Abstract
Accumulation of chromium (Cr) was determined in water, sediment, aquatic plants, invertebrates and fish in aquatic ecosystems receiving effluents from handloom textile industries in Ranaghat–Fulia region of West Bengal in India. Cr was determined in the samples by atomic absorption spectrophotometer and data were analyzed functionally by Genetic Algorithm to determine trend of depositions of Cr in the sediment and water. Area plot curve was used to represent accumulation of Cr in biota. The results indicate that the aquatic ecosystems receiving the effluents from handloom textile factories are heavily contaminated by Cr. The contamination is hardly reflected in the concentration of Cr in water, but sediment exhibits seasonal fluctuation in deposition of Cr, concentration reaching to as high as 451.0 μg g−1 during the peak production period. There is a clear trend of gradual increase in the deposition of Cr in the sediment. Aquatic weed, insect and mollusk specimens collected from both closed water bodies (S1 & S2) and riverine resources (S3 & S4) showed high rate of accumulation of Cr. Maximum concentration of Cr was detected in roots of aquatic weeds (877.5 μg g−1). Fish specimens collected from the polluted sites (S3 & S4) of river Churni showed moderate to high concentration of Cr in different tissues. Maximum concentration was detected in the liver of Glossogobius giuris (679.7 μg g−1) during monsoon followed by gill of Mystus bleekeri (190.0 μg g−1) and gut of G. giuris (123.7 μg g−1) during summer. Eutropiichthys vacha showed moderately high concentration of Cr in different tissues (65–99 μg g−1) while Puntius sarana showed relatively low concentration of Cr (below detection limit to 18.0 μg g−1) in different tissues except in gill (64.4 μg g−1).
Keywords: Chromium, Handloom, Textile, Pollution, Dye, Non-linear trend analysis
Introduction
Ranaghat, Fulia and Shantipur are three suburban towns, situated 90–110 km north of Kolkata on the bank of the river Churni and are famous for the clusters of handloom textile factories operating in Nadia district (West Bengal, India) and producing exquisite varieties of handloom clothes for many years. However, discharge of effluents from these textile factories into the river Churni as well as into many adjoining closed water bodies and its ecological hazards to local aquatic ecosystem remained largely unattended.
Textile factory effluents are serious offenders of aquatic environment. Use of synthetic dye is a part of textile processing for adding color to the raw materials or to the products as well as to prevent putrefaction of organic matters contained in the raw textile materials. As a result, textile factory effluents discharged into the environment at various stages of operation contain dye, which is a serious threat to aquatic lives due to presence of many toxic heavy metals in modern dyes [1]. Chromium (Cr) besides lead, cadmium and copper is widely used for the production of color pigments of textile dyes and is thus a common contaminant in textile factory effluents [2], [3], [4]. Soil contaminated by textile factory effluents has also been found to contain high concentration of Cr [4], [5]. In India, several other industrial effluents contribute to contamination of river and groundwater by Cr. These include tannery wastes [4], [6], discharge from thermal power plants [7] and sugar mill effluents [8].
The objective of the present study was to determine the trend of Cr contamination in the aquatic environment in and around the cluster of handloom textile industries operating in the Ranaghat–Fulia–Shantipur region due to discharge of handloom textile factory effluents. For this purpose we detected level of Cr in water, sediment soil, algae, macrophytes, invertebrates and fish specimens available in the water bodies receiving effluents of the handloom textile factories.
Concentrations of heavy metals in environment demonstrate seasonal pattern with increasing or decreasing trend. During monsoon the density of the Cr in water may be low due to rainfall and in summer the density may be high. Linear or quadratic regressions, in general, have been used previously to determine trend in aquatic ecosystem [9]. In this paper a nonlinear trend function was established analytically to identify the seasonal variation as well as increasing/decreasing trend of Cr with respect to time. By using MATHEMATICA-7 [10] we plotted the function over a period of 36 months succeeding 12 month period of observation to evaluate trend of Cr accumulation in environment.
