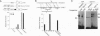Abstract
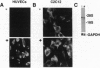
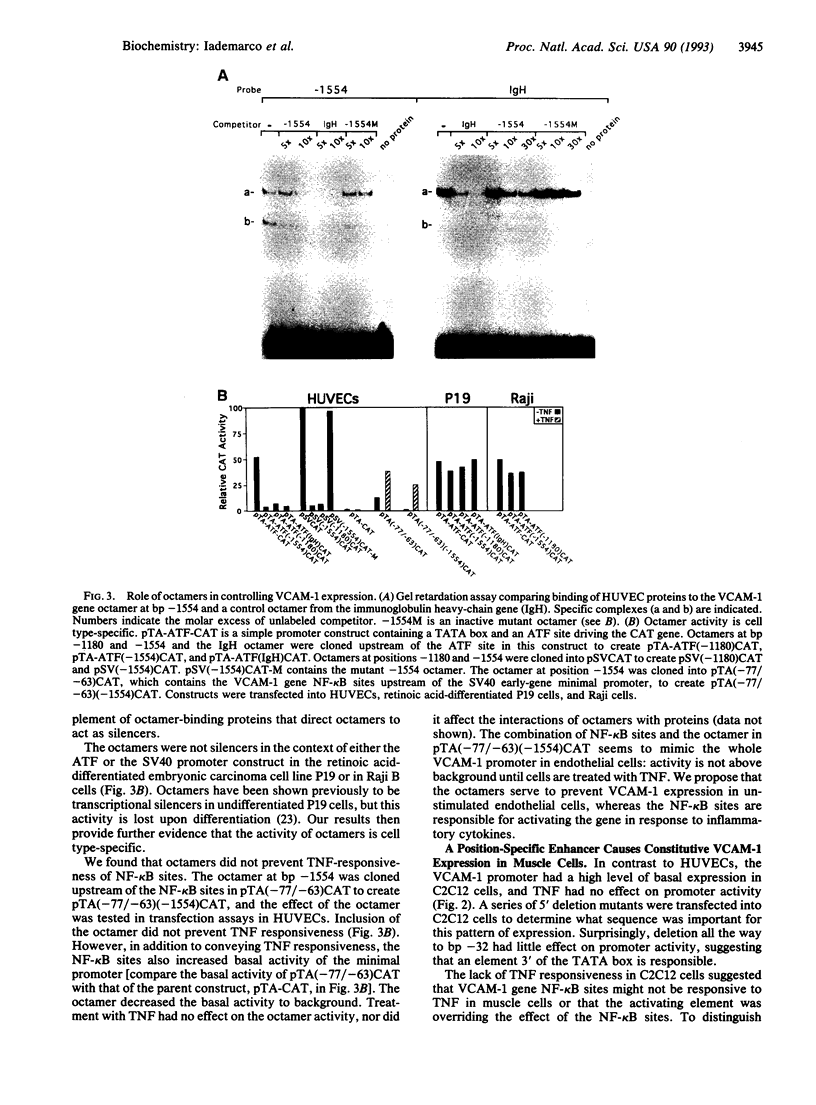
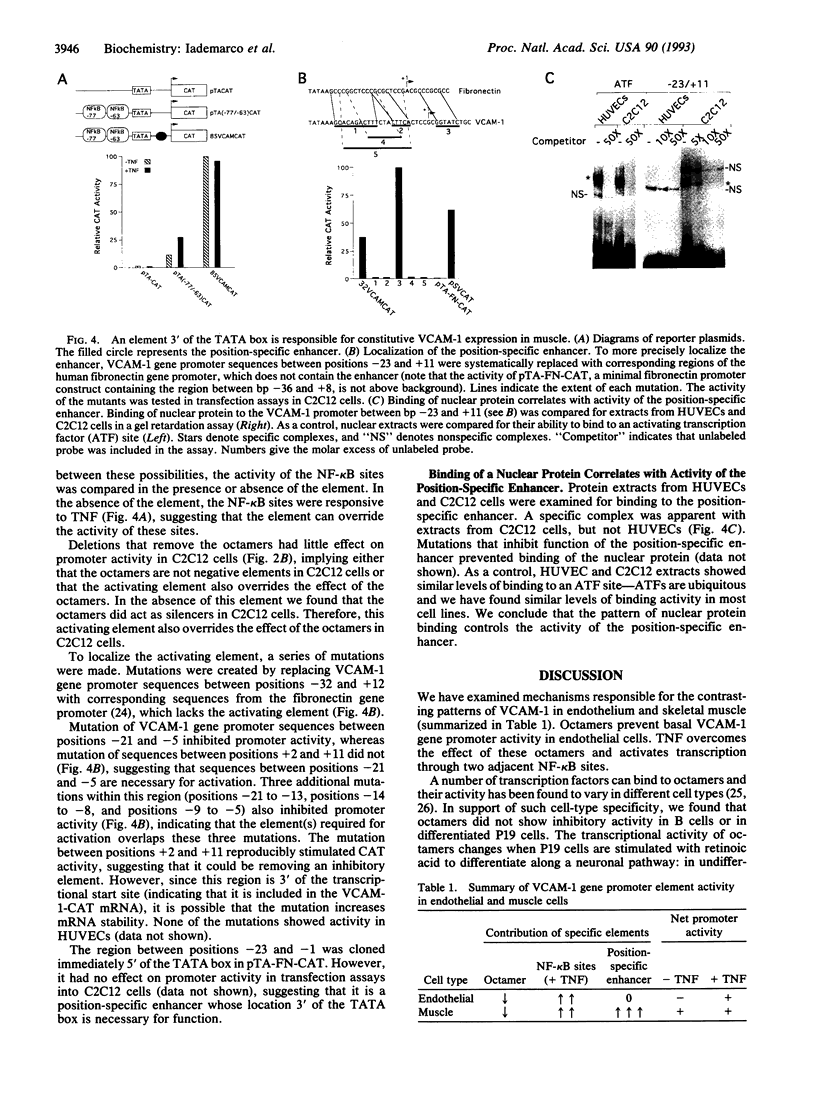
Interaction between vascular cell adhesion molecule 1 (VCAM-1), which appears on the surface of endothelial cells in response to inflammation, and its integrin counter receptor, alpha 4 beta 1, on immune cells is responsible for targeting these immune cells to cytokine-stimulated endothelium. In addition to its role in the immune system, VCAM-1 is also expressed in a developmentally specific pattern on differentiating skeletal muscle, where it mediates cell-cell interactions important for myogenesis through interaction with alpha 4 beta 1. In contrast to endothelium, there is high basal expression of VCAM-1 in skeletal muscle cells and the expression is not cytokine-responsive. Here, we examine the molecular basis for these contrasting patterns of expression in muscle and endothelium, using VCAM-1 promoter constructs in a series of transfection assays. In endothelial cells, octamer binding sites act as silencers that prevent VCAM-1 expression in unstimulated cells. Tumor necrosis factor alpha overcomes the negative effects of these octamers and activates the promoter through two adjacent NF-kappa B binding sites. In muscle cells, a position-specific enhancer located between bp -21 and -5 overrides the effect of other promoter elements, resulting in constitutive VCAM-1 expression. A nuclear protein binds the position-specific enhancer in muscle but not endothelial cells; thus the pattern of expression of this protein could control enhancer activity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkenmeier T. M., McQuillan J. J., Boedeker E. D., Argraves W. S., Ruoslahti E., Dean D. C. The alpha 5 beta 1 fibronectin receptor. Characterization of the alpha 5 gene promoter. J Biol Chem. 1991 Oct 25;266(30):20544–20549. [PubMed] [Google Scholar]
- Carlos T., Kovach N., Schwartz B., Rosa M., Newman B., Wayner E., Benjamin C., Osborn L., Lobb R., Harlan J. Human monocytes bind to two cytokine-induced adhesive ligands on cultured human endothelial cells: endothelial-leukocyte adhesion molecule-1 and vascular cell adhesion molecule-1. Blood. 1991 May 15;77(10):2266–2271. [PubMed] [Google Scholar]
- Dean D. C., Bowlus C. L., Bourgeois S. Cloning and analysis of the promotor region of the human fibronectin gene. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1876–1880. doi: 10.1073/pnas.84.7.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. C., McQuillan J. J., Weintraub S. Serum stimulation of fibronectin gene expression appears to result from rapid serum-induced binding of nuclear proteins to a cAMP response element. J Biol Chem. 1990 Feb 25;265(6):3522–3527. [PubMed] [Google Scholar]
