Abstract
Rationale: Annual computed tomography (CT) is now widely recommended for lung cancer screening in the United States, although concerns remain regarding the potential harms, including those from overdiagnosis.
Objectives: To examine the effect of airflow limitation on overdiagnosis by comparing lung cancer incidence, histology, and stage shift in a subgroup of the National Lung Screening Trial (NLST).
Methods: In an NLST subgroup (n = 18,714), screening participants were randomized to annual computed tomography (CT, n = 9,357) or chest radiograph (n = 9,357) screening and monitored for a mean of 6.1 years. After baseline prebronchodilator spirometry, to identify the presence of airflow limitation, 18,475 subjects (99%) were assigned as having chronic obstructive pulmonary disease (COPD) or no COPD. Lung cancer prevalence, incidence, histology, and stage shift were compared after stratification by COPD.
Measurements and Main Results: For screening participants with spirometric COPD (n = 6,436), there was a twofold increase in lung cancer incidence (incident rate ratio, 2.15; P < 0.001) and, when compared according to screening arm, no excess lung cancers and comparable histology. Compared with chest radiography, there was also a trend favoring reduced late-stage and increased early-stage cancers in the CT arm (P = 0.054). For those with normal baseline spirometry (n = 12,039), we found an excess of lung cancers during screening in the CT arm, almost exclusively early-stage adenocarcinoma-related cancers (histology shift and overdiagnosis). After correction for these excess cancers, stage shift was marginal (P = 0.077).
Conclusions: In the CT arm of the NLST-ACRIN (American College of Radiology Imaging Network) cohort, COPD status was associated with a doubling of lung cancer incidence, no apparent overdiagnosis, and a more favorable stage shift.
Keywords: National Lung Screening Trial, chronic obstructive pulmonary disease, airflow limitation, overdiagnosis, stage shift
At a Glance Commentary
Scientific Knowledge on the Subject
Although the National Lung Screening Trial (NLST) shows that computed tomographic (CT) screening for lung cancer reduces lung cancer mortality by 20%, concerns remain regarding the potential harms, including from overdiagnosis. In a post hoc analysis of the NLST, overdiagnosis is estimated to account for 18% of lung cancers identified during screening, so that for every one life saved, one person suffers overtreatment.
What This Study Adds to the Field
In this post hoc analysis of the NLST, we find that screening participants with airflow limitation have twofold greater lung cancer incidence, minimal overdiagnosis, and a more favorable stage shift compared with those with no airflow limitation, after correction for overdiagnosis. These results suggest the benefit-to-harm ratio of CT screening may differ across subgroups of those currently eligible for lung cancer screening.
On the basis of a 20% reduction in lung cancer deaths in the computed tomographic (CT) screening arm of the National Lung Screening Trial (NLST), yearly CT screening for lung cancer is now widely recommended (1–4). However, published reviews of the benefits and harms of cancer screening have raised concerns about the potential harms due to radiation exposure, unnecessary invasive workup, and overdiagnosis (5–7). Overdiagnosis is the identification and treatment of cancers that would not otherwise have caused death (8–10) and has been the basis of a review of existing screening programs for breast, colon, and prostate cancers (6). In an analysis of the NLST, it has been estimated that 18.5% of the cancers detected in the CT arm may represent overdiagnosis (8). For every 320 smokers undergoing CT screening, one life was saved from lung cancer and 1.38 cancers were “overtreated” (8). This raises the question, “Are there any biomarkers or patient characteristics that are associated with overdiagnosis in lung cancer screening?”
A central feature of overdiagnosed cancers is a long volume-doubling time (VDT) (11). In lung cancer this has been arbitrarily defined as greater than 365–400 days (10–12). Applying this criterion to the results from a single-arm CT screening trial of 3,642 smokers, Wilson and colleagues reported that 48% of non–small cell lung cancer cases, and 67% of prevalent cancers, were slow growing and potentially defined as overdiagnosed cancers (11). In a reanalysis of that data it was shown that compared with those with chronic obstructive pulmonary disease (COPD), defined as prebronchodilator airflow limitation on spirometry testing, screening participants with normal lung function had a twofold greater prevalence of lung cancers with a long VDT (slow growing) (13). A similar finding was reported by Veronesi and colleagues (14, 15). The relationship between airflow limitation (COPD) and overdiagnosis of lung cancer (13) has not yet been reported in the NLST.
Screening significantly reduces mortality by simultaneously increasing the absolute numbers of cancers diagnosed at an early treatable stage and reducing the absolute numbers of late-stage cancers (i.e., clinically relevant stage shift) (5–7, 16). This contrasts with merely increasing the proportion of early-stage cancers, many of which represent “excess cancers” identified through screening-related overdiagnosis (6–10). It is noteworthy that in all the large controlled CT lung cancer screening studies reported to date (7, 14, 17, 18), there has been an excess of cancers detected in the CT arm compared with the control arm. The majority of these excess cancers are early-stage cancers, predominantly of the adenocarcinoma (AC) or bronchioloalveolar subgroup (formerly known as BAC); these BAC-related cancers have been reclassified as adenocarcinoma in situ, minimally invasive adenocarcinoma, or invasive adenocarcinoma, lepidic predominant (8, 19). That CT screening identifies a significant excess of these “early-stage” adenocarcinoma-associated cancers, described previously as a “histology shift,” is a well-known feature of CT screening for lung cancer (9, 19). It is because of this histology shift, masquerading as a stage shift (increase in early-stage cancers), that single-arm CT studies cannot exclude overdiagnosis, or directly correlate stage shift with survival benefit (18, 20). To better understand the potential for histology shift and overdiagnosis in the NLST, we initially examined the full NLST results to determine lung cancer prevalence/incidence and lung cancer histology according to screening arm and interval (see the online supplement) (8, 21, 22). Using data from participants enrolled through the American College of Radiology Imaging Network (NLST-ACRIN cohort), which included 18,714 participants with spirometry testing, we examined the effect of airflow limitation (COPD) at baseline on lung cancer prevalence/incidence, histology shift, and clinical stage. Preliminary results from this study have been previously reported in the form of an abstract (23).
