Abstract
Background/Aims:
Gout is a common inf lammatory arthritis triggered by the crystallization of uric acid in the joints. Serum uric acid levels are highly heritable, suggesting a strong genetic component. Independent studies to confirm the genetic associations with gout in various ethnic populations are warranted. We investigated the association of polymorphisms in the ABCG2 and SLC2A9 genes with gout in Korean patients and healthy individuals.
Methods:
We consecutively enrolled 109 patients with gout and 102 healthy controls. The diagnosis of gout was based on the preliminary criteria of the America College of Rheumatology. Genomic DNA was extracted from whole blood samples. We identified single nucleotide polymorphism (SNP) changes in the ABCG2 and SLC2A9 genes using a direct sequencing technique. rs2231142 in ABCG2 and rs6449213 and rs16890979 in SLC2A9 and nearby regions were amplified by polymerase chain reaction.
Results:
Patients with gout had significantly higher A/A genotype (29.3% vs. 4.9%, respectively) and A allele (52.8% vs. 26.5%, respectively) frequencies of rs2231142 in ABCG2 than did controls (χ2 = 29.42, p < 0.001; odds ratio, 3.32; 95% confidence interval, 2.11 to 5.20). We found novel polymorphisms (c.881A>G and c.1002+78G>A) in the SLC2A9 gene. The univariate logistic regression analysis revealed that the c.881A>G and c.1002+78G>A SNPs were significantly higher in patients than in controls.
Conclusions:
We demonstrated a significant association between rs2231142 in the ABCG2 gene and gout and identified novel SNPs, c.881A>G and c.1002+78G>A, in the SLC2A9 gene that may be associated with gout in a Korean population.
Keywords: Gout, ABCG2, SLC2A9
INTRODUCTION
Gout is a common form of inflammatory arthritis triggered by the crystallization of uric acid within the joints [1]. The condition is characterized by painful joint inflammation, destructive tophi and can result in joint destruction and disability if untreated. A strong genetic predisposition to gout has been recognized for centuries; however, our understanding of the roles of specific genes was limited to families with uncommon mutations. These rare familial forms of gout and hyperuricemia include mutational defects in purine metabolic pathway constituents (e.g., X-linked superactivity of PRPS1 and deficiency of hypoxanthine-guanine phosphoribosyl transferase [HGPRT]) and those associated with hereditary renal disorders (e.g., autosomal dominant familial juvenile hyperuricemic nephropathy, medullary cystic kidney disease type 1 and 2) [2]. Recent genome-wide association studies (GWAS) have rapidly advanced understanding of the genetics underlying hyperuricemia and gout [3]. Recent GWAS have identified substantial associations between the single nucleotide polymorphisms (SNPs), rs2231142 in the ABCG2 gene and rs6449213 and rs16890979 in the SLC2A9 gene and uric acid concentration and gout in various ethnic groups [4,5]. We assessed the genetic associations of these SNPs and nearby regions with gout in a Korean population.
METHODS
Patient selection
A total of 109 patients with gout and 102 gout-free control subjects were recruited from the Chosun University Hospital and Daegu Catholic University Medical Center. The normal control subjects had selected by self-reported history of no arthritis, diabetes, and hypertension. All participants were interviewed using a structured questionnaire to obtain their personal history and demographic characteristics (age, sex, height, weight, and body mass index [BMI]). The gout diagnosis was made according to the 1977 preliminary criteria for the classification of gout published by the American College of Rheumatology for use in clinical settings and population-based epidemiological studies [6] and confirmed by experienced rheumatologists. The both ethics committees of the Institutional Review Board approved the study protocol and all participants provided written informed consent prior to participation in the study. The study was conducted in accordance with the principles of the current version of the Declaration of Helsinki.
Identification of single nucleotide polymorphisms
Serum was separated from peripheral venous blood samples obtained from each participant and stored at –70°C for the clinical chemistry assays. Polymerase chain reaction (PCR)-direct sequencing was performed to detect SNPs. rs2231142, rs6449213, rs16890979 and nearby regions were amplified by PCR, and the products were sequenced using the ABI 3730XL DNA sequencer (Applied Biosystems, Foster City, CA, USA) for mutational analysis. Genotype and allele frequencies were compared in patient and control samples.
