Two articles in this issue of the Journal, by Kumar and colleagues (pp. 998–1008) (1) and Le Hiress and colleagues (pp. 983–997) (2), provide new insights on how inflammatory processes can cause pulmonary arterial hypertension (PAH). The articles focus on the injury response in the pulmonary artery that causes pulmonary arterial remodeling with increased smooth muscle cells, a fibrotic response, and increases in the pulmonary artery and right ventricular systolic pressures.
The work of Le Hiress and colleagues (2) focused on how the responses of endothelial cells to injury can initiate inflammation in pulmonary hypertension (Figure 1). The injury was induced by hypoxia or monocrotaline exposure in rats. Previously, macrophage inhibitory factor (MIF) has been implicated in pulmonary artery remodeling and pulmonary hypertension (3–5). Le Hiress and colleagues (2) identified MIF and CD74 as critical regulators of inflammatory signals in endothelial cells, controlling the expression of specific adhesion molecules, cytokine mediator molecules, and leukocyte migration. The same molecular network of MIF/CD74 was up-regulated in the pulmonary artery tissues of humans with idiopathic or heritable PAH (iPAH, hPAH).
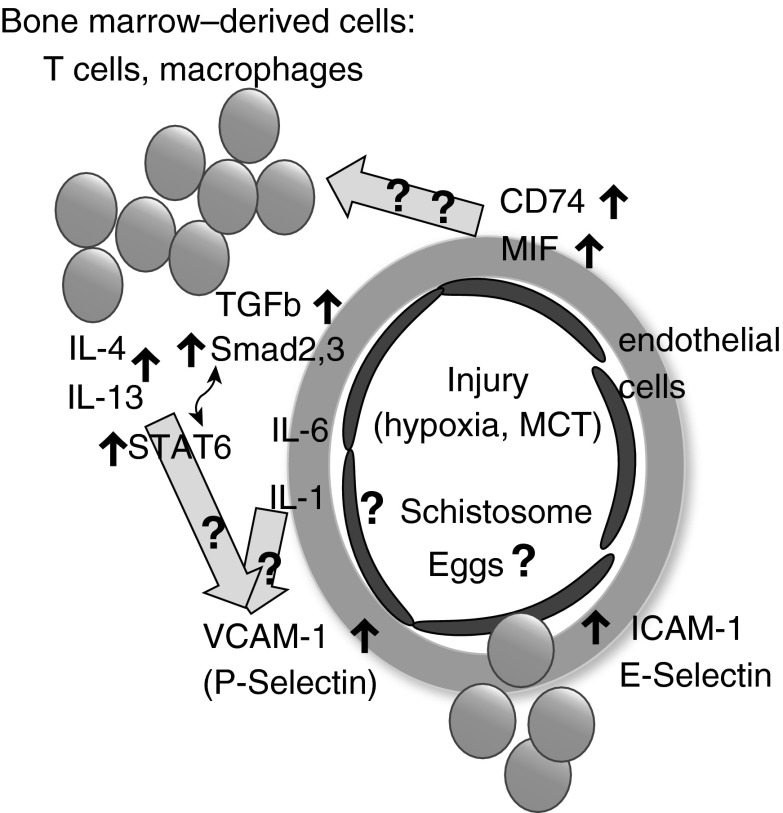
Figure 1.
Networks of mediators of inflammation in pulmonary arterial hypertension identified by Le Hiress and colleagues (2) and by Kumar and colleagues (1). The figure schematically shows several cell types in a pulmonary artery from pulmonary arterial hypertension lungs: endothelial cells (dark); leukocytes, among them T cells and macrophages (dark); and the remodeled artery wall of smooth muscle cells and connective tissue (gray shaded circle). The articles by Le Hiress and colleagues (2) and by Kumar and colleagues (1) focused on the contributions of the endothelial cell–CD74–MIF axis (2) and the bone marrow–derived leukocytes–IL-4–IL-13–transforming growth factor β axis (1), respectively. The groups identified shared cells (T cells), mediators (IL-6), and processes (leukocyte migration). Block arrows with question marks indicate potential additional links between the networks studied by the two research groups. Although these additional molecular links could exist, it is possible that the processes studied by Le Hiress and colleagues (2) and Kumar and colleagues (1) are distinct because the initializing events were distinct (hypoxia, monocrotaline, idiopathic pulmonary arterial hypertension, and heritable pulmonary arterial hypertension vs. Schistosoma-associated pulmonary arterial hypertension). ICAM-1 = intercellular adhesion molecule 1; MCT = monocrotaline; MIF = macrophage inhibitory factor; STAT6 = signal transducer and activator of transcription 6; TGF-β = transforming growth factor β; VCAM-1 = vascular cell adhesion protein 1.
