Abstract
Rationale: Roflumilast is a therapeutic agent in the treatment of chronic obstructive pulmonary disease (COPD). It has antiinflammatory effects; however, it is not known whether it can affect a biologic pathway implicated in COPD pathogenesis and progression. The self-propagating acetyl-proline-glycine-proline (AcPGP) pathway is a novel means of neutrophilic inflammation that is pathologic in the development of COPD. AcPGP is produced by extracellular matrix collagen breakdown with prolyl endopeptidase and leukotriene A4 hydrolase serving as the enzymes responsible for its production and degradation, respectively.
Objectives: We hypothesized that roflumilast would decrease AcPGP, halting the feed-forward cycle of inflammation.
Methods: We conducted a single-center, placebo-controlled, randomized study investigating 12 weeks of roflumilast treatment added to current therapy in moderate-to-severe COPD with chronic bronchitis. Subjects underwent sputum and blood analyses, pulmonary function testing, exercise tolerance, and quality-of-life assessment at 0, 4, and 12 weeks.
Measurements and Main Results: Twenty-seven patients were enrolled in the intention-to-treat analysis. Roflumilast treatment decreased sputum AcPGP by more than 50% (P < 0.01) and prolyl endopeptidase by 46% (P = 0.02), without significant improvement in leukotriene A4 hydrolase activity compared with placebo. Roflumilast also reduces other inflammatory markers. There were no significant changes in lung function, quality of life, or exercise tolerance between roflumilast- and placebo-treated groups.
Conclusions: Roflumilast reduces pulmonary inflammation through decreasing prolyl endopeptidase activity and AcPGP. As expected for lower AcPGP levels, markers of neutrophilic inflammation are blunted. Inhibiting this self-propagating pathway lessens the overall inflammatory burden, which may alter the natural history of COPD, including the risk of exacerbation.
Clinical trial registered with www.clinicaltrials.gov (NCT 01572948).
Keywords: COPD, roflumilast, neutrophil, proline-glycine-proline, prolyl endopeptidase
At a Glance Commentary
Scientific Knowledge on the Subject
Roflumilast is a therapeutic used in chronic obstructive pulmonary disease (COPD) in part because of its antiinflammatory effects. However, it is not known whether it can affect a biologic pathway implicated in COPD pathogenesis and progression. The self-propagating acetyl-proline-glycine-proline pathway is a novel means of neutrophilic inflammation that is pathologic in the development of COPD. Whether roflumilast treatment is effective in blunting this pathway is unknown.
What This Study Adds to the Field
Roflumilast reduces pulmonary inflammation through decreasing prolyl endopeptidase activity and acetyl-proline-glycine-proline. Inhibiting this self-propagating pathway lessens the overall inflammatory burden, which may alter the natural history of COPD, including the risk of exacerbation.
Chronic obstructive pulmonary disease (COPD) is currently the third leading cause of death and the sixth leading cause of disability in the United States (1). Chronic neutrophilic inflammation is a hallmark of the disease and directly impacts morbidity and mortality (2–4). At present, treatments are aimed primarily at the control of dyspnea and the prevention of complications, such as acute exacerbations (AECOPD), which drive most COPD-related morbidity and costs (5, 6). Inhaled therapies, including bronchodilators and inhaled corticosteroids (ICS), are the mainstay of this management strategy but are only modestly effective even when used in combination. No intervention other than smoking cessation and the use of supplemental oxygen in those patients with resting hypoxemia has been definitively shown to alter the natural history of the disease (7, 8). Major limitations to disease modification include the absence of any drug that attenuates the ongoing airway inflammation that persists even after smoking cessation (9, 10). Roflumilast is a selective phosphodiesterase-4 inhibitor, and the first medication approved exclusively for decreasing AECOPD, in part because of its role in reducing pulmonary inflammation and neutrophil burden (11–13).
Roflumilast increases intracellular cAMP in inflammatory cells, bronchial epithelia, and bronchial smooth muscle, and reduces leukotriene B4 (LTB4), reactive oxygen species, and tumor necrosis factor-α in vitro (14, 15). These antiinflammatory effects also occur in the short- and long-term where roflumilast has also been demonstrated to reduce sputum neutrophil count, eosinophil count, soluble IL-8, and neutrophil elastase, and lead to improvement in lung function and reduction in AECOPD risk (12).
However, neutrophil elastase, IL-8, and neutrophil burden are end products of several inflammatory pathways and are not specific for roflumilast’s mechanistic target. In contrast, proline-glycine-proline (PGP) and its acetylated form (AcPGP) are tripeptide collagen fragments generated from extracellular matrix breakdown that act as potent neutrophil chemoattractants, and are detected in sputum of patients with COPD (16–19). AcPGP and PGP are generated through a multistep proteolytic pathway with prolyl endopeptidase serving as the critical rate-limiting enzyme (19). Leukotriene A4 hydrolase (LTA4H) is the enzyme responsible for degrading PGP and terminating PGP-mediated neutrophilic influx in normal conditions (20). LTA4H is classically known as an epoxide hydrolase that converts LTA4 to LTB4 (21) and also has aminopeptidase activity specific for degrading PGP (20). Cigarette smoke selectively inactivates LTA4H’s aminopeptidase activity without affecting the hydrolase. This novel pathway of neutrophilic inflammation, unlike the “classic” mode associated with IL-8, has the potential to become self-propagating (17). In addition to its role in pathogenesis, PGP levels may also serve as a marker of AECOPD risk (22).