Material and methods
Study area
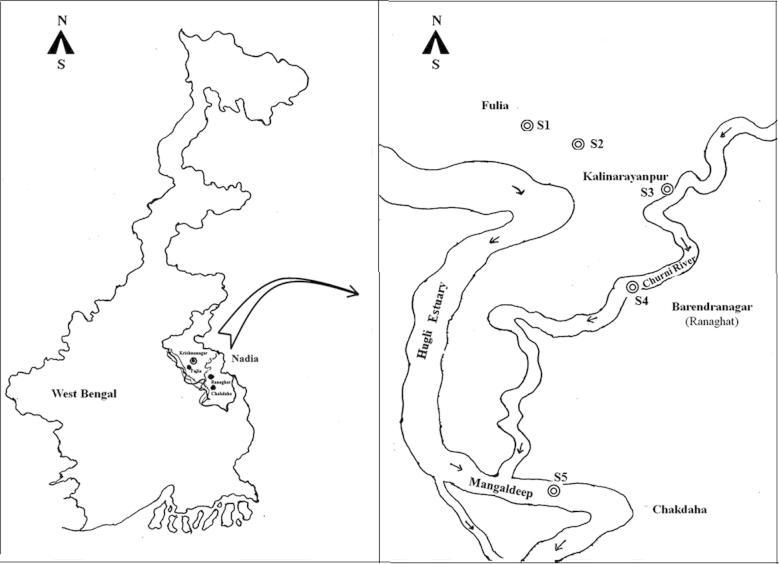
Studies were made on four sites in Ranaghat–Fulia–Shantipur area (23–23.24°N & 88.33–88.5°E) along the course of the river Churni, a 56 km long river originating from river Ichamati near Gede and terminating to Hugli estuary near Chakdaha (Fig. 1). Sites 1 and 2 (S1 and S2) were away from the river Churni and were closed water bodies receiving effluents directly from the handlooms, while sites 3 and 4 (S3 and S4) were located on river Churni, which receive effluents directly from several handloom textile factories. In addition, concentrations of Cr in water and sediment were determined in a fifth site (S5) 15 km downstream of S4 at the confluence of the river Churni with Hugli estuary and compared with those from sites 1 to 4. The site S5 was located on Hugli estuary and was free from direct discharge of any handloom textile factory. Previously Cr was detected in trace to moderate quantity in water (0.28–0.31 μg L−1) and in fish muscle (0.38–1.79 μg g−1) in further downstream industrial zones of Hugli estuary [11], but there was no record of Cr in water in this site. A recent study on 13 sites on Hugli estuary from Sagar island up to the confluence of river Churni indicated that concentrations of Cr and other metals in water were influenced by flux of suspended solids, depth of water and seasons and irrespective of the factors the concentrations of all metals associated with the suspended matters decreased from the industrial zone toward upstream and lowest concentrations were recorded near S5 of the present study [12].
Fig. 1.
Map of West Bengal and Ranaghat–Fulia region showing the sampling sites.
Sampling
Samples of water and sediment soil were collected from each study site during the first week of every month from October 2011 to September 2012. Random samples of water were collected early in the morning in two hundred ml reagent bottle from 50 m depth at two locations of each study site. Temperature, dissolved oxygen and pH of the water samples were determined immediately on the site. Rest of the water samples were brought to the laboratory for determination of total hardness, total alkalinity and Cr concentration of water. Turbidity of water was determined by Secchi disk on the site directly. Sediment soil samples were collected in acid soaked clean polythene packet and were brought to the laboratory. The samples were dried in a hot air oven at 85 °C, cooled and stored at −4 °C until used for determination of Cr level. Attempts were made to collect samples of fish, invertebrates, weeds and algae from the study sites during winter (December–January), summer (April–May) and monsoon (July–August) of the study period. However, fish could not be traced in S1 and S2 sites. Species of fish collected from S3 and S4 as well as other biota collected from all the sites varied from season to season. The samples of biota were collected in acid soaked clean polythene packet, brought to the laboratory and dried in a hot air oven at 85 °C. The samples were cooled and stored at −4 °C until used for digestion.
Analytical methods
Physico-chemical parameters
Dissolved oxygen, total hardness and total alkalinity of water were determined by titration using standard methods, while turbidity of water was determined by Secchi disk as mentioned above [13]. pH of the sampled water was determined by a direct reading digital pH meter (Hanna, Italy). A Celsius thermometer (scale ranging from −0 °C to 110 °C) was used to measure surface water temperature of water.
Determination of Cr
Concentrations of Cr in water, sediment soil and biota were determined by Atomic Absorption Spectrophotometer. Water samples were filtered and digested by strong nitric acid [13]. Sediment soil samples were digested by strong nitric acid and hydrochloric acid [14]. Samples of biota were digested by nitric acid, sulfuric acid and perchloric acid [15]. The digested samples were cooled, filtered, diluted by de-ionized water and were stored in acid washed glass bottles. Blanks for each sample type were prepared from de-ionized water following same digestion procedure. Concentration of Cr in the digested sample was determined in a flame AAS (Spectra AA-240, Agilent Technologies) using air-acetylene flame. Concentration of Cr in the sample was calibrated with standard solutions purchased from Agilent Technologies. Precision and accuracy was checked by repeated aspiration of standard solutions and recovery tests as outlined by Nafde et al. [16] and verified by Guhathakurta and Kaviraj [17]. Adopted analytical procedures yielded 94–97% recovery of the spiked metals from the samples tested. Detection limit of Cr, determined as three times the mean standard deviation of absorbance of 10 replicate blank samples, was set at 0.01 mg L−1.
