Abstract
Role of different mediators was described in the development of the granulomatous response and fibrosis observed in intestinal schistosomiasis. However, both Toll-like receptor 2 (TLR2) and nuclear factor kappa B (NF-κB) have not yet been investigated in intestinal schistosomiasis. This study aimed to characterize the role of TLR2 and NF-κB in the pathogenesis of intestinal schistosomiasis. Experimental animals were divided into two groups; group I: non-infected control group and group II: mice infected subcutaneously with S. mansoni cercariae. Colon samples were taken from infected mice, every two weeks, starting from the 6th week postinfection (PI) till 18th week PI. Samples were subjected to histopathological and immunohistochemical studies. Colon of S. mansoni infected mice showed histopathological changes in the form of mucosal degeneration, transmural mononuclear cellular infiltration and granulomas formation. Immunostained sections revealed significant increase in TLR2 and NF-κB positive cells in all layers of the colon, cells of the granuloma and those of the lymphoid follicles 10 weeks PI. All these changes decreased gradually starting from 12 weeks PI onward to be localized focally at 18 weeks PI. In conclusion, recruitment and activation of inflammatory cells to the colonic mucosa in intestinal schistosomiasis are multifactorial events involving TLR2 that can trigger the NF-κB pathways. Hence, down-regulation of both TLR2 and NF-κB could be exploited in the treatment of colonic schistosomiasis.
Keywords: Intestinal schistosomiasis, Nuclear factor kappa B, Toll-like receptor 2, Granuloma
Introduction
Intestinal schistosomiasis is the most common manifestation of infection with Schistosoma mansoni in endemic areas and if not diagnosed and treated early, it might lead to complications such as hepatosplenic schistosomiasis, which have high morbidity and mortality, and chronic intestinal schistosomiasis which may be presented by severe rectal bleeding or intussusceptions, pericolic or mesenteric granuloma, and intestinal obstruction [1].
This disease is caused mainly by the host’s immune response to schistosome eggs. The granulomas formed around the eggs aim to sequester or neutralize pathogenic egg antigens and also lead to fibrogenesis in host tissues [2]. Chronic morbidity in schistosomiasis develops when schistosome eggs lodge in the gut, liver and other organs causing extensive tissue damage. Immune responses to schistosome antigens manifest a striking shift from a moderate Th1 to a Th2-dominated response with the onset of egg laying around 5–6 weeks which is responsible for fibrosis and much of the pathology [3], [4].
Different mediators have been described to play a critical role in the development of the granulomatous response and the resulting fibrosis observed in schistosomiasis, e.g. IFN-γ, IL-10 [5] and TNF-α [6]. However, the role of TLR2 and nuclear factor (NF)-κB has not yet been investigated in intestinal schistosomiasis.
NF-κB is a transcription factor that regulates some processes such as inflammation, apoptosis, stress response, wound healing and angiogenesis [7]. NF-κB is markedly activated in the inflamed gut, especially in macrophages and epithelial cells. Sustained activation of NF-κB is detected in the intestinal lamina propria to the point that the degree of NF-κB activation correlates with the severity of intestinal inflammation [8].
Toll-like receptors (TLRs) belong to a family of receptors that can recognize all classes of pathogens, including parasitic invaders. TLRs are thought to play an important role in the rapid activation of innate immune responses in coordination with the adaptive immune response to eliminate pathogens [9]. Toll-like receptors are predominantly expressed on immune related cells such as monocytes, macrophages, neutrophils, dendritic cells, lymphocytes, and NK cells [10]. Moreover, it has been shown that TLRs are also expressed on other non-immune cells, especially in the epithelium, including epithelial cells of the gastrointestinal and respiratory tracts [11], [12]. The link between the activation of TLR2 and intestinal disease has been reported, both in the colon and in the ileum [13].
Whether TLR2 and NF-κB are involved in the pathogenesis of intestinal schistosomiasis is still to be elucidated. Their role to induce cellular activation and the mechanisms by which they can affect the pathogenesis of intestinal schistosomiasis needs to be studied.
Material and methods
Parasite
Laboratory bred Biomphalaria alexandrina snails were purchased from the Schistosome Biological Supply Program, Theodore Bilharz Research Institute (Giza, Egypt). According to Lewis et al. [14], the snails were placed in beakers containing dechlorinated water (1 ml/snail) and exposed to direct light at 28 °C for at least 4 h. S. mansoni cercariae shed from the snails were used to infect the experimental animals of the study. The cercarial suspension was adjusted to contain 50–60 cercariae/0.1 ml dechlorinated water.