We hypothesize the antiinflammatory activity of roflumilast is caused by direct and indirect effects on neutrophils by interfering with the AcPGP pathway. First, roflumilast may impair AcPGP production by decreasing prolyl endopeptidase, the oligopeptidase responsible for its release from extracellular matrix collagen fragments (19, 23). Second, it may also stimulate PGP degradation by direct interaction with the enzyme LTA4H and increasing its aminopeptidase function. Either one or both of these mechanisms would explain roflumilast’s capacity to blunt chronic neutrophilic inflammation in patients with moderate to severe COPD and reduce overall disease burden. We also posit that these meaningful reductions in biomarkers of pulmonary inflammation and sputum neutrophilia may trend with improvement in pulmonary function, sputum scores, and quality of life in stable moderate-to-severe COPD.
Methods
Patient Selection
Subjects were recruited from a single center to participate in a 12-week double-blinded, placebo-controlled, randomized control trial. The Institutional Review Board at the University of Alabama at Birmingham approved the conduct of the study (protocol number #F111129001) and the study is listed in ClinicalTrials.gov (NCT01572948). The study was granted an investigational new drug exemption by the Food and Drug Administration. Patients underwent informed consent and were included if they were greater than 40 years old, had a diagnosis of moderate-to-severe COPD as defined by Global Initiative for Chronic Obstructive Lung Disease criteria (24), were current or former cigarette smokers with more than 10 pack-years of total consumption, and had chronic bronchitis defined by chronic cough and sputum production lasting at least 3 months for 2 consecutive years,. Exclusion criteria include a diagnosis of asthma as defined by the American Thoracic Society/European Respiratory Society guidelines, clinically significant bronchiectasis, known sensitivity to roflumilast, the use of other methylxanthines (specifically theophylline) within 1 month of screening, changes to maintenance COPD therapy within 1 month of screening, and other factors as listed in the online supplement.
Study Protocol
The study was divided into six visits as outlined in Figure E1 in the online supplement. Visit 1 (Week −1) activities included informed consent, demographic and medical history evaluation, pulmonary function testing including prebronchodilator and post-bronchodilator FEV1 and FVC, and blood and induced sputum collection. Visit 2 (Week 0) included interval history; completion of St. George’s Respiratory Questionnaire (SGRQ) and Breath, Cough, and Sputum Scale (BCSS) questionnaires; 6-minute-walk distance (6MWD); and randomization by sealed envelope. Subjects were randomized to identical white tablets containing a 30-day supply of either roflumilast, 500 μg or placebo by a block randomization schema using a block size of four and an allocation ratio of 1:1. Block randomization was stratified by current smoking status and ICS use. This was done based on known associations between cigarette smoke use and increased PGP/AcPGP levels (17, 25) and uncertainty surrounding roflumilast use in the setting of ICS (11, 13, 26). The latter issue has been clarified somewhat by the recent publication of the REACT study demonstrating that as compared with placebo, roflumilast reduced the risk of exacerbations, including those requiring hospitalization, when used in conjunction with ICS in patients with chronic bronchitis (27).
Visit 3 (Week 4) included medical history review, spirometry, and blood and induced sputum collection. Visit 4 (Week 8) events included medical history update and spirometry. Visit 5 (Week 12) included medical history review, pulmonary function testing, quality-of-life questionnaires, blood and induced sputum collection, and 6MWD. Visit 6 (Week 14) was telephone follow-up after study completion to ensure no symptoms developed following investigational product cessation. Compliance was assessed by counting the number of pills participants returned at each follow-up visit.
Pulmonary Function and Exercise Tolerance Testing
Prebronchodilator and post-bronchodilator spirometry was performed on all subjects according to American Thoracic Society/European Respiratory Society standards (28) and 6MWD was performed according to American Thoracic Society standards (29).
Induced Sputum
Induced sputum using inhaled 3% hypertonic nebulized saline followed a well-established protocol by a trained respiratory therapist (12, 30, 31) and was used for all collections. Induced sputum was diluted in phosphate-buffered saline (1:4).
Quality-of-Life Determination
The SGRQ is a 50-item quality-of-life questionnaire with a minimal clinically important difference of a change in score of 4 points (32). The BCSS is a validated questionnaire examining the impact of cough- and sputum-related symptomatology with a substantial difference of a change of greater than 1.0 (33).
Plasma and Sputum Preparation
Phree columns (Phenomenex, Torrance, CA) were loaded with 1 ml of 60:40 methanol/acetonitrile. 13C15N-labeled AcPGP/13C15N-labeled PGP mix was added (final concentration of 10 ng/ml) directly onto the chromatography column with 200 μl of patient plasma or sputum to measure recovery. The recovery (mean ± SD) was 38.4 ± 10.3% for AcPGP and 35.7 ± 23.1% for PGP after filtration. The columns were covered and centrifuged at 1,500 × g for 60 minutes followed by an additional methanol/acetonitrile wash. The solution that had passed through the column was evaporated to dryness using a REACTI-VAP III system (Fisher Scientific, Pittsburgh, PA). The residue was dissolved in 100 μl of phosphate-buffered saline and used for liquid chromatography electrospray ionization tandem mass spectroscopy.
Biomarker Analyses
Sputum cell count was performed using a hemocytometer at ×40 magnification. AcPGP, PGP, and LTA4H aminopeptidase activity were detected by liquid chromatography electrospray ionization tandem mass spectroscopy as previously described (16, 17, 20, 25). LTA4H (Uscn, Wuhan, China), LTB4 (R&D Systems, Minneapolis, MN), IL-8 (CXCL8/IL-8 Quantikine kit; R&D Systems), and myeloperoxidase (MPO) (CalBiochem) were detected in induced sputum by commercially available enzyme immunoassays (17, 34, 35). Neutrophil elastase was detected by a commercially available chemiluminescence kit (CalBiochem, Billerica, MA). Prolyl endopeptidase activity was measured via immunofluorescence assay as previously described (19, 23, 36).