Methods
In the ACRIN cohort of the NLST, participants from 23 centers agreed to take part in the study, which included baseline prebronchodilator spirometry (21, 22). From this cohort of 18,714 ACRIN-based NLST participants, 768 patients with histology-confirmed lung cancer were diagnosed over the study period of 7.5 years. Data from the NLST-ACRIN cohort provide a unique opportunity to examine the effect of COPD status on CT screening for lung cancer (23).
Spirometry was measured at baseline with a SpiroPro spirometer (eResearchTechnology, GmbH, Estenfeld, Germany), taking the best maneuvers of acceptable blows. Consistent with other CT screening studies (11, 14, 17, 18), COPD was defined by the presence of airflow limitation based on prebronchodilator spirometry (FEV1/FVC < 0.70) and COPD severity (FEV1% predicted) according to the Global Initiative on Chronic Obstructive Lung Disease (GOLD) criteria grades 1–4 (www.gold.org; accessed June 18, 2014). Spirometry was performed only when the following criteria were met: no chest infection in the preceding 3 weeks and no use of a short-acting bronchodilator inhaler in the preceding 6 hours or long-acting bronchodilator in the preceding 24 hours. Those not meeting these criteria were rescheduled for spirometric testing at a later date. Patients with lung cancer were identified as those diagnosed after baseline screening and confirmed on histological sampling according to accepted international classification criteria (22). Of the 768 lung cancers identified, lung function results and lung cancer histology results were available for 758 patients with lung cancer (99% of total).
Statistical Analysis
Differences in lung cancer prevalence, incidence, incidence rates, and incidence rate ratios, stratified by screening arm, screening interval, and histology, were compared (see the Glossary in the online supplement). Differences in lung cancer prevalence according to screening arm and stratified by COPD were compared using 2 × 2 tables with Fisher’s exact test. Incidence rates were compared using incidence rate per 1,000 person-years, incidence rate ratio per 1,000 person-years, mid-P exact test (24), and exact confidence intervals. Differences in histology and clinical stage in the lung cancer cases were compared according to COPD status and screening arm, using 2 × 2 tables with Fisher’s exact test. Significance was defined as a two-tailed P < 0.05. All statistical analyses were performed with SAS (version 9.4; SAS Institute, Cary, NC) and STATA (StataCorp, College Station, TX) statistical software.
Results
Demographic Variables of the NLST-ACRIN Cohort
Table 1 shows a comparison of the demographic characteristics of the full NLST trial cohort (n = 53,452) and lung cancer cases (n = 2,058) compared with the NLST-ACRIN cohort (n = 18,714) and lung cancer cases (n = 768) (21, 22, 25). The NLST-ACRIN cohort participants are similar to the full NLST study participants with respect to the most important demographic variables of age, sex, pack-years, percent current smokers, and body mass index. On the basis of the pulmonary function testing in the NLST-ACRIN cohort (n = 18,714), 64.3% had no COPD, a further 27.4% had GOLD 1–2 COPD, 5.8% had GOLD 3 COPD, and 1.1% had GOLD 4 COPD (Table 1). There were missing data for 239 subjects.
Table 1.
Comparison of Baseline Demographics and Spirometric Data for NLST Study Subjects and NLST-ACRIN Cohort
| Screening Trial |
||||
|---|---|---|---|---|
| NLST (Main Study) |
NLST-ACRIN Cohort |
|||
| Screening Participants | Lung Cancer Cases | Total Cohort | Lung Cancer Cases | |
| Subject demographics | ||||
| Number | 53,452 | 2,058 | 18,714 | 768 |
| Mean (SD) age, yr | 61.4 (5.0) | 63.7 (5.3) | 61.6 (5.0) | 63.6 (5.2) |
| Male, % | 59 | 60 | 55 | 56 |
| Mean (SD) pack-years | 56.0 (23.9) | 64.9 (27.1) | 55.9 (23.5) | 63.9 (27.0) |
| Current smokers, % | 48 | 60 | 50 | 60 |
| Family history of lung cancer, % | 22 | 26 | 23 | 26 |
| Self-reported COPD,* % | 17 | 27 | 20 | 32 |
| Mean (SD) body mass index | 27.9 (5.0) | 26.8 (4.7) | 27.8 (5.1) | 26.9 (4.9) |
| Pulmonary function tests | ||||
| Total† | ND | ND | 18,714 | 768 |
| GOLD 1 | ND | ND | 1,607 (8.6%) | 78 (10.2%) |
| GOLD 2 | ND | ND | 3,528 (18.9%) | 213 (27.7%) |
| GOLD 3–4‡ | ND | ND | 1,294 (6.9%) | 109 (14.2%) |
| GOLD status unknown (due to missing data)§ | 7 (<1%) | 1 (<1%) | ||
| ALL COPD | ND | ND | 6,436 (34.4%) | 401 (52.2%) |
| No COPD | ND | ND | 12,039 (64.3%) | 357 (46.5%) |
| Missing spirometry data | ND | ND | 239 (1.3%) | 10 (1.3%) |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; GOLD = Global Initiative for Chronic Obstructive Lung Disease; ND = not done; NLST = National Lung Screening Trial; NLST-ACRIN = NLST participants enrolled through the American College of Radiology Imaging Network.
Self-reported COPD in the NLST was based on questionnaire responses referring to the past diagnosis of COPD, emphysema, chronic bronchitis, or a combination of these.
Pulmonary function results were available for 99% of screening participants and lung cancer cases.
Stage 4 COPD: 1.1% in total cohort and 2.7% in lung cancer cases.
COPD based on FEV1/FVC < 0.70 but percent predicted FEV1 not known owing to missing height.