Allele frequency was defined as the percentage of the individuals carrying the allele among the total number of the individuals. The SNP nomenclature used in this study was based on the Human Genome Variation Society recommendations and the National Center for Biotechnology Information SNP database.
Haplotype analysis
Linkage disequilibrium (LD) was measured using Lewontin’s D’ (|D’|) and r2 [7,8]. The haplotype of each individual was inferred using the expectation maximization (EM) algorithm (PHASE v 2.0) developed by Stephens et al. [9].
Statistical analysis
Statistical analyses were performed using the SPSS version 17 (SPSS Inc., Chicago, IL, USA). The data are expressed as mean ± standard deviation (SD). The chisquare test, t test, and multivariate logistic regression analysis were used for between-group comparisons. The associations of five polymorphisms and haplotypes (rs2231142 in ABCG2, and rs16890979, c.813-18A>G, c.881A>G, and c.1002+78G>A in SLC2A9) with the risk of gouty arthritis were assessed using logistic regression models with age (continuous value), sex, and BMI (continuous value) as covariates. The chi-square test was used to estimate the Hardy-Weinberg equilibrium. All statistical tests were conducted using SAS version 9.1 (SAS Institute, Cary, NC, USA) and SPSS version 17 was used to calculate the LD. In all analyses, p values < 0.05 were deemed to indicate statistical significance.
RESULTS
Participant demographic and clinical characteristics
The majority of the study participants (98.8%) were male. Mean uric acid levels were not significantly different between the patients with gout (5.8 ± 1.9 mg/dL) and controls (6.1 ± 1.2 mg/dL), possibly because most of the patients were likely to have received a uric acid-lowering agent such as allopurinol, febuxostat, or benzbromarone. Furthermore, we found no significant differences in age, height, weight, and BMI between the patient and control groups (Table 1).
Table 1.
Demographic and clinical characteristics of the study population
| Characteristic | Patients with gout (n = 109) | Control subjects (n = 102) | p value |
|---|---|---|---|
| Age, yr | 55.0 ± 12.1 | 51.8 ± 6.7 | 0.018a |
| Height, cm | 168.3 ± 6.4 | 169.4 ± 5.4 | 0.18 |
| Weight, kg | 69.9 ± 8.6 | 69.1 ± 8.7 | 0.54 |
| Body mass index, kg/m2 | 24.6 ± 2.7 | 24.0 ± 2.7 | 0.12 |
| Serum uric acid, mg/dL | 5.8 ± 1.9 | 6.1 ± 1.2 | 0.17 |
Values are presented as mean ± SD.
p < 0.05.
Identification of single nucleotide polymorphisms
Table 2 shows nine SNPs, three of which (rs2231142, rs6449213, and rs16890979) are associated with serum urate levels in patients with gout. rs2231142, also known as Q141K and C421A, is an SNP in the ABCG2 gene and, thus, a missense variant. The A allele of rs2231142 is associated with an increased risk of gout. The percentage of the A/A genotype (29.3% vs. 4.9%, respectively) and A allele (52.8% vs. 26.5%, respectively) frequency in the rs2231142 (c.421C>A) SNP were significantly higher in patients with gout than in control subjects and was significantly associated with gout (χ2 = 29.42, p < 0.001; odds ratio [OR], 3.32; 95% confidence interval [CI], 2.11 to 5.20) (Table 3). These findings are similar to those reported previously. Conversely, the genotype distributions of rs6449213 and rs16890979 did not differ significantly between patients and control subjects. rs6449213 is a surrogate for rs7442295 which is in a fairly tight linkage (r2 = 0.88) and, thus, associated with high serum urate levels. We found a high distribution of the A/A genotype in the rs6449213 SNPs of both patients and controls (98.2% vs. 99.0%, respectively). We identified the A/G genotype in two patients with gout and in one control subject (Table 4). Several previous independent studies have shown an association between rs16890979 and gout. rs16890979 may be a variation in the SLC2A9 gene, which is more commonly known as GLUT9. Furthermore, the distribution of the A/A genotype was high in the rs16890979 polymorphism in patients (100%) and controls (98.1%) (Table 5).