Statistical Analyses
The primary outcome for the study was a change in induced sputum AcPGP at 12 weeks post-randomization in an intention-to-treat analysis. The study was powered to detect a 50% reduction in sputum AcPGP based on reductions observed in the COPD Clinical Research Network Macrolide trial (22). To achieve these results with a power of 0.80 and an alpha of 0.05, 24 patients (n = 12 per group) were needed. Additional analyses were performed to compare the between-group changes in plasma AcPGP, sputum neutrophil counts, additional sputum biomarkers, the BCSS and SGRQ scores, and changes in post-bronchodilator FEV1 at the 12-week visit. All secondary analyses included all patients who were enrolled (intention-to-treat). Normal distribution was confirmed with the Shapiro-Wilk test. Bivariate analyses were conducted with the use of a two-tailed Fisher exact test for categorical data and two-tailed Student t test or Mann-Whitney U test for continuous data as appropriate. Repeated measures analysis of variance or Friedman test was used to determine changes in sputum and plasma biomarker values at the three time points (Weeks 0, 4, and 12) and paired Student t test or Wilcoxon matched-pair signed rank test was used to determine changes in sputum and plasma biomarker values at Weeks 0 and 12 for normally or nonnormally distributed values, respectively. All analyses were performed with SPSS Software (Version 22.0; IBM Corporation, Armonk, NY) and P values less than 0.05 were considered statistically significant. Figures were designed in Prism Version 5 (GraphPad Software Inc., La Jolla, CA).
Results
Patients
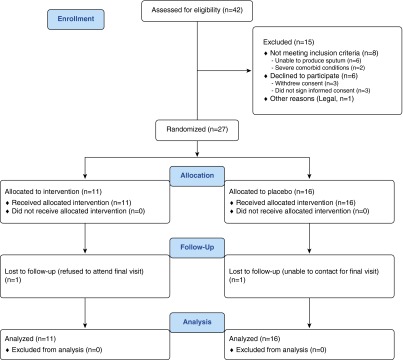
From 2012 to 2014, a total of 42 subjects were screened, of which 27 were enrolled. All patients received their study drug, and one participant in each study arm was lost to follow-up (Figure 1). Baseline characteristics were not statistically different between the two groups (Table 1). Subjects were 62 ± 7 years old, 63% male, 70% non-Hispanic white, with post-bronchodilator FEV1 of 44 ± 14% predicted. In total, 59% were current smokers with a 45 ± 22 pack-year history, 26% used supplemental oxygen, and 59% used either ICS alone or in combination with long-acting β-agonist therapy. Baseline quality-of-life scores (SGRQ), sputum scores (BCSS), and exercise capacity (6MWD) were comparable between placebo- and roflumilast-treated subjects. No participants developed AECOPD during the course of the study.
Figure 1.
Consolidated Standards of Reporting Trials flow diagram.
Table 1.
Baseline Characteristics
| Placebo (n = 16) | Roflumilast (n = 11) | |
|---|---|---|
| Age, yr | 61 ± 8 | 62 ± 7 |
| White race | 12 (75%) | 7 (64%) |
| Male sex | 10 (63%) | 7 (64%) |
| Current smoker | 10 (63%) | 6 (55%) |
| Lifetime pack-year history | 44 ± 19 | 47 ± 26 |
| GOLD stage, median (range) | 3 (2–4) | 3 (2–4) |
| Supplemental oxygen use | 4 (25%) | 3 (27%) |
| Supplemental oxygen amount, L/min | 2 ± 0 | 1.7 ± 0.6 |
| LAMA use | 8 (50%) | 6 (55%) |
| ICS or LABA/ICS use | 10 (63%) | 6 (55%) |
| Post-bronchodilator FEV1, % predicted | 44 ± 16 | 45 ± 12 |
| Post-bronchodilator FVC, % predicted | 69 ± 20 | 72 ± 17 |
| FEV1/FVC ratio | 0.48 ± 0.12 | 0.53 ± 0.12 |
| SGRQ total | 49 ± 13 | 56 ± 13 |
| BCSS | 4.6 ± 2.4 | 4.6 ± 2.7 |
| 6-min-walk distance, ft | 972 ± 255 | 739 ± 203 |
Definition of abbreviations: BCSS = Breathlessness Cough and Sputum Scale; GOLD = Global Initiative for Chronic Obstructive Lung Disease; ICS = inhaled corticosteroid; LABA = long-acting β-agonist; LAMA = long-acting muscarinic antagonist; SGRQ = St. George’s Respiratory Questionnaire.
All results are mean ± SD or number of patients (%).
Differences between groups for each category listed were not statistically different (P > 0.05).
