Abstract
Background/Aims:
In this study, the sensitivity-specificity of galactomannan-enzyme immunoassay (GM-EIA) with a cut-off value of 0.5 for a single, two, or three consecutive positivity in the diagnosis of invasive pulmonary aspergillosis (IPA) in neutropenic patients with hematological malignancy was investigated.
Methods:
IPA was classified as “proven,” “probable,” or “possible” as described in the guidelines prepared by the European Organization for Research and Treatment of Cancer and Mycoses Study Group.” Serum samples were collected from the patients twice a week throughout their hospitalization. A total of 1,385 serum samples, with an average of 8.3 samples per episode, were examined.
Results:
Based on the 165 febrile episodes in 106 patients, 80 (48.5%) were classified as IPA (4 proven, 11 probable, 65 possible) and 85 (51.5%) as non-IPA. The sensitivity/ specificity was 100%/27.1% for a single proven/probable IPA with the cut of value of GM-EIA ≥ 0.5, 86.7%/71.8% for two consecutive positive results, and 73.3%/85.9% for three consecutive positive results.
Conclusions:
With the galactomannan levels measured twice a week, consecutive sensitivity decreased and specificity increased. Therefore, an increase may be obtained in sensitivity-specificity by more frequent monitoring of GM-EIA starting from the first day of positivity is detected.
Keywords: Invasive aspergillosis, Galactomannan test, Cut-off value, Sensitivity and specificity, Enzyme-linked immunosorbent assay
INTRODUCTION
Despite the recent developments in the treatment of patients with hematologic malignancies who underwent hematopoietic stem cell transplantation and are undergoing heavy cytotoxic chemotherapy, invasive aspergillosis (IA) is still a significant cause of mortality. IA can cause invasive infection in many tissues/organs such as heart, brain, sinus, eye, skin, and ear. With increased use of immunosuppressive agents in recent years, invasive pulmonary aspergillosis (IPA) incidence rates have increased up to 30% in some centers [1-4]. Because the clinical symptoms of this infection are similar to those of other infectious diseases, the diagnosis is difficult. Moreover, since these patients are neutropenic, difficulties are experienced in the follow-up of these patients’ response to the treatment as well [5,6]. Galactomannan is a polysaccharide cell wall component of Aspergillus [7-9]. Today, progress has been achieved in the diagnosis via Aspergillus galactomannan (GM) antigen [10,11]. The follow-up of circulating GM level serves as an indicator of severity of the infection during the invasive disease course throughout the neutropenic period as much as in the diagnosis. Platelia Aspergillus enzyme immunoassay (EIA, Bio-Rad Laboratories, Hercules, CA, USA) is used to detect GM concentrations, and the result is expressed as galactomannan index (GMI) [7]. Through extensive studies, GMI measurement has been generally accepted as a non-invasive tool for IPA diagnosis among patients at-risk, and has also been considered one of the mycological criteria for a probable IPA case definition by the consensus group of the European Organization for Research and Treatment of Cancer/Mycosis Study Group (EORTC/MSG) [12]. Additionally, serial GMI measurements have also been recommended for monitoring treatment response or surrogate endpoint to assess clinical outcomes of IPA [13-15].
Recent studies conducted to identify the treatment process as much as the diagnosis recommended to increase the frequency of GM antigen controls [16]. Along with consecutive positive result required for the diagnosis, increased frequency of GMI checks will also lead to changes in sensitivity/specificity. In this study, we aimed to evaluate the effect of single, two, and three consecutive positive results in galactomannan tests, performed twice a week in series in patients with hematological malignancies who are at risk for IPA, on the sensitivity/specificity of GMI.
METHODS
Subjects
One hundred sixty-five consecutive febrile neutropenia episodes of 106 hematologic malignancy patients between the ages of 16 and 80 years, who had increased risk for IPA, were monitored proactively (except who underwent hematopoietic stem cell transplantation). Patients whose serum samples were collected twice a week starting on day 1 of their hospitalization but were lost due to other reasons before having fever and those whose consent could not be obtained were excluded from the study. The Institutional Ethics Committee approved the study.
Serum samples were collected from patients twice a week starting on day 1 of hospitalization for GMI measurements via enzyme-linked immunosorbent assay (ELISA; Platelia Aspergillus, Sanofi Diagnostics Pasteur, Marnes-La-Coquett, France) using the technique described by the producer company. The patients were closely monitored throughout their hospital stay for identification of sinopulmonary signs and symptoms. With this purpose, they were given daily physical examinations and their axillary body temperature measurements were done at least 4 times a day.