Trend analysis
Functional form of trend with respect to time was determined analytically as follows:
| (1) |
where A > 0 represents initial value, B represents amplitude of fluctuation, b represents time variation, w represents periodicity and ɛ is the epoch. b > 0 indicates increasing pattern and b < 0 indicates decreasing pattern. If the value of parameter b is very small we may neglect the higher order term of b and get a linear component instead of exponential component. The first components represent the increasing/decreasing trend and the second component represents seasonal variation. Since the trend considered in this paper was non-linear in nature, it was difficult to determine the values of these parameters by applying classical approach. Therefore, we applied Genetic Algorithm [18] to identify these parameters. Fitness function of Genetic Algorithm was considered as follows:
| (2) |
where Dt is the true observation of chromium (P = 2). To identify the efficiency of proposed function we used Mean Absolute Percentage Error (MAPE) as performance metric, which penalizes overestimation and underestimation efficiently.
If Ft is the amount counted by Eq. (1) after substituting estimated parameter values and Dt is the true value then
| (3) |
Results and discussion
Physico-chemical parameters of water
Average values of the physico-chemical parameters of water of the closed water bodies and river Churni have been given in Table 1. The closed water bodies were acidic in nature, but hardness was high, maximum value reaching as high as 400 mg L−1 during July. Dissolved oxygen was also low (5.63 ± 0.65) in the closed water bodies as compared to normal range (8.98 ± 1.69) observed in river water.
Table 1.
Physico-chemical parameters of water determined in the selected sites of closed water bodies and river Churni receiving effluents from handloom textile factories. Values are mean of 12 monthly observations ± SD.
| Parameters | Closed water bodies | River Churni |
|---|---|---|
| Temp (°C) | 33.42 ± 8.38 | 26.42 ± 7.99 |
| Turbidity (cm) | 16.25 ± 1.65 | 21.33 ± 2.87 |
| pH | 6.31 ± 1.04 | 8.04 ± 0.54 |
| Hardness (mg L−1) | 231.58 ± 55.88 | 148.37 ± 59.67 |
| Total alkalinity (mg L−1) | 107.29 ± 41.36 | 75.75 ± 33.67 |
| Dissolved O2 (mg L−1) | 5.63 ± 0.65 | 8.98 ± 1.69 |
Cr concentration in the sediment and water
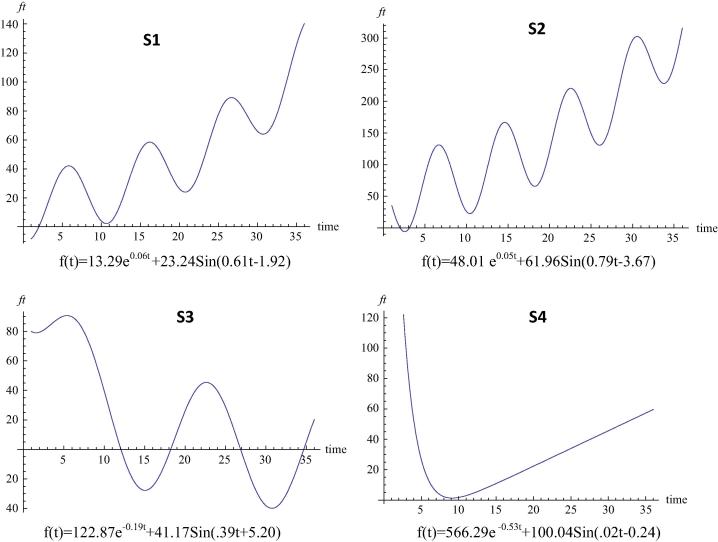
Monthly average concentrations of Cr in the sediment of the four sampling sites have been given in Table 2. By using trend analysis described above, we first estimated the value of five parameters in Eq. (1) for each data set. Then substituting the parameter values we obtained trend equations and corresponding Cr deposition in the sediment for 36 months (Fig. 2). It is revealed from Fig. 2 that Cr deposition is gradually increasing in both sites 1 and 2 (S1 & S2), which are closed water bodies and receive effluents regularly from the local dye factories associated with handloom textile industries. The trend curve for site 3 (S3, in river Churni) shows periodic fluctuation of Cr deposition in the sediment with two distinct peaks one during March–April and another during September–October. The trend of Cr deposition in the site 4 (S4, also in Churni), on the contrary shows a gradual increase of Cr in the sediment. Since Cr concentration in the sediment of S5 varied from below detection limit (BDL) to 0.03 mg kg−1 the trend curve for this site was not determined.
