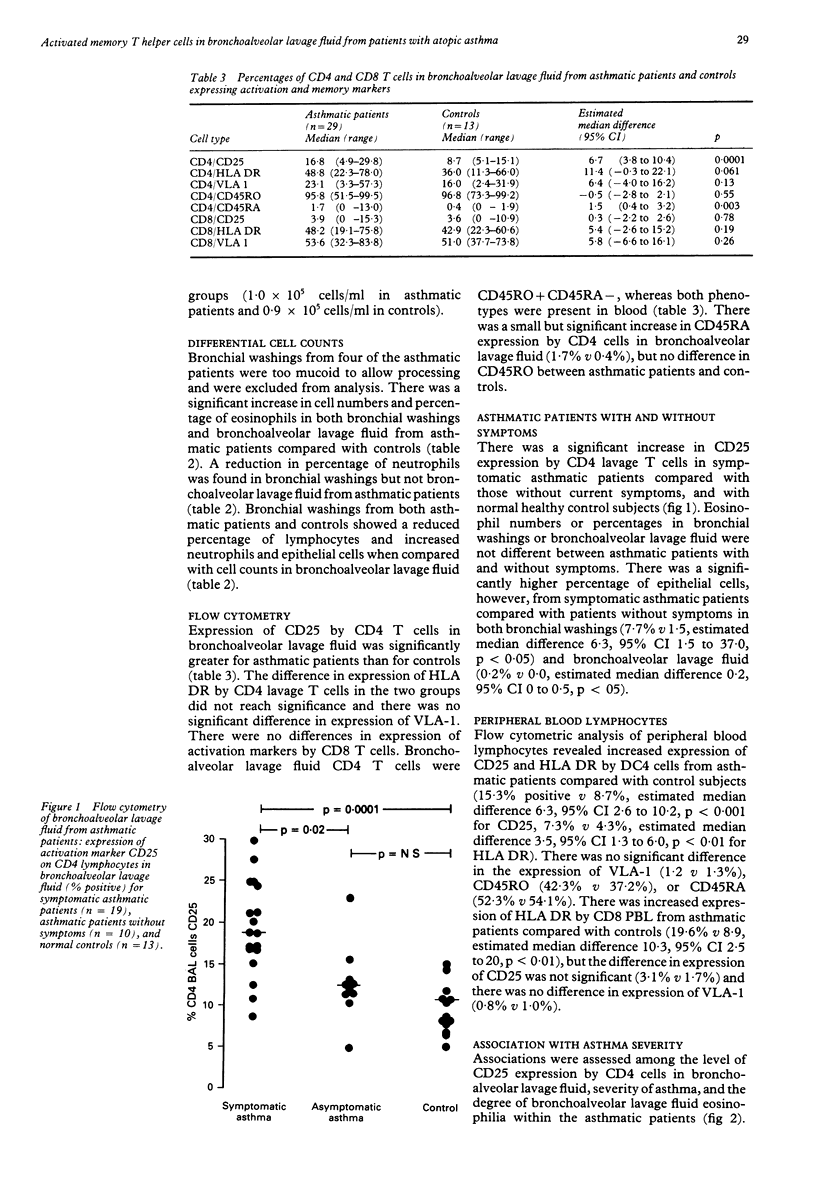
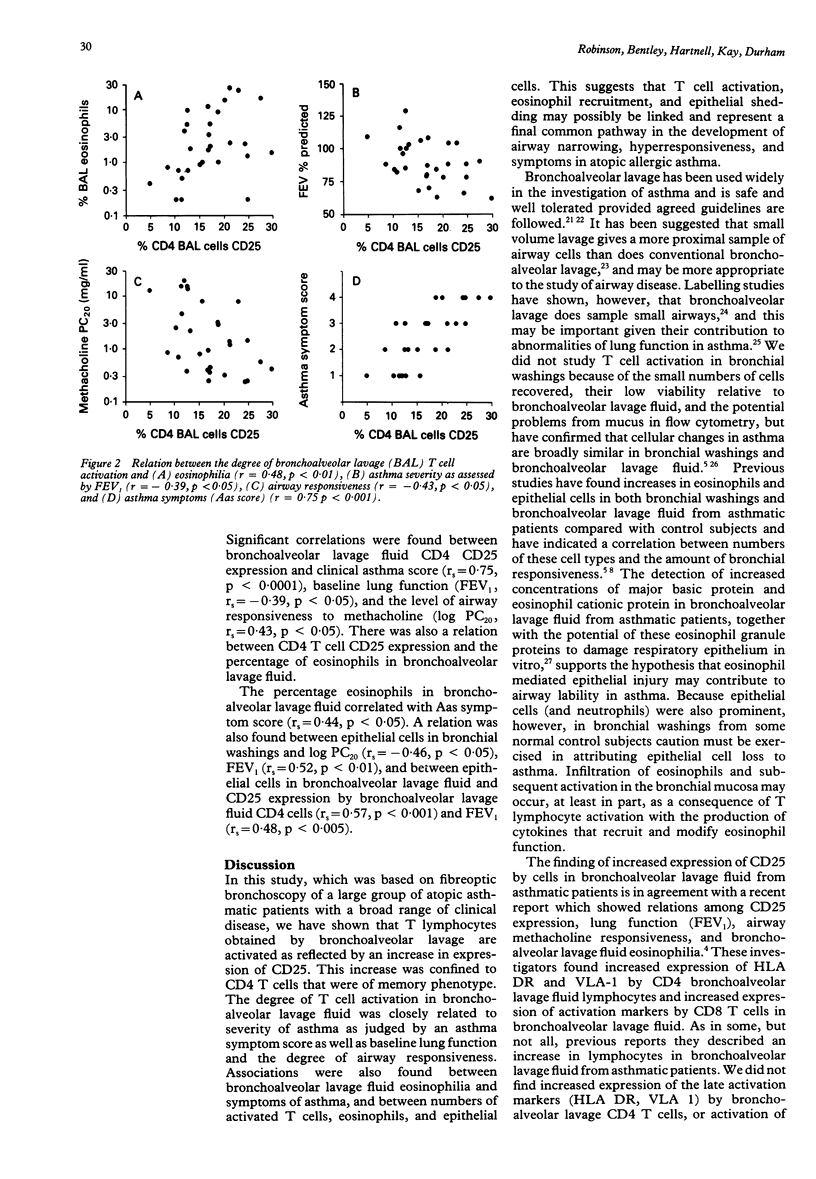
Abstract
BACKGROUND: Bronchial mucosal inflammation and epithelial damage are characteristic features of asthma. Activation of T helper lymphocytes may contribute to this process by mechanisms including the release of cytokines promoting eosinophil infiltration and activation. METHODS: Bronchial washings and bronchoalveolar lavage fluid were obtained from 29 atopic asthmatic patients (19 with current symptoms and 10 symptom free) and 13 normal volunteers. Flow cytometry was used to assess T cell phenotype and activation status in bronchoalveolar lavage fluid and peripheral blood, and differential cell counts were made on bronchial washings and bronchoalveolar lavage fluid. Findings were related to severity of disease as reflected by symptom scores, baseline lung function, and airway responsiveness. RESULTS: CD4 T lymphocytes in bronchoalveolar lavage fluid and blood from asthmatic patients were activated by comparison with controls (CD4 CD25, median 16.8% v 8.7% for bronchoalveolar lavage fluid, and 15.3% v 8.7% in blood). Bronchoalveolar lavage fluid CD4 T cells from both asthmatic patients and controls were of memory phenotype (95.8% and 96.8% CD45RO and 1.7% and 0.4% CD45RA respectively), whereas both CD45RO and CD45RA T cells were present in blood. Patients with asthma and current symptoms showed increased bronchoalveolar T cell activation compared with patients without symptoms (CD4 CD25 18.7% v 12.3%). Within the asthmatic group there was a significant association between CD4 CD25 lymphocytes and asthma symptom scores (rs = 0.75), airway methacholine responsiveness (log PC20, rs = -0.43) and baseline FEV1 (rs = -0.39). A correlation was also found between CD4 CD25 lymphocytes and eosinophils in bronchoalveolar lavage fluid (rs = 0.48). Eosinophils in bronchoalveolar lavage fluid were increased in asthmatic patients compared with controls and the percentage of eosinophils in bronchoalveolar lavage fluid correlated with asthma symptom score. A relation was found between percentage of epithelial cells in bronchoalveolar lavage fluid and FEV1 and methacholine PC20. CONCLUSION: These results support the hypothesis that selective activation of memory CD4 T cells contributes to eosinophil accumulation, bronchial hyperresponsiveness, and symptoms in asthma.