Abstract
Introduction
Interstitial cystitis/painful bladder syndrome (IC/PBS) is a chronic inflammatory condition of the bladder. Bladder instillation is one avenue of treatment but evidence for its effectiveness is limited. Chondroitin sulphate solution 2.0% (Urocyst) is a glycosaminoglycan (GAG) replenishment therapy instilled for patients with IC/PBS. We assessed its effectiveness for treating IC/PBS in Northern Ireland.
Methods
Patients with IC/PBS were assessed with the O'Leary-Sant interstitial cystitis index score and global response assessment questionnaire prior to commencing treatment. Assessment with these questionnaires was performed after 6 treatments (10 weeks) and again after 10 treatments (24 weeks). Assessment end points were pain, urgency, symptom score and problem score.
Results
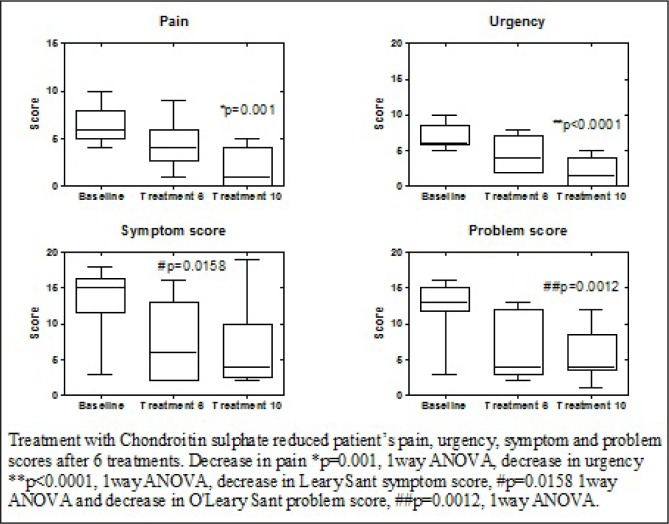
Data was collected on 10 patients, 9 female and 1 male. 6 patients had failed RIMSO-50 dimethyl sulphoxide (DMSO) 50% treatment prior. At baseline the mean pain score was 6.6, urgency score 7.00, symptom score 13.5 and problem score 12.5. After 24 weeks the mean pain score fell to 2.0, urgency score to 1.80, symptom score to 6.89 and problem score to 5.67. At 10 weeks the global response to treatment was 100%. Nocturia was the first symptom to improve with urgency and pain following. No side effects were noted during instillation and all patients tolerated the treatments.
Conclusion
IC/PBS is a difficult disease to treat. It requires a multimodal approach. We found that intravesical chondroitin sulphate reduced pain, urgency and O'Leary-Sant symptom and problem scores in patients with IC/PBS. All patients tolerated the treatment and no side effects were reported.
INTRODUCTION
Interstitial cystitis/painful bladder syndrome (IC/PBS), is a painful and debilitating chronic inflammatory disorder of the bladder. The disorder can have a significant impact on many aspects of a patient's life and some have suggested that the quality of life of IC/PBS patients is equivalent to those with end stage renal failure.
The disorder is characterized by suprapubic pain, often associated with urination, urinary frequency, urgency, and pressure in the bladder or pelvis. Patients may also experience nocturia and pelvic floor dysfunction making urination difficult and sexual intercourse painful. Symptoms can fluctuate from patient to patient, and from time to time for a given patient, making it a difficult condition to diagnose and manage.
Various different treatment modalities have been used to treat IC/PBS. Oral medications include pentosan polysulfate (Elmiron) and amitriptyline. Pain control is a major issue and some patients may need non-steroidal anti-inflammatory (NSAIDS) and/or narcotics. Second line treatments are bladder instillations or bladder coating therapies. Bladder coating treatments have shown positive results in the management of IC/PBS and are believed to replace the deficient glycosaminoglycan (GAG) layer on the bladder wall however the evidence for its effectiveness is limited. In this study we prospectively sought to determine the effectiveness of Chondroitin sulphate solution 2.0% (Urocyst) for the treatment of IC/PBS.