Table 2.
Concentration of Cr (μg g−1) in sediment soil sampled from sites S1–S4. Data are mean of three random observations ± SD.
| Months | S1 | S2 | S3 | S4 |
|---|---|---|---|---|
| October | 81.4 ± 4.1 | 79.3 ± 4.9 | 102.5 ± 3.5 | 241.6 ± 0.2 |
| November | BDL | BDL | 121 ± 1.4 | 260.1 ± 1.2 |
| December | 162.3 ± 8.4 | 134.7 ± 5.1 | 137.5 ± 3.5 | 302.0 ± 2.8 |
| January | 81.1 ± 2.6 | 29.2 ± 3.1 | 159.1 ± 0.5 | 389.3 ± 1.1 |
| February | 63.9 ± 6.5 | 136.6 ± 4.4 | 203.8 ± 5.3 | 451.0 ± 1.4 |
| March | 42.4 ± 1.2 | 110.6 ± 2.2 | 204.4 ± 6.2 | 436.5 ± 0.7 |
| April | 34.8 ± 3.4 | 102.3 ± 3.6 | 2.1 ± 0.1 | 4.4 ± 0.2 |
| May | 19.9 ± 1.9 | 86.1 ± 2.2 | 1.4 ± 0.0 | 2.4 ± 0.1 |
| June | 10.6 ± 1.3 | 67.7 ± 1.7 | 5.8 ± 0.1 | 2.4 ± 0.1 |
| July | 7.2 ± 0.5 | 29.9 ± 2.8 | 11.8 ± 0.1 | 1.5 ± 0.1 |
| August | 2.5 ± 0.3 | 25.1 ± 1.9 | 29.3 ± 4.3 | 46.8 ± 4.9 |
| September | 142.2 ± 3.2 | 98.8 ± 5.6 | 67.1 ± 5.9 | 96.01 ± 22.5 |
BDL = Below detection limit.
Fig. 2.
Trends of Cr deposition in the sediment of closed water bodies (S1 & S2) and river Churni (S3 & S4) receiving effluents from handloom textiles.
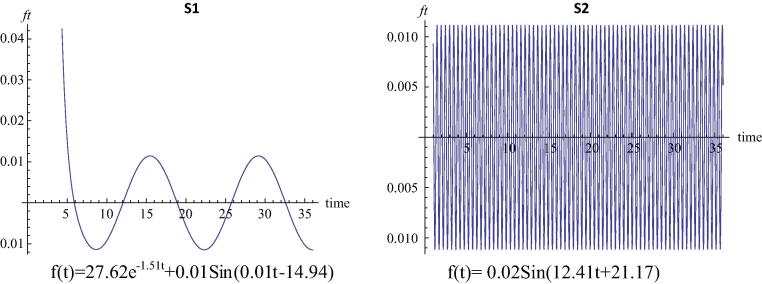
Concentration of Cr could not be detected in water in the two sampling sites (S3 and S4) in river Churni as well as in S5, 15 km downstream of S4 in the confluence of the river Churni with the Hugli estuary. On the other hand Cr concentration was detected in the closed water bodies receiving effluents from dye factories associated with handloom textile industries, but fluctuated seasonally from BDL to as high as 4.9 mg L−1 in S1 and BDL to 1.8 mg L−1 in S2. The trend curve (Fig. 3) shows a 36 month trend of Cr concentration in water in S1 similar to trend of Cr concentration in the sediment of this site (Fig. 2). However, in S2 Cr was hardly retained in water and was deposited in the sediment thereby exhibiting an increasing trend of Cr deposition in the sediment. The trend curve thus showed no trend of fluctuation of Cr concentration in water for this site (S2). Number of dye factories associated with handloom textile operating in a radius of five km of S1 and S2 are approximately 20 and 45 respectively. The trend curves and the equations presented in Fig. 2 also indicate that intensity of Cr pollution in S1 and S2 is influenced by the number of dye factories present around each.
Fig. 3.
Trends of Cr deposition in water of closed water bodies receiving effluents from handloom textiles.