Table 2.
ABCG2 and SLC2A9 single nucleotide polymorphisms
| Gene | Polymorphism (dbSNP no) | Polymorphism (HGVS nomenclature) | Genotype frequency in patients with gout (%) | Odds ratio |
|---|---|---|---|---|
| ABCG2 | rs2231142 | c.421C>A | AC (46.7) | 3.32a |
| ABCG2 | ∙ | c.871+386A>G | AA (95.4) | |
| SLC2A9 | rs6449213 | c.410+4190A>G | AA (98.2) | |
| SLC2A9 | ∙ | c.410+4124C>T | CC (98.2) | |
| SLC2A9 | ∙ | c.410+4242G>A | GG (99.1) | |
| SLC2A9 | rs16890979 | c.844A>G | AA (100) | |
| SLC2A9 | ∙ | c.881A>G | AA (47.7) | 1.54a |
| SLC2A9 | ∙ | c.1002 + 78G>A | GG (55.9) | 1.64a |
| SLC2A9 | ∙ | c.813-18A>G | AA (99.1) |
dbSNP, single nucleotide polymorphism database; HGVS, Human Genome Variation Society.
p < 0.05.
Table 3.
Genotype distribution and relative allele frequencies of the c.421C>A (rs2231142) polymorphism of ABCG2
| Group | No. | Genotype frequency, n (%) |
Allele frequency, n (%) |
|||
|---|---|---|---|---|---|---|
| C/C | C/A | A/A | C | A | ||
| Patient | 109 | 26 (23.8) | 51 (46.7) | 32 (29.3) | 103 (47.2) | 115 (52.8) |
| Control | 102 | 53 (51.9) | 44 (43.1) | 5 (4.9) | 150 (73.5) | 54 (26.5) |
| χ2 = 29.42, df = 2, p < 0.001a OR, 3.32; 95% CI, 2.11–5.20 | χ2 = 30.32, df =1, p < 0.001a OR, 3.10; 95% CI, 2.04–4.76 | |||||
OR, odds ratio; CI, confidence interval.
p < 0.05.
Table 4.
Genotype distribution and relative allele frequencies of the c.410+4190A>G (rs6449213) polymorphism in the SLC2A9 gene
| Group | No. | Genotype frequency, n (%) |
Allele frequency, n (%) |
|||
|---|---|---|---|---|---|---|
| A/A | A/G | G/G | A | G | ||
| Patient | 109 | 107 (98.2) | 2 (1.8) | 0 | 216 (99.0) | 2 (1.0) |
| Control | 102 | 101 (99.0) | 1 (1.0) | 0 | 203 (99.5) | 1 (0.5) |
| p > 0.05 | p > 0.05 | |||||
No significant difference in genotype distribution was found between patients with gout and control subjects.
Table 5.
Genotype distribution and relative allele frequencies of the c.844A>G (rs16890979) polymorphism in the SLC2A9 gene
| Group | No. | Genotype frequency, n (%) |
Allele frequency, n (%) |
|||
|---|---|---|---|---|---|---|
| A/A | A/G | G/G | A | G | ||
| Patient | 109 | 109 (100) | 0 | 0 | 218 (100) | 0 |
| Control | 102 | 100 (98.1) | 2 (1.9) | 0 | 202 (99.0) | 2 (1.0) |
| p = 0.232 | p = 0.232 | |||||
No significant difference in genotype was found between the gout and control groups.