Lung Cancer Histology According to Screening Arm Stratified by COPD Status
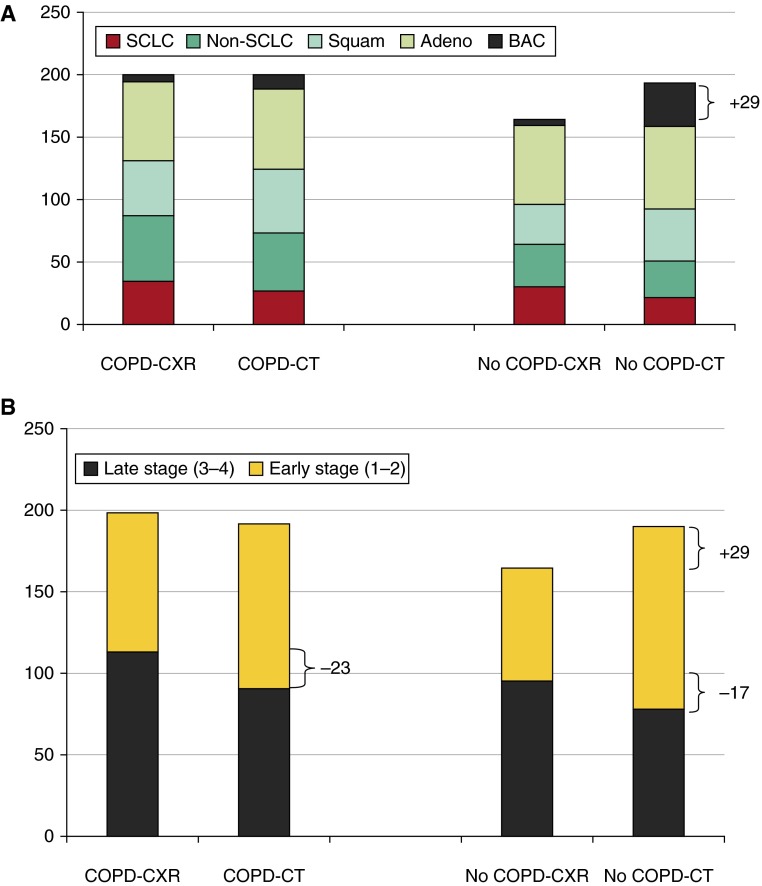
A comparison of the lung cancer prevalence, incidence rate, histology, and clinical stage, according to the presence or absence of COPD, is shown in Table 2. Regardless of the screening interval, patients with COPD were associated with a twofold greater lung cancer incidence rate than were those with normal lung function (P < 0.001 for screening and follow-up intervals; Table 2). COPD was also associated with significantly fewer BAC-related cancers and significantly more non–small cell lung cancer histology (Table 2). Table 3 and Figure 1 show a detailed breakdown of all lung cancers (n = 758) by histology, after stratification by COPD status and screening arm. In those diagnosed with lung cancer and spirometric COPD (n = 401), the lung cancer prevalence was nominally the same and the overall distribution of lung cancer histology was comparable in each screening arm with no evidence of a histology shift (Table 3 and Figure 1A). In contrast, in patients with lung cancer with no COPD at baseline (n = 357), there were an additional 29 lung cancers in the CT arm compared with the CXR arm (Table 3 and Figure 1), attributable to the 30 additional BAC-related cancers in the CT arm (Table 3). Of the 30 excess BAC lung cancers detected in the normal lung function group, 27 (90%) were identified in the screening (T0–T2) interval (comparable to findings in the full NLST; see Figure E2 in the online supplement).
Table 2.
Comparison of Lung Cancer Incidence Rate, Histology, and Clinical Stage According to Chronic Obstructive Pulmonary Disease Status in the NLST-ACRIN Cohort
| Characteristic | COPD | No COPD | Total | P Value |
|---|---|---|---|---|
| Total | 6,436 (34.8%) | 12,039 (65.2%) | 18,475* | |
| Lung cancer prevalence by screening arm | 401 (53%) | 357 (47%) | 758 | <0.0001 |
| CXR | 201 (50%) | 164 (46%) | 365 | 0.27 |
| CT | 200 (50%) | 193 (54%) | 393 | |
| Excess cancer | −1 | +29 | +28 | |
| Lung cancer incidence rate by interval (per 1,000 person-years) | ||||
| T0–T6 | 8.12 | 3.78 | 5.27 | <0.001† |
| T0–T2 | 12.73 | 6.01 | 8.33 | <0.001‡ |
| T3–T6 | 5.14 | 2.36 | 3.31 | <0.001§ |
| Lung cancer prevalence by histology | 0.0035 | |||
| Small cell | 60 (15%) | 51 (14%) | 111 (15%) | |
| Squamous cell | 95 (24%) | 73 (20%) | 168 (22%) | |
| Adenocarcinoma | 127 (32%) | 129 (36%) | 256 (34%) | |
| BAC | 19 (5%) | 40 (11%) | 59 (8%) | |
| Large cell | 16 (4%) | 14 (4%) | 30 (4%) | |
| Non–small cell|| | 81 (20%) | 50 (14%) | 131 (17%) | |
| Other | 3 (<1%) | 357 | 3 (<1%) | |
| Total | 401 | 758 | ||
| Lung cancer prevalence by clinical staging | 0.10 | |||
| Early | 187 (47%) | 181 (51%) | 368 (49%) | |
| Late | 203 (51%) | 173 (48%) | 376 (50%) | |
| Unknown | 11 (3%) | 3 (1%) | 14 (2%) | |
| Total | 401 | 357 | 758 |
Definition of abbreviations: BAC = bronchioloalveolar cancer; COPD = chronic obstructive pulmonary disease; CT = computed tomography; CXR = chest radiograph; NLST-ACRIN = National Lung Screening Trial participants enrolled through the American College of Radiology Imaging Network.
18,714 − missing data (239) = 18,475.
Incident rate ratio (IRR) = 2.15 (95% confidence interval [CI], 1.86–2.48) (T6 includes follow-up years 7 and 8).
IRR = 2.12 (95% CI, 1.76–2.55).
IRR = 2.18 (95% CI, 1.72–2.76).
Unspecified non–small cell lung cancer.
Table 3.
