Abstract
This study was carried out to investigate the prevalence and monthly intensity of Rhinoestrus (R) spp. among donkeys slaughtered at Giza Zoo abattoir, Egypt. A total of 144 donkeys were examined at postmortem through two visits per month from January 2010 to December 2010. All donkeys were infested with one or more larval stages during all months of the examination period (100%). The 1st and 2nd stage larvae (L1 and L2) were mostly observed in the turbinate bones and seldom in the nasal passages, whereas the 3rd stage larvae (L3) were observed mostly in ethmoid and lamina cribrosa and rarely in nasal passages and pharynx. The highest monthly intensity of infestation with the total number of larval stages was recorded in January and August, while the lowest occurred in September. L1 was observed during all months with two peaks in January and June. L2 occurred from February to April, July, and August. L3 was present from March to May, August, and September. The ranked size of infestation with the total number of the 3 larval stages of Rhinoestrus spp. showed that a total of 107 donkeys had 1–10 larvae; 34 had 11–30 larvae; and 3 harbored 31–50 larvae. The morphology and molecular characterization of the third stage larvae of Rhinoestrus spp. were investigated. Morphologically, two morphotypes (1 and 2) of Rhinoestrus spp. (R. usbekistanicus like and the other R. purpureus like) were reported. Whereas molecular sequencing of mitochondrial cytochrome-oxidase subunit I showed 99% homology with those of R. usbekistanicus. In conclusion, Rhinoestrus spp. present in Egypt is mainly R. usbekistanicus, which includes two morphotypes, R. usbekistanicus like and R. purpureus like.
Keywords: Donkeys, Rhinoestrus spp. larvae, Molecular and morphological identification, Egypt
Introduction
Rhinoestrosis is a parasitic diseases caused by larvae of Rhinoestrus (R) spp. (Diptera, Oestridae), which localize in nasal cavities, sinuses, and pharynx of horses, donkeys, and zebras [1]. This affection may induce local inflammation and causes clinical signs of varying intensity and severity ranging from inflammation to dyspnea, sneezing and cough [2], [3]. Also, it might cause reduction in horse performance and even death due to encephalomyelitis caused by the penetration of the larvae to the ethmoid and meanings [4]. Importantly, Rhinoestrus spp. may also cause ophthalmomyiasis externa and conjunctivitis in human [5]. Previous information on Rhinoestrus spp. prevalence, seasonal abundance, and life cycle in general are crucial for understanding its chronobiology, which will help in planning the critical period for its treatment and control. Valuable information on these topics is available in the literature concerning R. purpureus infesting donkeys in Egypt [6], [7], [8], [9]. However, the information is mostly out-dated. Furthermore, it is almost impossible to generalize the timing of the life cycle of Rhinoestrus spp. from other countries [3], [10], since it depends mainly on the area in which observation was carried out.
Originally R. purpureus and R. usbekistanicus were considered to be Palearctic species, which had reached several areas of African and Asiatic countries along with horses [1], [11]. In the past decade, myiasis caused by R. purpureus has been reported from donkeys in Egypt [6], [9]. R. usbekistanicus infest donkeys in Senegal [2], [12], Niger [13], horses and donkeys in Italy [3], [10], [14], and in France [15]. On the basis of key morphological characters (features of posterior spiracles and distribution of dorsal spines on the third segment) four different morphotypes were identified: R. usbekistanicus-like, R. purpureus-like and two morphotypes with shared features [14]. It was therefore concluded that these morphological characters could not be used to differentiate the two species and this was also confirmed by the analysis of gene encoding for the mitochondrial cytochrome c oxidase I (cox I) and for the ribosomal subunits 16S and 28S [14]. Studies on parasitic arthropods infesting donkeys in Egypt are scanty as well those on Rhinoestrus spp. [6], [7], [8], [9], without any documentation of its morphological and molecular identification. The aim of this research was to investigate the prevalence rate and monthly intensity of the 3 larval stages of Rhinoestrus spp. infesting donkeys in Egypt. Furthermore, morphological and molecular identification of Rhinoestrus spp. L3 were also reported and our findings were compared with previous studies.
