Abstract
The liquid chromatography–tandem mass spectrometric assay method for the simultaneous determination of rosuvastatin and amlodipine in human plasma using deuterated analogs as internal standards has been developed and validated. The analytes were extracted from 100 μL aliquots of human plasma via liquid–liquid extraction using a mixture of ethyl acetate and n-hexane (80:20, v/v) as an extraction solvent. The optimized mobile phase was composed of 0.1% formic acid in 5 mM ammonium acetate, methanol, and acetonitrile (20:20:60, v/v/v) and delivered at a flow rate of 0.75 mL/min. The calibration curve obtained was linear (R2 ⩾ 0.999) over the concentration range of 0.52–51.77 ng/mL for rosuvastatin and 0.10–10.07 ng/mL for amlodipine. A sample turnover rate of less than 2.5 min makes it an attractive procedure in high-throughput bioanalysis of rosuvastatin and amlodipine. The present method was found to be applicable to clinical studies and the results were authenticated by incurred sample reanalysis.
Keywords: Rosuvastatin, Amlodipine, Human plasma, LC/MS/MS, Method validation, Pharmacokinetics
Introduction
Hypertension and hyperlipidaemia are major risk factors for the development of atherosclerosis and its associated conditions such as coronary heart disease, ischemic cerebrovascular disease, and peripheral vascular disease. Calcium antagonists have been used for decades as antihypertensive agents. On the other hand, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) have been extensively used for the treatment of hyperlipidaemia because of their potent lipid lowering properties [1], [2], [3].
Rosuvastatin, a synthetic statin, was developed for the treatment of hyperlipidaemia [4], [5]. The dose dependent peak plasma concentration (Cmax) reached 3–5 h after oral administration of a 10- to 80-mg dose [6], [7], [8]. Amlodipine, a calcium antagonist, is prescribed for the treatment of hypertension and angina pectoris. It has a long elimination half-life and large volume of distribution. Low plasma concentrations (ng or pg) were reported after oral administration of amlodipine. The combination of rosuvastatin and amlodipine exerts more beneficial effects on cardiomyocyte hypertrophy and fibrosis [9], [10]. Compared with the co-administration of each drug, the convenience of a fixed dose combination (FDC) tablet has the potential outcome to improve patient adherence and the management of cardiovascular risk, thereby improving clinical outcomes.
Many liquid chromatography–tandem mass spectrometric (LC/MS/MS) methods have been reported for the determination of rosuvastatin [11], [12], [13], [14], [15], [16], [17], [18] individually or in combination with other drugs in biological samples. The major disadvantages of these methods include, less sensitivity [11], more sample volume (>0.25 mL) [11], [13], [14], [19], longer chromatographic run time (>4 min) [11], [12], [13], [14], [15], [16], [17], [18], complex with derivatization and expensive automated extraction procedure [13], [18], and narrow linearity range not suitable for bioequivalence/pharmacokinetic application in humans at higher dose (0.1–30 ng/mL) [13], [14]. Similarly, numerous LC/MS/MS methods are described in the literature to determine amlodipine in different biological fluids [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31]. Among the applied methods, either the chromatographic run time was long (>4 min) [19], [20], [22], [24], [25], [30], [31], the plasma volume was high (>0.25 mL) [19], [21], [22], [23], [24], [25], [30] or the method was insensitive for bioequivalence/pharmacokinetic application [20], [23], [25], [27], [30], [31].
Some methods [15], [21], [26], [27], [28], [29] which can be applied for quantitation of one drug in biological fluids selectively and sensitively, cannot be applied satisfactorily for simultaneous determination of rosuvastatin and amlodipine. To investigate the safety and tolerability of rosuvastatin and amlodipine fixed dose combination (FDCs) and/or for comparative bioavailability and bioequivalence studies of rosuvastatin associated with amlodipine, it is necessary to perform the quantitation of rosuvastatin and amlodipine simultaneously. An effective bio-analytical method should gratify in terms of sensitivity, efficient extraction process, rapid chromatography and specific. To our knowledge, no LC/MS/MS method has been reported for the simultaneous determination of amlodipine and rosuvastatin in human plasma. The present work describes a simple, selective and sensitive method, which employs liquid–liquid extraction (LLE) technique for sample preparation and liquid chromatography with electrospray ionization–tandem mass spectrometry for simultaneous quantitation of rosuvastatin and amlodipine in human plasma. The method uses isotope labeled compound rosuvastatin d6 and amlodipine d4 maleate as internal standards (IS) for the quantitation of rosuvastatin and amlodipine, respectively to avoid the potential matrix effect related problems and variability in recovery between analyte and IS. The suggested assay was applied to a clinical study in humans following oral administration of rosuvastatin and amlodipine. Furthermore, assay reproducibility is demonstrated by conducting incurred sample reanalysis (ISR).
Experimental
Reagents and chemicals
Reference standards of amlodipine besylate (purity 99.95%), amlodipine d4 maleate (IS1; purity 99.35%) and rosuvastatin d6 sodium salt (IS2; purity 99.87%) were purchased from Vivan Life Sciences Ltd. (Mumbai, India), while rosuvastatin calcium (purity 95.40%) was from Hetero Drugs Ltd. (Hyderabad, India). Water used for the LC/MS/MS analysis was prepared from Milli Q water purification system procured from Millipore (Bangalore, India). HPLC grade acetonitrile and methanol were purchased from J.T Baker (Phillipsburg, USA); while ethyl acetate and n-hexane were from Merck Ltd. (Mumbai, India). Analytical grade formic acid and ammonium acetate were also purchased from Merck (Mumbai, India). The control human plasma sample was procured from Deccan’s Pathological Labs (Hyderabad, India).
Preparation of stock and working solutions
Primary stock solutions (1 mg/mL) of rosuvastatin, amlodipine, IS1, and IS2 were prepared in methanol and these stocks were stored at 2–8 °C. Working solutions were prepared in a mixture of acetonitrile and water (50:50, v/v; diluent) for the purpose of plotting the calibration curve (CC) standards. Another set of working solutions were prepared in appropriate concentrations (using the same diluent) for quality control (QC) samples. A combined working solution for IS1 (500 ng/mL) and IS2 (50 ng/mL) was also prepared in diluent.