Distribution of Lung Cancer Histology and Stage in NLST-ACRIN Cohort, According to Screening Arm and Presence of Spirometry-defined Chronic Obstructive Pulmonary Disease at Baseline
| COPD: GOLD 1–4 (n = 401) |
No COPD (n = 357) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| CXR Arm | CT Arm | Abs Diff. | Total | CXR Arm | CT Arm | Abs Diff. | Total | Overall Total | |
| Histology | |||||||||
| Small cell | 34 (17%) | 26 (13%) | −8 | 60 (15%) | 30 (18%) | 21 (11%) | −9 | 51 (14%) | 111 |
| Squamous cell | 44 (22%) | 51 (26%) | +7 | 95 (24%) | 32 (20%) | 41 (21%) | +9 | 73 (20%) | 168 |
| Adenocarcinoma | 63 (31%) | 64 (32%) | +1 | 127 (32%) | 63 (38%) | 66 (34%) | +3 | 129 (36%) | 256 |
| BAC* | 7 (3%) | 12 (6%) | +5 | 19 (5%) | 5 (3%) | 35 (18%) | +30† | 40 (11%) | 59 |
| Large cell | 9 (4%) | 7 (4%) | −2 | 16 (4%) | 8 (5%) | 6 (3%) | −2 | 14 (4%) | 30 |
| Non–small cell | 43 (21%) | 38 (19%) | −5 | 81 (20%) | 26 (16%) | 24 (12%) | −2 | 50 (14%) | 131 |
| Other (carcinoid) | 1 (<1%) | 2 (1%) | +1 | 3 (0.8%) | 0 | 0 | 0 | 0 (0%) | 3 |
| Total | 201 | 200 | −1 | 401 | 164 | 193 | +29 | 357 | 758 |
| Clinical stage | |||||||||
| Stage 1–2 | 85 (43%) | 102 (53%) | +17 | 187 | 69 (42%) | 112 (59%) | +43 | 181 | 368 |
| Stage 3–4 | 113 (57%) | 90 (47%) | −23‡ | 203 | 95 (58%) | 78 (41%) | −17§ | 173 | 376 |
| Subtotal | 198 | 192 | -6 | 390 | 164 | 190 | +26 | 354 | 744 |
| Unknown stage | 3 | 8 | +5 | 11 | 0 | 3 | +3 | 3 | 14 |
| Total | 201 | 200 | -1 | 401 | 164 | 193 | +29 | 357 | 758 |
Definition of abbreviations: Abs Diff. = absolute difference; BAC = bronchioloalveolar cancer; COPD = chronic obstructive pulmonary disease; CT = computed tomography; CXR = chest radiograph; GOLD = Global Initiative for Chronic Obstructive Lung Disease; NLST-ACRIN = National Lung Screening Trial participants enrolled through the American College of Radiology Imaging Network.
BAC includes adenocarcinoma in situ, minimally invasive adenocarcinoma, and invasive adenocarcinoma, lepidic predominant.
Of the 35 excess BACs in the CT arm, 30 (86%) come from the “No COPD” group, 6-fold greater than in those with COPD. Of these 30 excess BACs, 27 were diagnosed during the T0–T2 interval.
In those with COPD, a trend toward a favorable stage shift toward early stage was seen in the CT arm compared with the CXR arm (odds ratio [OR], 1.51; 95% confidence interval [CI], 1.00–2.29; P = 0.054), which was associated with an absolute reduction in late-stage cancer (−23).
In those with no COPD, a favorable stage shift toward early stage was seen in the CT arm compared with the CXR arm, and a change in late-stage cancers (−17) was seen (OR, 1.97; 95% CI, 1.27–3.09; P = 0.002). When the excess lung cancers (+26 early-stage BAC from T0 to T2; see text) were excluded from the analysis, the stage shift was no longer significant (OR, 1.52; 95% CI, 0.96–2.40; P = 0.077). Excluding all 30 excess BACs in the CT versus CXR arm (T0–T6), P = 0.098.
Figure 1.
Absolute lung cancer numbers according to COPD status and screening arm in the NLST-ACRIN cohort, showing (A) excess early-stage BAC/Adeno and histology shift in the no-COPD subgroup and (B) no excess cancers but clinically significant stage shift in the COPD subgroup. Adeno = adenocarcinoma; BAC = bronchioloalveolar cancer; COPD = chronic obstructive pulmonary disease; CT = computed tomography; CXR = chest radiography; NLST-ACRIN = National Lung Screening Trial participants enrolled through the American College of Radiology Imaging Network; SCLC = small-cell lung cancer; Squam = squamous cell cancer.
Lung Cancer Staging According to Screening Arm Stratified by COPD Status
For those patients with lung cancer with spirometric COPD (Table 3), early-stage cancer (absolute numbers) was significantly greater for the CT arm (+17) compared with the CXR arm (53% vs. 43%) with a corresponding drop in late-stage cancer (−23) in the CT arm (47% vs. 57%). This difference in reduced late-stage cancers (P = 0.054) corresponds to a clinically relevant stage shift in which an absolute reduction in late-stage cancers occurs together with an increase in early-stage cancers, where the latter does not include excess or overdiagnosed lung cancers (see below).
The previously described finding differs from that of the lung cancer cases found in participants with no COPD (Table 3), where there was an excess of cancers in the CT arm compared with the CXR arm (also observed in the full study and shown in Figures E1 and E2). This is associated with a shift toward early-stage cancers (59% vs. 42%) in the CT arm (+43) and a downward shift (−17) in late-stage cancers (41% vs. 58%, respectively; P = 0.0020). On a reanalysis of the no-COPD data, when 26 of the 29 “excess” cancers in the CT arm (Table 3) are excluded from the analysis, representing those early-stage BAC-related cancers from the T0–T2 screening interval, the stage shift is now no longer significantly in favor of the CT arm (P = 0.077). After removing these 26 BACs (potentially overdiagnosed cancers), we found that the reduction in late-stage cancers was less in absolute numbers in favor of CT screening than CXR (increase in early-stage cancer of +17 and decrease in late-stage cancer of −17) for no COPD (Table 3). This is less than we found for those with COPD, for whom there was an increase in early stage of +17 and a decrease in late stage of −23 (Table 3). Given that smokers with COPD account for only 35% of all screening participants in the NLST-ACRIN cohort but 53% of all lung cancers (Table 2), we estimate that nearly two-thirds of the reduced late-stage shift from CT screening may come from those with underlying COPD. This estimate is based on the stage shift data in Table 3: (1) similar reductions in late-stage cancers (with CT in COPD, −23/201 CXR cancers [11.4%]; with CT in no COPD, −17/164 CXR cancers [10.4%]) and (2) COPD prevalence in the whole study and in those with lung cancer (35 and 53%, respectively; i.e., 0.35 ÷ 0.53 = 0.66 or 66%).
Discussion
In an analysis of the NLST-ACRIN cohort, we found significant differences in lung cancer incidence and lung cancer histology according to COPD status. The annual lung cancer incidence, regardless of screening interval, was twofold greater in participants with COPD compared with those with normal lung function. In those patients with lung cancer with COPD at baseline, there were no excess cancers in comparing the CT and CXR arms, with comparable histology and a nearly significant stage shift in favor of early-stage cancer over late-stage cancer (Table 3). In those lung cancers found in screening subjects with no COPD at baseline, we found an excess of cancers attributed entirely to early-stage cancers of the BAC-related subgroup (Table 3). When these were excluded, we found that the stage shift favoring early-stage over late-stage cancers was no longer significant. This suggests that identifying lung cancer by CT screening in smokers with COPD may result in more cancers per person screened while minimizing overdiagnosis.