Material and methods
Collection of Rhinoestrus spp. larvae
During the period from January–December, 2010, 144 donkeys (12 donkeys each month) were examined at postmortem in Giza Zoo abattoir (Giza, Egypt) through bimonthly visits, for the detection of infestation with Rhinoestrus spp. larvae. The donkeys were obtained from four governorates (Giza (48), Fayoum (48), BaniSweif (24), and Monofia (24)). The first 3 governorates were located south of Cairo city at a distance of 2.5, 103, and119 km, respectively while, the fourth was situated at 72 km north of Cairo. The animals were field working, aged between 4–8 years, fed on green ration, and never received any antiparasitic medications. The mean monthly temperature and relative humidity in Giza governorate was reported during the experimental period. All Institutional and National Guidelines for the care and use of animals were followed.
The head of each animal was separated from the rest of the body, cut in sagittal section and the nasal passages and pharynx were examined by naked eyes. The L2 and L3 were collected from each donkey, placed in a separate vial containing saline solution (0.9% Na Cl) and labeled with the locality, sex, and age. The two turbinate bones of each donkey were placed in plastic bag and labeled with the same information. The materials were examined on the same day of collection at the Parasitology Department, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt for further studies.
Collection of L1 from turbinate bones
In the laboratory; the turbinates were immediately examined; each turbinate was washed several times in a petri dish containing warm normal saline (50 mL) at 37–40 °C, with careful watch for the migrating L1. After approximately 10 min, the turbinates were removed and the saline examined under stereoscopic microscope for the detection of first instar larvae. The collected L1 were counted and identified
Statistical analysis
Prevalence of infection was compared between paired donkeys using 2 × 2X2 contingency tables and 95% confidence intervals (CI) for prevalence and analyzed using Chi square test. Significance was considered when P ⩽ 0.05. All analyses were performed using the SPSS v.11.0.
Data analysis
Epidemiological indexes (Intensity, % of larvae) were calculated according to (Bush et al. [16]).
Morphological identification of L3
Thirty L3 were chosen from infested donkeys of 4 governorates (Giza (8), Fayoum (8), BaniSweif (7), and Monofia (7)) for light microscopic examination. The larvae were washed several times with saline solution then incubated in 10 mL of 5% sodium hydroxide (NaOH) and left overnight at room temperature. The larvae were emptied from its contents, washed with water, and then dehydrated through ascending serial concentrations of ethanol 70%, 80%, 90%, and 100% for one hour each. Finally, they were cleared in clove oil followed by xylene for few minutes, then mounted in Canada balsam and left in an oven at 40 °C to dry for 24 h. The morphological parameters of L3 and their dimensions were reported using stereoscopic microscope (100× and 200×) and identified using morphological keys previous reported [1], [15].
For scanning electron microscopy, 10 L3 were chosen from the material representing the four governorates. They were prepared by serial washing in saline solution and fixed in 2.5% glutaraldehyde as previously described [9]. Specimens were then dehydrated through ascending ethanol series, dried in CO2 critical point drier (Autosamdri-815, Germany), and glued over stubs and coated with 20 nm gold in a sputter coater (Spi-Module sputter Coater, UK). Specimens were examined and photographed with scanning electron microscope at magnifications ranging from 35× to 500×(JSM 5200, Electron prob Microanalyzer, Jeol, Japan).
Molecular identification of L3
Forty Rhinoestrus spp. L3 were randomly collected from infested donkeys obtained from Giza, Fayoum, BaniSweif, and Monofia governorates (ten larvae from each governorate).The larvae were processed for molecular identification without previous morphological identification of its morphotype to avoid any morphological change (as removal of spines covering the surface) during dissection and sampling of its internal organs. The larvae were processed as previously reported by Otranto et al. [14]. Briefly, Genomic DNA was extracted from ∼20 mg of larval internal organs with a commercial kit (Quantum Prep, Aqua Pure Genomic DNA Kit, Bio-Rad, Hercules, CA). All the DNA extracts (n = 40) were subjected to polymerase chain reaction (PCR) to specifically amplify the most variable part of cytochrome oxidase I (COI) gene (i.e.: 688bP) encoding for E4-COOH region. Two fragments of COI gene encoding for E4-COOH region which overlapped on the internal region were separately amplified by the primer sets UEA7-UEA8 and UEA9-UEA10 [17], [18]. Sequences were determined in both directions (using the same primers individually as for PCR), the electrophotogram verified by eye and molecular analysis of sequence data was conducted using MEGA version.