Primary Efficacy Variables
Sputum AcPGP and PGP
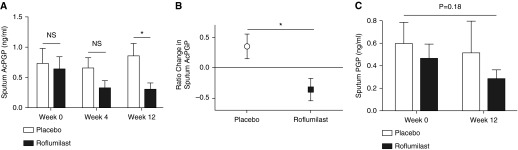
Baseline sputum AcPGP (0.64 ± 0.21 vs. 0.74 ± 0.25 ng/ml; P = 0.76) and PGP (0.42 ± 0.14 vs. 0.59 ± 0.19 ng/ml; P = 0.45) were not statistically different among subjects treated with roflumilast or placebo, respectively. As seen in Figure 2A, roflumilast treatment reduced sputum AcPGP at 12 weeks of therapy (0.30 ± 0.11 compared with 0.70 ± 0.18 ng/ml in placebo; P = 0.04), corresponding to a greater than 50% reduction in sputum AcPGP (P = 0.01 by Wilcoxon matched-pair signed rank test) (Figure 2B). In a separate, per-protocol analysis, sputum AcPGP was decreased after 12 weeks of roflumilast treatment (0.27 ± 0.11 compared with 0.81 ± 0.21 ng/ml in placebo; P = 0.03). Although sputum PGP levels were decreased after 12 weeks of roflumilast therapy, these were not statistically different from findings in the placebo group (Figure 2C) (0.29 ± 0.08 compared with 0.52 ± 0.28 ng/ml in placebo; P = 0.44).
Figure 2.
Roflumilast reduces sputum acetyl-proline-glycine-proline (AcPGP). Sputa were analyzed in randomized patients. (A) Roflumilast treatment results in lower AcPGP levels compared with placebo after 12 weeks of therapy. (B) Roflumilast reduces sputum AcPGP by >50%. Following 12 weeks of treatment with (C) roflumilast or placebo, there were no statistical changes in sputum proline-glycine-proline (PGP). Open bars and circle represent the placebo group (n = 16), and solid bars and square represent the roflumilast group (n = 11). Values expressed as mean ± SEM. *P < 0.05. NS = not significant.
Secondary Efficacy Variables
Effects on AcPGP/PGP generation in sputum
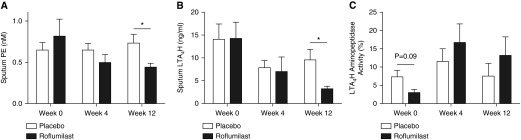
Baseline sputum prolyl endopeptidase was not statistically different between roflumilast- and placebo-treated subjects (0.81 ± 0.62 vs. 0.65 ± 0.34 nM, respectively; P = 0.25). As seen in Figure 3A, 12 weeks of roflumilast treatment significantly reduces prolyl endopeptidase compared with placebo (0.44 ± 0.15 vs. 0.73 ± 0.42 nM; P = 0.02). Sputum prolyl endopeptidase activity had a modest correlation with sputum AcPGP (r = 0.39; P = 0.059).
Figure 3.
Roflumilast affects enzymes critical to the acetyl-proline-glycine-proline/proline-glycine-proline pathway. (A) Roflumilast decreases prolyl endopeptidase (PE), the enzyme critical in the generation of proline-glycine-proline. (B) Likewise, roflumilast treatment reduces leukotriene A4 hydrolase (LTA4H) amount in sputum compared with placebo. (C) Although there was an increase in LTA4H aminopeptidase activity in roflumilast treatment after 12 weeks, there were no statistical differences in this group or placebo compared with baseline values. Open bars represent the placebo group (n = 16), and solid bars represent the roflumilast group (n = 11). Values expressed as mean ± SEM. *P < 0.05.
Effects on PGP degradation in sputum
Baseline LTA4H (14.4 ± 3.5 vs. 14.1 ± 3.4 ng/ml; P = 0.96) and LTA4H aminopeptidase activity (2.7 ± 1.0 vs. 7.4 ± 1.8 ng/ml/min per ng/enzyme; P = 0.09) were comparable between roflumilast- and placebo-treated subjects, respectively. As seen in Figure 3B, roflumilast treatment results in a reduction of sputum LTA4H (3.2 ± 0.6 vs. 9.6 ± 2.3 ng/ml in placebo; P = 0.04). Although there was a 4.5-fold improvement in aminopeptidase activity in the roflumilast-treated group (Figure 3C; P = 0.08), there were no statistical differences in aminopeptidase activity between roflumilast- and placebo-treated subjects (13.3 ± 5.0 vs. 7.6 ± 3.5 ng/ml/min per ng/enzyme; P = 0.36).
Plasma PGP/AcPGP markers
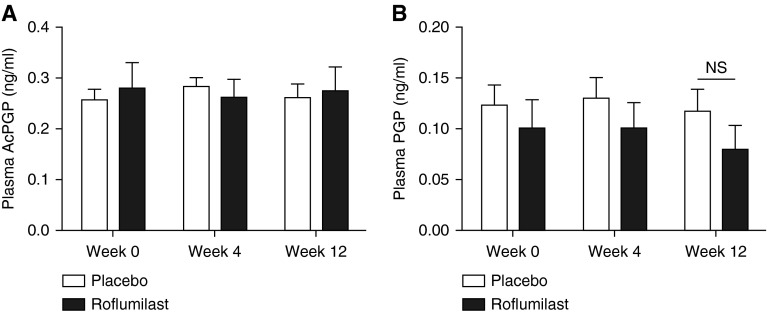
Although the presence and importance of sputum PGP/AcPGP have been described, little is known about its presence in the systemic circulation. We measured plasma PGP and AcPGP according to roflumilast intervention compared with placebo. Plasma AcPGP (0.28 ± 0.05 vs. 0.26 ± 0.02 ng/ml; P = 0.69) and PGP (0.10 ± 0.03 vs. 0.12 ± 0.02 ng/ml; P = 0.51) were comparable at baseline between placebo and roflumilast groups. As seen in Figure 4, roflumilast treatment did not result in reductions of either PGP or AcPGP in plasma.