It is well accepted that overdiagnosis is an issue for cancer screening programs and that CT screening identifies many lung cancers that may not be life-threatening (8–13, 26). In an analysis of the NLST study, it was estimated that overdiagnosis accounted for about 18.5% of all lung cancers detected in the CT arm (8). However, although there was a 35% excess of lung cancers in the CT arm compared with the CXR arm during screening (T0–T2) in the full NLST (see data in the online supplement, including Figure E2 and Table E1), there was approximately double that reported in the Danish Lung Cancer Screening Trial, where there was an excess of 65% in the CT arm compared with no screening (17–19). The latter suggests that compared with no screening, the potential for overdiagnosis in CT-based lung cancer screening may be even higher than previously anticipated (8). That a significant excess of lung cancers detected by CT during the screening period then became a significant deficit for the next 2–3 years of follow-up (see Figures E1 and E2) suggests many of the cancers detected during screening (T0–T2) had long doubling times (>365 d) (9, 11, 12, 26, 27). This is particularly relevant in Figure E2 showing that in the CT arm, the incidence of BAC-related cancers reduces dramatically during follow-up, while the excess non–small cell lung cancer (NSCLC) during screening (T0–T2) becomes a deficit during follow-up (T3–T6). This contrasts with the results for those randomized to the CXR arm, where lung cancer incidence (and histology) was relatively stable across the screening and follow-up intervals (∼130/yr; Figures E1 and E2, and Table E1). That the BAC-related cancer prevalence during CT follow-up (T3–T6) is comparable to both CXR intervals (Table E1) suggests that the vast majority are overdiagnosed and mostly indolent, consistent with the findings of Patz and colleagues (8). In contrast to the SCLC/other histology group, in which prevalence is comparable irrespective of screening arm or interval, for the NSCLC group the excess during CT screening (T0–T2) is 1.5-fold the deficit (+171/−113) during 4 years of follow-up (Table E1). We suggest that this large excess of NSCLC during screening in the full NLST (8), followed by a disproportionately smaller deficit during follow-up, indicates that a large proportion of NSCLC detected in the CT arm reflects a histology shift and overdiagnosis, identifying lung cancers behaving in either an “indolent” or “less aggressive” manner (9–12, 26, 27). On the basis of these findings, we would reframe the question posed by Detterbeck in his editorial on overdiagnosis (26), that is, “Is the detection of these cancers purely ‘tangential’ to the implementation of CT screening?” as “Is it better to focus on (or target) more aggressive lung cancers?”
Consistent with other CT screening studies, the excess lung cancers in the NLST were primarily of the BAC-related and AC histological subtypes (Figure 1 and Figures E1 and E2) (8). These findings concur with preliminary data from the European CT screening studies, specifically, Continuous Observation of Smoking Subjects (COSMOS), Danish Lung Cancer Screening Trial (DLST), and Dutch–Belgian Lung Cancer Screening Trial (NELSON) (14, 17, 18). Screening studies reporting VDT have observed that most cancers with a VDT equal to or exceeding 400 days are either BAC-related cancers or AC (11, 12, 27). Pastorino states that “when there is an increase above the expected number of BAC-related cancers and AC lung cancers found on screening, then overdiagnosis should be considered” (9). The data we report here show for the first time that these excess cancers are limited to those with normal lung function (discussed further later). It has been suggested that CT screening identifies a distinct type of “adenocarcinoma” with more indolent behavior, represented by either BAC (now classified as a subgroup of adenocarcinomas) or slow-growing adenocarcinoma (27). Just what proportion of these excess cancers represents truly indolent disease remains uncertain, but it is clear that overdiagnosis is an important issue in lung cancer screening by CT and may place at harm a significant number of asymptomatic (otherwise “healthy”) smokers (8). The question then becomes “what biomarkers or strategies are available that might help minimize overdiagnosis and maximize the stage shift during screening?”
When we stratified the lung cancer cases in the NLST-ACRIN cohort by COPD status, we found that in those with COPD, there were no excess cancers and no histology shift in the CT arm compared with the CXR arm (i.e., no apparent histology shift or overdiagnosis). However, we did find a nearly significant stage shift toward early-stage lung cancer with reduced late-stage cancers (Table 2 and Figure 1). True stage shift, wherein screening simultaneously leads to an increase in early-stage and reduction in late-stage cancers, likely underlies the mortality reduction gained from screening (16, 26). Indeed, in the CT arm of the NLST-ACRIN cohort, we report an 11.5% reduction (23/200) in late-stage cancers with COPD, which contributes to the overall 20% reduction in mortality reported in the NLST (22). In contrast, among those with normal lung function, excess cancers primarily of the BAC-related histology were found. More importantly, the increase in early-stage cancers in those with no COPD, after correction for this excess, resulted in an 8.8% reduction in late-stage cancers (17/193) with CT, wherein the stage shift was no longer significant (Table 3 and Figure 1). That these excess cancers were almost exclusively identified by CT during the screening period (T0–T2; Figure 1 and Figure E2) indicates that the tendency to overdiagnosis (and potential harm from overtreatment) is most relevant to those with normal lung function. This also suggests that COPD appears to be associated with more aggressive forms of CT-detected lung cancer, concordant with findings from other screening studies (11, 13). We note, in this study, that 68% of all BAC-related lung cancers (40/59) were identified in those with no COPD (Table 3). This is important because many of these will be identified as ground-glass opacities, with indolent (nonprogressive) behavior, that could be better managed with serial CT scans. Such an approach will reduce the potential for harm from unnecessary intervention (i.e., reduce overdiagnosis). In a small study of patients with mild-to-moderate COPD undergoing yearly CT screening compared with an unscreened COPD group (28), the mortality reduction was nearly 10-fold greater with screening compared with no screening. Although this finding requires confirmation in a larger and better powered study, it suggests that significant gains come from screening those with mild to moderate COPD (29). It is noteworthy that 79% of those with COPD in the ACRIN-NLST cohort had mild-to-moderate COPD, previously associated with a significant risk of lung cancer (30–33). Although some argue that competing causes of death might dilute the mortality gains of screening smokers with COPD (34), the data do not bear this out (33). In all three studies that address this issue, after stratifying by the presence or absence of COPD on presurgery spirometry, comparable 5-year survival outcomes were found after surgical removal of early-stage NSCLC (35–37). If the differences in stage shift we found between COPD and no COPD were translated directly to mortality reduction, with no effect from competing causes of death or perioperative death from COPD, we estimate that the mortality reduction would be nearly twofold greater in those with COPD than in those with no COPD (66 and 34%, respectively). On the basis of these observations, the differential effects of spirometry-defined airflow limitation (COPD) on mortality reduction in the NLST-ACRIN cohort are currently the subject of a detailed analysis.