Animals and experimental design
A total of 115 laboratory bred male Swiss albino mice, 6–8 weeks old, weighing 20–25 g were purchased from Theodore Bilharz Research Institute (Giza, Egypt). The experiment was conducted in accordance with the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978). Mice were housed in appropriate cages and allowed ad libitum for a commercial rodent chow and tap water. They were divided into two groups; group I: 10 non-infected mice (control group) sacrificed once 6 weeks from the start of the experiment, group II: mice infected by subcutaneous injection of 0.1 ml cercarial suspension as described by Peter and Warren [15] then 15 mice were sacrificed every two weeks postinfection (PI) starting from 6 week PI till 18 week PI. From each mouse, colon was removed and preserved for histopathological and immunohistochemical studies.
Histopathological studies
The distal 3 cm of the colon was cut and washed with saline and fixed in 4% formol saline. The specimen was dehydrated in ascending grades of ethanol and cleared in xylene then embedded into paraffin. Serial sections from colonic tissues of 5 μm thickness were obtained and stained with hematoxylin and eosin (H&E). Four colonic sections were examined for each mouse [16]. Histological score in these sections was determined according to Dieleman et al. [17] with modifications. The following items were assessed: the degree (0–3) and extent (0–3) of inflammation, crypt damage (0–4) and the area involved (0–4) (Table 1). The score of each parameter was multiplied by four and the sum of these multiples was the final score. For the estimation of the number and size of granuloma, morphometric analysis was performed using Leica microscope with built-in camera (Leica Image System Ltd, Cambridge, UK) in Histology Department, Faculty of Medicine, Tanta University to assess the mean number and size of granuloma in five randomly selected fields at 400× magnification [18].
Table 1.
Histological scoring system of colitis according to Dieleman et al. [17] with modification.
| Histological changes | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Degree of inflammation | None | Mild | Moderate | Severe | – |
| Extent of inflammation | None | Mucosa | Mucosa | Submucosa | Transmural |
| Crypt damage | None | Damage of basal 1/3 | Damage of basal 2/3 | Intact surface epithelium only | Loss of entire epithelium and crypt |
| Ulcer | Absence of ulcer | 1 or 2 foci of ulceration | 3 or 4 foci of ulceration | More than 4 foci of ulceration | Confluent & extensive ulceration |
Immunohistochemical staining
Briefly, paraffin-embedded sections were deparaffinized, rehydrated, and microwave heated for 15 min in 0.01 mol/L citric buffer (pH 6.0) for antigen retrieval. Then, 3% hydrogen peroxide was applied to block endogenous peroxidase activity. After 30 min of blocking with normal serum (Invitrogen, Carlsbad, CA), the primary antibodies were added and incubated overnight at 4 °C. They were in the form of TLR2 (a mouse monoclonal antibody; Dako) and the primary rabbit anti-phospho-NF-κB p65 ser276 antibody (NF kappa B p65) (henceforth pp65, Cell Signaling, Danvers, MA). Slides were washed thrice with phosphate buffer solution (PBS), each for 5 min. The biotinylated secondary antibody and the streptavidin–biotin complex were applied, each for 60 min incubation at room temperature. After rinsing with PBS, the slides were immersed for 10 min in 3,39-diaminobenzidine (Sigma, St. Louis, MO) solution (0.4 mg/mL with 0.003% hydrogen peroxide), monitored under the microscope and the reaction was terminated by adding distilled water. Slides were then counterstained with hematoxylin, dehydrated, and coverslipped. Immunoreactivity of NF-κB appeared as brown cytoplasmic and nuclear staining of varying degrees of intensity in epithelial and inflammatory cells. Immunoreactivity of TLR2 appeared as brown cytoplasmic staining of varying degrees of intensity in epithelial cells and inflammatory cells. For negative control, the primary antibody was replaced by PBS [19]. For the estimation of number of TLR2 and NF-κB positive cells, morphometric analysis was performed on immunostained sections to measure the number of TLR2 and NF-κB positive cells whether nuclear or cytoplasmic. Ten random nonoverlapping fields in each slide were examined and digitally imaged at magnification of 400×.
Statistical analysis
Data were presented as mean ± standard deviation (SD). The data were analyzed by One Way-ANOVA with Scheffe post-test to determine significance of differences between groups. The results were considered statistically significant if P < 0.05 and highly significant if P < 0.001. The statistical analyses were processed using Statistical Program of Social Sciences (SPSS) software for windows, version 14.0.
Results
Histopathological studies
Hematoxylin and eosin stained colonic sections of the control mice were formed of mucosa, submucosa and muscularis externa. The colonic mucosa was formed of two types of cells; simple columnar enterocytes with apical regular eosinophilic brush border and many goblet cells in-between. The surface was punctated with pits leading to packed deep crypts of Lieberkuhn. Crypts were embedded in the lamina propria of highly cellular connective tissue and extended down to the muscularis mucosa. The submucosa was formed of connective tissue. The muscularis externa was formed of inner circular and outer longitudinal muscle layers (Fig. 1A).