Patient definitions and classifications for IPA
The patients were classified as “proven,” “probable,” or “possible” IPA as described in EORTC/MSG guidelines [12]. But GM test, which is included in mycologic criteria in EORT/MSG guidelines, were not used for patients classification. Thus statistical calculations could be done for galactomannan [17].
Statistical analysis
Sensitivity was calculated based on the results of proven/probable IPA patients and specificity was calculated based on those measured in non-IPA patients. Positive and negative predictive values were estimated based on the combination of both groups. Sensitivity/specificity calculations were made in three different conditions: ≥ 1 in the first evaluation, ≥ 2 in the second evaluation, and ≥ 3 consecutive positive serum GMIs in the third evaluations using a threshold for positivity of ≥ 0.5.
RESULTS
Febrile neutropenia episodes during the study
According to EORTC/MSG criteria, four episodes were defined as proven IPA (2.4%), 11 as probable IPA (6.7%), 65 were possible IPA (39.4%), and the remaining 85 were defined as non-IPA (51.5%) episodes.
Patient characteristics
One hundred sixty-five episodes of 106 patients were included in the study. Mean age was 42.5 ± 17 [16-18]. Mean duration of neutropenia was 36.4 ± 22.2 days. There were 30 patients (18.1%) with acute lymphoblastic leukemia, 107 (64.8%) with acute myelocytic leukemia, 13 (7.8%) with lymphoma, five (3%) with myelodysplastic syndrome, five (3%) with chronic lymphocytic leukemia, and five (3%) with other hematologic malignancies. Antifungal therapy targeting IPA was used in 111 (67.2%) of the episodes. The features of febrile neutropenia episodes are presented in Table 1.
Table 1.
Features of febrile neutropenic episodes
| Variable | Proven IA | Probable IA | Possible IA | Non-IA | Total |
|---|---|---|---|---|---|
| No. of episodes | 4 | 11 | 65 | 85 | 165 |
| Age, yr | 52 ± 16 | 49 ± 17 | 42 ± 16 | 41 ± 17 | 42 ± 17 |
| No. of deaths | 3 | 8 | 35 | 6 | 52 |
| Duration of episode, day | 24 ± 14 | 49 ± 29 | 41 ± 23 | 31 ± 18 | 36 ± 22 |
| Episodes with antifungal use | 4 | 11 | 60 | 36 | 111 |
IA, invasive aspergillosis.
Patients diagnosed with IPA
Among the 165 episodes, 15 patients (9%) were diagnosed with IPA, among which four were proven and 11 were probable IPA cases. Hyphae invasion was observed in the open lung biopsy, performed via postmortem thoracotomy, of the first patient diagnosed with proven IPA. Aspergillosis could not be cultured in the collected tissue but hyphae were observed in direct inspection of the collected pleural fluid and aspergillosis had developed from the catheter while the patient was alive. The second patient was surgically operated and aspergillosis was cultured from the postoperative biopsy materials. Aspergillosis was cultured in the blindly collected postmortem lung biopsy sample of the third patient but hyphae invasion could not be demonstrated histopathologically. Aspergillosis was cultured in the pleural fluid collected postmortem from the fourth patient.
Aspergillus flavus was cultured in 7 episodes, Aspergillus fumigatus in 9 episodes, and Aspergillus niger in 1 episode. These were obtained from phlegm (n = 9 episodes), tracheal aspirate fluid (n = 5 episodes), bronchoalveolar lavage fluid (n = 2 episodes), catheter tip (n = 1 episode), pleural effusion obtained postmortem (n = 1 episode), lung tissue obtained during operation (n = 1 episode), and postmortem lung biopsy (n = 1 episode) specimens.
GM results
Throughout the study, GM antigen levels were checked in 1,385 serum samples using the double-sandwich ELISA method. When all episodes considered, an average of 8.3 serum samples per episode were studied (Table 2).
Table 2.
Number of serum for galactomannan assay
| Variable | Proven IA | Probable IA | Possible IA | Non-IA | Total |
|---|---|---|---|---|---|
| No. (%) of episodes | 4 (2.5) | 11 (6.6) | 65 (39.4) | 85 (51.5) | 165 (100) |
| No. of serum | 26 | 121 | 633 | 605 | 1,385 |
| Mean no. of serum | 6.6 | 11 | 9.7 | 7.1 | 8.3 |
| Positive serum for GM | |||||
| ODI ≥ 1.5 | 10 | 19 | 39 | 28 | 96 |
| ODI ≥ 1.0 | 15 | 22 | 59 | 49 | 145 |
| ODI ≥ 0.5 | 21 | 55 | 193 | 168 | 437 |
IA, invasive aspergillosis; GM, galactomannan; ODI, optical density index.