Results
Prevalence and percentage of each larval stage from the total larvae of Rhinoestrus spp.
All donkeys (100%) were infested with one or more larval stages of Rhinoestrus spp. Out of 144 donkeys L1, L2, and L3 infest 132 (91.6%), 43 (29.8%) and 52 (36.1%) donkeys. During the period of examination a total number of 1344 larvae were collected, among these larvae the percentage of L1, L2 and L3 were 66.1%, 12.3%, and 21.6% respectively.
Localization of the 3 larval stages
L1 and L2 were mostly observed in the turbinates and seldom in nasal passages, while L3 occurred mostly in ethmoid, lamina cribrosa, and turbinates, rarely in nasal passages and pharynx.
Intensity of infestation with the 3 larval stages
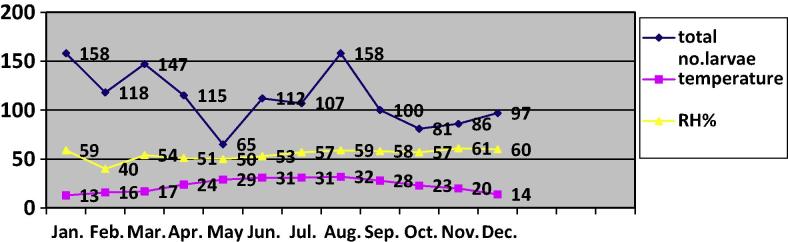
The monthly intensity of infestation with the 3 larval stages showed two peaks in January and August (Fig. 1). The total number of the larvae decreased significantly (P < 0.001) to reach its lowest value in May (65 larvae). Then, the number increased significantly (P < 0.05) to reach its second peak in August. During September, October, November, and December a significant decrease (P < 0.05) was observed in these months without significant differences during the last 3 months.
Fig. 1.
The intensity of infestation with the 3 larval stages of Rhinoestrus spp. in donkeys compared to the mean temperature (°C) and relative humidity (RH%) during January–December.
Temperature and humidity during the tested period
The mean monthly temperature prevailing during the study period showed variations from a lowest degree during January (13 °C) and December (14 °C) to highest during June, July (31 °C) and August (32 °C). The relative humidity showed minor variations (40–60%) during the study period (Fig. 1).
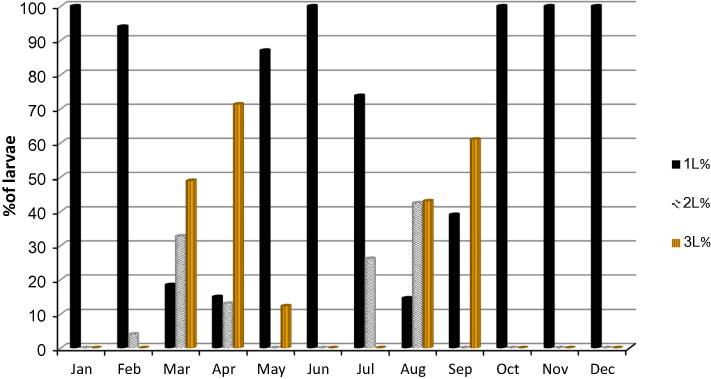
Percentages of L1, L2, and L3 of Rhinoestrus spp. larvae
L1 was the only larval stage reported (100%) during January, June, and from October to December (Fig. 2). The highest percentage of L2 was in March (32.7%) and August (42.4%). L3 reached its highest value in April (71.3%) and September (61.0%).
Fig. 2.
Percentages of the 1st, 2nd and 3rd stage larvae of Rhinoestrus spp. during January–December.