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aas K. Heterogeneity of bronchial asthma. Sub-populations--or different stages of the disease. Allergy. 1981 Jan;36(1):3–14. doi: 10.1111/j.1398-9995.1981.tb01818.x. [DOI] [PubMed] [Google Scholar]
- Akbar A. N., Terry L., Timms A., Beverley P. C., Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988 Apr 1;140(7):2171–2178. [PubMed] [Google Scholar]
- Azzawi M., Bradley B., Jeffery P. K., Frew A. J., Wardlaw A. J., Knowles G., Assoufi B., Collins J. V., Durham S., Kay A. B. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am Rev Respir Dis. 1990 Dec;142(6 Pt 1):1407–1413. doi: 10.1164/ajrccm/142.6_Pt_1.1407. [DOI] [PubMed] [Google Scholar]
- Beasley R., Roche W. R., Roberts J. A., Holgate S. T. Cellular events in the bronchi in mild asthma and after bronchial provocation. Am Rev Respir Dis. 1989 Mar;139(3):806–817. doi: 10.1164/ajrccm/139.3.806. [DOI] [PubMed] [Google Scholar]
- Bottomly K. A functional dichotomy in CD4+ T lymphocytes. Immunol Today. 1988 Sep;9(9):268–274. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- Bousquet J., Chanez P., Lacoste J. Y., Barnéon G., Ghavanian N., Enander I., Venge P., Ahlstedt S., Simony-Lafontaine J., Godard P. Eosinophilic inflammation in asthma. N Engl J Med. 1990 Oct 11;323(15):1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- Cockcroft D. W., Killian D. N., Mellon J. J., Hargreave F. E. Bronchial reactivity to inhaled histamine: a method and clinical survey. Clin Allergy. 1977 May;7(3):235–243. doi: 10.1111/j.1365-2222.1977.tb01448.x. [DOI] [PubMed] [Google Scholar]
- Corrigan C. J., Hartnell A., Kay A. B. T lymphocyte activation in acute severe asthma. Lancet. 1988 May 21;1(8595):1129–1132. doi: 10.1016/s0140-6736(88)91951-4. [DOI] [PubMed] [Google Scholar]
- Del Prete G., Maggi E., Parronchi P., Chrétien I., Tiri A., Macchia D., Ricci M., Banchereau J., De Vries J., Romagnani S. IL-4 is an essential factor for the IgE synthesis induced in vitro by human T cell clones and their supernatants. J Immunol. 1988 Jun 15;140(12):4193–4198. [PubMed] [Google Scholar]