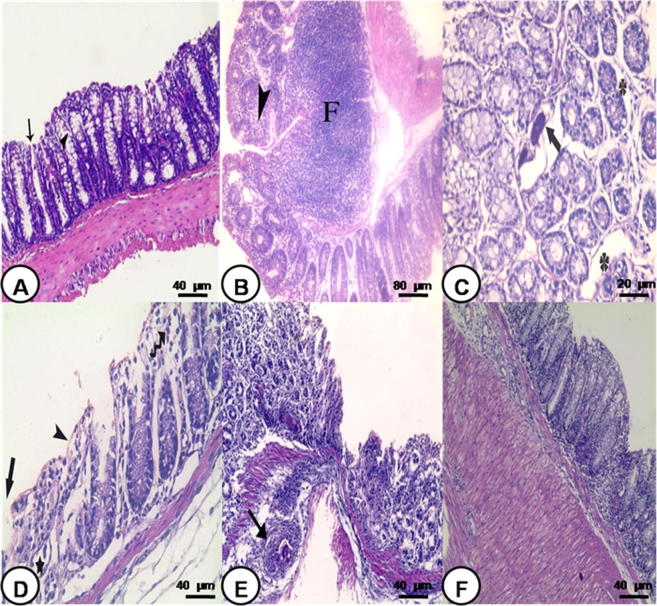
Fig. 1.
Photomicrograph of colon: (A) control noninfected group showing mucosa with many goblet cells in-between (arrow head). Notice crypts of Lieberkuhn (arrow) with underlying normal submucosa and muscularis externa (H&E 200×), (B) S. mansoni infected group 6 weeks PI showing large sized coalescent follicles (F) with reactive germinal center in the submucosa extending into the lamina propria and mononuclear cellular infiltration in-between the crypts of Lieberkuhn (arrow head) (H&E 100×), (C) S. mansoni infected group 8 weeks PI showing ova deposition (arrow) and edema (star) (H&E 400×), (D) S. mansoni infected group 10 weeks PI showing superficial mucosal ulceration (arrow), degenerated enterocytes (arrow head) with disrupted crypts (star). Notice mononuclear cellular infiltration (wavy arrow) (H&E 200×), (E) S. mansoni infected group 10 weeks PI showing loss of architecture, damaged crypts, cellular infiltration and granuloma formation (arrow) (H&E 200×), and (F) S. mansoni infected group 18 weeks PI showing mild cellular infiltration in mucosa and submucosa and thickening of the muscularis externa (H&E 200×).
The colon of S. mansoni infected mice showed different degrees of microscopical changes in varying severity depending on the period postinfection, being highly severe during 6 and 8 weeks PI and reached its peak at 10 weeks PI and decreased gradually starting from 12 weeks PI onward. The colon of mice obtained 6 and 8 weeks PI showed transmural mononuclear cellular infiltration involving all layers of the colon. Many large sized and coalescent follicles with reactive germinal center were seen in the submucosa and extending into the lamina propria (Fig. 1B). The crypt of Lieberkuhn showed ova deposition in association with interstitial edema (Fig. 1C). The colon showed the most extensive morphological alterations at 10 weeks PI. These changes were in the form of loss of the architecture, decrease thickness of the mucosa. Enterocytes showed degeneration with vacuolation of the cytoplasm and irregularity or loss of their nuclei in association with superficial mucosal ulceration and loss of goblet cells. Many crypts showed dilatation with loss of the cellular lining or disruption of their wall (Fig. 1D). Massive mononuclear cell infiltration disrupting all layers of the colon in association with granuloma formation was observed (Fig. 1E).
The colonic mucosa of mice at 12, 14 and 16 weeks PI displayed decrease of most of the microscopic changes. However, medium sized lymphoid aggregates located below intact surface epithelium in the proximity of normal looking crypts and in nearly all layers of the colon were still seen. The colonic mucosa of infected mice 18 weeks PI showed regeneration of a surface epithelium and complete crypt formation. Musculosa was thickened and many smooth muscles showed vacuolation in association with localized mononuclear cellular infiltrate (Fig. 1F). The histological scoring system confirmed the histological findings. It showed statistically significant increase at 6 week PI and highly significant increase at 8, 10, 12 and 14 weeks PI then it started to decrease gradually from 14 weeks PI onward. At 18 weeks PI, there was no significant difference in comparison with control group (Table 2).
Table 2.
The histological scoring system for the different studied groups.
| Control | 6 weeks PI | 8 weeks PI | 10 weeks PI | 12 weeks PI | 14 weeks PI | 16 weeks PI | 18 weeks PI | |
|---|---|---|---|---|---|---|---|---|
| Mean | 2.72 | 22 | 47.84 | 50.72 | 36.48 | 34.07 | 28.53 | 11.84 |
| ±SD | 0.635 | 5.63 | 7.58 | 12.35 | 10.32 | 8.30 | 9.64 | 3.10 |
| F. test | 6.889 | |||||||
| P value | – | 0.002⁎ | 0.001⁎⁎ | 0.001⁎⁎ | 0.001⁎⁎ | 0.001⁎⁎ | 0.003⁎ | 0.190 |
P value <0.05 means that difference is statistically significant.