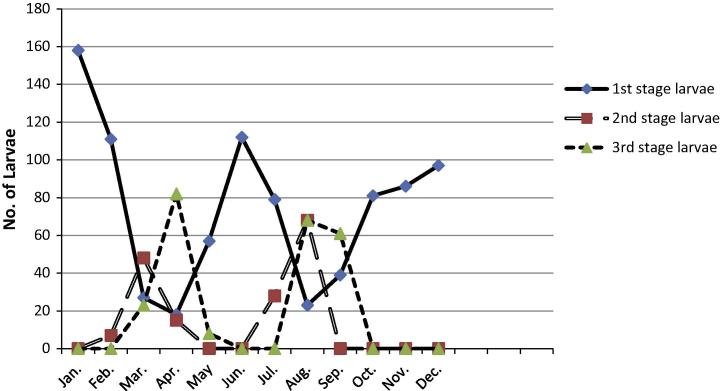
Intensity of L1, L2 and L3 throughout the tested period
L1
L1 was present during all months of the year with a peak value (158 larvae) in January followed by a significant decrease in March and April (P < 0.001). A second peak occurred in June with significant increase (P < 0.05) followed by a significant decrease in August (P < 0.05). A nonsignificant increase (P > 0.1) was observed during the period from September to December (Fig. 3).
Fig. 3.
Intensity of infestation with 1st, 2nd and 3rd stage larvae of Rhinoestrus spp. in donkeys during January–December.
L2
L2 was present only from February to April, in July and August. Two significant peaks (P < 0.05) were observed in March and August (Fig. 3).
L3
L3 was present from March to May, in August and September (Fig. 3). Two peaks were also observed for L3; the first one occurred in April and the 2nd in August and both showed significant increase (P < 0.05).
Size of infestation with Rhinoestrus spp. in donkeys
The ranked sizes of infestation with the total number of the 3 larval stages among 144 infested donkeys were as follows: a total of 107 (74.3%) donkeys had 1–10 larvae, 34 (23.6%) had 11–30 larvae, and 3 (2.1%) harbored 31–50 larvae.
Morphological identification of L3
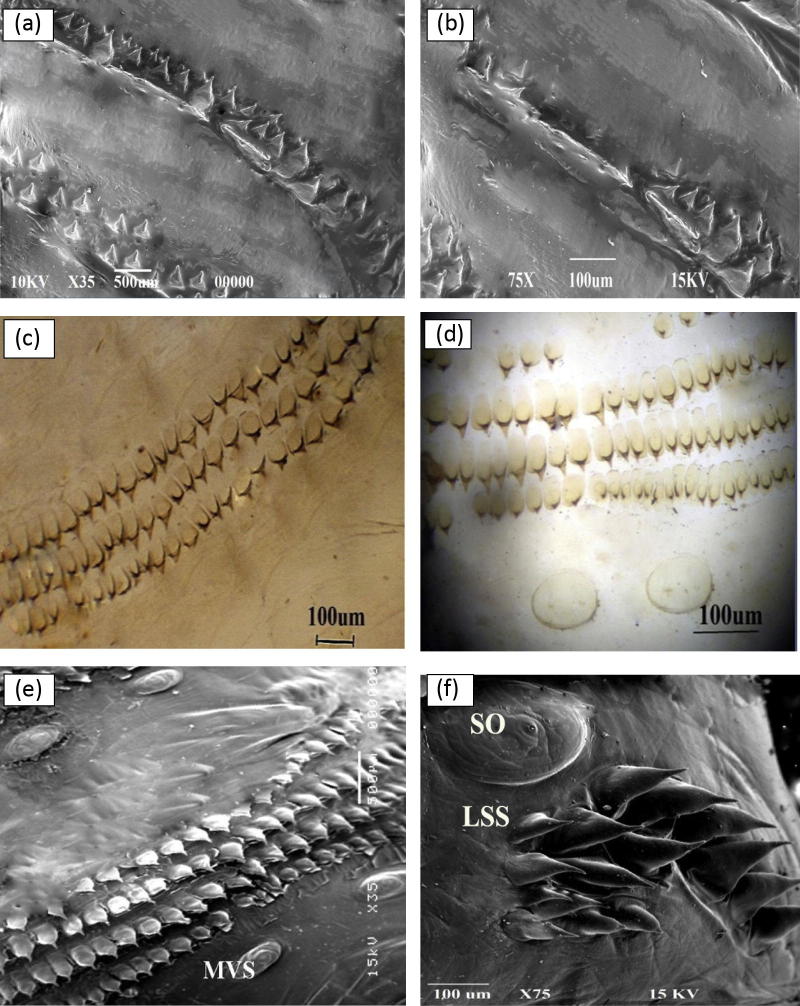
Light and scanning electron microscopic description of L3 revealed two morphologically different morphotypes of Rhinoestrus spp. (morphotype 1 and 2). The morphological differences were presented (Table 1). Briefly, they were concerned with spines on the dorsal surface of segments 3 and 5 and on ventral surface of 2nd, 3rd, 4th and 5th–10th segments together with the posterior spiracles (Fig. 4).
