infection after renal transplantation: use of immunostaining as a guide to diagnosis. Transplantation. 2001;71:896–899. doi: 10.1097/00007890-200104150-00013. [DOI] [PubMed] [Google Scholar]
- 12.Barri YM, Ahmad I, Ketel BL, et al. Polyoma viral infection in renal transplantation: the role of immunosuppressive therapy. Clin Transplant. 2001;15:240–246. doi: 10.1034/j.1399-0012.2001.150404.x. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch HH, Mohaupt M, Klimkait T. Prospective monitoring of BK virus load after discontinuing sirolimus treatment in a renal transplant patient with BK virus nephropathy. J Infect Dis. 2001;184:1494–1495. doi: 10.1086/324425. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch HH, Knowles W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347:488–496. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 15.Babel N, Fendt J, Karaivanov S, et al. Sustained BK viruria as an early marker for the development of BKV-associated nephropathy: analysis of 4128 urine and serum samples. Transplantation. 2009;88:89–95. doi: 10.1097/TP.0b013e3181aa8f62. [DOI] [PubMed] [Google Scholar]
- 16.Viscount HB, Eid AJ, Espy MJ, et al. Polyomavirus polymerase chain reaction as a surrogate marker of polyomavirus-associated nephropathy. Transplantation. 2007;84:340–345. doi: 10.1097/01.tp.0000275205.41078.51. [DOI] [PubMed] [Google Scholar]
- 17.Randhawa P, Brennan DC. BK virus infection in transplant recipients: an overview and update. Am J Transplant. 2006;6:2000–2005. doi: 10.1111/j.1600-6143.2006.01403.x. [DOI] [PubMed] [Google Scholar]
- 18.Wiseman AC. Polyomavirus nephropathy: a current perspective and clinical considerations. Am J Kidney Dis. 2009;54:131–142. doi: 10.1053/j.ajkd.2009.01.271. [DOI] [PubMed] [Google Scholar]
- 19.Toupance O, Bouedjoro-Camus MC, Carquin J, et al. Cytomegalovirus-related disease and risk of acute rejection in renal transplant recipients: a cohort study with case-control analyses. Transpl Int. 2000;13:413–419. doi: 10.1007/s001470050723. [DOI] [PubMed] [Google Scholar]
- 20.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 21.Kang YN, Han SM, Park KK, Jeon DS, Kim HC. BK virus infection in renal allograft recipients. Transplant Proc. 2003;35:275–277. doi: 10.1016/s0041-1345(02)03954-4. [DOI] [PubMed] [Google Scholar]
- 22.Mengel M, Marwedel M, Radermacher J, et al. Incidence of polyomavirus-nephropathy in renal allografts: influence of modern immunosuppressive drugs. Nephrol Dial Transplant. 2003;18:1190–1196. doi: 10.1093/ndt/gfg072. [DOI] [PubMed] [Google Scholar]
- 23.Drachenberg CB, Papadimitriou JC, Hirsch HH, et al. Histological patterns of polyomavirus nephropathy: correlation with graft outcome and viral load. Am J Transplant. 2004;4:2082–2092. doi: 10.1046/j.1600-6143.2004.00603.x. [DOI] [PubMed] [Google Scholar]
- 24.Kiberd BA. Screening to prevent polyoma virus nephropathy: a medical decision analysis. Am J Transplant. 2005;5:2410–2416. doi: 10.1111/j.1600-6143.2005.01034.x. [DOI] [PubMed] [Google Scholar]
- 25.Randhawa P, Ho A, Shapiro R, et al. Correlates of quantitative measurement of BK polyomavirus (BKV) DNA with clinical course of BKV infection in renal transplant patients. J Clin Microbiol. 2004;42:1176–1180. doi: 10.1128/JCM.42.3.1176-1180.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim YJ, Jeong JC, Koo TY, et al. Impact of combined acute rejection on BK virus-associated nephropathy in kidney transplantation. J Korean Med Sci. 2013;28:1711–1715. doi: 10.3346/jkms.2013.28.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]