P value <0.001 means that difference is statistically highly significant.
As regards the granuloma, different forms of variables sized granulomas were seen situated either in the lamina propria in-between the crypts of Lieberkuhn, or in the submucosa and rarely in the musculosa. Some granulomas presented ova only (Fig. 1C) as in 8 weeks PI, while others displayed ova surrounded with inflammatory cells with or without fibrosis at 10 weeks PI (Fig. 1E). Some granulomas were seen without ova at 16 weeks PI. However, absence of granuloma was observed in some colonic sections at 18 weeks PI. The most common inflammatory cells seen were in the form lymphocytes, histiocytes (macrophages), eosinophils and few neutrophils. Those surrounded with fibrosis showing fibroblast and collagen fibers. The colonic mucosa 10 weeks PI showed the maximum mean numbers and size of granulomas per colonic sections in comparison with others which started to decrease gradually onward with time (Table 3).
Table 3.
The characters of the granuloma in the different studied groups.
| Control | 6 weeks PI | 8 weeks PI | 10 weeks PI | 12 weeks PI | 14 weeks PI | 16 weeks PI | 18 weeks PI | |
|---|---|---|---|---|---|---|---|---|
| Number of granuloma/colonic sections (mean ± SD) | – | 0.76 ± 0.12 | 1.36 + 0.34 | 3.84 + 0.85 | 1.48 + 0.63 | 1.16 + 0.27 | 1.52 + 0.42 | 1.04 + 0.35 |
| P value | – | – | 0.035⁎ | 0.014⁎ | 0.010⁎ | 0.019⁎ | 0.024⁎ | 0.047⁎ |
| Diameter of the granuloma in μm (mean ± SD) | – | 49.74 ± 13.65 | 79.61 ± 11.95 | 101.25 ± 25.97 | 59.95 ± 20.23 | 61.81 ± 18.37 | 39.81 ± 14.63 | 29.93 ± 12.02 |
| P value | – | – | 0.352 | 0.085 | 0.635 | 0.424 | 0.158 | 0.335 |
| Type of the granuloma | – | – | Cellular granuloma (Ova without granuloma) | Cellular granuloma (Ova with and without fibrosis) | Fibrocellular granuloma (Ova with fibrosis) | Fibrocellular granuloma (Ova with fibrosis) | Fibrotic granuloma (Without ova) | – |
| Site of the granuloma | – | – | Mucosa (Lamina propria) | Submucosa & musculosa | Submucosa | – |
Significant.
Immunohistochemical staining
Expression of TLR2 in colon tissues
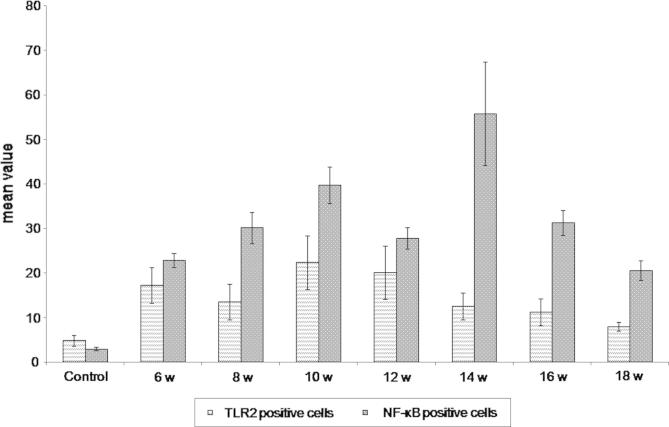
Immunostained sections of control group showed mild positive immunoreaction in the cytoplasm of crypt epithelial cells and in some sporadic cells of the lamina propria. However, this expression decreased at the luminal surface (Fig. 2A). The colon of S. mansoni infected mice showed increased expression of TLR2 in both surface and crypt epithelium reaching all layers of the colon. Also, some inflammatory cells showed expression of TLR2 mainly lymphocytes and few macrophages. It showed strong reaction in the lymphoid cells close to the crypt rather than the luminal surface of the epithelium (Fig. 2B). TLR2 expression was also detected in the granuloma cells (Fig. 2C). The immune reaction was diffuse involving nearly all layers of the colon at 10 and 12 weeks PI then decreased gradually and became localized at 18 weeks PI. The mean number of TLR2 positive cells in the colonic sections was significantly increased after 6 and 8 weeks PI to be highly significantly increased 10 and 12 weeks PI and became insignificant at 18 weeks PI as compared to the control group (P < 0.001) (Fig. 3).