Figure 4.
Roflumilast therapy does not affect systemic proline-glycine-proline (PGP). There were no statistical differences in plasma (A) acetyl-proline-glycine-proline (AcPGP) and (B) PGP, suggesting the antiinflammatory effect is most pronounced in the lung. Open bars represent the placebo group (n = 16), and solid bars represent the roflumilast group (n = 11). Values expressed as mean ± SEM. NS = not significant.
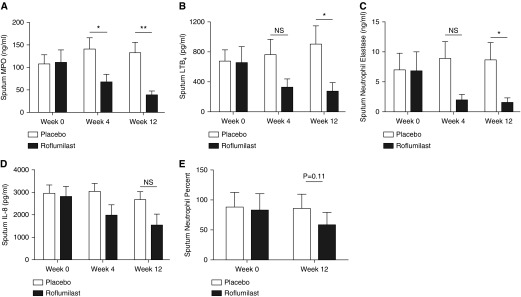
Sputum neutrophil cell count and inflammatory markers
Sputum MPO (110 ± 29 vs. 108 ± 21 ng/ml; P = 0.94), LTB4 (658 ± 214 vs. 675 ± 158 pg/ml; P = 0.94), IL-8 (2,811 ± 460 vs. 2,950 ± 385 pg/ml; P = 0.82), and neutrophil elastase (6.9 ± 3.1 vs. 7.1 ± 2.7 ng/ml; P = 0.96) were similar at baseline between roflumilast and placebo groups, respectively. As seen in Figure 5, roflumilast treatment at 12 weeks results in significant reduction of MPO (39 ± 9 vs. 133 ± 23 ng/ml; P = 0.003), LTB4 (176 ± 77 vs. 903 ± 248 pg/ml; P = 0.03), and neutrophil elastase (1.6 ± 0.8 vs. 8.7 ± 2.8 ng/ml; P = 0.03), with a trend toward lower IL-8 (1,530 ± 512 vs. 2,680 ± 348 pg/ml; P = 0.08) levels in sputum. The concomitant reductions in LTA4H and LTB4 with relative sparing of aminopeptidase activity reinforce prior observations of selective LTA4H inhibition. Although there was a reduction in overall sputum neutrophil burden after 12 weeks of therapy, it did not reach statistical significance (Figure 5E) (58 ± 24 vs. 86 ± 22%; P = 0.11). However, sputum MPO (r = 0.36; P = 0.001), LTB4 (r = 0.24; P = 0.03), IL-8 (r = 0.40, P < 0.001), and neutrophil elastase (r = 0.62, P < 0.001) correlate well with sputum neutrophil counts, highlighting their biologic relevance.
Figure 5.
Roflumilast reduces other markers of pulmonary inflammation. (A) Myeloperoxidase (MPO) was significantly reduced at 4 and 12 weeks. Roflumilast use is associated with a reduction in (B) leukotriene B4 (LTB4) and (C) neutrophil elastase but not (D) IL-8. (E) Neutrophil counts were reduced, although no statistical difference was observed. Open bars represent the placebo group (n = 16), and solid bars represent the roflumilast group (n = 11). Values expressed as mean ± SEM. *P < 0.05; **P < 0.01. NS = not significant.
Relationship between roflumilast treatment and clinical outcomes
Lung function, quality of life, and exercise tolerance were evaluated over the 12-week study period. Baseline values are reported in Table 1. As seen in Figure E3A, mean post-bronchodilator FEV1 did not significantly improve compared with baseline or with placebo, but the 41-ml increase observed with roflumilast treatment was similar to what has previously been reported (37). Total SGRQ score (see Figure E3B), BCSS score (see Figure E3C), and 6MWD (see Figure E3D) showed trends toward improvement, although none reached statistical significance.
Safety and side-effect profile
The incidence of treatment-related adverse effects as assessed by the investigator was 45% in the roflumilast group and 25% in the placebo group (P = 0.24) (Table 2). Adverse effects of special interest that developed in the roflumilast group were gastrointestinal including nausea, diarrhea, and weight loss of 10 pounds (27%); upper respiratory infection (9%); cough (9%); and insomnia (9%). No significant adverse effect, death, or hospitalization occurred in the study. No adverse effects led to discontinuation of study drug.
Table 2.
Adverse Effects
| Placebo (n = 16) | Roflumilast (n = 11) | |
|---|---|---|
| Any AE | 4 (25%) | 5 (45%) |
| Any significant AE | 0 | 0 |
| Treatment-related AE | ||
| Any gastrointestinal | 0 | 3 (27%) |
| Nausea | 0 | 1 (9%) |
| Diarrhea | 0 | 1 (9%) |
| Weight loss of 10 lb | 0 | 1 (9%) |
| Any sinopulmonary | 4 (25%) | 2 (18%) |
| Upper respiratory infection | 2 (13%) | 1 (9%) |
| Cough | 0 | 1 (9%) |
| Pleurisy | 1 (6%) | 0 |
| Pneumonia | 1 (6%) | 0 |
| Insomnia | 0 | 1 (9%) |
Definition of abbreviation: AE = adverse effect.
P = 0.24 for any AE differences between placebo and roflumilast groups.
All results are number of patients (%).