Preparation of calibration curve standards and quality control samples
Calibration samples were prepared by spiking 950 μL of control human plasma with the appropriate working standard solution of the each analyte (50 μL combined dilution of rosuvastatin and amlodipine). Calibration curve (CC) standards of analytes in blank plasma were prepared by spiking with an appropriate volume of the working solutions, giving final concentrations of 0.52, 1.04, 2.59, 5.19, 10.37, 20.75, 31.06, 41.41, and 51.77 ng/mL for rosuvastatin and 0.10, 0.20, 0.51, 1.01, 2.02, 4.04, 6.04, 8.06, and 10.07 ng/mL for amlodipine. The CC samples were analyzed along with the quality control (QC) samples for each batch of plasma samples. The QC samples were prepared at five different concentration levels of 0.52 (lower limit of quantification, LLOQ), 1.49 (low quality control, LQC), 6.19 (middle quality control, MQC-1), 25.78 (MQC-2) and 46.03 (high quality control, HQC) ng/mL for rosuvastatin and 0.10 (LLOQ), 0.29 (LQC), 1.20 (MQC-1), 5.02 (MQC-2) and 8.96 (HQC) ng/mL for amlodipine. All the prepared plasma samples were stored at −70 ± 10 °C.
Sample processing
All frozen subject samples, calibration standards and quality control samples were thawed and allowed to equilibrate at room temperature prior to analysis. The samples were vortexed for 10 s prior to spiking. A 100 μL aliquot of human plasma sample was mixed with 25 μL of the internal standard working solution (500 ng/mL of IS1 and 50 ng/mL of IS2). After vortexing for 15 s, a 4 mL of extraction solvent (ethyl acetate and n-hexane, 80:20, v/v) was added using Dispensette Organic (Brand GmbH, Wertheim, Germany). The sample was shaken for 10 min using a reciprocating shaker (Scigenics Biotech, Chennai, India) and then centrifuged for 5 min at 4000 rpm on Megafuse 3SR (Heraeus, Germany). The clear organic layer (3 mL) was transferred to a 5 mL glass test tube and evaporated at 45 °C under a gentle stream of nitrogen. The dried extract was reconstituted with 250 μL of the mobile phase and a 20 μL aliquot of it was injected into the LC/MS/MS system.
Chromatographic conditions
An HPLC system (Shimadzu, Kyoto, Japan) consisting of a Zorbax SB C18 column (50 × 4.6 mm, 3.5 μm; Agilent Technologies, Santa Clara, CA, USA), a binary LC-20AD prominence pump and an autosampler (SIL-HTc), and a solvent degasser (DGU-20A3) were used for the study. Aliquots of the processed samples (20 μL) were injected into the column, which was kept at ambient temperature (25 ± 5 °C). An isocratic mobile phase of a mixture of 0.1% formic acid in 5 mM ammonium acetate, methanol and acetonitrile (20:20:60, v/v/v) was delivered at a rate of 0.75 mL/min into the electrospray ionization chamber of the mass spectrometer.
Mass spectrometry conditions
Quantitation was achieved with MS/MS detection in positive ion mode for both the analytes and the internal standards using an AB Sciex API-4000 mass spectrometer (Foster City, CA, USA) equipped with a Turboionspray™ interface at 550 °C. The ion spray voltage was set at 5500 V. The source parameters viz. the nebulizer gas (GS1), auxiliary gas (GS2), curtain gas (CUR) and collision gas (CAD) were set at 45, 40, 40 and 8 psi, respectively. The compound parameters viz. the declustering potential (DP), collision energy (CE), entrance potential (EP) and collision cell exit potential (CXP) were 110, 47, 10, 15 V for rosuvastatin, 35, 15, 10, 13 V for amlodipine, 46, 47, 10, 15 V for IS1 and 35, 15, 10, 13 V for IS2. Detection of the ions was carried out in the multiple reaction monitoring (MRM) mode by monitoring the transition pairs of m/z 482.1 precursor ion to the m/z 258.3 for rosuvastatin, m/z 409.4 precursor ion to the m/z 238.1 for amlodipine, m/z 488.1 precursor ion to the m/z 264.2 for the IS1 and m/z 413.2 precursor ion to the m/z 238.0 product ion for the IS2. Quadrupoles Q1 and Q3 were set on unit resolution. The analysis data obtained were processed by Analyst software™ (version 1.6.1).
Method validation
A through method validation was carried out as per US FDA and EMEA guidelines [32], [33]. The parameters included carry over, selectivity, specificity, sensitivity, matrix effect, linearity, precision and accuracy, recovery, dilution integrity, stability and run size evaluation.
Pharmacokinetic study design and incurred sample reanalysis
A single dose pharmacokinetic study was performed in healthy South Indian male subjects (n = 12). The Ethics Committee (Samkshema Independent Ethics Committee, Hyderabad, India) approved the protocol and the volunteers provided with written informed consent. All the subjects were fasted for 12 h before the drug formulation administration. Twelve healthy male subjects with an age group of 20–40 years and body-mass index (BMI) of ⩾18.5 kg/m2 and ⩽24.9 kg/m2, with body weight not less than 50 kg were chosen for the study. They were randomly assigned to two groups and took a single oral dose of 40 mg rosuvastatin and 10 mg amlodipine tablets, respectively. Blood samples were collected at 1, 2, 2.33, 2.67, 3, 3.33, 3.67, 4, 4.33, 4.67, 5, 5.33, 5.67, 6, 6.5, 7, 8, 10, 12, 24, 48, 72 and 96 h for rosuvastatin and 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, 12, 16, 24, 36, 48, 72, 96 and 120 h for amlodipine in K2 EDTA vacutainer (5 mL) collection tubes (BD, Franklin, NJ, USA). A predose sample was also collected before administration of each drug formulation. All the tubes were centrifuged at 3200 rpm for 10 min and the supernatant plasma was collected and stored at −70 ± 10 °C till their use. Plasma samples were spiked with the IS dilution and processed as per the procedure described under sample processing. WinNonlin Version 5.2 software was used to calculate main pharmacokinetic parameters of rosuvastatin and amlodipine by non-compartmental model. As per FDA [34] recommendations it is necessary to perform ISR using dosed subject samples. ISR is to verify the reliability and reproducibility of the reported subject sample analyte concentrations. Hence, an ISR was performed by selecting 2 samples from each subject (a total of 12 samples for each analyte) near Cmax and the elimination phase in the pharmacokinetic profile of both the drugs. The reanalyzed values were compared with the initial values. The percent change deviation allowed is ±20% [35].