- Dent C. L., Lillycrop K. A., Estridge J. K., Thomas N. S., Latchman D. S. The B-cell and neuronal forms of the octamer-binding protein Oct-2 differ in DNA-binding specificity and functional activity. Mol Cell Biol. 1991 Aug;11(8):3925–3930. doi: 10.1128/mcb.11.8.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Erle D. J., Rüegg C., Sheppard D., Pytela R. Complete amino acid sequence of an integrin beta subunit (beta 7) identified in leukocytes. J Biol Chem. 1991 Jun 15;266(17):11009–11016. [PubMed] [Google Scholar]
- Freedman A. S., Munro J. M., Rice G. E., Bevilacqua M. P., Morimoto C., McIntyre B. W., Rhynhart K., Pober J. S., Nadler L. M. Adhesion of human B cells to germinal centers in vitro involves VLA-4 and INCAM-110. Science. 1990 Aug 31;249(4972):1030–1033. doi: 10.1126/science.1697696. [DOI] [PubMed] [Google Scholar]
- Haggarty A., Camato R., Paterno G., Cohen L., Hiscott J., Skup D. A developmentally regulated octamer-binding activity in embryonal carcinoma cells which represses beta-interferon expression. Cell Growth Differ. 1991 Oct;2(10):503–510. [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Schwarz L. The VLA protein family. Characterization of five distinct cell surface heterodimers each with a common 130,000 molecular weight beta subunit. J Biol Chem. 1987 Mar 5;262(7):3300–3309. [PubMed] [Google Scholar]
- Iademarco M. F., McQuillan J. J., Rosen G. D., Dean D. C. Characterization of the promoter for vascular cell adhesion molecule-1 (VCAM-1). J Biol Chem. 1992 Aug 15;267(23):16323–16329. [PubMed] [Google Scholar]
- Kemler I., Bucher E., Seipel K., Müller-Immerglück M. M., Schaffner W. Promoters with the octamer DNA motif (ATGCAAAT) can be ubiquitous or cell type-specific depending on binding affinity of the octamer site and Oct-factor concentration. Nucleic Acids Res. 1991 Jan 25;19(2):237–242. doi: 10.1093/nar/19.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
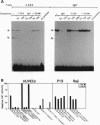
- McBurney M. W., Rogers B. J. Isolation of male embryonal carcinoma cells and their chromosome replication patterns. Dev Biol. 1982 Feb;89(2):503–508. doi: 10.1016/0012-1606(82)90338-4. [DOI] [PubMed] [Google Scholar]
- Miyake K., Medina K., Ishihara K., Kimoto M., Auerbach R., Kincade P. W. A VCAM-like adhesion molecule on murine bone marrow stromal cells mediates binding of lymphocyte precursors in culture. J Cell Biol. 1991 Aug;114(3):557–565. doi: 10.1083/jcb.114.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K., Weissman I. L., Greenberger J. S., Kincade P. W. Evidence for a role of the integrin VLA-4 in lympho-hemopoiesis. J Exp Med. 1991 Mar 1;173(3):599–607. doi: 10.1084/jem.173.