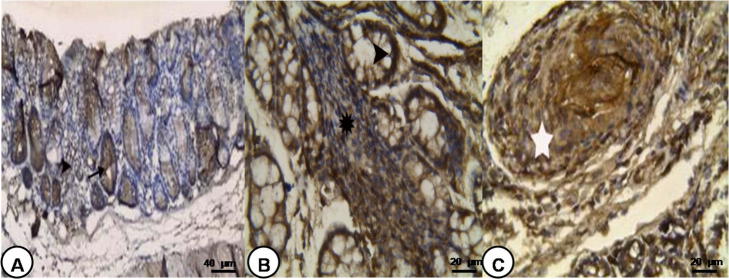
Fig. 2.
Photomicrograph of TLR2 immunostaining of the colon: (A) control noninfected group showing positive immunoreaction in the cytoplasm of crypt epithelial cells (arrow) and in some sporadic cells of the lamina propria (arrow head) (TLR2 immunostaining 200×), (B) S. mansoni infected group 8 weeks PI showing positive immunoreaction in the cytoplasm of crypt epithelium (arrow head) and many cells of the lamina propria and lymph follicle (star) (TLR2 immunostaining 400×), and (C) S. mansoni infected group 10 weeks PI showing positive immunoreaction in the granuloma (star) (TLR2 immunostaining 400×).
Fig. 3.
Mean number of TLR2 and NF-κB positive cells in colonic mucosa of the studied groups at different durations. The mean number of TLR2 and NF-κB positive cells in the colonic sections was significantly increased at 6 and 8 week PI to be highly significantly increased at 10 weeks PI as compared to the control group (P < 0.001).
Expression of NF-κB in colon tissues
Light microscopic examination of colonic immunostained sections of control group showed negative immunoreaction for NF-κB in all layers. Colon of S. mansoni infected mice displayed various degrees of immunoexpression for NF-κB in the cytoplasm and also in the nuclei of many cells. Surface epithelium, cells of the crypts and separate cells of the lamina propria, submucosa and muscularis externa were common sites for immunoexpression (Fig. 4A). Also, NF-κB was expressed in cells of the granuloma and those of lymphoid follicles mainly in lymphocytes and few macrophages (Fig. 4B and C). At 6 weeks PI, the colon of the infected mice showed positive reaction which continue up to 16 weeks PI while at 18 weeks PI, the colon showed decreased expression but it did not disappear completely (Fig. 4D). Morphometric analysis showed significant increase in the mean number of NF-κB positive cells in the colonic sections at 6 week PI to be highly significantly at 10 weeks PI up to 16 weeks PI which then decreased at 18 weeks PI as compared to the control group (Fig. 4E) (P < 0.001) (Fig. 3).
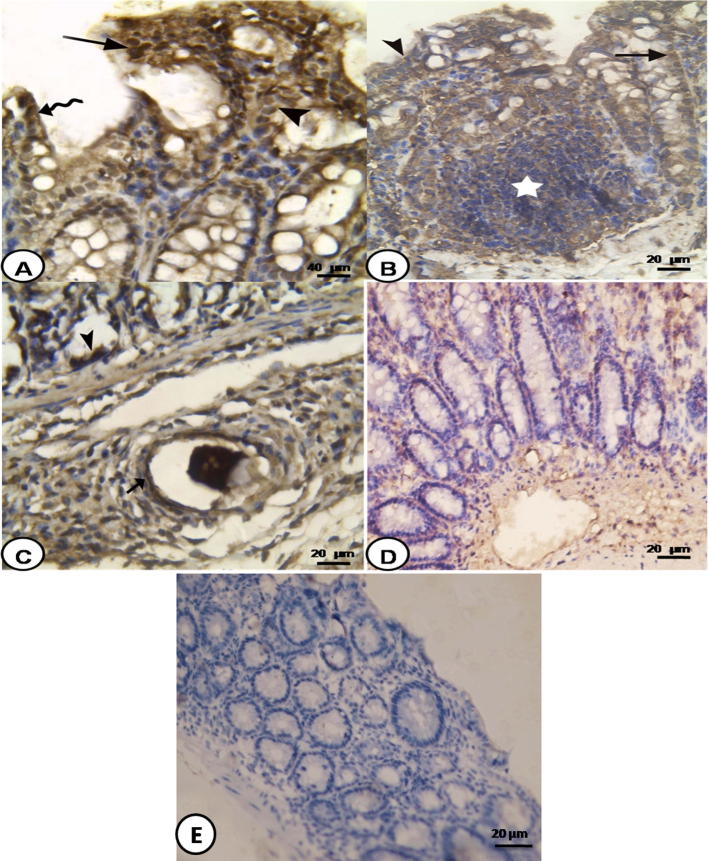
Fig. 4.