Results and Discussion
Optimization of chromatographic conditions
It was difficult to set chromatographic conditions that produced sharp peak shape and adequate response for rosuvastatin and amlodipine due to their different physicochemical properties. To develop a selective and sensitive analytical method in biological samples requires the judicious selection of column, mobile phase and organic solvent. These parameters should be suitably monitored to produce the better resolution from endogenous components which in turn affect sensitivity and reproducibility of the analytical method. Once the above mentioned parameters were optimized the flow rate, column temperature and buffer type and concentration can be altered for optimal response. Separation was attempted using organic solvents such as methanol and acetonitrile in different volume ratios with buffers such as ammonium formate, ammonium acetate (2–15 mM) as well as acid additives such as acetic acid and formic acid (0.1–0.4%) in varying strength on different columns such as C8 and C18 of different makes (Hypurity advance 75 × 4.6, 5 μm; Zorbax SB C18, 50 × 4.6, 3.5 μm; Kromasil 100-5C18, 100 × 4.6, 5 μm; Ace 3 C18 150 × 4.6, 3 μm; Alltima HP C18 50 × 4.6, 3 μm; Zorbax XDB-phenyl 75 × 4.6, 3.5 μm; Discovery HS C18 50 mm × 4.6 mm, 5 μm). It was observed that 0.1% formic acid in 5 mM ammonium acetate, methanol and acetonitrile (20:20:60, v/v/v) as the mobile phase was most appropriate to give best sensitivity, efficiency and peak shape for both analytes and the internal standards. Among the various chromatographic columns tested for their suitability Zorbax SB C18, 50 × 4.6 mm, 3.5 μm column gave good peak shape and response even at lowest concentration level for both the analytes. In addition, the effect of flow rate was also studied from 0.25 to 1.0 mL/min, which was also responsible for acceptable chromatographic peak shape and short run time and finally was set at 0.75 mL/min. The retention time of rosuvastatin, amlodipine, IS1 and IS2 (1.3, 1.7, 1.3 and 1.7 min, respectively) was low enough allowing a small run time of 2.5 min.
Mass spectrometry
The present study was carried out using ESI as the ionization source. The mass parameters were optimized using 100 ng/mL of tuning solution of analytes in positive and negative ionization modes. However, the response observed was much higher in positive ionization mode for the analytes compared to the negative mode due to their basic nature. To develop sensitive and selective assay method for the quantification of rosuvastatin and amlodipine different options were evaluated to optimize detection and chromatography parameters. The source dependent parameters and compound dependent parameters were suitably optimized to get better sensitivity and selectivity. As earlier publications have discussed the details of fragmentation patterns of rosuvastatin [12] and amlodipine [22], we are not presenting the data pertaining to this. LC-MRM technique was used for the quantification of analytes since it provides sensitivity and selectivity.
Optimization of sample extraction procedure
Single step extraction of rosuvastatin and amlodipine from plasma was difficult due to their physiochemical properties and polarities. Initially, solid phase extraction (SPE) was tried with Oasis HLB, Starata polymeric sorbent, Bond Elut Plexa and Orpheus C18 extraction cartridges with/without acidic buffer addition to obtain the clean sample and to remove the interference from endogenous components. But, the recovery results obtained for amlodipine were in-consistent at different QC levels. Thus, LLE was carried out using solvents like dichloromethane, ethyl acetate, hexane, diethyl ether, chloroform and methyl tert-butyl ether (MTBE), alone and in combination with and without addition of acidic/basic buffers. But although MTBE in combination with dichloromethane gave promising results, the recovery was not consistent for amlodipine at LQC level. Poor recovery results were obtained with diethyl ether and dichloromethane. Finally promising results were obtained with ethyl acetate and n-hexane (80:20, v/v), which can produce a clean chromatogram for a blank sample and yields the highest recovery for the analyte from the plasma. Stable labeled isotope standards of the analyte as an internal standard is suggested for bioanalytical assays to increase assay precision and limit variable recovery between analyte and the IS [36]. Hence, rosuvastatin d6 and amlodipine d4 maleate were selected for the quantification of rosuvastatin and amlodipine, respectively.
Selectivity and chromatography
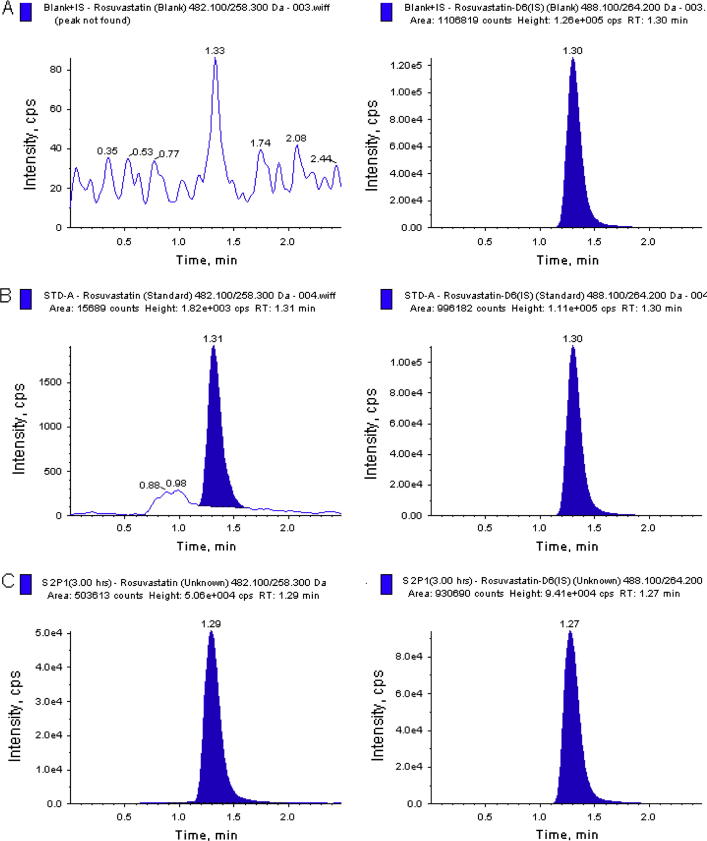
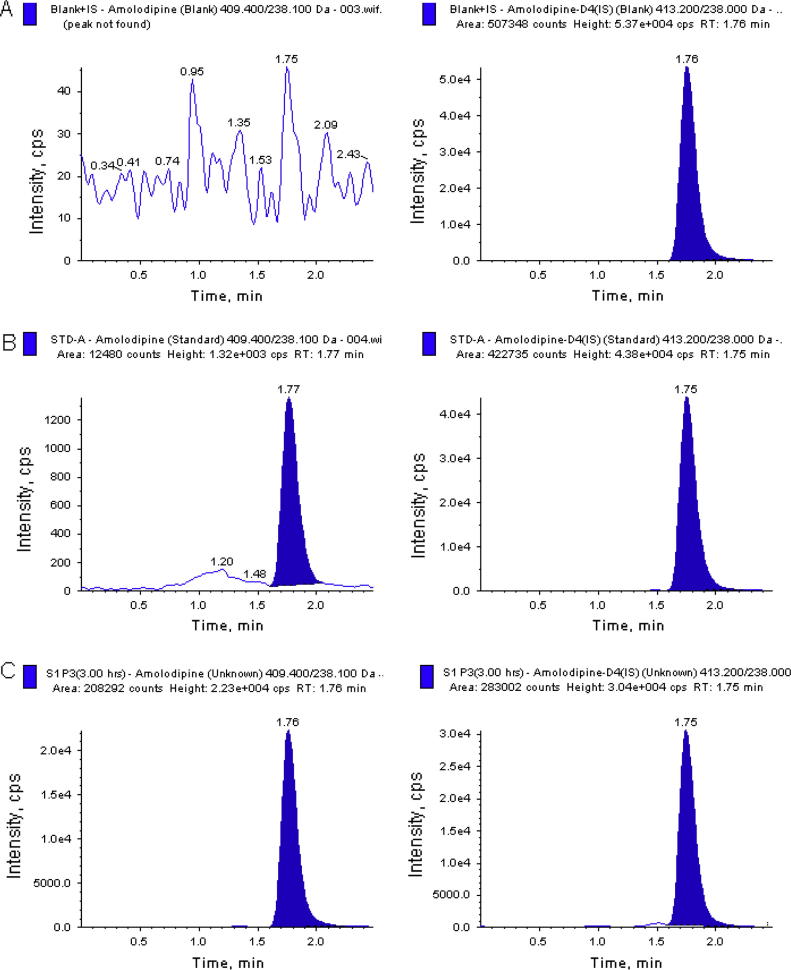