Discussion
This study is the first evidence of roflumilast’s ability to lower sputum AcPGP levels over a 12-week period that coincides with reductions in pulmonary inflammation in patients with moderate to severe COPD with chronic bronchitis. The reduction in sputum AcPGP by more than 50% achieved the primary end point of the study. We also demonstrate for the first time that reducing prolyl endopeptidase, the rate-limiting enzyme responsible for PGP/AcPGP release from collagen fragments, is affected by pharmacologic intervention. This is an example of translation of observations from in vitro and animal models to human subjects, highlighting the pathologic importance of AcPGP in the development of COPD (17) into a proof of concept clinical trial. Our findings suggest that the antiinflammatory effects of roflumilast are most pronounced in the lung and these effects are more pronounced than roflumilast’s other properties as a bronchodilator and airway modulator. Roflumilast was well tolerated in this study.
The significant reduction in sputum AcPGP following 12 weeks of therapy with roflumilast occurred primarily because of inhibition of its generating pathway specifically by decreasing prolyl endopeptidase. We have previously demonstrated that prolyl endopeptidase is expressed on human macrophages, airway epithelial cells, and neutrophils, where it is increased 25-fold in subjects with COPD compared with healthy control subjects (38). We have also previously described the crucial role of prolyl endopeptidase in the de novo production of PGP and AcPGP from collagen fragments and shown that it is a viable therapeutic target using in vitro and animal models of COPD (25, 36, 39).
The current study highlights the importance of prolyl endopeptidase as a key enzyme in regulating neutrophilic inflammation in COPD and warrants further investigation. The 4.5-fold improvement in LTA4H aminopeptidase function was not statistically significant. This may be caused in part by severe baseline disease in the patient population as evidenced by marked suppression of aminopeptidase function or may be a result of our small sample size. As we have previously shown, subjects with COPD have dramatically impaired aminopeptidase activity compared with healthy control and control smoking subjects (17).
In addition to the reductions in AcPGP, roflumilast also lowers other markers of inflammation. Here, total neutrophil count was reduced by 57%, IL-8 by 37%, and neutrophil elastase amount by 70%. These are comparable with the respective approximately 40%, approximately 38%, and approximately 30% reductions observed by Grootendorst and coworkers (12). We also found significant reductions in MPO and LTB4, highlighting roflumilast’s ability to reduce multiple markers of neutrophilic inflammation.
COPD exacerbations are a major target for novel therapeutic agents, including roflumilast and azithromycin. Although effective at reducing exacerbations, these drugs are given long-term and use is hampered by drug intolerance and other side effects. We have previously evaluated the association between sputum PGP, azithromycin use, and acute exacerbations (22). We found that azithromycin use for longer than 6 months was correlated to lower sputum PGP amounts. Here, we show that roflumilast treatment results in lowering of sputum AcPGP in 3 months, suggesting a more rapid and sustained treatment effect compared with azithromycin in severe COPD. Both the current study and the MACRO trial (40) enrolled COPD subjects with moderate to severe airflow obstruction that were at risk for COPD exacerbations. However, there were two major differences in the study populations: we specifically evaluated patients with chronic bronchitis in the current study, and subjects enrolled in the current study were more often current smokers (59% vs. ∼22% in the MACRO trial). Taking these differences into context and supported by recent findings suggesting daily azithromycin therapy is most effective in nonsmoking subjects with COPD with milder airflow obstruction (41) may help explain observed differences in efficacy. Although a head-to-head trial is necessary to adequately distinguish the risk-benefit ratio for using roflumilast or azithromycin in different COPD populations, our data support the use of roflumilast as a treatment for moderate to severe COPD with chronic bronchitis by reducing pulmonary inflammation, even in the setting of ongoing cigarette use.
The present study is limited by its small sample size and limited follow-up. These limitations increase the likelihood that true findings, such as improvement in FEV1 or quality of life, were not observed. Despite these limitations, the important findings of reductions in sputum AcPGP and other markers of neutrophilic inflammation with chronic roflumilast treatment highlight the overall robust nature of these associations. An additional limitation was unequal randomization: a greater number of subjects randomized to placebo compared with roflumilast. Our randomization scheme used block randomization to account for smoking status and inhaled steroid use and this happened to result in the discrepancy. Finally, given the heterogeneity of COPD, novel computed tomography–based metrics are increasingly being used to subphenotype COPD, including chronic bronchitis (42, 43). We did not have access to computed tomography imaging in this study, but further studies should be performed to link the AcPGP-pathway with computed tomography findings of chronic bronchitis.
In summary, this study suggests for the first time that administration of roflumilast for 12 weeks can reduce lung inflammation through mechanistically inhibiting a pathway integral to the pathogenesis of COPD and implicated with COPD exacerbations. The beneficial clinical effect of treatment seen in patients with moderate-to-severe COPD at risk for acute exacerbations is most likely caused by these reductions in pulmonary prolyl endopeptidase, AcPGP, and neutrophilic inflammation. This study was powered on the basis of reductions in sputum AcPGP, therefore further adequately powered studies focusing on sputum and plasma AcPGP and clinical end points, such as exacerbation frequency or severity, are needed. Considering the present results together with previous findings with azithromycin, both PGP and AcPGP show promise as clinically relevant biomarkers for COPD.
Footnotes
Supported by Forest Laboratories, Inc. The University of Alabama at Birmingham Targeted Metabolomics and Proteomics Laboratory receives key support from S10 RR17261, P30 DK079337-07, R01 AG043076-03, and 4R00 HL111322-03.