Table 1.
Morphological differences between morphotype 1 and 2 of Rhinoestrus spp. third stage larvae.
| Main difference | Morphotype 1 | Morphotype 2 |
|---|---|---|
| Two rows of spines on dorsal surface of 3rd segment | Interrupted | Complete Fig. 4a |
| Dorsal surface of 5th segment | Devoid from lateral spines | Have lateral spines Fig. 4b |
| 1st row of spines on ventral surface of 2nd and 3rd segments | Incomplete | Complete Fig. 4c |
| Spines on ventral surface of 4th segment | 3 rows | 4 rows |
| 1st row of spines on ventral surface of segment 5–10 | Interrupted Fig. 4d | No interruption Fig. 4e |
| Posterior spiracles | Length similar to width (370–430 pores) | Length longer than width (290–350 pores) |
Fig. 4.
Third stage larvae of Rhinoestrus spp., (a) third segment; dorsal surface (morphotype 2), note two complete uninterrupted rows of spines on 1st and 2nd segments. Scanning electron micrograph (SEM), Scale bar: 500 μm. (b) Fifth segment; dorsal surface; (morphotype 2) note group of lateral spines on each side (SEM); Scale bar: 500 μm. (c) Third segment; ventral surface; (morphotype 2) note complete 1st row of spines; light microscopic picture, Scale bar: 100 μm. (d) Seventh segment; ventral surface; Scale bar: 100 μm. note incomplete 1st row of spines; light microscopic picture. (e) Sixth segment; ventral surface; (morphotype 2) note 4 complete rows of spines; note medioventral sensorial structure (MVS); (SEM); Scale bar: 500 μm. (f) Seventh segment; ventral surface; (both morphotypes) note lateral sensorial structure (so) and lateral spines behind them (Lss); (SEM); Scale bar: 500 μm.
Both morphotypes revealed the presence of lateral sensorial structure (SO) on both sides of segments 2–10 together with group of spines (LSS, 15–25 in numbers) behind each structure (Fig. 4f).
The result of morphological examination of 40 L3 (30 light and 10 scanning electron microscope) revealed that 30 belong to morphotype1 and 10 to morphotype 2. None of the examined L3 showed shared morphological characters of both morphotypes.
Molecular characterization
DNA was extracted from 40 L3 and PCR amplification was performed using specific primer. In each PCR, the primer combination yielded amplicons with a sequence of 689 bp. Each specimen examined resulted nearly 99% homologous to R. usbekistanicus (Genbank ™, accession number: AF497771). This was the only sequence to which the obtained sequences were compared. It was registered in the Gene bank for R. usbekistanicus obtained from donkey in Nigeria [18]. No insertions or deletions were detected in the sequences and none of the sequences exhibited any unusual mutations. The molecular analysis evidenced 18 identical and 22 representative sequences with an overall intraspecific pairwise divergence ranging from 0.15% to 0.78%. The geographical provenience of Rhinoestrus spp. specimens did not discriminate the nucleotide variability among all the samples herein examined.
The comparison of the 22 representative cox1 sequences with that of R. usbekistanicus revealed 4 missense substitutions. Two samples showed identical nucleotide sequences.