The degree of interference by endogenous plasma components with the analytes and the internal standards was assessed by inspection of chromatograms derived from processed blank plasma sample. As shown in Fig. 1, Fig. 2, no significant direct interference in the blank plasma traces was observed from endogenous substances in drug-free plasma at the retention time of the analytes. Also, no significant interferences were found from both the internal standards to the MRM channel of the analytes. Similarly, no interference was observed from over-the-counter (OTC) drugs such as paracetamol, nicotine, pantoprazole, ibuprofen, caffeine, diphenhydramine, dicyclomine and pseudoephedrine (data not shown).
Fig. 1.
Typical MRM chromatograms of rosuvastatin (left panel) and IS (right panel) in human plasma spiked with IS (A), a LLOQ sample along with IS (B), and 3 h subject plasma sample (C), after the administration of a 40 mg oral single dose of rosuvastatin tablet. The sample concentration was determined to be 28.28 ng/mL.
Fig. 2.
Typical MRM chromatograms of amlodipine (left panel) and IS (right panel) in human plasma spiked with IS (A), a LLOQ sample along with IS (B), and 3 h subject plasma sample (C), after the administration of a 10 mg oral single dose of amlodipine tablet. The sample concentration was determined to be 1.51 ng/mL.
Sensitivity
The lowest limit of reliable quantification (LLOQ) for the rosuvastatin and amlodipine was set at the concentration of 0.52 ng/mL and 0.10 ng/mL, respectively. At this concentration, the precision and accuracy results were found to be 16.69% and 110.15% and 12.53% and 110.07% for rosuvastatin and amlodipine, respectively.
Matrix effect
Matrix effect experiment was conducted in six different sources of plasma lots at LQC and HQC level. The precision and accuracy for rosuvastatin at LQC concentration were found to be 1.24% and 110.73%, and at HQC level they were 1.32% and 92.13%, respectively. Similarly, the precision and accuracy for amlodipine at LQC concentration were found to be 3.21% and 94.44%, and at HQC level they were 2.13% and 93.47%, respectively.
Also, the average matrix factor valve calculate as the response of the post spiked sample/response of neat sample for rosuvastatin at LQC and HQC concentration were 0.99 and 1.00, respectively and for amlodipine were 1.02 and 1.01, respectively which indicated negligible suppression or enhancement.
Calibration curve and linearity
Five calibration curves generated for rosuvastatin and amlodipine were linear over the concentration range of 0.52–51.77 ng/mL and 0.10–10.07 ng/mL with a determination coefficient (R2) ⩾ 0.9992 and 0.9994, respectively. The mean linear equation obtained for rosuvastatin and amlodipine was y = (0.034760 ± 0.001474)x + (0.000714 ± 0.000631) and y = (0.287600 ± 0.017530)x + (0.001480 ± 0.001247), respectively where y is the peak area ratio of the analyte/IS and x the concentration of the analyte.
Precision and accuracy
The results for intra-day and inter-day precision and accuracy in plasma quality control samples are summarized in Table 1. These results are well within the acceptance limits [32], [33].
Table 1.
Intra-day and inter-day precision and accuracy data for rosuvastatin and amlodipine.
| QC |
Intra-day precision and accuracy (n = 12; 6 from each batch) |
Inter-day precision and accuracy (n = 30; 6 from each batch) |
|||||
|---|---|---|---|---|---|---|---|
| Analyte | Concentration spiked (ng/mL) | Concentration found (mean; ng/mL) | Precision (%) | Accuracy (%) | Concentration found (mean; ng/mL) | Precision (%) | Accuracy (%) |
| Rosuvastatin | 0.52 | 0.51 ± 0.01 | 2.82 | 97.69 | 0.52 ± 0.02 | 4.67 | 100.50 |
| 1.49 | 1.47 ± 0.02 | 1.35 | 98.79 | 1.50 ± 0.03 | 2.11 | 100.68 | |
| 6.19 | 6.03 ± 0.09 | 1.51 | 97.41 | 6.15 ± 0.13 | 2.19 | 99.41 | |
| 25.78 | 25.45 ± 0.35 | 1.36 | 98.73 | 25.88 ± 0.47 | 1.81 | 100.41 | |
| 46.03 | 44.67 ± 0.40 | 0.89 | 97.05 | 45.26 ± 0.61 | 1.34 | 98.32 | |
| Amlodipine | 0.10 | 0.10 ± 0.00 | 2.22 | 99.59 | 0.10 ± 0.00 | 2.87 | 100.40 |
| 0.29 | 0.29 ± 0.01 | 2.19 | 100.55 | 0.30 ± 0.01 | 2.42 | 102.45 | |
| 1.20 | 1.20 ± 0.02 | 1.31 | 99.47 | 1.22 ± 0.03 | 2.46 | 101.65 | |
| 5.02 | 5.01 ± 0.06 | 1.19 | 99.80 | 5.12 ± 0.11 | 2.23 | 102.01 | |
| 8.96 | 8.87 ± 0.10 | 1.18 | 99.00 | 9.00 ± 0.15 | 1.69 | 100.48 | |
Recovery and dilution integrity
The recoveries of analytes and the internal standards were good and reproducible. The mean overall recoveries (with the precision range) of rosuvastatin and amlodipine were 79.53 ± 3.68% (1.19–8.56%) and 76.85 ± 4.73% (1.36–7.57%), respectively. Similarly, the mean recovery of the IS1 and IS2 was 80.35% and 79.28%, respectively.