Author Contributions: Conception and design, J.M.W., W.C.B., M.T.D., and J.E.B. Analysis and interpretation, J.M.W., P.L.J., L.V., S.P.B., J.G., G.H., R.W.K., X.X., M.T.D., and J.E.B. Drafting the manuscript for important intellectual content, J.M.W., P.L.J., L.V., S.P.B., J.G., G.H., R.W.K., X.X., A.G., W.C.B., M.T.D., and J.E.B.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201503-0543OC on July 7, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System. 2013;61:1–117. [PubMed] [Google Scholar]
- 2.Celli BR, Locantore N, Yates J, Tal-Singer R, Miller BE, Bakke P, Calverley P, Coxson H, Crim C, Edwards LD, et al. ECLIPSE Investigators. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185:1065–1072. doi: 10.1164/rccm.201110-1792OC. [DOI] [PubMed] [Google Scholar]
- 3.Stănescu D, Sanna A, Veriter C, Kostianev S, Calcagni PG, Fabbri LM, Maestrelli P. Airways obstruction, chronic expectoration, and rapid decline of FEV1 in smokers are associated with increased levels of sputum neutrophils. Thorax. 1996;51:267–271. doi: 10.1136/thx.51.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stockley RA. Neutrophils and the pathogenesis of COPD. Chest. 2002;121(Suppl. 5):151S–155S. doi: 10.1378/chest.121.5_suppl.151s. [DOI] [PubMed] [Google Scholar]
- 5.Mannino DM. Chronic obstructive pulmonary disease: definition and epidemiology. Respir Care. 2003;48:1185–1191; discussion 1191–1183. [PubMed] [Google Scholar]
- 6.Ferguson GT. Recommendations for the management of COPD. Chest. 2000;117(Suppl. 2):23S–28S. doi: 10.1378/chest.117.2_suppl.23s. [DOI] [PubMed] [Google Scholar]
- 7.Kim V, Benditt JO, Wise RA, Sharafkhaneh A. Oxygen therapy in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:513–518. doi: 10.1513/pats.200708-124ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scanlon PD, Connett JE, Waller LA, Altose MD, Bailey WC, Buist AS, Tashkin DP Lung Health Study Research Group. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease: The Lung Health Study. Am J Respir Crit Care Med. 2000;161:381–390. doi: 10.1164/ajrccm.161.2.9901044. [DOI] [PubMed] [Google Scholar]
- 9.Miller M, Cho JY, Pham A, Friedman PJ, Ramsdell J, Broide DH. Persistent airway inflammation and emphysema progression on CT scan in ex-smokers observed for 4 years. Chest. 2011;139:1380–1387. doi: 10.1378/chest.10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Retamales I, Elliott WM, Meshi B, Coxson HO, Pare PD, Sciurba FC, Rogers RM, Hayashi S, Hogg JC. Amplification of inflammation in emphysema and its association with latent adenoviral infection. Am J Respir Crit Care Med. 2001;164:469–473. doi: 10.1164/ajrccm.164.3.2007149. [DOI] [PubMed] [Google Scholar]
- 11.Calverley PM, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:154–161. doi: 10.1164/rccm.200610-1563OC. [DOI] [PubMed] [Google Scholar]
- 12.Grootendorst DC, Gauw SA, Verhoosel RM, Sterk PJ, Hospers JJ, Bredenbröker D, Bethke TD, Hiemstra PS, Rabe KF. Reduction in sputum neutrophil and eosinophil numbers by the PDE4 inhibitor roflumilast in patients with COPD. Thorax. 2007;62:1081–1087. doi: 10.1136/thx.2006.075937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabbri LM, Calverley PM, Izquierdo-Alonso JL, Bundschuh DS, Brose M, Martinez FJ, Rabe KF M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374:695–703. doi: 10.1016/S0140-6736(09)61252-6. [DOI] [PubMed] [Google Scholar]
- 14.Antoniu SA. New therapeutic options in the management of COPD: focus on roflumilast. Int J Chron Obstruct Pulmon Dis. 2011;6:147–155. doi: 10.2147/COPD.S7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatzelmann A, Schudt C. Anti-inflammatory and immunomodulatory potential of the novel PDE4 inhibitor roflumilast in vitro. J Pharmacol Exp Ther. 2001;297:267–279. [PubMed] [Google Scholar]
- 16.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 17.Wells JM, O’Reilly PJ, Szul T, Sullivan DI, Handley G, Garrett C, McNicholas CM, Roda MA, Miller BE, Tal-Singer R, et al. An aberrant leukotriene A4 hydrolase-proline-glycine-proline pathway in the pathogenesis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190:51–61. doi: 10.1164/rccm.201401-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardison MT, Galin FS, Calderon CE, Djekic UV, Parker SB, Wille KM, Jackson PL, Oster RA, Young KR, Blalock JE, et al. The presence of a matrix-derived neutrophil chemoattractant in bronchiolitis obliterans syndrome after lung transplantation. J Immunol. 2009;182:4423–4431. doi: 10.4049/jimmunol.0802457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaggar A, Jackson PL, Noerager BD, O’Reilly PJ, McQuaid DB, Rowe SM, Clancy JP, Blalock JE. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol. 2008;180:5662–5669. doi: 10.4049/jimmunol.180.8.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snelgrove RJ, Jackson PL, Hardison MT, Noerager BD, Kinloch A, Gaggar A, Shastry S, Rowe SM, Shim YM, Hussell T, et al. A critical role for LTA4H in limiting chronic pulmonary neutrophilic inflammation. Science. 2010;330:90–94. doi: 10.1126/science.1190594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haeggström JZ. Leukotriene A4 hydrolase/aminopeptidase, the gatekeeper of chemotactic leukotriene B4 biosynthesis. J Biol Chem. 2004;279:50639–50642. doi: 10.1074/jbc.R400027200. [DOI] [PubMed] [Google Scholar]