The level of contamination by Cr in the selected sites under the present study could hardly be detected from the concentration of Cr in water. The metal seemed to be precipitated quickly over the sediment, which acted as the sink of Cr. Deepali and Gangwar [4] observed 568.0 μg g−1 Cr in the sediment soil of effluent ponds of textile industries in Hardwar. Sediment serves as a sink of pollutants entering into aquatic ecosystem and reflects anthropogenic activities around it. Number of handloom textile dye factories discharging effluents directly into river Churni within 500 m upstream and downstream of S3 and S4 are respectively 30 and 52. Production in the factories present around S4 continues throughout the year in contrast to seasonal production in the factories present around S3. The trend curve and equation presented in Fig. 2 also reflect continuous increase in deposition of Cr in the sediment of S4 in contrast to seasonal fluctuation of Cr concentration in the sediment of S3. The maximum level of Cr detected in the sediment at S4 is close to the level observed by Deepali and Gangwar [4]. The seasonal fluctuation of Cr concentration in the sediment observed in S3 as well as in the two closed water bodies (S1 and S2) showed two peaks, which coincided with the local festivals and intensity in production schedule in the factories, one during March–April (Bengali New year) and another during September–October (Durga Puja). The trend equation derived from the observed data in the present study clearly indicates that there is an increasing trend of Cr deposition in the sediment, irrespective of the aquatic ecosystem, which if unchecked may attain a serious situation in near future. Continuous discharge of tannery waste has resulted in similar increase in the concentration of Cr in the sediment (147 mg kg−1) of river Ganga near Kanpur [19]. Continuous deposition of the metal in sediment may also result in contamination of underground water. Concentration of Cr in groundwater has been reported to exceed permissible limit in many cities in India due to sustained industrial activities [20]. Dumping of tannery wastes and subsequent contamination of groundwater by chromium in the city of Kanpur in Northern India have been found as the root cause of severe health hazards among the inhabitants [6].
Periodical high concentration of Cr detected in water of the two closed water bodies (S1 and S2) in the present investigation is also of great concern for public health. Provisional WHO guideline value of total Cr in drinking water is 0.05 mg L−1 [21]. Concentration of Cr occasionally increased to level much higher than the WHO guideline value in water of these two closed water bodies. In the present study, Cr was not detected in the two sampling sites of the river Churni. But this metal is frequently detected in water at level 0.1–0.21 mg L−1 in different rivers of India [7], [8]. Lessle et al. [22] observed that increasing contamination of Cr in river may adversely affect aquatic life in river at community level.
Cr Concentration in biota
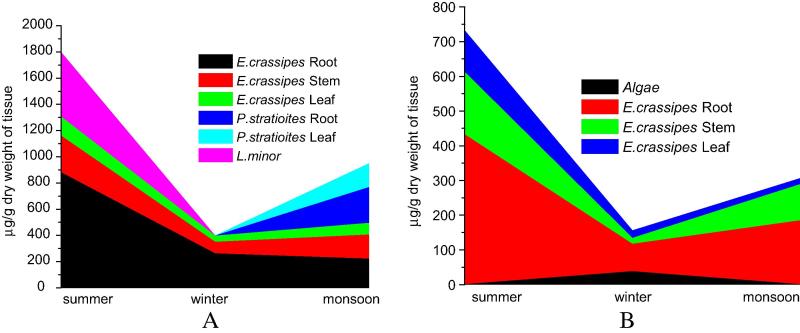
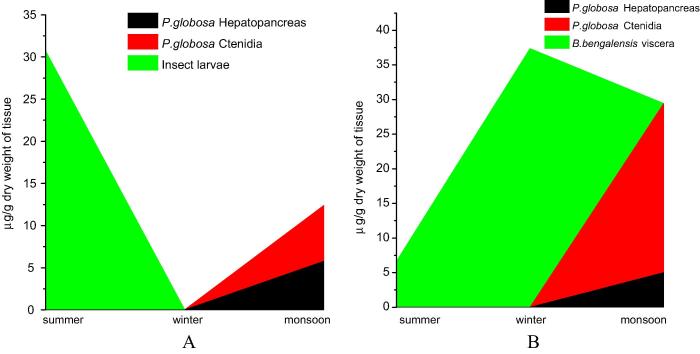
Observed data on concentrations of Cr in the weeds, algae, invertebrates and fish sampled from S1 to S4 during summer, monsoon and winter have been presented as area plots in Fig. 4, Fig. 5, Fig. 6. The aquatic weed Eichhornia crassipes, Pistia stratiotes and Lemna minor were available from S1 and S2, while only E. crassipes was available from S3 and S4. Floating filamentous algae (unidentified) were also available from S3 and S4. It is revealed from Fig. 4 that Cr is detected in E. crassipes root in all seasons, but is concentrated in high concentration (877.5 μg g−1) during summer (April–May) as compared to other two seasons. In L. minor (pink) Cr is found only during summer. It is also observed that Cr accumulation in the roots of E. crassipes is higher than stems and leaves. P. stratiotes was available only during monsoon (July–August) and showed high concentration of Cr in leaves (181.3 μg g−1) and roots (273.1 μg g−1). The filamentous algae however accumulated moderate level of Cr (37.5 μg g−1) only during winter. Fig. 5 represents concentration of Cr in insect larvae and mollusk specimens sampled from the sites. In the closed water bodies, insect larvae were available only during summer and showed high concentration of Cr. The apple snail Pila globosa was available from the closed water bodies only during monsoon. Cr concentration was determined separately in hepatopancreas and ctenidia of the apple snail during this period and 5.75 and 6.62 μg g−1 Cr were recorded respectively in these two tissues. These specimens were not available during winter. In riverine sites (S3 and S4) P. globosa was available only during monsoon and showed high level of Cr accumulation particularly in ctenidia (24.42 μg g−1). On the other hand, Bellamya bengalensis, another species of snail was available in river Churni (S3 and S4) during summer and winter and exhibited moderate to high concentration of Cr in the viscera, winter (December–January) showing the higher concentration of Cr (37.35 μg g−1; Fig. 5).