We identified novel polymorphisms in regions near rs16890979. The genotype frequencies of the c.881A>G and c.1002+78G>A SNPs in the SLC2A9 gene were signifcantly different between patients with gout and controls. The c.881A>G variant in the patient group had significantly higher frequencies of the G/G genotype (20.1% vs. 5.8%, respectively) and G allele (36.2% vs. 25.0%, respectively) than did the control group (χ2 = 9.36, p < 0.001; OR, 1.54; 95% CI, 1.03 to 2.28) (Table 6). Moreover, the distribution of the A/A genotype (10.1% vs. 2.9%, respectively) and A allele frequency (27.1% vs. 17.2%, respectively) for c.1002+78G>A was significantly higher in the patient than in the control group (χ2 = 5.93, p = 0.05; OR, 1.64; 95% CI, 1.03 to 2.62) (Table 7). Conversely, the genotype frequencies of c.871+386A>G in the ABCG2 gene and c.410+4124C>T and c.410+4242G>A in the SCL2A9 gene were not significantly different between patients with gout and control subjects. c.813-18A>G, which is located near rs16890979 in SLC2A9, had an A/A genotype distribution of 99.1%. Notably, none of the minor alleles of the four SNPs were observed in the patients with gout; thus, the OR could not be calculated.
Table 6.
Genotype distribution and relative allele frequencies of the c.881A>G polymorphism in the SLC2A9 gene
| Group | No. | Genotype frequency, n (%) |
Allele frequency, n (%) |
|||
|---|---|---|---|---|---|---|
| A/A | A/G | G/G | A | G | ||
| Patient | 109 | 52 (4 7.7) | 35 (32.1) | 22 (20.1) | 139 (63.7) | 79 (36.2) |
| Control | 102 | 57 (55.8) | 39 (38.2) | 6 (5.8) | 153 (75.0) | 51 (25.0) |
| χ2 = 9.36, df = 2, p < 0.001a OR, 1.54; 95% CI, 1.03–2.28 | χ2 = 6.24, df =1, p = 0.015a OR, 1.70; 95% CI, 1.12–2.60 | |||||
OR, odds ratio; CI, confidence interval.
p < 0.05.
Table 7.
Genotype distribution and relative allele frequencies of the c.1002+78G>A polymorphism in the SLC2A9 gene
| Group | No. | Genotype frequency, n (%) |
Allele frequency, n (%) |
|||
|---|---|---|---|---|---|---|
| A/A | A/G | G/G | A | G | ||
| Patient | 109 | 11 (10.1) | 37 (33.9) | 61 (55.9) | 59 (27.1) | 159 (72.9) |
| Control | 102 | 3 (2.9) | 29 (28.4) | 70 (68.6) | 35 (17.2) | 169 (82.8) |
| χ2 = 5.93, df = 2, p = 0.011a OR, 1.64; 95% CI, 1.03–2.26 | χ2 = 5.96, df =1, p = 0.015a OR, 1.78; 95% CI, 1.11–2.86 | |||||
OR, odds ratio; CI, confidence interval.
p < 0.05.
Haplotype association analysis
Of the four SNPs in the SLC2A9 gene, c.881A>G and c.1002+78G>A showed tight linkage (|D’| = 1, r2 = 0.672), whereas the linkage between rs16890979 and c.813-18A>G was low due to their extremely low frequencies (Fig. 1); thus, c.881A>G and c.1002+78G>A were used for haplotype construction. Haplotypes of SLC2A9 were constructed using PHASE (v 2.1), and three haplotypes were used for further statistical analysis. We observed no linkage with rs2231142 in ABCG2.
Figure 1.
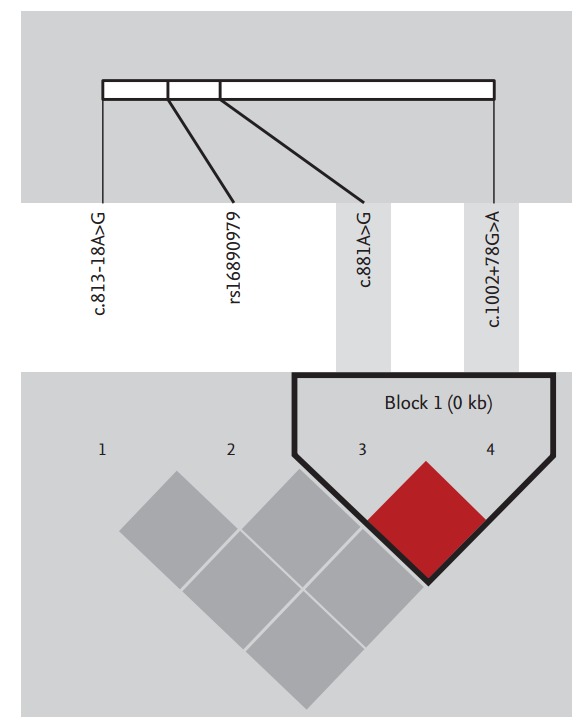
Among the four single nucleotide polymorphisms in the SLC2A9 gene, c.881A>G and c.1002+78G>A showed a tight linkage (|D’| = 1, r2 = 0.672).