- 22.O’Reilly PJ, Jackson PL, Wells JM, Dransfield MT, Scanlon PD, Blalock JE. Sputum PGP is reduced by azithromycin treatment in patients with COPD and correlates with exacerbations. BMJ Open. 2013;3:e004140. doi: 10.1136/bmjopen-2013-004140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Reilly PJ, Hardison MT, Jackson PL, Xu X, Snelgrove RJ, Gaggar A, Galin FS, Blalock JE. Neutrophils contain prolyl endopeptidase and generate the chemotactic peptide, PGP, from collagen. J Neuroimmunol. 2009;217:51–54. doi: 10.1016/j.jneuroim.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 25.O’Reilly P, Jackson PL, Noerager B, Parker S, Dransfield M, Gaggar A, Blalock JE. N-alpha-PGP and PGP, potential biomarkers and therapeutic targets for COPD. Respir Res. 2009;10:38. doi: 10.1186/1465-9921-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calverley PM, Martinez FJ, Fabbri LM, Goehring UM, Rabe KF. Does roflumilast decrease exacerbations in severe COPD patients not controlled by inhaled combination therapy? The REACT study protocol. Int J Chron Obstruct Pulmon Dis. 2012;7:375–382. doi: 10.2147/COPD.S31100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet. 2015;385:857–866. doi: 10.1016/S0140-6736(14)62410-7. [DOI] [PubMed] [Google Scholar]
- 28.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 29.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 30.Sutherland ER, Pak J, Langmack EL, Silkoff PE, Martin RJ. Safety of sputum induction in moderate-to-severe chronic obstructive pulmonary disease. Respir Med. 2002;96:482–486. doi: 10.1053/rmed.2002.1342. [DOI] [PubMed] [Google Scholar]
- 31.in ’t Veen JC, de Gouw HW, Smits HH, Sont JK, Hiemstra PS, Sterk PJ, Bel EH. Repeatability of cellular and soluble markers of inflammation in induced sputum from patients with asthma. Eur Respir J. 1996;9:2441–2447. doi: 10.1183/09031936.96.09122441. [DOI] [PubMed] [Google Scholar]
- 32.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation: the St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 33.Leidy NK, Rennard SI, Schmier J, Jones MK, Goldman M. The breathlessness, cough, and sputum scale: the development of empirically based guidelines for interpretation. Chest. 2003;124:2182–2191. doi: 10.1378/chest.124.6.2182. [DOI] [PubMed] [Google Scholar]
- 34.Ko FW, Leung TF, Wong GW, Ngai J, To KW, Ng S, Hui DS. Measurement of tumor necrosis factor-alpha, leukotriene B4, and interleukin 8 in the exhaled breath condensate in patients with acute exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2009;4:79–86. [PMC free article] [PubMed] [Google Scholar]
- 35.Bollinger JG, Thompson W, Lai Y, Oslund RC, Hallstrand TS, Sadilek M, Turecek F, Gelb MH. Improved sensitivity mass spectrometric detection of eicosanoids by charge reversal derivatization. Anal Chem. 2010;82:6790–6796. doi: 10.1021/ac100720p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdul Roda M, Sadik M, Gaggar A, Hardison MT, Jablonsky MJ, Braber S, Blalock JE, Redegeld FA, Folkerts G, Jackson PL. Targeting prolyl endopeptidase with valproic acid as a potential modulator of neutrophilic inflammation. PLoS One. 2014;9:e97594. doi: 10.1371/journal.pone.0097594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685–694. doi: 10.1016/S0140-6736(09)61255-1. [DOI] [PubMed] [Google Scholar]
- 38.Overbeek SA, Braber S, Koelink PJ, Henricks PA, Mortaz E, LoTam Loi AT, Jackson PL, Garssen J, Wagenaar GT, Timens W, et al. Cigarette smoke-induced collagen destruction; key to chronic neutrophilic airway inflammation? PLoS One. 2013;8:e55612. doi: 10.1371/journal.pone.0055612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braber S, Koelink PJ, Henricks PA, Jackson PL, Nijkamp FP, Garssen J, Kraneveld AD, Blalock JE, Folkerts G. Cigarette smoke-induced lung emphysema in mice is associated with prolyl endopeptidase, an enzyme involved in collagen breakdown. Am J Physiol Lung Cell Mol Physiol. 2011;300:L255–L265. doi: 10.1152/ajplung.00304.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA, Jr, Criner GJ, Curtis JL, Dransfield MT, Han MK, Lazarus SC, et al. COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689–698. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han MK, Tayob N, Murray S, Dransfield MT, Washko G, Scanlon PD, Criner GJ, Casaburi R, Connett J, Lazarus SC, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Respir Crit Care Med. 2014;189:1503–1508. doi: 10.1164/rccm.201402-0207OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim V, Davey A, Comellas AP, Han MK, Washko G, Martinez CH, Lynch D, Lee JH, Silverman EK, Crapo JD, et al. COPDGene® Investigators. Clinical and computed tomographic predictors of chronic bronchitis in COPD: a cross sectional analysis of the COPDGene study. Respir Res. 2014;15:52. doi: 10.1186/1465-9921-15-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim V, Han MK, Vance GB, Make BJ, Newell JD, Hokanson JE, Hersh CP, Stinson D, Silverman EK, Criner GJ COPDGene Investigators. The chronic bronchitic phenotype of COPD: an analysis of the COPDGene Study. Chest. 2011;140:626–633. doi: 10.1378/chest.10-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]