Fig. 4.
Concentration of Cr in the flora sampled from S1 and S2 is grouped together in A and that from S3 and S4 is grouped together in B.
Fig. 5.
Concentration of Cr in invertebrate specimens sampled from S1 and S2 is grouped together in A and that from S3 and S4 is grouped together in B.
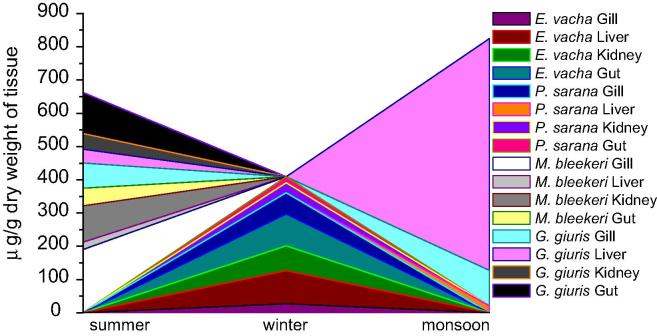
Fig. 6.
Concentration of Cr in fish specimens sampled from river Churni (S3 and S4).
Fish specimens were available only from the riverine sites (S3 and S4). Four species of fish, sampled during summer, winter and monsoon included Eutropiichthys vacha, Puntius sarana, Mystus bleekeri and Glossogobius giuris. Cr was detected in moderate to high concentration in different organs of these species. Maximum concentration of Cr was detected in the liver of G. giuris (679.5 μg g−1) during monsoon followed by gill of M. bleekeri (190.0 μg g−1) and gut of G. giuris (123.7 μg g−1) during summer (Fig. 6). High concentration of Cr can cause severe histopathological damages to liver, kidney and gill of fish [23]. Cr was detected in moderately high concentration in kidney, gill and gut of E. vacha during winter (65–99 μg g−1), gill of G. giuris during monsoon and summer (75.5–106.6 μg g−1), gill of P. sarana during winter (64.4 μg g−1), gut of M. bleekeri during summer (53.0 μg g−1) and kidney (47.5 μg g−1) and liver (41.3 μg g−1) of G. guiris during summer. Cr deposited in relatively low concentration in liver, kidney and gut of P. sarana during winter (below detection limit to 18.0 μg g−1), liver of E. vacha during winter (27 μg g−1) and liver of M. bleekeri during summer (22.0 μg g−1).
Cr is hardly retained in water. In the present study it was found either quickly deposited over sediment or accumulated by aquatic plants. The uptake of metals by plants depends on the chemical form of the metal and life form of the macrophytes [24]. The present study reveals that the free floating aquatic plants such as E. crassipes and P. stratiotes accumulate high concentration of Cr, roots of E. crassipes contributing major share of the accumulation. E. crassipes is a metal hyper accumulator plant and is efficient in absorbing Cr from solution [25], roots contributing the major share [26]. P. stratiotes is also efficient in removing Cr from solution [27]. Both species of aquatic plants grow profusely in the natural water bodies of West Bengal and serve as good agents for sequestration of metals from water. In addition, it is revealed from the present study that the free floating aquatic plant L. minor and the filamentous algae can also remove significant amount of Cr from the water.