The upper concentration limits can be extended to 83.43 ng/mL for rosuvastatin and 16.24 ng/mL for amlodipine by 1/2 and 1/4 dilutions with screened human blank plasma. The precision and accuracy for rosuvastatin at 1/2 dilution were found to be 1.60% and 98.78%, and at 1/4 dilution they were 0.89% and 99.49%, respectively. Similarly, the precision and accuracy for amlodipine at LQC concentration were found to be 1.04% and 100.01%, and at HQC level they were 1.42% and 99.33%, respectively.
Stability studies
In various stability experiments carried out namely bench top stability (12 h), autosampler stability (80 h), repeated freeze–thaw cycles (5 cycles), reinjection stability (42 h), wet extract stability (75 h at 2–8 °C) and long-term stability at −70 °C for 68 days the mean% nominal values of the analytes were found to be within ±15% of the predicted concentrations for the analytes at their LQC and HQC levels (Table 2). Thus, the results were found to be within the acceptable limits during the entire validation.
Table 2.
Stability data for rosuvastatin and amlodipine (n = 6).
| Analyte | Stability test | QC (spiked concentration (ng/mL) | Mean ± SD (ng/mL) | Accuracy/Stability (%) | Precision (%) |
|---|---|---|---|---|---|
| Rosuvastatin | Processa | 1.49 | 1.53 ± 0.02 | 102.77 | 1.14 |
| 46.03 | 45.77 ± 0.39 | 99.44 | 0.85 | ||
| Processb | 1.49 | 1.52 ± 0.01 | 102.56 | 0.95 | |
| 46.03 | 46.11 ± 0.34 | 100.19 | 0.73 | ||
| Bench topc | 1.49 | 1.53 ± 0.02 | 103.10 | 1.22 | |
| 46.03 | 46.08 ± 0.44 | 100.12 | 0.95 | ||
| FTd | 1.49 | 1.52 ± 0.02 | 102.56 | 1.48 | |
| 46.03 | 45.71 ± 0.49 | 99.31 | 1.07 | ||
| Reinjectione | 1.49 | 1.48 ± 0.02 | 99.93 | 1.56 | |
| 46.03 | 44.65 ± 0.39 | 97.01 | 0.87 | ||
| Long-termf | 1.49 | 1.52 ± 0.02 | 102.05 | 1.13 | |
| 46.03 | 46.12 ± 0.23 | 100.20 | 0.50 | ||
| Amlodipine | Processa | 0.29 | 0.30 ± 0.01 | 104.90 | 3.21 |
| 8.96 | 9.11 ± 0.27 | 101.71 | 2.91 | ||
| Processb | 0.29 | 0.30 ± 0.00 | 104.15 | 1.63 | |
| 8.96 | 9.31 ± 0.10 | 103.91 | 1.11 | ||
| Bench topc | 0.29 | 0.30 ± 0.01 | 105.31 | 3.32 | |
| 8.96 | 9.01 ± 0.35 | 100.58 | 3.91 | ||
| FTd | 0.29 | 0.31 ± 0.01 | 105.54 | 4.81 | |
| 8.96 | 9.28 ± 0.15 | 103.61 | 1.66 | ||
| Reinjectione | 0.29 | 0.30 ± 0.01 | 103.06 | 1.90 | |
| 8.96 | 8.89 ± 0.05 | 99.26 | 0.53 | ||
| Long-termf | 0.29 | 0.31 ± 0.01 | 106.23 | 4.21 | |
| 8.96 | 9.08 ± 0.24 | 101.38 | 2.59 | ||
After 80 h in autosampler at 10 °C.
After 75 h in refrigerator at 2–8 °C.
After 12 h at room temperature.
After 5 freeze and thaw cycles.
After 42 h of Reinjection.
At −70 °C for 68 days.
Stock solutions of rosuvastatin, amlodipine and internal standards were found to be stable for 8 days at 2–8 °C in refrigerator. The percentage stability (with the precision range) of rosuvastatin, amlodipine, IS1 and IS2 was 101.04% (1.21–1.48%), 99.95% (1.42–2.36%), 99.93% (1.14–1.34%) and 98.32% (1.20–2.17%), respectively.
Run size evaluation
Run size evaluation was carried out to assess the integrity of the samples analyzed in a long run during study sample analysis. Thirty sets of each of LQC, MQC1, MQC2 and HQC samples stored at −70 ± 10 °C were processed and analyzed for run size evaluation along with freshly spiked calibration curve standards and quality control samples (Low, Middle and High QC samples). 120 QC’s out of 120 QC’s of run size evaluation and 24 QC’s out of 24 QC’s of freshly prepared QCs for rosuvastatin were within 15% of their respective nominal (theoretical) values. Similarly, 120 QC’s out of 120 QC’s of run size evaluation and 24 QC’s out of 24 QC’s of freshly prepared QCs for amlodipine were within 15% of their respective nominal (theoretical) values.
