Abstract
Patient: Female, 19
Final Diagnosis: Lung adenocarcinoma
Symptoms: Chest pain
Medication: —
Clinical Procedure: Ct scan and pet-ct
Specialty: Oncology
Objective:
Unusual clinical course
Background:
Lung cancer in young patients is quite uncommon; clinical presentation and outcome in this population compared to the older group are not yet well defined and data about this setting are mostly single-institutional retrospective analyses.
Case Report:
We report here a case of a very young woman with diagnosis of early-stage lung adenocarcinoma harboring EML4-ALK rearrangement; she underwent radical surgery and adjuvant chemotherapy according to the pathologic stage. Potential risk factors for lung cancer in our patient are discussed and clinico-pathologic features and outcomes of lung cancer in the young population compared to the elderly are reviewed through discussing studies with sample sizes larger than 100 patients.
Conclusions:
A wide clinical overview should be performed when lung cancer is diagnosed in a young patient. Large-population studies are required to define the molecular signature and clinical behavior of lung cancer in young patients.
MeSH Keywords: Lung Neoplasms, Patient Outcome Assessment, Risk Factors, Young Adult
Background
According to the Surveillance, Epidemiology, and End Results Program (SEER) registry based on 2007–2011 new cases, lung cancer (LC) is more frequently diagnosed among people aged 65–74 with only 1.6% of all cases occurring in patients younger than 45 years [1]. Most published data about LC in young populations are single-institutional retrospective analyses and few report on very young patients specifically. Previous data suggest that LC in young adults may be an entity with distinct characteristics compared to LC in older patients; however, data are not always consistent among all series [2–11]. In addition, age limits ranging from 40 to 50 years have been variably chosen by different authors to define younger cohorts of patients. We report a case of a young woman with early-stage EML4-ALK rearranged lung adenocarcinoma who underwent surgery followed by adjuvant chemotherapy. We also consider possible susceptibility factors for LC in our patient and review the majority of clinical studies with a sample size larger than 100 patients, in order to highlight and discuss LC patterns in young versus old patients [2–11].
Case Report
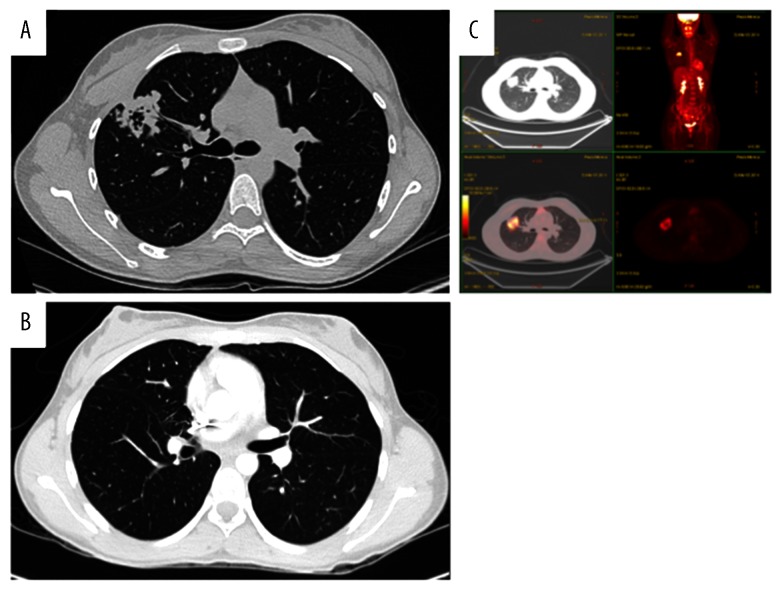