For a single proven/probable IPA with the cut of value of GM-EIA ≥ 0.5, the obtained sensitivity/specificity was 100%/27.1% with a positive predictive value of 19.5%, a negative predictive value of 100%, and 38% accuracy. For the two consecutive positive GM-EIA ≥ 0.5, sensitivity/specificity were 86.7%/71.8%, positive predictive value 35.1%, negative predictive value 96.8%, and accuracy 74%. For three consecutive positive GM-EIA ≥ 0.5, sensitivity/specificity was 73.3%/85.9%, with a positive predictive value of 47.8%, a negative predictive value of 94.8%, and accuracy of 84% (Table 3).
Table 3.
The diagnostic values of the serum-galactomannan assays with different sequence
| Variable | Single positive results ODI ≥ 0.5 | Two consecutive positive results ODI ≥ 0.5 | Three consecutive positive results ODI ≥ 0.5 |
|---|---|---|---|
| Proven+propable, positive/negative | 15/0 | 13/2 | 11/4 |
| Non-IPA, positive/negative | 62/23 | 24/61 | 12/73 |
| Sensitivity, % | 100 | 86.7 | 73.3 |
| Specificity, % | 27.1 | 71.8 | 85.9 |
| Positive predictive value, % | 19.5 | 35.1 | 47.8 |
| Negative predictive value, % | 100 | 96.8 | 94.8 |
| Accuracy, % | 38 | 74 | 84 |
ODI, optical density index; IPA, invasive pulmonary aspergillosis.
DISCUSSION
IPA diagnosis still constitutes a significant issue for immunosuppressed patients today. Though tissue sample collection for histopathologic investigation purposes is a golden standard for the diagnosis, it is often not possible due to deep cytopenia. It is often too late to have successful outcomes even if a treatment is initiated as soon as a specific image is obtained radiologically. On the other hand, an antifungal therapy is initiated empirically on each patient suspected to have IPA and the unnecessary treatments lead to increased costs. Therefore, it is highly important to consecutively monitor GM antigen tests via serological methods. Many studies around the world determined that identifying GM antigen serially in high-risk neutropenia patients helps diagnosing IPA. This has shown that identification may sometimes be possible before clinical symptoms or the radiologic markers are apparent. Sensitivity-specificity varies between 30% to 100% in these studies [7,16,18-21].
The sensitivity in this study was 100% for single positive result, 86.7% for two consecutive positive results, and 73.3% for three consecutive positive results. This decline in sensitivity may be due to “false negative” results. Such conditions have been reported for proven cases in other studies [22]. It has been associated with limited angioinvasion, high antibody titer, and very low amounts of GM secretion from the fungus. Additionally, in proven animal models, prophylactic or preemptive use of amphotericin B has been shown to reduce mycelial growth and suppress GM secretion [23]. Another reason for this decline in the sensitivity may be insufficient frequency of sampling. More frequent sampling may increase sensitivity.
NPV was high (100%) for single positive GM assay. However, NPV for two consecutive and three consecutive positive GM assays were 96.8% and 94.8% (still high enough to rule out the diagnosis), respectively, despite of decreasing sensitivity (Table 3). This is because IPA has a low incidence of 5% to 15% [24]. Thus increasing the number of consecutive positivity causes a decrease in sensitivity but not the same proportional decrease in NPV.
Considering that more tolerable antifungal agents are available today, the high mortality rate among diagnosed patients is more crucial than handling the relatively lower number of false positives. The increasing antigenemia is more important than a single positive result after the initiation of antifungal treatment.
Specificity was 27.1% for single positivity, 71.8% for two consecutive positivity, and 85.9% for consecutive three positivity results. This increase in specificity, provided via consecutive monitoring of GM levels, can ensure quitting the empiric antifungal treatment, playing a crucial role in reducing the side effects and cost of the medication.
Antifungal therapy was administered to 60 of the 65 possible IPA patients and 36 of the 85 non-IPA patients in this study. There were 55 cases who had single positivity, 36 two consecutive positive results, and 20 three consecutive positive results in the possible IPA patients group. Consecutive GM positivity is more useful for managing the continuation or the discontinuation of the antifungal therapy than for early diagnosis because the length of antifungal therapy with empirical or treatment intents is still an issue.