Photomicrograph of S. mansoni infected colon: (A) 8 weeks PI showing positive immunoreaction for NF-κB in the nuclei of enterocytes (wavy arrow), epithelial crypts (arrow head) and cells of lamina propria (arrow)(NF-κB immunostaining 200×), (B) 6 weeks PI showing positive immunoreaction for NF-κB in the surface epithelium (arrow head), cells of the crypts (arrow), and lymphoid follicles (star) (NF-κB immunostaining 400×), (C) 8 weeks PI showing positive immunoreaction for NF-κB in crypt epithelial cells (arrow head) and cells of the granuloma (arrow) (NF-κB immunostaining 400×), (D) 16 weeks PI showing decreased immunoreaction for NF-κB in the cells of the crypts and of the lamina propria (NF-κB immunostaining 400×), and (E) negative control showed no immune reaction (NF-κB immunostaining 400×).
Discussion
Intestinal schistosomiasis is caused by Schistosoma egg induced pathology and granulomatous response [2]. Histopathological results showed that S. mansoni infected colons showed different degrees of microscopical changes with increasing severity peaked at 10 weeks PI. Those changes were variable from transmural mononuclear cellular infiltration, granuloma formation, ova deposition in association with interstitial edema superficial mucosal ulceration, loss of goblet cells and loss of architecture. The colonic mucosa of infected mice started regeneration at 16 and 18 weeks PI. The same results were obtained by Ross et al. [20]. They found that eggs retained in the intestinal wall cause an inflammatory response which may lead to hyperplasia, ulceration, microabscess, and granuloma formation. Few reports described colorectal polyposis associated with S. mansoni. The polyps showed granulomatous inflammation around multiple Schistosoma eggs [21]. Godyn et al. [22] and Cao et al. [23] observed that microscopic examination showed that schistosomal colonic mucosa have generally preserved crypt architecture with moderate inflammatory infiltrate in mucosa. Intact Schistosoma ova were deposited in lamina propria with infiltration of eosinophils and neutrophilic granulocytes in case of acute schistosomal colitis whereas calcified ova were surrounded by infiltration of lymphocytes and plasma cells in chronic schistosomal colitis. Some areas with distorted crypt architecture and intraluminal leukocytes were noted, usually next to disintegrating eggs. Also, submucosal hyperblastosis and fibrosis could also be found in chronic cases.
In the present study, the granulomas decreased in number and size with time. The same trend was observed by Akdis et al. [24]. They showed that in vivo treatment with LP40, a TLR2 ligand, significantly decreases granuloma size in the S. mansoni-egg-induced lung model. In the chronic stage of infection, Layland et al. [25] demonstrated that lyso-PS from S. mansoni eggs could stimulate DCs to induce IL-10-producing Treg cells in a TLR2-dependent manner thus regulate and ameliorate of chronic inflammation of Schistosoma infection.
As regards TLR2, we found that it is more highly expressed in a diffuse manner involving nearly all layers of the colon at 10 and 12 weeks PI. Its expression decreased gradually and became localized after 18 weeks PI. These results coincide with those of Belmonte et al. [26]. They showed that TLR2 was present in the crypts and luminal surface and also they localize its expression in epithelial cells and promote an increased production of mucosal proinflammatory cytokines which could indicate the potential role of epithelial cells in the inflammatory process.
Activation of TLRs leads to the production of proinflammatory cytokines (such as IFN-γ and TNF-α), and synthesis of reactive oxygen and nitrogen intermediates via the activation of several transcription factors, particularly NF-κB [27], [28]. Studies have demonstrated that NF-κB is a key transcription factor of lymphocytes and macrophages with important regulatory functions on the inflammatory processes [29]. Characteristically, NF-κB proteins are sequestered in the cytoplasm as a result of retention by a class of inhibitory proteins, referred to as the IKB family. On stimulation, IKB is phosphorylated and proteolytic degradation takes place rapidly. The free NF-κB is then translocated to the nucleus and regulates transcriptional activity by binding to specific DNA sequences in promoter/enhancer regions of inflammation genes [29], [30]. NF-κB is markedly activated in case of inflammation to the point that the degree of NF-κB activation correlates with the severity of intestinal inflammation [8]. We therefore investigated the expression of NF-κB protein and found that increased amounts of NF-κB protein in nuclei of colonic cells of mice infected with S. mansoni in comparison normal control mice. Positive reaction continued up to 16 weeks PI. At 18 weeks PI, the colon showed decrease expression of NF-κB protein but it not completely disappeared. It was found that the expression of both TLR2 and NF-κB was decreased after the 12 weeks PI even though the parasite burden is still high. Cheng et al. [31] found that the expressions of TLRs are suppressed after the deposition of Schistosoma eggs and that Schistosoma modulates dendritic cells via TLRs-mediated signals, and subsequently influences generation of different inflammatory mediators. The same trend was also described by van der Klei et al. [32]. They showed that chronic infection with S. haematobium leads to down-regulated responses to TLR ligands that may be due to continuous stimulation of the immune system leading to tolerance in the innate immune system. Similarly, Wang et al. [33] suggested that down-regulation of TLR expression may be due to tolerance to TLR-activating molecules. In addition to that, the activation of NF-κB results in a shift from the cytoplasm to the nucleus so its expression disappeared from cytoplasm.