In summary, we found a strong association of rs2231142 in the ABCG2 gene with gouty arthritis. Moreover, we identified a novel association between the c.881A>G and c.1002+78G>A polymorphisms in the SLC2A9 gene and gout. Thus, we compared the association of genotype and allele frequencies of three SNPs (rs2231142 in ABCG2, c.881A>G, and c.1002+78G>A in SLC2A9) in Korean patients with gout and healthy control subjects.
DISCUSSION
Gout is a common inflammatory joint disease that may lead to joint destruction and disability if untreated [10]. Estimates indicate that up to 2% of males over the age of 30 years and females over the age of 50 years will develop gout at some time [1]. Hyperuricemia is associated with a variety of adverse health risks including gout. Emerging evidence suggests that gout is strongly associated with metabolic syndrome and may lead to cardiovascular disease, diabetes, and premature death [11-14].
GWAS have emerged as a comprehensive and powerful approach for identifying genetic variants related to complex diseases. Since 2007, several systematic, well-powered, GWAS have been performed worldwide to explore the relationships between common sequence variations and disease predisposition [15]. These genetic data have considerably advanced our understanding of the pathogenesis of hyperuricemia and gout. Several recent GWAS have identified genes that may be causally associated with uric acid levels, including SLC2A9/GLUT9, ABCG2 (BCRP), SLC13A3, SLC17A1 (NPT1), SLC17A3 (NPT4), SLC17A4 (named as NPT5 provisionally), SLC22A11 (OAT4), SLC22A12 (URAT1), and SLC16A9 (MCT9) as well as the urate transport-related scaffolding protein PDXK1 [16-23]. Given the urate transporter activity of SLC2A9 and ABCG2 and the strong associations between their genetic variants and serum uric acid levels, these genes appear to be important modulators of uric acid levels and potential determinants of the risk of developing gout [17,19]. Several recent studies have found that the rs6449213, rs16890979, rs7442295, rs6855911, and rs734553 SNPs in the SLC2A9 gene are highly associated with gout [17,24-26]. Furthermore, rs2231142, the missense SNP in ABCG2 (Q141K) has been associated with hyperuricemia and gout in Caucasian, African-American, Japanese, and Han Chinese samples [27-29]. The investigation of the ABCG2 variant (rs2231142) in Asian populations such as Han Chinese and Japanese [29-31] was particularly noteworthy.
We conducted a replication study of the associations of the rs6449213 and rs16890979 SNPs in SLC2A9 and rs2231142 in ABCG2 with gout. Our findings for the rs2231142 variant are similar to those reported previously. However, in contrast to previous studies, we found no differences in the distribution of the rs6449213 and rs16890979 genetic variants between the gout and control groups and no association with gout. This disparity may be explained by differences in the genetic variant among the various ethnic groups. In the search for novel SNPs, we compared allele and genotype distributions in regions near the target SNPs in the ABCG2 and SLC2A9 genes in the 109 Korean patients with gout and 102 gout-free controls. The genotype frequency of the c.881A>G and c.1002+78G>A variants differed significantly between gout and control groups. Moreover, c.881A>G and c.1002+78G>A showed a high LD in the haplotype analysis.
Our study had several limitations. First, the sample size was not sufficient to detect a potential correlation between the genetic associations of the SNPs and gout in a Korean population. Second, because only 2% of our participants were female, we were unable to perform a statistical analysis according to sex. Thus, the generalizability of our findings is limited to male patients with gout. Third, uric acid levels were not significantly different between the patient and control groups, possibly because the majority of patient with gout would have received a uric acid-lowering agent such as allopurinol, febuxostat, or benzbromarone. Despite these limitations, our study is the first to show an association between gout and SNPs (rs2231142 in ABCG2, and rs6449213 and rs16890979 in SLC2A9) among Koreans. Although previous studies have investigated ABCG2 and SLC2A9 variants in various ethnic populations, our study is the first to investigate the presence and effects of these SNPs in a Korean population.