Pharmacokinetic study results
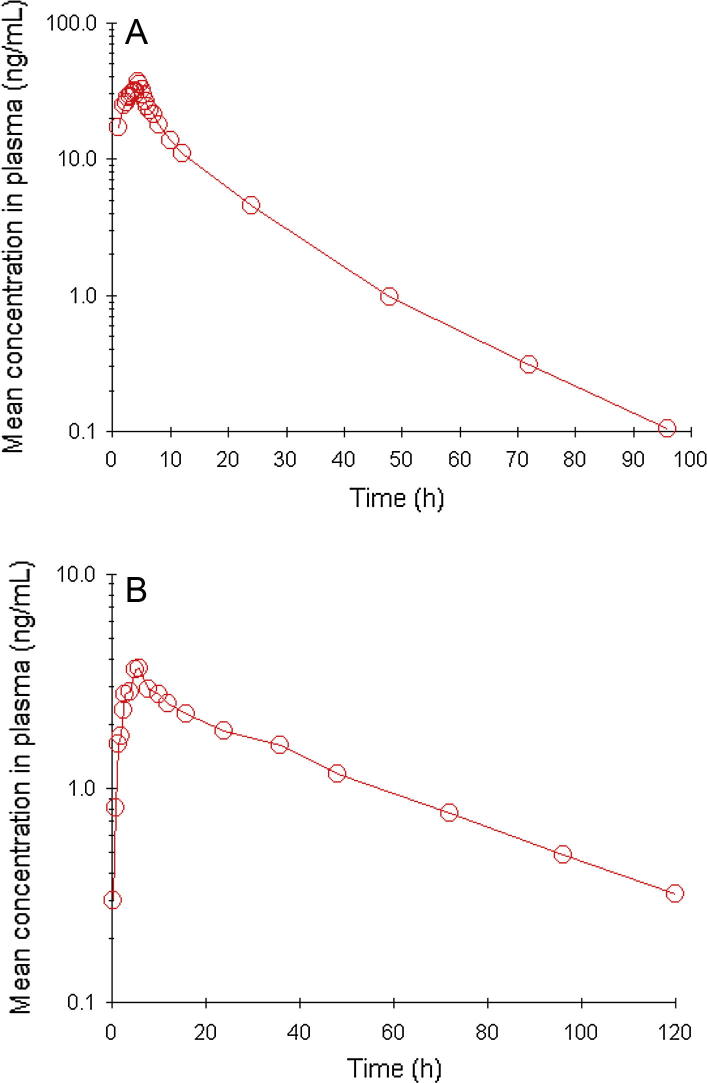
The sensitivity and selectivity of proposed method was verified by applying real time subject sample analysis for a pharmacokinetic study in humans (n = 12). The mean plasma concentration vs time profile of rosuvastatin and amlodipine is shown in Fig. 3 and the corresponding pharmacokinetic parameters are listed in Table 3. These pharmacokinetic parameters are essential for therapeutic drug monitoring studies and to study the relationship between drug dosage regimens and concentration-time profiles. The precision and accuracy results obtained for quality control samples analyzed along with unknown subject plasma samples are summarized in Table 4. These results indicate the reproducibility of the proposed method and reliability of the study data.
Fig. 3.
Mean plasma concentration-time profile of rosuvastatin (A), amlodipine (B), in human plasma following oral dosing of rosuvastatin (40 mg) and amlodipine (10 mg) tablet to healthy volunteers (n = 6).
Table 3.
Pharmacokinetic parameters of rosuvastatin and amlodipine (n = 6, Mean ± SD).
| Parameter | Rosuvastatin | Amlodipine |
|---|---|---|
| Cmax (ng/mL) | 39.32 ± 5.25 | 3.68 ± 0.46 |
| tmax (h) | 3.78 ± 1.38 | 5.83 ± 0.41 |
| AUC0–t (ng h/mL) | 429.26 ± 224.35 | 141.88 ± 10.99 |
| AUC0–inf (ng h/mL) | 431.64 ± 225.48 | 160.72 ± 14.91 |
| t1/2 (h) | 12.68 ± 3.53 | 38.80 ± 8.78 |
| Kel (h−1) | 0.06 ± 0.01 | 0.02 ± 0.00 |
Table 4.
Precision and accuracy data of quality control samples analyzed along with unknown samples (n = 12; 6 from each batch).
| Analyte | QC concentration spiked (ng/mL) | QC concentration found (mean; ng/mL) | Precision (%) | Accuracy (%) |
|---|---|---|---|---|
| Rosuvastatin | 1.50 | 1.44 ± 0.04 | 2.51 | 95.84 |
| 6.25 | 5.68 ± 0.19 | 3.29 | 91.02 | |
| 26.03 | 24.58 ± 0.36 | 1.46 | 94.44 | |
| 46.48 | 43.13 ± 0.90 | 2.10 | 92.79 | |
| Amlodipine | 0.29 | 0.26 ± 0.01 | 2.37 | 90.49 |
| 1.21 | 1.12 ± 0.03 | 2.31 | 92.97 | |
| 5.04 | 4.86 ± 0.03 | 0.65 | 96.42 | |
| 8.99 | 8.60 ± 0.09 | 1.03 | 95.59 | |
The authenticity of the study data is demonstrated through ISR. The differences in concentrations between the ISR and the initial values for all the tested samples were less than 15% (Table 5), indicating good reproducibility of the present method.
Table 5.
Incurred samples re-analysis data of rosuvastatin and amlodipine.
| Subject no. | Rosuvastatin |
Amlodipine |
||||||
|---|---|---|---|---|---|---|---|---|
| Sampling point (h) | Initial conc. (ng/mL) | Re-assay conc. (ng/mL) | Differencea (%) | Sampling point (h) | Initial conc. (ng/mL) | Re-assay conc. (ng/mL) | Differencea (%) | |
| 1 | 5 | 35.06 | 33.16 | 5.56 | 5 | 2.81 | 3.02 | −7.24 |
| 1 | 24 | 3.21 | 3.37 | −4.68 | 120 | 0.40 | 0.38 | 4.83 |
| 2 | 4.33 | 35.34 | 38.25 | −7.89 | 5 | 3.11 | 3.05 | 2.11 |
| 2 | 24 | 4.87 | 5.40 | −10.24 | 120 | 0.42 | 0.40 | 4.15 |
| 3 | 4.67 | 29.39 | 27.36 | 7.15 | 5 | 4.01 | 4.52 | −11.87 |
| 3 | 12 | 4.26 | 4.52 | −5.87 | 96 | 0.48 | 0.52 | −8.78 |
| 4 | 3.67 | 36.55 | 33.45 | 8.87 | 6 | 3.83 | 3.96 | −3.39 |
| 4 | 24 | 2.30 | 2.01 | 13.39 | 120 | 0.39 | 0.38 | 1.57 |
| 5 | 5 | 42.40 | 43.23 | −1.94 | 5 | 3.91 | 3.48 | 11.63 |
| 5 | 48 | 3.10 | 2.92 | 6.02 | 96 | 0.37 | 0.32 | 13.33 |
| 6 | 4.33 | 36.45 | 38.92 | −6.57 | 5 | 3.36 | 3.20 | 4.88 |
| 6 | 12 | 2.92 | 3.22 | −9.72 | 96 | 0.40 | 0.41 | −1.72 |
Expressed as [(initial conc.-re-assay conc.)/average] × 100%.