METHODS
A prospective interventional single centre study of 10 patients with IC/PBS was conducted. Eligible patients were male or female patients, 18 years or older with a diagnosis of IC/PBS confirmed by cystoscopy, hydrodistension and bladder biopsy. Exclusion criteria included recurrent urinary tract infection (UTI), overactive bladder, bladder cancer or cystitis of another aetiology. Patients who had failed a previous intravesical therapy were included in the study.
This study was conducted in compliance with Good Clinical Practice guidelines. Chondroitin sulphate solution is given directly into the bladder by urinary catheter by a urology nurse specialist. Patients were then commenced on instillations of chondroitin sulphate solution 2.0% intravesical therapy for 24 weeks. Six treatments were administered in the first 10 weeks and 4 treatments were administered over the next 14 weeks. At each treatment 20ml of the solution was instilled with patients were instructed to hold it in the bladder for at least 30 minutes.
The co–primary efficacy end points were change from baseline in the number of pain and urgency episodes as assessed by interstitial cystitis symptom index responders at week 10 and week 24. Secondary outcome measure is global response assessment (GRA) responders at week 10 and at week 24. Baseline assessment of symptoms severity was performed by assessment with O'Leary-Sant interstitial cystitis index score. This is a questionnaire filled out by the patient encompassing symptoms of urgency, frequency, nocturia and pain/burning. It has been shown to be a valid and reliable method of measuring change in IC symptoms. Response to treatment was assessed with the global response assessment questionnaire. The GRA measures overall improvement with therapy. It is now used as the primary end point in clinical trials of therapies for IC/PBS. Responders to treatment is indicated by a “marked” or “moderate” improvement as assessed on the 7 point scale. Unless otherwise stated, data is represented as mean (interquartile range: IQR). Differences in distribution of clinical data and the development of a SSI were evaluated using 1way ANOVA. P value less than 0.05 was assumed to be significant. All calculations were done using Prism version 5.0 (GraphPad Software, Inc., La Jolla, CA).
RESULTS
Patient demographics and baseline IC/PBS related variables
There were 10 patients included in the study; 9 (90%) female and 1 (10%) male. Mean age was 52 (27 – 81) years. Baseline assessments showed 7 patients (70%) had moderate IC/PBS and 3 patients (30%) had severe IC/PBS. There were no patients with mild IC/PBS. Mean urinary frequency prior to treatment was 12.6 IRQ (8.4-15.5) voids per day and all patients had remained symptomatic on oral medical therapies (5 patients (50%) were taking the tricyclic anti-depressant amitriptyline). 5 patients (50%) were taking hormone replacement therapy (HRT). No patient was taking antihistamines, antibiotics, anticholinergics or Pentosan polysulphate (Elimiron). 6 (60%) patients had failed previous intravesical RIMSO-50 dimethyl sulphoxide (DMSO) 50% w/w treatment prior to instillation of chondroitin sulphate. No patients had been treated with Hyaluronate (Cystistat), Chondroitin sulphate 0.2% or Heparin (Table 1). Baseline mean pain score was 6.6 IRQ (5-8), urgency score 7.00 IRQ (5.75-8.5), O'Leary-Sant symptom score 13.5(11.5-16.3) and O'Leary-Sant problem score 12.5(11.75-16) (Table 2).
Table 1.
Demographics and IC related variables at baseline
| Variable | Total (N) % |
| Age of patient (mean, IRQ) | 52 (27–81) |
| Sex of patient | |
| Male | 9 (90%) |
| Female | 1 (10%) |
| Severiaty of IC | |
| Mild | 0 (0%) |
| Moderate | 7 (70%) |
| Severe | 3 (30%) |
| Urinary frequency, (mean, IRQ) | 12.6 (8.4-15.5) |
| Oral theraphy. | |
| Antide pressants | 5 (50) |
| Antihi stamines | 0 (0%) |
| Hormonal agonists or antagonist* | 5 (50%) |
| Pentosan polysulphate (Elimiron) | 0 (0%) |
| Antimicrobials | 0 (0%) |
| Anticholinergies | 0 (0%) |
| Prior intravesical therapy: | |
| Hyaluronaic acid (Cystistat) | 0 (0%) |
| Herparin 10,000 IU | 0 (0%) |
| RIMSO-50 dimethyl sulphoxide (DMSO) | 6 (60%) |
N indicates number of patients; IQR, interquartile range; IU, International units.