In conclusion, rs2231142 in the ABCG2 gene and c.881A>G, c.1002+78G>A in the SLC2A9 gene are potential candidate variants underlying the pathogenesis of gout in Korean individuals. In-depth investigations of these SNPs in functional experiments are warranted to elucidate the precise mechanisms underlying the pathogenesis of gout.
KEY MESSAGE
1. Our study in a Korean population demonstrated a strong association of rs2231142 in the ABCG2 gene with gout. Furthermore, we identified novel single nucleotide polymorphisms, c.881A>G and c.1002+78G>A, in the SLC2A9 gene that are associated with gout.
Acknowledgments
This study is supported by fund of the Soonchunhyang University.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Choi HK, Mount DB, American College of Physicians. American Physiological Society Pathogenesis of gout. Ann Intern Med. 2005;143:499–516. doi: 10.7326/0003-4819-143-7-200510040-00009. [DOI] [PubMed] [Google Scholar]
- 2.Zaka R, Williams CJ. New developments in the epidemiology and genetics of gout. Curr Rheumatol Rep. 2006;8:215–223. doi: 10.1007/s11926-996-0028-0. [DOI] [PubMed] [Google Scholar]
- 3.Choi HK, Zhu Y, Mount DB. Genetics of gout. Curr Opin Rheumatol. 2010;22:144–151. doi: 10.1097/BOR.0b013e32833645e8. [DOI] [PubMed] [Google Scholar]
- 4.Wang B, Miao Z, Liu S, et al. Genetic analysis of ABCG2 gene C421A polymorphism with gout disease in Chinese Han male population. Hum Genet. 2010;127:245–246. doi: 10.1007/s00439-009-0760-4. [DOI] [PubMed] [Google Scholar]
- 5.Dehghan A, Kottgen A, Yang Q, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet. 2008;372:1953–1961. doi: 10.1016/S0140-6736(08)61343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]
- 7.Hedrick PW. Gametic disequilibrium measures: proceed with caution. Genetics. 1987;117:331–341. doi: 10.1093/genetics/117.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan N, Weir BS. Expected behavior of conditional linkage disequilibrium. Am J Hum Genet. 1992;51:333–343. [PMC free article] [PubMed] [Google Scholar]
- 9.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JW, Ko DJ, Yoo JJ, et al. Clinical factors and treatment outcomes associated with failure in the detection of urate crystal in patients with acute gouty arthritis. Korean J Intern Med. 2014;29:361–369. doi: 10.3904/kjim.2014.29.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007;57:109–115. doi: 10.1002/art.22466. [DOI] [PubMed] [Google Scholar]
- 12.Krishnan E, Baker JF, Furst DE, Schumacher HR. Gout and the risk of acute myocardial infarction. Arthritis Rheum. 2006;54:2688–2696. doi: 10.1002/art.22014. [DOI] [PubMed] [Google Scholar]
- 13.Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation. 2007;116:894–900. doi: 10.1161/CIRCULATIONAHA.107.703389. [DOI] [PubMed] [Google Scholar]
- 14.Choi HK, De Vera MA, Krishnan E. Gout and the risk of type 2 diabetes among men with a high cardiovascular risk profile. Rheumatology (Oxford) 2008;47:1567–1570. doi: 10.1093/rheumatology/ken305. [DOI] [PubMed] [Google Scholar]
- 15.McCarthy MI, Abecasis GR, Cardon LR, et al. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat Rev Genet. 2008;9:356–369. doi: 10.1038/nrg2344. [DOI] [PubMed] [Google Scholar]
- 16.van der Harst P, Bakker SJ, de Boer RA, et al. Replication of the five novel loci for uric acid concentrations and potential mediating mechanisms. Hum Mol Genet. 2010;19:387–395. doi: 10.1093/hmg/ddp489. [DOI] [PubMed] [Google Scholar]