Patients, who had fever despite intravenous antibiotics for more than 5 days and also who were suspicious for IPA (proven, probable, or possible for IPA) have been added antifungal therapy (n = 75). GM tests were performed for all patients before and after antifungal therapy. Fig. 1 (time chart) has shown the days of single, two consecutive and three consecutive positive results of GM in “proven” patients (the time of initiation of antifungal therapy has been termed as zeroth day). Cases 1, 2, and 4 have three consecutive positive results before starting of antifungal therapy. Case 1 died 6 days after the beginning of antifungal therapy and his two GM tests—performed after the onset of antifungal therapy—were higher than 0,5. Case 2 did not die and his GM tests results remained higher than 0.5 for 7 days (next 7 days test results were below 0.5). Case 3 died 13 days after the beginning of antifungal therapy and his all GM tests—performed after the onset of antifungal therapy—were higher than 0.5 (the last test result was 19.7). Case 4 died 3 days after the beginning of antifungal therapy and GM test could not be applied to her after the onset of antifungal therapy.
Figure 1.
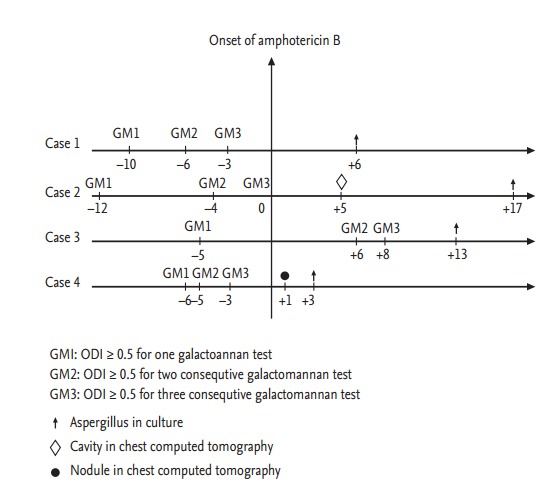
The days of single, two consecutive and three consecutive positive results of galactomannan (GM) in “proven” patients. ODI, optical density index.
We observed no false negatives in the proven IPA group but when this group was combined with the probable IPA group, false negative rate has become 13%. This rate was higher than in the autopsy-controlled study (4.5%) conducted by Machetti et al. [18]. However, this may be due to the fact that we could not cross-check the diagnosis of the probable IPA patients in our study via autopsy. Even if types of aspergillosis are developed in all of the probable IPA patients, lack of a histological diagnosis will prevent the ability to differentiate between invasive disease and colonization. Serum GM antigen test will result negative in case of colonization.
KEY MESSAGE
1. Because use of culturing and imaging methods in specific invasive pulmonary aspergillosis diagnosis takes time, it is often too late for the treatment when a diagnosis is reached. The current recommendation for diagnosis is the twice a week serum galactomannan (GM) test.
2. In our study, based on the twice a week controls, the sensitivity was observed to decline in three consecutive positive results cases compared to two consecutive positive results while specificity increased.
3. More frequent GM monitoring than twice a week controls starting from the first positivity may ensure discontinuation of unnecessary therapies and reduce the side effects and increased costs associated with medication.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Dykewicz CA, Centers for Disease Control and Prevention (U.S.) Infectious Diseases Society of America. American Society of Blood and Marrow Transplantation Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin Infect Dis. 2001;33:139–144. doi: 10.1086/321805. [DOI] [PubMed] [Google Scholar]
- 2.Verweij PE, Stynen D, Rijs AJ, de Pauw BE, Hoogkamp-Korstanje JA, Meis JF. Sandwich enzyme-linked immunosorbent assay compared with Pastorex latex agglutination test for diagnosing invasive aspergillosis in immunocompromised patients. J Clin Microbiol. 1995;33:1912–1914. doi: 10.1128/jcm.33.7.1912-1914.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pagano L, Caira M, Candoni A, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. 2006;91:1068–1075. [PubMed] [Google Scholar]
- 4.Ozcelik T, Ozkalemkas F, Kocaeli H, et al. Successful treatment of neuroaspergillosis in a patient with acute lymphoblastic leukemia: role of surgery, systemic antifungal therapy and intracavitary therapy. Mikrobiyol Bul. 2009;43:499–506. [PubMed] [Google Scholar]
- 5.Lehrnbecher T, Frank C, Engels K, Kriener S, Groll AH, Schwabe D. Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J Infect. 2010;61:259–265. doi: 10.1016/j.jinf.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Arikan S. Clinical microbiology and infectious diseases. Management of fungal infections. IDrugs. 2001;4:746–749. [PubMed] [Google Scholar]