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Rice G. E., Munro J. M., Bevilacqua M. P. Inducible cell adhesion molecule 110 (INCAM-110) is an endothelial receptor for lymphocytes. A CD11/CD18-independent adhesion mechanism. J Exp Med. 1990 Apr 1;171(4):1369–1374. doi: 10.1084/jem.171.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. E., Munro J. M., Corless C., Bevilacqua M. P. Vascular and nonvascular expression of INCAM-110. A target for mononuclear leukocyte adhesion in normal and inflamed human tissues. Am J Pathol. 1991 Feb;138(2):385–393. [PMC free article] [PubMed] [Google Scholar]
- Rosen G. D., Birkenmeier T. M., Dean D. C. Characterization of the alpha 4 integrin gene promoter. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4094–4098. doi: 10.1073/pnas.88.10.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen G. D., Sanes J. R., LaChance R., Cunningham J. M., Roman J., Dean D. C. Roles for the integrin VLA-4 and its counter receptor VCAM-1 in myogenesis. Cell. 1992 Jun 26;69(7):1107–1119. doi: 10.1016/0092-8674(92)90633-n. [DOI] [PubMed] [Google Scholar]
- Rüegg C., Postigo A. A., Sikorski E. E., Butcher E. C., Pytela R., Erle D. J. Role of integrin alpha 4 beta 7/alpha 4 beta P in lymphocyte adherence to fibronectin and VCAM-1 and in homotypic cell clustering. J Cell Biol. 1992 Apr;117(1):179–189. doi: 10.1083/jcb.117.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheeren R. A., Koopman G., Van der Baan S., Meijer C. J., Pals S. T. Adhesion receptors involved in clustering of blood dendritic cells and T lymphocytes. Eur J Immunol. 1991 May;21(5):1101–1105. doi: 10.1002/eji.1830210503. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Newman W., Gopal T. V., Horgan K. J., Graber N., Beall L. D., van Seventer G. A., Shaw S. Four molecular pathways of T cell adhesion to endothelial cells: roles of LFA-1, VCAM-1, and ELAM-1 and changes in pathway hierarchy under different activation conditions. J Cell Biol. 1991 Jun;113(5):1203–1212. doi: 10.1083/jcb.113.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale S. T., Baltimore D. The "initiator" as a transcription control element. Cell. 1989 Apr 7;57(1):103–113. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Herr W. Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell. 1990 Feb 9;60(3):375–386. doi: 10.1016/0092-8674(90)90589-7. [DOI] [PubMed] [Google Scholar]
- Weintraub S. J., Dean D. C. Interaction of a common factor with ATF, Sp1, or TATAA promoter elements is required for these sequences to mediate transactivation by the adenoviral oncogene E1a. Mol Cell Biol. 1992 Feb;12(2):512–517. doi: 10.1128/mcb.12.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]