In this subanalysis of the NLST, we have shown that lung cancer incidence rates were twofold greater in those with COPD than in those with normal lung function (Table 2). This replicates the results from other screening studies showing greater lung cancer detection (or diagnosis) rates in those with COPD (29, 33, 38, 39). We and others have previously noted that NLST-based eligibility criteria for lung cancer screening, limited to age and pack-year exposure, have low sensitivity, excluding between 50 and 70% of all lung cancers from screening (39–41). In the study by Sanchez-Salcedo and colleagues, sensitivity of the eligibility criteria was substantially increased when the presence of airflow limitation or emphysema was added to the current NLST-based eligibility criteria (39). Collectively, these findings suggest that when risk stratifying current and former smokers for their lung cancer risk, variables related to susceptibility to COPD (age, smoking history, self-reported COPD, low body mass index, genetic factors, CT emphysema, or lung function) can be combined to derive a more precise overall lung cancer risk (23, 25, 38, 42–45).
There are several potential limitations of our study. We note that the NLST-ACRIN cohort participants make up only 35% of the full NLST study. However, the NLST-ACRIN cohort appears to be representative of the wider NLST population as the demographic variables of this subgroup are comparable to those of the full study (Table 1). Consistent with other CT screening studies (11, 14, 17, 18, 39, 45), only prebronchodilator airflow limitation was used in this study to define COPD status. Such an approach allows for greater comparability with these existing studies and better reflects the community-based (epidemiological) use of spirometry as a screening tool for airflow limitation. Although a misdiagnosis of asthma (or fully reversible airflow limitation) is possible in those we assigned as COPD, it is likely to be modest (conferring a dilutional effect) in this group of older heavy smokers (mean age, 62 yr; mean pack-years, 56). A further limitation of the study is that data on VDTs are not available to confirm the results of others suggesting many of these “excess cancers” are indeed indolent and not life-threatening (11, 13). Data from the NELSON trial suggest that volumetric-based determination of VDT may reduce both false positive rates (46) and overdiagnosis (47). Last, we have not assessed the presence of emphysema in those with and without airflow limitation or lung cancer, and it remains a hotly debated issue as to whether airflow limitation or emphysema is the more important manifestation of COPD linked to an increased risk of lung cancer (33, 39, 48). On this basis, we cannot exclude the possibility that some BAC-related cancers, corresponding to ground-glass opacities during screening, are more difficult to identify on CT in the presence of emphysema.
We conclude that the presence of COPD identifies smokers at greatest risk of lung cancer (30–32) and that it is associated with an increased lung cancer incidence rate in CT screening studies (29, 33, 38, 39). COPD is also associated with more aggressive cancers and significantly less (or minimal) overdiagnosis (13, 28, 29). Although this finding requires replication (17, 18), our observations argue in favor of the routine use of screening spirometry in asymptomatic smokers at risk of lung cancer either in general (49), or specifically as part of assessing the harm-to-benefit ratio of CT screening (31, 33, 39). We believe that preselection of eligible smokers for possible CT screening requires greater appraisal as current criteria include many low-risk smokers for whom the harm may substantially outweigh the benefit of screening (25, 43, 50). The results of this study suggest that overdiagnosis is a significant issue in CT screening for lung cancer and is found exclusively in those with normal lung function at lower risk. We conclude that lung cancer risk assessment requires the inclusion of variables underlying COPD risk, and that such an approach may help to better optimize the benefit-to-harm ratio of CT screening.
Footnotes
Supported by a grant from Johnson & Johnson and by grants U01-CA-80098 and U01-CA-79778 from the Division of Cancer Prevention, National Cancer Institute, National Institutes of Health, to the American College of Radiology Imaging Network.
The sponsors or funders for the study reported in this manuscript played no role in the design and conduct of the study, nor in the collection, analysis, and interpretation of the data, or in the preparation, review, approval, or decision to submit the manuscript.