In January 2014, a 19-year-old white, never-smoker woman experienced chest pain; a chest X-ray and a computed tomography (CT)-scan showed a cavitating right lung lesion in the upper lobe without enlargement of mediastinal lymph nodes (Figure 1A). A bronchoscopy was performed and the evaluation of cell block prepared from bronchial brushings led to the diagnosis of adenocarcinoma. A positron emission tomography/computerized tomography (PET/CT) scan excluded additional disease localizations (Figure 1B). In March 2014 the patient underwent right upper lobectomy with systematic lymphadenectomy by video-assisted thoracic surgery (VATS); a diagnosis of primary pulmonary adenocarcinoma with papillary predominant pattern was made. Immunohistochemistry showed that tumor cells were positive for TTF-1 and negative for p63; Ki67 was 70%. Molecular analysis showed no EGFR, KRAS, and BRAF gene mutations by Sanger’s direct sequencing, whereas fluorescent in situ hybridization (FISH) showed the presence of EML4-ALK rearrangement in 57% of cells (Figure 2). The patient was then referred to our Institution in April 2014. Clinical examination showed Eastern Cooperative Oncology Group (ECOG) performance status 0, and no additional findings. As previous medical history, the patient referred a general discomfort occurring between May and October 2013, characterized by nausea, vomiting, diarrhea, and skin rash. Blood test results are reported in Table 1. In November 2013, the patient underwent an esophagogastroduodenoscopy with multiple biopsies, leading to the diagnosis of celiac disease. A gluten-free diet induced symptoms regression. The cancer family history revealed that the patient’s father died of renal cell carcinoma in 2007. A genetic test on a blood sample did not show TP53 mutations and the constitutional karyotype was normal. According to the pathologic stage (pT2a N1, stage IIA), she received adjuvant chemotherapy with 4 cycles of cisplatin-pemetrexed from May to July 2014. Before starting chemotherapy, the patient underwent ovarian tissue cryopreservation and gonadotropin-releasing hormone analogue was administered during the adjuvant treatment. Because of persistence of elevated gamma-glutamyl-transpeptidase (GGT) and transaminases before and during chemotherapy, the patient had a specialist opinion, which resulted in the diagnosis of autoimmune hepatitis. Currently, the patient remains on oncological and hepatologic follow-up visits. At the last follow-up visit, in February 2015, a CT-scan showed no disease recurrence (Figure 1C).
Figure 1.
Computed tomography (CT) scan at diagnosis and positron emission tomography and CT-scan (PET/CT) at diagnosis, before surgery (A, B). CT scan after surgery at last follow-up visit (C).
Figure 2.
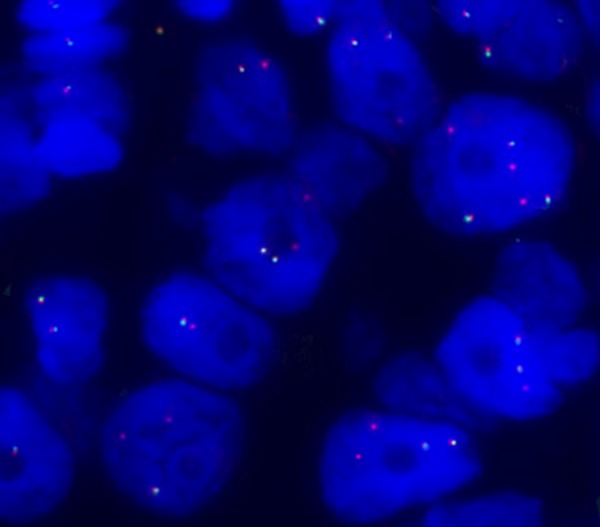
FISH analysis was performed with ALK dual-color break-apart probe labelled with SpectrumOrange (3’end) and SpectrumGreen (5’end) (Abbott Molecular). The predominant ALK-positive FISH pattern observed in the sample was isolated red signal.
Table 1.