Cr deposited over sediment can cause serious threats to bottom organisms. This is evident from the high level of Cr detected in mollusk specimens sampled from both closed water bodies and river in the present study. Glossogobius giuris, a fish known to feed on detritus on the bottom of river [28] was found to accumulate a very high concentration of Cr in its liver during monsoon. M. bleekeri and E. vacha were also found to accumulate high level of Cr. The selected sites of Churni river are apparently non-industrial apart from the handloom textile factories operating along the bank of the river near these sites. Fish harvested from these two sites of the river Churni were found contaminated by Cr, which probably originated from the discharge of effluents from handloom dye factories into the river. Liver and gill tissues are most common targets of Cr deposition in fish [29]. Results of the present study reveal that Cr is also deposited in high level in the gut and kidney tissue of some species. However, muscle tissue has been found inactive for accumulation of Cr [29]. As per dietary practice existing in the area gill and gut are removed before cooking and consumption of fish, while significant part of liver and kidney tissues remains in the fish when it is taken as food. Most of the average daily dietary Cr intake estimates representing various populations living in different countries range between 30 and 60 μg [30], while the estimated safe and adequate daily dietary intake (ESADDI) of Cr as proved by the Food and Nutrition Board of the US National Academy of Science in 1989 for adult man is 50–20 μg/day [31].
Conclusions
It is concluded from the present study that the aquatic ecosystems in Ranaghat–Fulia region are heavily contaminated by Cr, which originate from the handloom textile factories operating in the area. There is a clear trend of gradually increasing deposition of Cr in the aquatic ecosystems. The study also indicates possible human health hazards through consumption of Cr contaminated fish.
Conflict of Interest
The authors have declared no conflict of interest.
Acknowledgments
The authors acknowledge partial financial support received from DST PURSE and Personal Research Grant of the University of Kalyani for this research.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Gopalakrishnan K., Jeyadoss T. Comparative study on biosorption of Zn, Cu and Cr from textile dye effluent using activated rice husk and activated coconut fibre. Indian J Chem Technol. 2011;18(1):61–66. [Google Scholar]
- 2.Ugoji E.O., Aboaba O. Biological treatments of textile industrial effluents in Lagos Metropolis, Nigeria. J Environ Biol. 2004;25(4):497–502. [PubMed] [Google Scholar]
- 3.Manzoor S., Shah M.H., Shaheen N., Khalique A., Jaffar M. Multivariate analysis of trace metals in textile effluents in relation to soil and groundwater. J Hazard Mater. 2006;137(1):31–37. doi: 10.1016/j.jhazmat.2006.01.077. [DOI] [PubMed] [Google Scholar]
- 4.Deepali, Gangwar K.K. Metals concentration in textile and tannery effluent, associated soil and ground water. N Y Sci J. 2010;3(4):82–89. [Google Scholar]
- 5.Malarkodi M., Krishnasamy R., Kumaraperumal R., Chitdeshwari T. Characterization of heavy metal contaminated soils of Coimbatore district in Tamil Nadu. J Agron. 2007;6(1):147–151. [Google Scholar]
- 6.Sharma P., Bihari V., Agarwal S.K., Verma V., Kesavachandran C.N., Pangtey B.S., Mathur N., Singh K.P., Srivastava M., Goel S.K. Groundwater contaminated with hexavalent Chromium [Cr (VI)]: a health survey and clinical examination of community inhabitants (Kanpur, India) PLoS ONE. 2012;7(10):e47877. doi: 10.1371/journal.pone.0047877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Javed M., Usmani N. Assessment of heavy metal (Cu, Ni, Fe Co, Mn, Cr, Zn) pollution in effluent dominated rivulet water and their effect on glycogen metabolism and histology of Mastacembelus armatus. Springer Plus. 2013;2:390. doi: 10.1186/2193-1801-2-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Javed M., Usmani N. Investigation on accumulation of toxicants and health status of freshwater fish Channa punctatus, exposed to sugar mill effluent. Int J Zoo Res. 2013;3(1):43–48. [Google Scholar]
- 9.Mondal D.K., Kaviraj A., Saha S. Water quality parameters and fish biodiversity indices as measures of ecological degradation: a case study in two floodplain lakes of India. J. Water Resour Prot. 2010;2:85–92. [Google Scholar]