Kumar and colleagues (1) focused on Schistosoma-induced PAH in humans and the experimental model in mice. Th2 responses, with IL-4 and IL-13 as prominent mediators, are known to be an important component of the immune response to infection with schistosome parasites (6) and have been previously reported to be important for PAH in the experimental model (7, 8). Kumar and colleagues (1) studied the inflammatory cell aspect of the process (Figure 1) and showed that bone marrow cell-derived IL-4 and IL-13 were critical determinants of the pulmonary hypertension phenotype in mice. Further, the authors showed that the IL-4/IL-13 axis is increased in human schistosomiasis-associated PAH lungs.
Kumar and colleagues (1) demonstrated that transforming growth factor β (TGF-β) signaling was amplified by the excess IL-4/IL-13 produced in response to Schistosoma egg exposures. It is of note that schistosomiasis-associated PAH can persist in humans even after antihelminthic treatment and an inability to detect active infection (9).
We do not know whether and how the mechanisms of inflammation in PAH studied by Le Hiress (2) and colleagues and Kumar and colleagues (1) are linked. The two research groups studied separate experimental models and separate human PAH forms. For example, it is entirely possible that in the IL-4/IL-13–dependent process that causes PAH, the CD74/MIF axis has no critically important role, and vice versa. The two studies (1, 2) identified shared cell types (T cells), processes (inflammatory cell migration), and mediators (IL-6). Figure 1 illustrates several additional potential connections. CD74 has at least two cellular functions: it is the invariant chain of major histocompatibility complex class II (MHCII) protecting the MHCII molecule during assembly in the endoplasmic reticulum, and it is a receptor for MIF. MIF is also a multifunctional soluble mediator that helps to retain macrophages in the tissue and that controls inflammation. Le Hiress and colleagues (2) identified T cells in the lung tissue of patients with PAH as the producers of MIF. Kumar and colleagues (1) found that macrophages responded to the IL-4/IL-13–initiated process by activating TGF-β. Previous studies have shown that mice deficient in CD74 or MIF, or mice treated with a MIF inhibitor, have highly significantly depressed IL-4/IL-13 responses (10, 11).
The molecular networks studied by Le Hiress and colleagues (2) and Kumar and colleagues (1) could also be linked via the adhesion molecules that were up-regulated in the endothelial cells (Figure 1). Le Hiress and colleagues (2) found that the MIF/CD74 axis controlled the increased expression of several adhesion molecules, among them vascular cell adhesion protein 1 (VCAM-1), and the authors detected increased P-selectin expression by pulmonary artery endothelial cells from iPAH and hPAH lungs. IL-4 and IL-13 signaling, the critical event for PAH studied by Kumar and colleagues (1), is known to significantly induce both VCAM-1 (12) and P-selectin (13) on endothelial cells. Further, Kumar and colleagues (1) found that the migration of bone marrow–derived leukocytes that were capable of producing IL-4 and IL-13 was necessary for the development of experimental PAH. Le Hiress and colleagues (2) showed that MIF/CD74–induced up-regulation of adhesion molecules on endothelial cells facilitated the adhesion of leukocytes to the endothelial cell layer and the transmigration of the cells to chemotactic stimuli.
An intriguing observation is that many different kinds of injuries can trigger the process. In the studies by Kumar and colleagues (1), the initializing agent was Schistosoma eggs in immunized mice; in humans with schistosomiasis-associated PAH, the parasite-initiated process is maintained by an unknown process after antihelmintic treatment and the inability to detect Schistosoma antigens in the lungs (9). Hypoxia and monocrotaline were the experimental triggers in the studies by Le Hiress and colleagues (2). We do not know the triggers of inflammation in the patients with iPAH and hPAH. Previous studies (14, 15) showed that even triggers given via the airways can induce the pulmonary hypertension phenotype via IL-13–associated inflammation.
In conclusion, the articles (1, 2) in the current issue of the Journal shed exciting new light on the mechanisms of inflammation that can contribute to PAH. These articles also highlight the gaps in our understanding that future research will need to address.
Footnotes
Supported by a National Institutes of Health research grant (NHLBI R01 HL095764-01; G.G.) and grants from Turkiye Bilimsel Arastirma Kurumu and Hacettepe University (N.D.).