Discussion
This study revealed that all of the examined donkeys during one year period harbored one or more larval stage of Rhinoestrus spp. (100%). The higher prevalence in the present study compared with previous investigation from Egypt (61.11%) [8] could be due to the changes in the locality and hence the epidemiological environment prevailing around the sampled donkeys. Tuzer and Tan [19] in Turkey reported 100% prevalence from horses. Varied prevalences were reported in other countries, including Ethiopia 0.002% [20], Italy 6.13% and 4.16% in the Apulia and in Sicily regions, respectively [3]. Both of two donkeys examined by [3] in Italy were positive. Different values were reported from horses in other countries, Niger, 8.1% [13], Turkey, 13.6% [21], Senegal, 48% [2] and in Sardinia, Italy 49% [10]. Generally, the variation in the prevalence in Egypt from other countries could be attributed to climatic variations, unhygienic conditions, immune-suppression due to infection with other microorganisms and/or other parasites.
The total number of Rhinoestrus spp. larvae reached its peak value in January and August. The variations in the total number of larvae during different month of the year were mainly due to the significant variations in the mean monthly atmospheric temperature while the variations in relative humidity and rainfall were nonsignificantly correlated with the total number of larvae.
Analyses of the monthly trends of percentage of L1, L2 and L3 indicated that L1 only constituted the 1st peak (January) of the total larvae while the 2nd peak (August) was formed mainly from L2 and L3 and to a lesser extent to L1. The monthly prevalence of L1, L2 and L3 was of primary interest in this study as it gave an idea of the duration of various stages of the life cycle of Rhinoestrus spp. and the number of generation occurring per year. L1 was present during all months of the year with two peaks in January and June. The first peak (January) was due to the accumulation of L1 in the animal during the period from September to December of the preceding year due to the gradual decrease of the atmospheric temperature. The second peak of L1 (June) was due to decreasing numbers of L3 during May and its release from the animal with the emergence of adult stage which deposit L1 in June. This conclusion was reached in view of previous record [1] mentioned that the newly emerged female gives its L1 15 days after mating. L2 had two peaks of infestation in March and August. This indicates that the L1 molt to L2 in late February and March (1st peak) and late July and August (2nd peak). This conclusion was reached from our finding of a significant decrease of the 1st stage larvae in February and July. L3 had two peaks in April (1st peak) and August (2nd peak); then the number of the L3 reached its lowest value in October indicating its release from the animal and formation of pupa in the ground followed by emergence of adult stage. Therefore, the number of 1st stage larvae started to increase in October, November and December.
Mula et al. [10] studied the dynamics of Rhinoestrus spp. larval stages in Italy and considered 3 periods in its chronobiology. The diapause (September–February) characterized by an absolute prevalence of L1; the active phase of the endogenous phase (February–September) with an increase in the percentage of L2 and L3, and the exit phase (May–September) pointed by further increase of L1.
It could be concluded that Rhinoestrus spp. infesting donkeys in Egypt had two generations through the year (January and June) indicating that the newly emerged fly occurs mostly during these two months. Zayed et al., [8] reported two generations in the year with two peaks of infestation for both the 1st stage larvae and the total number of larvae occurring during March and June.
Our study describes for the first time the morphology and molecular identification of Rhinoestrus spp. L3 infesting donkeys in Egypt. This investigation indicated that Rhinoestrus spp. present in Egypt is mainly R. usbekistanicus which include two morphotypes, R. usbekistanicus like (no. 1) and R. purpureus like (no. 2) [1], [15]. Our study revealed that both morphotypes possessed a lateral sensorial structure with group of spines on segments 2–10 which were not previously reported. The differences between both morphotypes in the sensorial structure and perimeter lengths are in line to previous studies [15], [10].