- 17.Vitart V, Rudan I, Hayward C, et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet. 2008;40:437–442. doi: 10.1038/ng.106. [DOI] [PubMed] [Google Scholar]
- 18.Doring A, Gieger C, Mehta D, et al. SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat Genet. 2008;40:430–436. doi: 10.1038/ng.107. [DOI] [PubMed] [Google Scholar]
- 19.Woodward OM, Kottgen A, Coresh J, Boerwinkle E, Guggino WB, Kottgen M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci U S A. 2009;106:10338–10342. doi: 10.1073/pnas.0901249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shima Y, Teruya K, Ohta H. Association between intronic SNP in urate-anion exchanger gene, SLC22A12, and serum uric acid levels in Japanese. Life Sci. 2006;79:2234–2237. doi: 10.1016/j.lfs.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 21.Charles BA, Shriner D, Doumatey A, et al. A genome-wide association study of serum uric acid in African Americans. BMC Med Genomics. 2011;4:17. doi: 10.1186/1755-8794-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuo H, Chiba T, Nagamori S, et al. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am J Hum Genet. 2008;83:744–751. doi: 10.1016/j.ajhg.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anzai N, Jutabha P, Amonpatumrat-Takahashi S, Sakurai H. Recent advances in renal urate transport: characterization of candidate transporters indicated by genome-wide association studies. Clin Exp Nephrol. 2012;16:89–95. doi: 10.1007/s10157-011-0532-z. [DOI] [PubMed] [Google Scholar]
- 24.Preitner F, Bonny O, Laverriere A, et al. Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy. Proc Natl Acad Sci U S A. 2009;106:15501–15506. doi: 10.1073/pnas.0904411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuo H, Yamamoto K, Nakaoka H, et al. Genome-wide association study of clinically defined gout identifies multiple risk loci and its association with clinical subtypes. Ann Rheum Dis. 2015 Feb 2; doi: 10.1136/annrheumdis-2014-206191. [Epub]. http://dx.doi.org/10.1136/annrheumdis-2014-206191. [DOI] [PMC free article] [PubMed]
- 26.Hu M, Tomlinson B. Gender-dependent associations of uric acid levels with a polymorphism in SLC2A9 in Han Chinese patients. Scand J Rheumatol. 2012;41:161–163. doi: 10.3109/03009742.2011.637952. [DOI] [PubMed] [Google Scholar]
- 27.Dong Z, Guo S, Yang Y, et al. Association between ABCG2 Q141K polymorphism and gout risk affected by ethnicity and gender: a systematic review and meta-analysis. Int J Rheum Dis. 2015;18:382–391. doi: 10.1111/1756-185X.12519. [DOI] [PubMed] [Google Scholar]
- 28.Matsuo H, Takada T, Nakayama A, et al. ABCG2 dysfunction increases the risk of renal overload hyperuricemia. Nucleosides Nucleotides Nucleic Acids. 2014;33:266–274. doi: 10.1080/15257770.2013.866679. [DOI] [PubMed] [Google Scholar]
- 29.Lv X, Zhang Y, Zeng F, et al. The association between the polymorphism rs2231142 in the ABCG2 gene and gout risk: a meta-analysis. Clin Rheumatol. 2014;33:1801–1805. doi: 10.1007/s10067-014-2635-x. [DOI] [PubMed] [Google Scholar]
- 30.Matsuo H, Takada T, Ichida K, et al. Common defects of ABCG2, a high-capacity urate exporter, cause gout: a function-based genetic analysis in a Japanese population. Sci Transl Med. 2009;1:5ra11. doi: 10.1126/scitranslmed.3000237. [DOI] [PubMed] [Google Scholar]
- 31.Yamagishi K, Tanigawa T, Kitamura A, et al. The rs2231142 variant of the ABCG2 gene is associated with uric acid levels and gout among Japanese people. Rheumatology (Oxford) 2010;49:1461–1465. doi: 10.1093/rheumatology/keq096. [DOI] [PubMed] [Google Scholar]