- Djukanović R., Wilson J. W., Lai C. K., Holgate S. T., Howarth P. H. The safety aspects of fiberoptic bronchoscopy, bronchoalveolar lavage, and endobronchial biopsy in asthma. Am Rev Respir Dis. 1991 Apr;143(4 Pt 1):772–777. doi: 10.1164/ajrccm/143.4_Pt_1.772. [DOI] [PubMed] [Google Scholar]
- Dominique S., Bouchonnet F., Smiéjan J. M., Hance A. J. Expression of surface antigens distinguishing "naive" and previously activated lymphocytes in bronchoalveolar lavage fluid. Thorax. 1990 May;45(5):391–396. doi: 10.1136/thx.45.5.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers S., Smith K. A. Differentiation of T cell lymphokine gene expression: the in vitro acquisition of T cell memory. J Exp Med. 1991 Jan 1;173(1):25–36. doi: 10.1084/jem.173.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleich G. J., Frigas E., Loegering D. A., Wassom D. L., Steinmuller D. Cytotoxic properties of the eosinophil major basic protein. J Immunol. 1979 Dec;123(6):2925–2927. [PubMed] [Google Scholar]
- Gleich G. J. The eosinophil and bronchial asthma: current understanding. J Allergy Clin Immunol. 1990 Feb;85(2):422–436. doi: 10.1016/0091-6749(90)90151-s. [DOI] [PubMed] [Google Scholar]
- Hamid Q., Barkans J., Robinson D. S., Durham S. R., Kay A. B. Co-expression of CD25 and CD3 in atopic allergy and asthma. Immunology. 1992 Apr;75(4):659–663. [PMC free article] [PubMed] [Google Scholar]
- Jeffery P. K., Wardlaw A. J., Nelson F. C., Collins J. V., Kay A. B. Bronchial biopsies in asthma. An ultrastructural, quantitative study and correlation with hyperreactivity. Am Rev Respir Dis. 1989 Dec;140(6):1745–1753. doi: 10.1164/ajrccm/140.6.1745. [DOI] [PubMed] [Google Scholar]
- Laitinen L. A., Heino M., Laitinen A., Kava T., Haahtela T. Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis. 1985 Apr;131(4):599–606. doi: 10.1164/arrd.1985.131.4.599. [DOI] [PubMed] [Google Scholar]
- Lopez A. F., Sanderson C. J., Gamble J. R., Campbell H. D., Young I. G., Vadas M. A. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J Exp Med. 1988 Jan 1;167(1):219–224. doi: 10.1084/jem.167.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkenschlager M., Terry L., Edwards R., Beverley P. C. Limiting dilution analysis of proliferative responses in human lymphocyte populations defined by the monoclonal antibody UCHL1: implications for differential CD45 expression in T cell memory formation. Eur J Immunol. 1988 Nov;18(11):1653–1661. doi: 10.1002/eji.1830181102. [DOI] [PubMed] [Google Scholar]