Hormone replacement therapy
Table 2.
Treatment response symptom scores
| End points | Base line | Treatment 6 | Treatment 10 | *P Value |
|---|---|---|---|---|
| Pain, mean (IRQ) | 6.60 (5-8) | 4.30 (2.75-6) | 2.00(0-4) | 0.0001 |
| Urgency, mean (IRQ) | 7.00 (5.73-8.55) | 4.40 (2-7) | 1.80 0-4) | < 0.0001 |
| Symptom score, mean (IRQ) | 13.50(11.5-16.2) | 7.50 (2-13) | 6.89 (2.5-10) | 0.0158 |
| Problem score, mean (IRQ) | 12.50 (11.75-15) | 6.50(3-12) | 5.67 (3.5-8.5) | 0.0012 |
| GRA | 0% | 80% | 70% |
Treatment response to chondroitin sulphate, baseline symptoms and symptoms after 6 treatments and 10 treatments. IRQ, interquartile range; GRA, global assessment response.
All assessed with the 1way ANOVA.
Fig 1.
Treatment response
10 week assessment
After 6 intravesical chondroitin sulphate treatments the mean pain score fell to 4.3 (2.75-6), urgency score to 4.4 (2-7), O'Leary-Sant symptom score 7.5 (2-13) and O'Leary-Sant problem score to 6.5 (3-12). The global response to treatment was 80% (i.e. patients had a marked” or “moderate” improvement) (Table 2). Patients reported that nocturia was the first symptom improved followed by urgency and pain.
24 week assessment
After 10 intravesical chondroitin sulphate 2.0% treatments the mean pain score fell to 2.0 (0-4), urgency score to 1.8 (0-4), O'Leary-Sant symptom score 6.89 (2.5-10) and the O'Leary-Sant problem score 5.67 (3.5-8.5) (Table 1). The fall in score was significant compared to the baseline assessment; decrease in pain score p=0.001, urgency, p<0.001, O'Leary-Sant symptom score p=0.0158 and O'Leary-Sant problem score p=0.0012 (1way ANOVA). The global response to treatment was 70% (Table 2). No side effects were reported during instillation and all patients tolerated the drug for the required length of time.
DISCUSSION
IC/PBS is a chronic condition defined by the International Society of Bladder Pain Syndrome (ESSIC) as “the complaint of suprapubic pain related to bladder filling, accompanied by other symptoms, such as increased daytime and night-time frequency in the absence of proven urinary infection or other obvious pathology”. Patients with cystoscopic features such as glomerulations and Hunner's ulcer are classified as IC and those without as having painful bladder syndrome (PBS); both conditions are encompassed by the term bladder pain syndrome (BPS)1. ESSIC have proposed a classification system based on cystoscopy, hydrodistension and biopsy results.4 IC/PBS has been reported to affect up to 6.53% of the population and is associated with other diseases including IBS, fibromyalgia, depression, vulvodynia, migraine and SLE. 2, 3
There is currently no definitive pathogenesis for IC/PBS however it is generally thought that an initial insult to the bladder triggers endocrine, inflammatory and neurological changes which may result in defects in the urothelial GAG layer of the bladder wall; exposing submucosal nerve fibres to the toxic constituents of urine. Additionally a ten-fold increase in the number of mast cells in the bladder tissue has been noted in a subset of IC/PBS patients. Neurogenic inflammation can result in bladder wall pain with an increased density of peripheral nerves and neuromediator release confirmed in several studies.3
Management of IC/PBS is multidisciplinary and multi-faceted. In addition to lifestyle modification pain control is the first line of treatment with various studies showing amitriptyline, cimetidine and oral pentosan polysulphate to improve pain control.5 Second line treatments include a wide range of intravesical therapies that have been developed with the aim of replacing the deficient GAG layer of the bladder wall. These include sodium hyaluronate (Cystostat), pentosan polysulphate (Elmiron) and chondroitin sulphate 2.0% (Uracyst). Surgical intervention is often deferred until all other available therapies have failed to control symptoms or improve quality of life.