- 7.Mennink-Kersten MA, Donnelly JP, Verweij PE. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. Lancet Infect Dis. 2004;4:349–357. doi: 10.1016/S1473-3099(04)01045-X. [DOI] [PubMed] [Google Scholar]
- 8.Stynen D, Sarfati J, Goris A, et al. Rat monoclonal antibodies against Aspergillus galactomannan. Infect Immun. 1992;60:2237–2245. doi: 10.1128/iai.60.6.2237-2245.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stynen D, Goris A, Sarfati J, Latge JP. A new sensitive sandwich enzyme-linked immunosorbent assay to detect galactofuran in patients with invasive aspergillosis. J Clin Microbiol. 1995;33:497–500. doi: 10.1128/jcm.33.2.497-500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yucesoy M, Ergon MC. Investigation of Aspergillus galactomannan levels in antimicrobial agents. Mikrobiyol Bul. 2007;41:565–570. [PubMed] [Google Scholar]
- 11.Agca H, Ener B, Yilmaz E, et al. Comparative evaluation of galactomannan optical density indices and culture results in bronchoscopic specimens obtained from neutropenic and non-neutropenic patients. Mycoses. 2014;57:169–175. doi: 10.1111/myc.12126. [DOI] [PubMed] [Google Scholar]
- 12.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maertens J, Buve K, Theunissen K, et al. Galactomannan serves as a surrogate endpoint for outcome of pulmonary invasive aspergillosis in neutropenic hematology patients. Cancer. 2009;115:355–362. doi: 10.1002/cncr.24022. [DOI] [PubMed] [Google Scholar]
- 14.Marr KA. Aspergillus galactomannan index: a surrogate end point to assess outcome of therapy? Clin Infect Dis. 2008;46:1423–1425. doi: 10.1086/528715. [DOI] [PubMed] [Google Scholar]
- 15.Miceli MH, Grazziutti ML, Woods G, et al. Strong correlation between serum Aspergillus galactomannan index and outcome of aspergillosis in patients with hematological cancer: clinical and research implications. Clin Infect Dis. 2008;46:1412–1422. doi: 10.1086/528714. [DOI] [PubMed] [Google Scholar]
- 16.Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis. 2006;42:1417–1427. doi: 10.1086/503427. [DOI] [PubMed] [Google Scholar]
- 17.Ilstrup DM. Statistical methods in microbiology. Clin Microbiol Rev. 1990;3:219–226. doi: 10.1128/cmr.3.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Machetti M, Feasi M, Mordini N, et al. Comparison of an enzyme immunoassay and a latex agglutination system for the diagnosis of invasive aspergillosis in bone marrow transplant recipients. Bone Marrow Transplant. 1998;21:917–921. doi: 10.1038/sj.bmt.1701206. [DOI] [PubMed] [Google Scholar]
- 19.Maertens J, Verhaegen J, Demuynck H, et al. Autopsy-controlled prospective evaluation of serial screening for circulating galactomannan by a sandwich enzyme-linked immunosorbent assay for hematological patients at risk for invasive Aspergillosis. J Clin Microbiol. 1999;37:3223–3228. doi: 10.1128/jcm.37.10.3223-3228.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maertens J, Verhaegen J, Lagrou K, Van Eldere J, Boogaerts M. Screening for circulating galactomannan as a noninvasive diagnostic tool for invasive aspergillosis in prolonged neutropenic patients and stem cell transplantation recipients: a prospective validation. Blood. 2001;97:1604–1610. doi: 10.1182/blood.v97.6.1604. [DOI] [PubMed] [Google Scholar]
- 21.Maertens J, Van Eldere J, Verhaegen J, Verbeken E, Verschakelen J, Boogaerts M. Use of circulating galactomannan screening for early diagnosis of invasive aspergillosis in allogeneic stem cell transplant recipients. J Infect Dis. 2002;186:1297–1306. doi: 10.1086/343804. [DOI] [PubMed] [Google Scholar]
- 22.Tanriover MD, Ascioglu S, Altun B, Uzun O. Galactomannan on the stage: prospective evaluation of the applicability in routine practice and surveillance. Mycoses. 2010;53:16–25. doi: 10.1111/j.1439-0507.2008.01652.x. [DOI] [PubMed] [Google Scholar]
- 23.Rohrlich P, Sarfati J, Mariani P, et al. Prospective sandwich enzyme-linked immunosorbent assay for serum galactomannan: early predictive value and clinical use in invasive aspergillosis. Pediatr Infect Dis J. 1996;15:232–237. doi: 10.1097/00006454-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood. 2002;100:4358–4366. doi: 10.1182/blood-2002-05-1496. [DOI] [PubMed] [Google Scholar]