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kumar R, Mickael C, Chabon J, Gebreab L, Rutebemberwa A, Rodriguez Garcia A, Koyanagi DE, Sanders L, Gandjeva A, Kearns MT, et al. The causal role of IL-4 and IL-13 in Schistosoma mansoni pulmonary hypertension. Am J Respir Crit Care Med. 2015;192:998–1008. doi: 10.1164/rccm.201410-1820OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Hiress M, Tu L, Ricard N, Phan C, Thuillet R, Fadel E, Dorfmüller P, Montani D, de Man F, Humbert M, et al. Proinflammatory signature of the dysfunctional endothelium in pulmonary hypertension: role of the macrophage migration inhibitory factor/CD74 complex. Am J Respir Crit Care Med. 2015;192:983–997. doi: 10.1164/rccm.201402-0322OC. [DOI] [PubMed] [Google Scholar]
- 3.Becker H, Willeke P, Schotte H, Domschke W, Gaubitz M. Macrophage migration inhibitory factor may contribute to vasculopathy in systemic sclerosis. Clin Rheumatol. 2008;27:1307–1311. doi: 10.1007/s10067-008-0960-7. [DOI] [PubMed] [Google Scholar]
- 4.Zhang B, Luo Y, Liu ML, Wang J, Xu DQ, Dong MQ, Liu Y, Xu M, Dong HY, Zhao PT, et al. Macrophage migration inhibitory factor contributes to hypoxic pulmonary vasoconstriction in rats. Microvasc Res. 2012;83:205–212. doi: 10.1016/j.mvr.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Talwar A, Tsang D, Bruchfeld A, Sadoughi A, Hu M, Omonuwa K, Cheng KF, Al-Abed Y, Miller EJ. Macrophage migration inhibitory factor mediates hypoxia-induced pulmonary hypertension. Mol Med. 2012;18:215–223. doi: 10.2119/molmed.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson MS, Mentink-Kane MM, Pesce JT, Ramalingam TR, Thompson R, Wynn TA. Immunopathology of schistosomiasis. Immunol Cell Biol. 2007;85:148–154. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham BB, Mentink-Kane MM, El-Haddad H, Purnell S, Zhang L, Zaiman A, Redente EF, Riches DW, Hassoun PM, Bandeira A, et al. Schistosomiasis-induced experimental pulmonary hypertension: role of interleukin-13 signaling. Am J Pathol. 2010;177:1549–1561. doi: 10.2353/ajpath.2010.100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham BB, Chabon J, Gebreab L, Poole J, Debella E, Davis L, Tanaka T, Sanders L, Dropcho N, Bandeira A, et al. Transforming growth factor-β signaling promotes pulmonary hypertension caused by Schistosoma mansoni. Circulation. 2013;128:1354–1364. doi: 10.1161/CIRCULATIONAHA.113.003072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham BB, Chabon J, Bandeira A, Espinheira L, Butrous G, Tuder RM. Significant intrapulmonary Schistosoma egg antigens are not present in schistosomiasis-associated pulmonary hypertension. Pulm Circ. 2011;1:456–461. doi: 10.4103/2045-8932.93544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das R, Moss JE, Robinson E, Roberts S, Levy R, Mizue Y, Leng L, McDonald C, Tigelaar RE, Herrick CA, et al. Role of macrophage migration inhibitory factor in the Th2 immune response to epicutaneous sensitization. J Clin Immunol. 2011;31:666–680. doi: 10.1007/s10875-011-9541-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang B, Huang X, Wolters PJ, Sun J, Kitamoto S, Yang M, Riese R, Leng L, Chapman HA, Finn PW, et al. Cutting edge: deficiency of macrophage migration inhibitory factor impairs murine airway allergic responses. J Immunol. 2006;177:5779–5784. doi: 10.4049/jimmunol.177.9.5779. [DOI] [PubMed] [Google Scholar]
- 12.Sironi M, Sciacca FL, Matteucci C, Conni M, Vecchi A, Bernasconi S, Minty A, Caput D, Ferrara P, Colotta F, et al. Regulation of endothelial and mesothelial cell function by interleukin-13: selective induction of vascular cell adhesion molecule-1 and amplification of interleukin-6 production. Blood. 1994;84:1913–1921. [PubMed] [Google Scholar]
- 13.Woltmann G, McNulty CA, Dewson G, Symon FA, Wardlaw AJ. Interleukin-13 induces PSGL-1/P-selectin-dependent adhesion of eosinophils, but not neutrophils, to human umbilical vein endothelial cells under flow. Blood. 2000;95:3146–3152. [PubMed] [Google Scholar]
- 14.Park SH, Chen WC, Esmaeil N, Lucas BEG, Marsh LM, Reibman J, Grunig G. Interleukin 13- and interleukin 17A-induced pulmonary hypertension phenotype due to inhalation of antigen and fine particles from air pollution. Pulm Circ. 2014;4:654–668. doi: 10.1086/678511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daley E, Emson C, Guignabert C, de Waal Malefyt R, Louten J, Kurup VP, Hogaboam C, Taraseviciene-Stewart L, Voelkel NF, Rabinovitch M, et al. Pulmonary arterial remodeling induced by a Th2 immune response. J Exp Med. 2008;205:361–372. doi: 10.1084/jem.20071008. [DOI] [PMC free article] [PubMed] [Google Scholar]