Author Contributions: All authors contributed to the conception and design; acquisition, analysis, and interpretation; drafting and review for important intellectual content; and final approval of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as 10.1164/rccm.201505-0894OC on July 22, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, Byers T, Colditz GA, Gould MK, Jett JR, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307:2418–2429. doi: 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer NetworkGuidelines for lung cancer screening, version 1. 2012 [accessed 2013 Nov]. Available from: www.NCCN.org
- 3.Jaklitsch MT, Jacobson FL, Austin JH, Field JK, Jett JR, Keshavjee S, MacMahon H, Mulshine JL, Munden RF, Salgia R, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg. 2012;144:33–38. doi: 10.1016/j.jtcvs.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 4.Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guides. Chest. 2013;143(Suppl):e78S–e92S. doi: 10.1378/chest.12-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humphrey LL, Deffebach M, Pappas M, Baumann C, Artis K, Mitchell JP, Zakher B, Fu R, Slatore CG. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive Services Task Force recommendation. Ann Intern Med. 2013;159:411–420. doi: 10.7326/0003-4819-159-6-201309170-00690. [DOI] [PubMed] [Google Scholar]
- 6.Esserman LJ, Thompson IM, Jr, Reid B. Overdiagnosis and overtreatment in cancer: an opportunity for improvement. JAMA. 2013;310:797–798. doi: 10.1001/jama.2013.108415. [DOI] [PubMed] [Google Scholar]
- 7.Aberle DR, Abtin F, Brown K. Computed tomography screening for lung cancer: has it finally arrived? Implications of the National Lung Screening Trial. J Clin Oncol. 2013;31:1002–1008. doi: 10.1200/JCO.2012.43.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patz EF, Jr, Pinsky P, Gatsonis C, Sicks JD, Kramer BS, Tammemägi MC, Chiles C, Black WC, Aberle DR NLST Overdiagnosis Manuscript Writing Team. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med. 2014;174:269–274. doi: 10.1001/jamainternmed.2013.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pastorino U. Lung cancer screening. Br J Cancer. 2010;102:1681–1686. doi: 10.1038/sj.bjc.6605660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Detterbeck FC. Cancer, concepts, cohorts and complexity: avoiding oversimplification of overdiagnosis. Thorax. 2012;67:842–845. doi: 10.1136/thoraxjnl-2012-201779. [DOI] [PubMed] [Google Scholar]
- 11.Wilson DO, Ryan A, Fuhrman C, Schuchert M, Shapiro S, Siegfried JM, Weissfeld J. Doubling times and CT screen–detected lung cancers in the Pittsburgh Lung Screening Study. Am J Respir Crit Care Med. 2012;185:85–89. doi: 10.1164/rccm.201107-1223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Infante M, Berghmans T, Heuvelmans MA, Hillerdal G, Oudkerk M. Slow-growing lung cancer as an emerging entity: from screening to clinical management. Eur Respir J. 2013;42:1706–1722. doi: 10.1183/09031936.00186212. [DOI] [PubMed] [Google Scholar]
- 13.Young RP, Hopkins RJ. Estimating overdiagnosis of lung cancer. Ann Intern Med. 2013;158:635–636. doi: 10.7326/0003-4819-158-8-201304160-00013. [DOI] [PubMed] [Google Scholar]
- 14.Veronesi G, Maisonneuve P, Bellomi M, Rampinelli C, Durli I, Bertolotti R, Spaggiari L. Estimating overdiagnosis in low-dose computed tomography screening for lung cancer: a cohort study. Ann Intern Med. 2012;157:776–784. doi: 10.7326/0003-4819-157-11-201212040-00005. [DOI] [PubMed] [Google Scholar]
- 15.Maisonneuve P, Veronesi G, Bertolotti R. Estimating overdiagnosis of lung cancer—reply. Ann Intern Med. 2013;158:635–636. doi: 10.7326/0003-4819-158-8-201304160-00014. [DOI] [PubMed] [Google Scholar]
- 16.Midthun DE, Gould MK. Favorable stage distribution in the NELSON trial: promise or hype? Am J Respir Crit Care Med. 2013;187:792–793. doi: 10.1164/rccm.201302-0314ED. [DOI] [PubMed] [Google Scholar]
- 17.Saghir Z, Dirksen A, Ashraf H, Bach KS, Brodersen J, Clementsen PF, Døssing M, Hansen H, Kofoed KF, Larsen KR, et al. CT screening for lung cancer brings forward early disease: the randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax. 2012;67:296–301. doi: 10.1136/thoraxjnl-2011-200736. [DOI] [PubMed] [Google Scholar]
- 18.Horeweg N, van der Aalst CM, Thunnissen E, Nackaerts K, Weenink C, Groen HJ, Lammers JW, Aerts JG, Scholten ET, van Rosmalen J, et al. Characteristics of lung cancers detected by computer tomography screening in the randomized NELSON trial. Am J Respir Crit Care Med. 2013;187:848–854. doi: 10.1164/rccm.201209-1651OC. [DOI] [PubMed] [Google Scholar]
- 19.Young RP, Hopkins RJ. Stage shift in computed tomography screening: possible role of indolent cancers, “histology shift,” and overdiagnosis. Am J Respir Crit Care Med. 2013;188:1034–1035. doi: 10.1164/rccm.201305-0832LE. [DOI] [PubMed] [Google Scholar]
- 20.Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS International Early Lung Cancer Action Program Investigators. Survival of patients with stage 1 lung cancer detected on CT screening. N Engl J Med. 2006;355:1763–1771. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 21.Aberle DR, Adams AM, Berg CD, Clapp JD, Clingan KL, Gareen IF, Lynch DA, Marcus PM, Pinsky PF National Lung Screening Trial Research Team. Baseline characteristics of participants in the randomized National Lung Screening Trial. J Natl Cancer Inst. 2010;102:1771–1779. doi: 10.1093/jnci/djq434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young RP, Chiles C, Duan F, Hopkins RJ, Gamble GD, Aberle DH. Stage-shift and histology-shift in NLST according to COPD status: a sub-analysis of the ACRIN-biomarker arm of the NLST [abstract] Am J Respir Crit Care Med. 2014;189:A6306. [Google Scholar]
- 24.Lancaster HO. Significance tests in discrete distributions. J Am Stat Assoc. 1961;56:223–234. [Google Scholar]
- 25.Tammemägi MC, Katki HA, Hocking WG, Church TR, Caporaso N, Kvale PA, Chaturvedi AK, Silvestri GA, Riley TL, Commins J, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368:728–736. doi: 10.1056/NEJMoa1211776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Detterbeck FC. Overdiagnosis during lung cancer screening: is it an overemphasised, underappreciated, or tangential issue? Thorax. 2014;69:407–408. doi: 10.1136/thoraxjnl-2014-205140. [DOI] [PubMed] [Google Scholar]