Conclusions
In ultimate analysis it can be vouchsafed that, we have developed and validated a sensitive, selective and rapid LC/MS/MS method in MRM mode for the simultaneous determination of rosuvastatin and amlodipine in human plasma. This method utilizes deuterated analogs as internal standards for the quantification to avoid the potential matrix effect related problems and variability in recovery between analyte and IS. This is the first LC/MS/MS report for the simultaneous determination of rosuvastatin and amlodipine in any of the biological matrices. The proposed method is rapid with the chromatographic run time of 2.5 min and suitable for high-throughput bioanalysis of rosuvastatin and amlodipine simultaneously. Moreover, the method showed suitability for clinical studies in humans. In addition, assay reproducibility is effectively proved by incurred sample reanalysis.
Conflict of Interest
The authors have declared no conflict of interest.
Acknowledgments
The authors gratefully acknowledge PCR Laboratories, Hyderabad for providing necessary facilities to carry out this work.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Shepherd J. Preventing coronary artery disease in the West Scotland: implications for primary prevention. Am J Cardiol. 1998;82:57T–59T. doi: 10.1016/s0002-9149(98)00728-0. [DOI] [PubMed] [Google Scholar]
- 2.Williams D., Feely J. Pharmacokinetic–pharmacodynamic drug interactions with HMG-CoA reductase inhibitors. Clin Pharmacokinet. 2002;41:343–370. doi: 10.2165/00003088-200241050-00003. [DOI] [PubMed] [Google Scholar]
- 3.Jukema J.W., Zeinderman A.H., van Boven A.J., Reiber J.H., Van der Laarse A., Lie K.I. Evidence for a synergistic effect of calcium channel blockers with lipid-lowering therapy in retarding progression of coronary atherosclerosis in symptomatic patients with normal to moderately raised cholesterol levels. The REGRESS study group. Arterioscler Thromb Vasc Biol. 1996;16:425–430. doi: 10.1161/01.atv.16.3.425. [DOI] [PubMed] [Google Scholar]
- 4.Olsson A.G., McTaggart F., Raza A. Rosuvastatin: a highly effective new HMG-CoA reductase inhibitor. Cardiovasc Drug Rev. 2002;20:303–328. doi: 10.1111/j.1527-3466.2002.tb00099.x. [DOI] [PubMed] [Google Scholar]
- 5.Blasetto J.W., Stein E.A., Brown W.V., Chitra R., Raza A. Efficacy of rosuvastatin compared with other statins at selected starting doses in hypercholesterolemic patients and in special population groups. Am J Cardiol. 2003;91:3C–10C. doi: 10.1016/s0002-9149(03)00003-1. [DOI] [PubMed] [Google Scholar]
- 6.Martin P.D., Dane A.L., Nwose O.M., Schneck D.W., Warwick M.J. No effect of age or gender on the pharmacokinetics of rosuvastatin: a new HMG-CoA reductase inhibitor. J Clin Pharmacol. 2002;42:1116–1121. doi: 10.1177/009127002401382722. [DOI] [PubMed] [Google Scholar]
- 7.Martin P.D., Mitchell P.D., Schneck D.W. Pharmacodynamic effects and pharmacokinetics of a new HMG-CoA reductase inhibitor, rosuvastatin, after morning or evening administration in healthy volunteers. Br J Clin Pharmacol. 2002;54:472–477. doi: 10.1046/j.1365-2125.2002.01688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin P.D., Warwick M.J., Dane A.L., Brindley C., Short T. Absolute oral bioavailability of rosuvastatin in healthy white adult male volunteers. Clin Ther. 2003;25:2553–2563. doi: 10.1016/s0149-2918(03)80316-8. [DOI] [PubMed] [Google Scholar]
- 9.Jukema J.W., van der Hoorn J.W. Amlodipine and atorvastatin in atherosclerosis: a review of the potential of combination therapy. Expert Opin Pharmacother. 2004;5:459–468. doi: 10.1517/14656566.5.2.459. [DOI] [PubMed] [Google Scholar]
- 10.Kang B.Y., Wang W., Palade P., Sharma S.G., Mehta J.L. Cardiac hypertrophy during hypercholesterolemia and its amelioration with rosuvastatin and amlodipine. J Cardiovas Pharmacol. 2009;54:327–334. doi: 10.1097/FJC.0b013e3181b76713. [DOI] [PubMed] [Google Scholar]
- 11.Singh S.S., Sharma K., Patel H., Jain M., Shah H., Gupta S. Estimation of rosuvastatin in human plasma by HPLC tandem mass spectroscopic method and its application to bioequivalence study. J Brazilian Chem Soc. 2005;16:944–950. [Google Scholar]
- 12.Xu D.-H., Ruan Z.R., Zhou Q., Yuan H., Jiang B. Quantitative determination of rosuvastatin in human plasma by liquid chromatography with electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:2369–2375. doi: 10.1002/rcm.2542. [DOI] [PubMed] [Google Scholar]
- 13.Hull C.K., Penman A.D., Smith C.K., Martin P.D. Quantification of rosuvastatin in human plasma by automated solid phase extraction using tandem mass spectrometric detection. J Chromatogr B. 2002;772:219–228. doi: 10.1016/s1570-0232(02)00088-0. [DOI] [PubMed] [Google Scholar]
- 14.Lan K., Jiang X., Li Y., Wang L., Zhou J., Jiang O. Quantitative determination of rosuvastatin in human plasma by ion pair liquid–liquid extraction using liquid chromatography with electrospray ionization tandem mass spectrometry. J Pharm Biomed Anal. 2007;44:540–546. doi: 10.1016/j.jpba.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Gao J., Zhong D., Duan X., Chen X. Liquid chromatography/negative ion electrospray tandem mass spectrometry method for the quantification of rosuvastatin in human plasma: application to a pharmacokinetic study. J Chromatogr B. 2007;856:35–40. doi: 10.1016/j.jchromb.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Lee H.K., Ho C.S., Hu M., Tomlinson B., Wong C.K. Development and validation of a sensitive method for simultaneous determination of rosuvastatin and N-desmethyl rosuvastatin in human plasma using liquid chromatography/negative electrosprayionization/tandem mass spectrometry. Biomed Chromatogr. 2013;17:1369–1374. doi: 10.1002/bmc.2944. [DOI] [PubMed] [Google Scholar]