- 10.Wolfram S. 5th ed. Wolfram Media; Cambridge: 2005. The mathematica book. [Google Scholar]
- 11.Bhattacaharya A.K., Mandal S.N., Das S.K. Heavy metals accumulation in water, sediment and tissues of different edible fishes in upper stretch of Gangetic West Bengal. Trends Appl Sci Res. 2008;3:61–68. [Google Scholar]
- 12.Mukherjee D.P. Dynamics of metal ions in suspended sediments in Hugli estuary, India and its importance towards sustainable monitoring program. J Hydrol. 2014;517:762–776. [Google Scholar]
- 13.APHA (American Public Health Association) American public health association, American water works association and water pollution control federation; Washington: 1995. Standard methods for the examination of water and wastewater. [Google Scholar]
- 14.Vanloon J.C. Academic press; New York: 1980. Analytical atomic absorption spectroscopy. [Google Scholar]
- 15.Churnoff B. A method for wet digestion of mullet tissue for heavy metal analysis. Trans Am Fish Soc. 1975;104:803–804. [Google Scholar]
- 16.Nafde A.S., Kondwar V.K., Hasan M.Z. Precision and accuracy control in the determination of heavy metals in sediments and water by AAS. J Ind Assoc Environ Manage. 1998;25:83–91. [Google Scholar]
- 17.Guhathakurta H., Kaviraj A. Heavy metal concentration in water, sediment, shrimp (Penaeus monodon) and mullet (Liza parsia) in some brackish water ponds of Sunderban, India. Mar Pollut Bull. 2000;40(11):914–920. [Google Scholar]
- 18.Michalewicz Z. 3rd (extended) ed. Springer; New York: 1992. Genetic Algorithms + Data Structures = Evolution Programs. [Google Scholar]
- 19.Beg K.R., Ali S. Chemical contaminants and toxicity of Ganga river sediment from up and down stream area at Kanpur. Am J Environ Sci. 2008;4(4):362–367. [Google Scholar]
- 20.Ramesh A., Nagendra Praksh B.S., Sivapullaiaih P.V., Sadhashivaiah A.S. Assessment of groundwater quality in designated Pennya industrial area and estate, Bangalore, India – a case study. Int J Environ Prot. 2012;2(6):21–25. [Google Scholar]
- 21.WHO (World Health Organization) 3rd ed. WHO; Geneva: 2008. Guidelines for drinking water quality, Vol I: recommendations. [Google Scholar]
- 22.Leslie H.A., Pavluk T.I., Vaate A., Kraak M.H. Triad assessment of the impact of chromium contamination on benthic macro invertebrates in the Chusovaya River (Urals, Russia) Arch Environ Contam Toxicol. 1999;37(2):182–189. doi: 10.1007/s002449900504. [DOI] [PubMed] [Google Scholar]
- 23.Parvathi K., Sivakumar P., Sarasu C. Effects of chromium on histological alterations of gill, liver and kidney of freshwater teleost Cyprinus carpio (L.) J Fish Int. 2011;6(1):1–5. [Google Scholar]
- 24.Mishra V.K., Tripathi B.D. Accumulation of chromium and zinc from aqueous solutions using water hyacinth (Eichhornia crassipes) J Hazard Mater. 2009;64(2–3):1059–1063. doi: 10.1016/j.jhazmat.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 25.Sabale S., Jadhav V., Jadhav D., Mohite B.S., Patil K.J. Lake contamination by accumulation of heavy metal ions in Eichhornia crassipes: a case study of Rankala lake, Kolhapur (India) J Environ Sci Eng. 2010;52(2):155–156. [PubMed] [Google Scholar]
- 26.Mishra K., Gupta K., Rai U.N. Bioconcentration and phytotoxicity of chromium in Eichhornia crassipes. J Environ Biol. 2009;30(4):521–526. [PubMed] [Google Scholar]
- 27.Odjegba V.J., Fasidi I.O. Accumulation of trace elements by Pistia stratiotes: implications for phytoremediation. Ecotoxicology. 2004;13(7):637–646. doi: 10.1007/s10646-003-4424-1. [DOI] [PubMed] [Google Scholar]
- 28.Islam M.N. Ecobiology of freshwater gobi, Glossogobius giuris (Hamilton) of the river Padma in relation to its fishery: a review. J Biol Sci. 2004;4(6):780–793. [Google Scholar]
- 29.Ciftci N., Cicik B., Erdem C., Ay O., Gunalp C. Accumulation of chromium in liver, gill and muscle tissue of Oreochromis niloticus. J Anim Vet Adv. 2010;9(14):1958–1960. [Google Scholar]
- 30.Krejpcio Z. Essentiality of chromium for human nutrition and health. Pol J Environ Stud. 2001;10(6):399–404. [Google Scholar]
- 31.Anderson R.A., Brydenn A., Polansky M.M. Dietary chromium intake: freely chosen diets, institutional diets and individual foods. Biol Trace Elem Res. 1992;32:117–123. doi: 10.1007/BF02784595. [DOI] [PubMed] [Google Scholar]