- Metzger W. J., Richerson H. B., Worden K., Monick M., Hunninghake G. W. Bronchoalveolar lavage of allergic asthmatic patients following allergen bronchoprovocation. Chest. 1986 Apr;89(4):477–483. doi: 10.1378/chest.89.4.477. [DOI] [PubMed] [Google Scholar]
- Rennard S. I., Ghafouri M., Thompson A. B., Linder J., Vaughan W., Jones K., Ertl R. F., Christensen K., Prince A., Stahl M. G. Fractional processing of sequential bronchoalveolar lavage to separate bronchial and alveolar samples. Am Rev Respir Dis. 1990 Jan;141(1):208–217. doi: 10.1164/ajrccm/141.1.208. [DOI] [PubMed] [Google Scholar]
- Robinson D. S., Hamid Q., Ying S., Tsicopoulos A., Barkans J., Bentley A. M., Corrigan C., Durham S. R., Kay A. B. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992 Jan 30;326(5):298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- Saltini C., Kirby M., Trapnell B. C., Tamura N., Crystal R. G. Biased accumulation of T lymphocytes with "memory"-type CD45 leukocyte common antigen gene expression on the epithelial surface of the human lung. J Exp Med. 1990 Apr 1;171(4):1123–1140. doi: 10.1084/jem.171.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. M., Liu M. C., Weinmann G. G., Permutt S., Bleecker E. R. Peripheral lung resistance in normal and asthmatic subjects. Am Rev Respir Dis. 1990 Mar;141(3):584–588. doi: 10.1164/ajrccm/141.3.584. [DOI] [PubMed] [Google Scholar]
- Walker C., Kaegi M. K., Braun P., Blaser K. Activated T cells and eosinophilia in bronchoalveolar lavages from subjects with asthma correlated with disease severity. J Allergy Clin Immunol. 1991 Dec;88(6):935–942. doi: 10.1016/0091-6749(91)90251-i. [DOI] [PubMed] [Google Scholar]
- Wardlaw A. J., Dunnette S., Gleich G. J., Collins J. V., Kay A. B. Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Relationship to bronchial hyperreactivity. Am Rev Respir Dis. 1988 Jan;137(1):62–69. doi: 10.1164/ajrccm/137.1.62. [DOI] [PubMed] [Google Scholar]
- Weller P. F. The immunobiology of eosinophils. N Engl J Med. 1991 Apr 18;324(16):1110–1118. doi: 10.1056/NEJM199104183241607. [DOI] [PubMed] [Google Scholar]