The most important aspect of this study is its potential application in the treatment of colonic schistosomiasis after being sure from the similarities between human and mouse TLR2 pathways. Immunological and inflammatory pathways regulating NF-κB and its downstream processes can be exploited, e.g. via the use of TLR2mAb. Dong et al. [34] suggested that TLR2mAb can suppress induced colitis. Atreya et al. [35] stated that NF-κB is considered an excellent target for the development of pharmacological agents for inflammatory bowel disease.
Conclusions
Recruitment and activation of inflammatory cells to the colonic mucosa in intestinal schistosomiasis are multifactorial events involving TLR2 that can trigger the NF-κB pathways. Hence, down-regulation of both TLR2 and NF-κB could be exploited in the treatment of colonic schistosomiasis.
Conflict of Interests
The authors have declared no conflict of interest.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.El-Shiekh A., Al Karawi M.A., Yasawy M.I. Schistosomal colonic disease. Gut. 1990;31:439–442. doi: 10.1136/gut.31.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson M.S., Mentink-Kane M.M., Pesce J.T., Ramalingam T.R., Thompson R., Wynn T.A. Immunopathology of schistosomiasis. Immunol Cell Biol. 2007;85:148–154. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearce E.J., MacDonald A.S. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 4.Reiman R.M., Thompson R.W., Feng C.G., Hari D., Knight R., Cheever A.W. Interleukin-5 (IL-5) augments the progression of liver fibrosis by regulating IL-13 activity. Infect Immun. 2006;74:1471–1479. doi: 10.1128/IAI.74.3.1471-1479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann K.F., Cheever A.W., Wynn T.A. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol. 2000;164:6406–6416. doi: 10.4049/jimmunol.164.12.6406. [DOI] [PubMed] [Google Scholar]
- 6.Booth M., Mwatha J.K., Joseph S., Jones F.M., Kadzo H., Ireri E. Periportal fibrosis in human Schistosoma mansoni infection is associated with low IL-10, low IFN-gamma, high TNF-alpha, or low RANTES, depending on age and gender. J Immunol. 2004;172:1295–1303. doi: 10.4049/jimmunol.172.2.1295. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava S., Ramana K.V. Focus on molecules: nuclear factor-kappa B. Exp Eye Res. 2009;88:2–3. doi: 10.1016/j.exer.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogler G., Brand K., Vogl D., Page S., Hofmeister R., Andus T. Nuclear factor Kappa B is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–369. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- 9.Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 10.McGettrick A.F., O’Neill L.A. Toll-like receptors: key activators of leucocytes and regulator of haematopoiesis. Br J Haematol. 2007;139:185–193. doi: 10.1111/j.1365-2141.2007.06802.x. [DOI] [PubMed] [Google Scholar]
- 11.Ortega-Cava C.F., Ishihar S., Rumi M.A., Kawashima K., Ishimura N., Kazumori H. Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J Immunol. 2003;170:3977–3985. doi: 10.4049/jimmunol.170.8.3977. [DOI] [PubMed] [Google Scholar]
- 12.Yu L., Chen S. Toll-like receptors expressed in tumor cells: targets for therapy. Cancer Immunol Immunother. 2008;57:1271–1278. doi: 10.1007/s00262-008-0459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szebeni B., Veres G., Dezsofi A., Rusai K., Vannay A., Bokodi G. Increased mucosal expression of Toll-like receptor (TLR)2 and TLR4 in coeliac disease. J Pediatr Gastroenterol Nutr. 2007;45:187–193. doi: 10.1097/MPG.0b013e318064514a. [DOI] [PubMed] [Google Scholar]
- 14.Lewis F.A., Stirewalt M.A., Souza C.P., Gazzinelli G. Large-scale laboratory maintenance of Schistosoma mansoni, with observations on three schistosome snail host combination. J Parasitol. 1986;72(6):813–829. [PubMed] [Google Scholar]
- 15.Peter P.A., Warren K.S. A rapid method for infecting mice and other laboratory animals with subcutaneous injection. J Parasitol. 1969;131:558–561. [Google Scholar]
- 16.Marilyn G. The hematoxylin and eosin. In: Bancroft J.D., Gample M., editors. Theory and practice of histological technique. 6th ed. Elsevier; China: 2008. p. 121. [Google Scholar]