We assessed chondroitin sulphate as an intravesical treatment for IC/PBS. The goal of treatment is to replace the deficient GAG layer in the bladder and improve symptom control. Using the O'Leary-Sant symptom and problem score as well as mean pain and urgency score we showed an improvement of the scores with intravesical chondroitin sulphate 2.0% treatment in 10 patients with moderate/severe IC/PBS. A response to treatment was noted in patients who had failed a different intravesical bladder therapy (DMSO). Reduction in symptoms was noted after 6 treatments (10 weeks) and continued treatment course reduced symptoms further. All symptom reduction scores were statistically significant, however the reduction in pain and urgency scores was considerably more than O'Leary-Sant scores. The global response to treatment was 80% at 10 weeks and 70% at 24 weeks indicating that that the majority of patients noted a marked or moderate response to the administration of chondroitin sulphate. No patient reported any significant side effects or symptom deterioration due to the treatment. All patients tolerated the catherisation.
These findings are in agreement with the current literature. A study by Steinhoff et al with a group of 18 patients treated with chondroitin sulphate showed a response to treatment in 12 of 13 patients.6 Another trial by Sorenson et al showed an average improvement in symptoms in 73.1% of patients with refractory IC/PBS with a more concentrated solution needed in 8 patients.7
Chondroitin sulphate is priced at £87.50 per instillation which is cheaper than Cystistat (sodium hyaluronate £98 per instillation) and Rimso-50 (DMSO - £101+VAT per instillation). Our findings suggest that chondroitin sulphate 2.0% is a valuable treatment for the short term management of symptoms due to IC/PBS, particularly for patients troubled with pain and urgency.
CONCLUSION
Management of IC/PBS is difficult due to the multifactorial aetiology and diagnostic uncertainty as well as multiple treatment options. A multimodal approach is often necessary; intravesical treatments aimed at replenishing the GAG layer of the bladder are one option for treatment. Although our study has small numbers we demonstrated that intravesical chondroitin sulphate 2.0% is an effective treatment which is well tolerated by patients with no side effects reported.
REFERENCES
- 1.van de Merwe JP, Nordling J, Bouchelouche P, Bouchelouche K, Cervigni M, Daha LK, et al. Diagnostic criteria, classification and nomenclature for painful bladder syndrome/interstitial cystitis: an ESSIC proposal. Eur Urol. 2008;53(1):60–7. doi: 10.1016/j.eururo.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 2.Rourke W, Khan SAA, Ahmed K, Masood PD, Dasgupta P, Khan MS. Painful bladder syndrome/interstitial cystitis: Aetiology, evaluation and management. Arch Ital Urol Androl. 2014;86(2):126–31. doi: 10.4081/aiua.2014.2.126. [DOI] [PubMed] [Google Scholar]
- 3.Fall M, Baranowski AP, Elneil S, Engeler D, Hughes J, Messelink EJ, et al. EAU guidelines on chronic pelvic pain. Eur Urol. 2010;57(1):35–48. doi: 10.1016/j.eururo.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 4.Lubeck DP, Whitmore K, Sant GR, Alvarez-Horine S, Lai C. Psychometric validation of the O'leary-Sant interstitial cystitis symptom index in a clinical trial of pentosan polysulfate sodium. Urology. 2001;57(6 Suppl 1):62–6. doi: 10.1016/s0090-4295(01)01126-8. [DOI] [PubMed] [Google Scholar]
- 5.Hanno PM, Burks DA, Clemens JQ, Dmochowski RR, Erickson D, Fitzgerald MP, et al. AUA guideline diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol. 2011;185(6):2162–70. doi: 10.1016/j.juro.2011.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinhoff G, Ittah B, Rowan S. The efficacy of chondroitin sulphate in treating interstitial cystitis. Can J Urol. 2002;9(1):1454–8. [PubMed] [Google Scholar]
- 7.Sorenson RB. Chondroitin sulphate in the treatment of interstitial cystitis and chronic inflammatory disease of the urinary bladder. Eur Urol Suppl. 2003;2(Suppl 4):16–8. [Google Scholar]