- 27.Chirieac LR, Flieder DB. High-resolution computed tomography screening for lung cancer: unexpected findings and new controversies regarding adenocarcinogenesis. Arch Pathol Lab Med. 2010;134:41–48. doi: 10.5858/134.1.41. [DOI] [PubMed] [Google Scholar]
- 28.de-Torres JP, Casanova C, Marín JM, Zagaceta J, Alcaide AB, Seijo LM, Campo A, Carrizo S, Montes U, Cordoba-Lanus E, et al. Exploring the impact of screening with low-dose CT on lung cancer mortality in mild to moderate COPD patients: a pilot study. Respir Med. 2013;107:702–707. doi: 10.1016/j.rmed.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Young RP, Hopkins RJ. CT screening in COPD: the impact on lung cancer mortality [letter] Respir Med. 2014;108:813–814. doi: 10.1016/j.rmed.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 30.Young RP, Hopkins RJ, Christmas T, Black PN, Metcalf P, Gamble GD. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J. 2009;34:380–386. doi: 10.1183/09031936.00144208. [DOI] [PubMed] [Google Scholar]
- 31.Calabrò E, Randi G, La Vecchia C, Sverzellati N, Marchianò A, Villani M, Zompatori M, Cassandro R, Harari S, Pastorino U. Lung function predicts lung cancer risk in smokers: a tool for targeting screening programmes. Eur Respir J. 2010;35:146–151. doi: 10.1183/09031936.00049909. [DOI] [PubMed] [Google Scholar]
- 32.de Torres JP, Marín JM, Casanova C, Cote C, Carrizo S, Cordoba-Lanus E, Baz-Dávila R, Zulueta JJ, Aguirre-Jaime A, Saetta M, et al. Lung cancer in patients with chronic obstructive pulmonary disease— incidence and predicting factors. Am J Respir Crit Care Med. 2011;184:913–919. doi: 10.1164/rccm.201103-0430OC. [DOI] [PubMed] [Google Scholar]
- 33.Young RP, Hopkins RJ. Diagnosing COPD and targeted lung cancer screening. Eur Respir J. 2012;40:1063–1064. doi: 10.1183/09031936.00070012. [DOI] [PubMed] [Google Scholar]
- 34.Bach PB. Computed tomographic screening for lung cancer: reply [letter] JAMA. 2012;308 doi: 10.1001/2012.jama.11892. [DOI] [PubMed] [Google Scholar]
- 35.Ueda K, Jinbo M, Li TS, Yagi T, Suga K, Hamano K. Computed tomography–diagnosed emphysema, not airway obstruction, is associated with the prognostic outcome of early-stage lung cancer. Clin Cancer Res. 2006;12:6730–6736. doi: 10.1158/1078-0432.CCR-06-1196. [DOI] [PubMed] [Google Scholar]
- 36.Sekine Y, Behnia M, Fujisawa T. Impact of COPD on pulmonary complications and on long-term survival of patients undergoing surgery for NSCLC. Lung Cancer. 2002;37:95–101. doi: 10.1016/s0169-5002(02)00014-4. [DOI] [PubMed] [Google Scholar]
- 37.Lee SJ, Lee J, Park YS, Lee C-H, Lee SM, Yim JJ, Yoo CG, Han SK, Kim YW. Impact of chronic obstructive pulmonary disease on the mortality of patients with non-small-cell lung cancer. J Thorac Oncol. 2014;9:812–817. doi: 10.1097/JTO.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 38.Young RP, Hopkins RJ. Targeted CT image screening and its effect on lung cancer detection rate. Chest. 2013;144:1419–1420. doi: 10.1378/chest.13-1321. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez-Salcedo P, Wilson DO, de-Torres JP, Weissfeld JL, Berto J, Campo A, Alcaide AB, Pueyo J, Bastarrika G, Seijo LM, et al. Improving selection criteria for lung cancer screening: the potential role of emphysema. Am J Respir Crit Care Med. 2015;191:924–931. doi: 10.1164/rccm.201410-1848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young RP, Hopkins RJ. Lung cancer risk prediction to select smokers for screening CT [letter] Cancer Prev Res (Phila) 2012;5:697–698, author reply 699. doi: 10.1158/1940-6207.CAPR-11-0531. [DOI] [PubMed] [Google Scholar]
- 41.Pinsky PF, Berg CD. Applying the National Lung Screening Trial eligibility criteria to the US population: what percent of the population and of incident lung cancers would be covered? J Med Screen. 2012;19:154–156. doi: 10.1258/jms.2012.012010. [DOI] [PubMed] [Google Scholar]
- 42.Young RP, Hopkins RJ, Whittington CF, Hay BA, Epton MJ, Gamble GD. Individual and cumulative effects of GWAS susceptibility loci in lung cancer: associations after sub-phenotyping for COPD. PLoS One. 2011;6:e16476. doi: 10.1371/journal.pone.0016476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young RP, Hopkins RJ, Midthun DE. Computed tomographic screening for lung cancer [letter] JAMA. 2012;308:1320. doi: 10.1001/2012.jama.11892. [DOI] [PubMed] [Google Scholar]
- 44.Tammemagi MC, Lam SC, McWilliams AM, Sin DD. Incremental value of pulmonary function and sputum DNA image cytometry in lung cancer risk prediction. Cancer Prev Res (Phila) 2011;4:552–561. doi: 10.1158/1940-6207.CAPR-10-0183. [DOI] [PubMed] [Google Scholar]
- 45.de-Torres JP, Wilson DO, Sanchez-Salcedo P, Weissfeld JL, Berto J, Campo A, Alcaide AB, García-Granero M, Celli BR, Zulueta JJ. Lung cancer in patients with chronic obstructive pulmonary disease: development and validation of the COPD Lung Cancer Screening Score. Am J Respir Crit Care Med. 2015;191:285–291. doi: 10.1164/rccm.201407-1210OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horeweg N, van der Aalst CM, Vliegenthart R, Zhao Y, Xie X, Scholten ET, Mali W, Thunnissen E, Weenink C, Groen HJ, et al. Volumetric computed tomography screening for lung cancer: three rounds of the NELSON trial. Eur Respir J. 2013;42:1659–1667. doi: 10.1183/09031936.00197712. [DOI] [PubMed] [Google Scholar]
- 47.Revel M-P. Avoiding overdiagnosis in lung cancer screening: the volume doubling time strategy. Eur Respir J. 2013;42:1459–1463. doi: 10.1183/09031936.00157713. [DOI] [PubMed] [Google Scholar]
- 48.Sekine Y, Katsura H, Koh E, Hiroshima K, Fujisawa T. Early detection of COPD is important for lung cancer surveillance. Eur Respir J. 2012;39:1230–1240. doi: 10.1183/09031936.00126011. [DOI] [PubMed] [Google Scholar]
- 49.Young RP, Hopkins RJ. A clinical practice guideline update on the diagnosis and management of stable chronic obstructive pulmonary disease [letter] Ann Intern Med. 2012;156:68–69, author reply 69. doi: 10.7326/0003-4819-156-1-201201030-00021. [DOI] [PubMed] [Google Scholar]
- 50.Bach PB, Gould MK. When the average applies to no one: personalized decision making about potential benefits of lung cancer screening. Ann Intern Med. 2012;157:571–573. doi: 10.7326/0003-4819-157-8-201210160-00524. [DOI] [PubMed] [Google Scholar]