- 17.Dieleman L.A., Palmen M.J.H.J., Akol H.E., Pen A.S.A., Meuwissen S.G.M., Vanrees E.P. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali S.A., Hamed M.A. Effect of Ailanthus altissima and Ziziphus spina Christi on bilharzial infestation in mice. Histological and Histopathological studies. J Appl Sci. 2006;6(7):1437–1446. [Google Scholar]
- 19.Jackson P., Blythe D. Immunohistochemical techniques. In: Bancroft J.D., Gample M., editors. Theory and practice of histological technique. 6th ed. Elsevier; China: 2008. p. 423. [Google Scholar]
- 20.Ross A.G., Bartley P.B., Sleigh A.C., Olds G.R., Li Y., Williams G.M., McManus D.P. Schistosomiasis. N Engl J Med. 2002;346:1212–1220. doi: 10.1056/NEJMra012396. [DOI] [PubMed] [Google Scholar]
- 21.Mesquita N.F., Silva R.A., Brandão C.L., Dinis-Ribeiro M.J., Fernandes N.S., Lomba-Viana H. Schistosomal colonic polyposis. Gastrointest Endosc. 2003;58:910–911. doi: 10.1016/s0016-5107(03)02276-4. [DOI] [PubMed] [Google Scholar]
- 22.Godyn J.J., Siderits R., Hazra A. Schistosoma mansoni in colon and liver. Arch Pathol Lab Med. 2005;129:544–545. doi: 10.5858/2005-129-544-SMICAL. [DOI] [PubMed] [Google Scholar]
- 23.Cao J., Liu W.J., Xu X.Y., Zou X.P. Endoscopic findings and clinicopathologic characteristics of colonic schistosomiasis: a report of 46 cases. World J Gastroenterol. 2010;16(6):723–727. doi: 10.3748/wjg.v16.i6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akdis C.A., Kussebi F., Pulendran B., Akdis M., Lauener R.P., Schmidt-Weber C.B. Inhibition of T helper 2-type responses, IgE production and eosinophilia by synthetic lipopeptides. Eur J Immunol. 2003;33:2717–2726. doi: 10.1002/eji.200323329. [DOI] [PubMed] [Google Scholar]
- 25.Layland L.E., Rad R., Wagner H., da Costa C.U. Immunopathology in schistosomiasis is controlled by antigen-specific regulatory T cells primed in the presence of TLR2. Eur J Immunol. 2007;37:2174–2184. doi: 10.1002/eji.200737063. [DOI] [PubMed] [Google Scholar]
- 26.Belmonte L., Beutheu Youmba S., Bertiaux-Vandaële N., Antonietti M., Lecleire S., Zalar A. Role of toll like receptors in irritable bowel syndrome: differential mucosal immune activation according to the disease subtype. PLoS ONE. 2012;7(8):e42777. doi: 10.1371/journal.pone.0042777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Werling D., Jungi T.W. Toll-like receptors linking innate and adaptive immune response. Vet Immunol Immunopathol. 2003;91:1–12. doi: 10.1016/s0165-2427(02)00228-3. [DOI] [PubMed] [Google Scholar]
- 28.Kawai T., Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 29.Baldwin A.S. The transcription factor NF-κB and human disease. J Clin Invest. 2001;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karin M., Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 31.Cheng P.C., Lin C.N., Peng S.Y., Li L.L., Luo T.Y., Fan C.K. A study of immunomodulatory genes responses to macrophages of Schistosoma japonicum infection during different stages by microarray analysis. Acta Trop. 2013;127(3):251–260. doi: 10.1016/j.actatropica.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 32.van der Kleij D., van den Biggelaar A.H., Kruize Y.C., Retra K., Fillie Y., Schmitz M. Responses to Toll-like receptor ligands in children living in areas where schistosome infections are endemic. J Infect Dis. 2004;189(6):1044–1051. doi: 10.1086/382089. [DOI] [PubMed] [Google Scholar]
- 33.Wang J.H., Doyle M., Manning B.J., Di Wu Q., Blankson S., Redmond H.P. Induction of bacterial lipoprotein tolerance is associated with suppression of toll-like receptor 2 expression. J Biol Chem. 2002;277:36068–36075. doi: 10.1074/jbc.M205584200. [DOI] [PubMed] [Google Scholar]
- 34.Dong L., Li J., Liu Y., Yue W., Luo X. Toll-like receptor 2 monoclonal antibody or/and Toll-like receptor 4 monoclonal antibody increase counts of Lactobacilli and Bifidobacteria in dextran sulfate sodium-induced colitis in mice. J Gastroenterol Hepatol. 2012;27(1):110–119. doi: 10.1111/j.1440-1746.2011.06839.x. [DOI] [PubMed] [Google Scholar]
- 35.Atreya I., Atreya R., Neurath M.F. NF-kappa B in inflammatory bowel disease. J Intern Med. 2008;263:591–596. doi: 10.1111/j.1365-2796.2008.01953.x. [DOI] [PubMed] [Google Scholar]