- 17.Macwan J.S., Ionita I.A., Akhlaghi F. A simple assay for the simultaneous determination of rosuvastatin acid, rosuvastatin-5S-lactone, and N-desmethyl rosuvastatin in human plasma using liquid chromatography–tandem mass spectrometry (LC/MS/MS) Anal Bioanal Chem. 2012;402:1217–1227. doi: 10.1007/s00216-011-5548-4. [DOI] [PubMed] [Google Scholar]
- 18.Oudhoff K.A., Sangster T., Thomas E., Wilson I.D. Application of microbore HPLC in combination with tandem MS for the quantification of rosuvastatin in human plasma. J Chromatogr B. 2006;832:191–196. doi: 10.1016/j.jchromb.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y., Li J., He X., Jia M., Liu M., Li H. Development and validation of a liquid chromatography–tandem mass spectrometry method for simultaneous determination of amlodipine, atorvastatin and its metabolites ortho-hydroxy atorvastatin and para-hydroxy atorvastatin in human plasma and its application in a bioequivalence study. J Pharm Biomed Anal. 2013;83:101–107. doi: 10.1016/j.jpba.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 20.Massaroti P., Moraes L.A.B., Marchioretto M.A.M., Cassiano N.M., Bernasconi G., Calafatti S.A. Development and validation of a selective and robust LC/MS/MS method for quantifying amlodipine in human plasma. Anal Bioanal Chem. 2005;382:1049–1054. doi: 10.1007/s00216-005-3227-z. [DOI] [PubMed] [Google Scholar]
- 21.Nirogi R.V., Kandikere V.N., Mudigonda K., Shukla M., Maurya S. Sensitive and rapid liquid chromatography/tandem mass spectrometry assay for the quantification of amlodipine in human plasma. Biomed Chromatogr. 2006;20:833–842. doi: 10.1002/bmc.600. [DOI] [PubMed] [Google Scholar]
- 22.Bhatt J., Singh S., Subbaiah G., Shah B., Kambli S., Ameta S. A rapid and sensitive liquid chromatography–tandem mass spectrometry (LC/MS/MS) method for the estimation of amlodipine in human plasma. Biomed Chromatogr. 2007;21:169–175. doi: 10.1002/bmc.730. [DOI] [PubMed] [Google Scholar]
- 23.Ma Y., Qin F., Sun X., Lu X., Li F. Determination and pharmacokinetic study of amlodipine in human plasma by ultra performance liquid chromatography–electrospray ionization mass spectrometry. J Pharm Biomed Anal. 2007;43:1540–1545. doi: 10.1016/j.jpba.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Streel B., Laine C., Zimmer C., Sibenaler R., Ceccato A. Enantiomeric determination of amlodipine in human plasma by liquid chromatography coupled to tandem mass spectrometry. J Biochem Biophys Methods. 2002;54:357–368. doi: 10.1016/s0165-022x(02)00133-1. [DOI] [PubMed] [Google Scholar]
- 25.Sarkar A.K., Ghosh D., Das A., Selvan P.S., Gowda K.V., Mandal U. Simultaneous determination of metoprolol succinate and amlodipine besylate in human plasma by liquid chromatography–tandem mass spectrometry method and its application in bioequivalence study. J Chromatogr B. 2008;873:77–85. doi: 10.1016/j.jchromb.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 26.Kallem R.R., Mullangi R., Hotha K.K., Ravindranath L.K., Spoorthy Y.N., Seshagirirao J.V. Simultaneous estimation of amlodipine and atenolol in human plasma: a sensitive LC/MS/MS method validation and its application to a clinical PK study. Bioanalysis. 2013;5:827–837. doi: 10.4155/bio.13.39. [DOI] [PubMed] [Google Scholar]
- 27.Chang H., Li J., Li J., Guan X., Sun F., Qian Z. Simultaneous determination of amlodipine and bisoprolol in rat plasma by a liquid chromatography/tandem mass spectrometry method and its application in pharmacokinetic study. J Pharm Biomed Anal. 2012;71:104–110. doi: 10.1016/j.jpba.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Karra V.K., Pilli N.R., Inamadugu J.K., Rao J.V. Simultaneous determination of losartan, losartan acid and amlodipine in human plasma by LC/MS/MS and its application to a human pharmacokinetic study. Pharm Methods. 2012;3:18–25. doi: 10.4103/2229-4708.97711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravi V.B., Inamadugu J.K., Pilli N.R. Simultaneous determination of telmisartan and amlodipine in human plasma by LC/MS/MS and its application in a human pharmacokinetic study. J Pharm Anal. 2012;2:319–326. doi: 10.1016/j.jpha.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sirikatitham A., Panrat K., Tanmanee N. Determination of amlodipine in human plasma by electrospray ionization LC/MS/MS method: validation and its stability studies. Songklanakarin J Sci Technol. 2004;30:455–462. [Google Scholar]
- 31.Yacoub M., Awwad A.A., Alawi M., Arafat T. Simultaneous determination of amlodipine and atorvastatin with its metabolites; ortho and para hydroxy atorvastatin; in human plasma by LC/MS/MS. J Chromatogr B. 2013;917–918:36–47. doi: 10.1016/j.jchromb.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 32.US DHHS. FDA and CDER. Guidance for industry: bioanalytical method validation. Rockville (MD): US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research and Center for Veterinary Medicine; 2001.
- 33.Guideline on bioanalytical method validation. Science and Medicinal Health. European Medicines Agency (EMEA). EMEA/CHMP/EWP/192217/2009; 21 July 2011.
- 34.De Boer T., Wieling J. Incurred sample accuracy assessment: design of experiments based on standard addition. Bioanalysis. 2011;3:983–992. doi: 10.4155/bio.11.36. [DOI] [PubMed] [Google Scholar]
- 35.Fast D.M., Kelley M., Viswanathan C.T., O’Shaughnessy J., King S.P., Chaudhary A. Workshop report and follow-up—AAPS workshop on current topics in GLP bioanalysis: assay reproducibility for incurred samples—implications of crystal city recommendations. AAPS J. 2009;11:238–241. doi: 10.1208/s12248-009-9100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viswanathan C.T., Bansal S., Booth B., DeStefano A.J., Rose M.J., Sailstad J. Quantitative bioanalytical methods validation and implementation: best practices for chromatographic and ligand binding assays. Pharm Res. 2007;24:1962–1973. doi: 10.1007/s11095-007-9291-7. [DOI] [PubMed] [Google Scholar]