The molecular analysis of COI gene of forty L3 evidenced 18 identical and 22 representative sequences within an overall intraspecific pairwise divergence ranging from 0.15% to 0.78%. Low intraspecific variation of COI gene sequence (0.14–0.43%) was reported for the four morphotypes of Rhinoestrus spp. [14] as typical for a single species. Mula et al. [10] reported a pairwise distance ranging between 0.4 and 0.6 for a sequence of COI of R. usbekistanicus. The same COI gene sequences of other taxonomically well defined Oestridae ranked within the same genus showed interspecific divergence constantly higher than 6% and an intraspecific nucleotide divergence below 1% [18]. In the molecular investigation on three Przhevalskiana species, the percentage of interspecific variability of the COI ranged from 0.19% to 0.29% [22], which falls within the range of intraspecific differences in the Oestridae family [18]. Moreover, the most variable region of the 28S rDNA gene of P. silensus, P. aegagri, and P. crassii showed 100% homology, thus confirming they are morphotypes of the same species [22]. The current study confirmed the presence of one species only in Egypt (R. usbekistanicus). Similar studies were carried out by Otranto et al. [14] who detected 4 morphologically different Rhinoestrus spp. (R. purpureus, R. Purpureus like, R. usbekistanicus and R. usbekistanicus like) from horses in Italy while molecular examination of the same material confirmed the presence of one unique species. Furthermore, Mula et al. [10] studied Rhinoestrus spp. infesting horses in Italy, their results indicated that 3 morphotypes were found, R. purpureus (8%), R. usbekistanicus (8%) and 84% evidenced intermediate features. Contrastingly, molecular analysis of COI gene of the larvae confirmed uniformity at genetic level in the Mediterranean area.
Conclusions
Since this study demonstrated that Rhinoestrus spp. had two generations per year with maximum total larval number during January and August, a twice yearly treatment of donkeys is recommended during these months. This investigation indicated that Rhinoestrus spp. infesting donkeys in Egypt was molecularly identified mainly as R. usbekistanicus which includes two morphotypes (1 and 2), one is R. usbekistanicus like and the other R. purpureus like.
Conflict of interest
The authors have declared no conflict of interest.
Acknowledgments
We are grateful to Dr. D. Otranto and F. Dantes-Torres, Faculty of Veterinary Medicine, University of Bari, Valenzano, Italy, regarding their help in molecular characterization of our materials.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Zumpt F. Butterworths; London: 1965. Myiasis in man and animals in the old world. [Google Scholar]
- 2.Deconinck P., Pangui L.J., Githego A., Dorchies P. Prevalence of Rhinoestrus usbekistanicus (Gan 1947) in donkeys (Equus asinus) in Senegal. Rev Elev Med Vet Pays Trop. 1996;49:38–40. [PubMed] [Google Scholar]
- 3.Otranto D., Colwell D.D., Milillo P., Di Marco V., Paradies P., Napoli C. Report in Europe of nasal myiasis by Rhinoestrus spp. in horses and donkeys: seasonal patterns and taxonomical consideration. Vet Parasitol. 2004;122:79–88. doi: 10.1016/j.vetpar.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Di Marco V, Riili S, Vullo S, Capecchio MT, Dorchies P. One case of equine myiasis caused by Rhinoestrus usbekistanicus. In: Proceeding of the 18th international conference of the World Association for the Advancement of Veterinary Parasitology (WAAVP), Palm Cove, Australia, August 2001. vol. 190. p. 26–30.
- 5.Peyresblanques J. Myases oculares. Ann d’ Oculistic, Paris; 1964, vol. 197. p. 271–95. [PubMed]
- 6.Zayed A.A. Studies on Rhinoestrus purpureus (Diptera: Oestridae) larvae infesting donkeys (Equus asinus) in Egypt. III. Pupal duration under controlled conditions. Vet Parasitol. 1992;44:285–290. doi: 10.1016/0304-4017(92)90123-q. [DOI] [PubMed] [Google Scholar]
- 7.Zayed A.A., Hilali M. Studies on Rhinoestrus purpureus larvae infecting donkeys in Egypt. J Equine Vet Sci. 1993;13:92–95. [Google Scholar]
- 8.Zayed A.A., Hilali M., El Metenawy T.M. Studies on Rhinoestrus purpureus (Diptera: Oestridae) larvae infesting donkeys (Equus asinus) in Egypt. Incidence and seasonal variations. J Equine Vet Sci. 1993;13:46–49. [Google Scholar]
- 9.Zayed A.A., Abdel-Shafy S., El- Khateeb R.M. Surface ultrastructure of posterior abdominal spiracles of third instars of nasal bots of Cephalopina titillator, Oestrus ovis and Rhinoestrus purpureus (Diptera: Oestridae) infesting camels, sheep and donkeys in Egypt. Res J Parasitol. 2008;3:1–11. [Google Scholar]
- 10.Mula P., Pilo C., Solinas C., Pipia A.P., Varcasia A., Francisco I. Epidemiology, chronobiology and taxonomic updates of Rhinoestrus spp. infestation in horses of Sardinia Isle, Western Mediterranean (Italy) Vet Parasitol. 2013;192:240–246. doi: 10.1016/j.vetpar.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Otranto D., Stevens J.R., Brianti E., Dorchies P. Human and live stock migrations: a history of bot fly biodiversity in the Mediterranean region. Trends Parasitol. 2006;22:209–213. doi: 10.1016/j.pt.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Kaboret Y., Deconinck P., Pangui J., Akakpo J., Dorchies P. Lesion in Rhinoestrus usbekistanicus (Gan 1947) infection of donkeys (Equus asinus) in Senegal. Rev Med Vet. 1997;148:123–126. [PubMed] [Google Scholar]
- 13.Tibayrence R., Garba D., Dorchies P. Prevalence de Rhinoestrus usbekistanicus (Gan 1947) chez I’ ane (Equus asinus) dans la region de Niamey, Niger. Rev Elev Med Vet Pays Trop. 1999;52:113–115. [PubMed] [Google Scholar]
- 14.Otranto D., Milillo P., Traversa D., Colwell D.D. Morphological variability and genetic identity in Rhinoestrus spp. causing horse nasal myiasis. Med Vet Entomol. 2005;19:96–100. doi: 10.1111/j.0269-283X.2005.00553.x. [DOI] [PubMed] [Google Scholar]
- 15.Guitton C., Dorchies P., Morand S. Scanning electron microscopy of larval instars and imago of Rhinoestrus usbekistanicus Gan, 1947 (Oestridae) Parasite. 1996;3(2):155–159. doi: 10.1051/parasite/2001082155. [DOI] [PubMed] [Google Scholar]
- 16.Bush A.O., Laffrty K.D., Lotz J.M., Shostak A.W. Parasitology meets ecology on its own terms. J Parasitol. 1997;83:575–583. [PubMed] [Google Scholar]
- 17.Lunt D.H., Zhang D.X., Szymura J.M., Hewitt G.M. The insect cytochrome oxidase I gene: evolutionary patterns and conserved primers for phylogenetic studies. Insect Mol Biol. 1996;5:153–165. doi: 10.1111/j.1365-2583.1996.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 18.Otranto D., Traversa D., Guida B., Tarsitano E., Fiorente P., Stevens J.R. Molecular characterization of mitochondrial cytochrome oxidase I gene of Oestridae species causing obligate myiasis. Med Vet Parasitol. 2003;17:307–315. doi: 10.1046/j.1365-2915.2003.00442.x. [DOI] [PubMed] [Google Scholar]
- 19.Tuzer E., Tan H. Rhinoestrosis in race horses in Istanbul and the use of endoscopy for its diagnosis. Veteriner Fakultesi Dergisi (Istanbul) 1994;20(2–3):173–175. [Google Scholar]
- 20.Getachew M., Trawford A., Feseha G., Reid S. Gastrointestinal parasites of working donkeys of Ethiopia. Trop Anim Health Prod. 2010;42(1):27–33. doi: 10.1007/s11250-009-9381-0. [DOI] [PubMed] [Google Scholar]
- 21.Tan H., Akdogan Kaymaz A., Yilgin C., Gonul R. Disturbances observed by endoscopic examination of the upper respiratory airway tract in horses. Turk Veteriner likve Hayvanclk Dergisi. 1999;23(4):657–663. [Google Scholar]
- 22.Otranto D., Traversa D. Molecular evidence indicating that Przhevalskiana silensus, Przhevalskiana aegagri and Przhevalskiana acrossii (Diptera, Oestridae) are one species. Acta Parasitologia. 2004;49:173–176. [Google Scholar]