Significant selection of blood tests performed before diagnosis of lung cancer.
| Blood test | Result | Normal values |
|---|---|---|
| Alkaline Phosphatase (ALP) | 95 | 42–98 (U/L) |
| Lactate Dehydrogenase (LDH) | 175 | 135–214 (U/L) |
| Aspartate Transaminase (AST) | 215 | 10–35 (U/L) |
| Alanine Transaminase (ALT) | 197 | 7–35 (U/L) |
| Gamma-glutamyl transferase (GGT) | 470 | 3–45 (U/L) |
| Gamma Globulins | 29.6 | 11–20 (%) |
| Immunoglobulin (Ig) G | 288 | 65–165 (ug/dL) |
| Anti-endomysial IgM | Positive | Negative |
| Anti-transglutaminase IgA | 17.3 | <4 (U/mL) |
| Anti-Nucleus Antibodies (ANA) | Positive | Negative |
| Anti-HBc IgM | Negative | Negative |
| Anti-HBc | Negative | Negative |
| Anti-HCV | Negative | Negative |
| Anti-HBsAg | 56 | Negative <10 UI/L, positive ≥10 UI/L |
| HBsAg | Negative | Negative |
| Anti-Cytomegalovirus IgG | Positive | Negative |
| Epstein-Barr Virus (EBV) Viral Capside Antigen (VCA) Ig G | Positive | Negative |
| EBV VCA Ig M | Positive | Negative |
| EBV Nuclear Antigen (EBNA) antibodies | Positive | Negative |
| Anti-Extractable Nuclear Antigens (ENA) antibodies | Negative | Negative |
| anti-Liver Kidney Microsomal (LKM) antibodies | Negative | Negative |
| Anti-Smooth Muscle Antibodies (ASMA) | Negative | Negative |
| Anti-Mithocondrial Antibodies (AMA) | Negative | Negative |
| Soluble Liver Antigen (SLA) antibodies | Negative | Negative |
| Anti-Neutrophil Cytoplasmic Antibodies (ANCA) | Negative | Negative |
| Anti-Saccharomyces Cerevisiae Antibodies (ASCA) | Negative | Negative |
| CEA | 3.9 | 0.0–5.0 (ug/L) |
| CYFRA 21.1 | 1.2 | 0.0–3.3 (ug/L) |
Discussion
Occurrence of LC in young adults is quite uncommon and is characterized by peculiar epidemiological, clinical, and prognostic features. To date, the pathogenesis of this disease in young people is still very unclear. None of the known risk factors for LC could explain the early onset of the malignancy and no specific genomic alteration has been detected in this subgroup of patients.
According to the SEER registry, the proportion of African-Americans, Asian, and Pacific Islanders was higher among younger than older patients [1]. This epidemiologic discrepancy may be due to differences in carcinogens exposure or to biologic differences such as inefficient cell cycle arrest and DNA damage accumulation or cytochrome polymorphisms [9,12,13]. Regarding the clinico-pathologic features of LC in the young population, most retrospective series with sample sizes of more than 100 patients (Table 2) reported a higher proportion of women and adenocarcinoma in younger groups compared to older patients [3,4,6–11], but some cases of very young patients with squamous cell LC have also been reported in recent years [14–16]. Data on the proportion of asymptomatic patients at diagnosis in young and old groups are discordant. However, chest pain is definitively the most frequent symptom of younger patients in comparative analysis [6,8], such as in our patient. Finally, lower occurrence of early-stage disease in young people, described in most of the retrospective series, could be due to more aggressive disease or a delayed diagnosis due to a low degree of suspicion of cancer in young patients [4,9–11].
Table 2.
Studies comparing young lung cancer patients to older patients with sample size of more of 100 patients: data about clinico-pathologic features.
| N (% of total) | Country, Year | Age cut-off | Smoking status* | Cancer cases in family | % of females | Histological type | Stage | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Young (%) | Old (%) | P | Young (%) | Old (%) | P | Young (%) | Old (%) | P | Young (%) | Old (%) | P | Young (%) | Old (%) | P | ||||
| Roviaro, 1985 [2] | 155 (10%) | Italy, 1967–1980 | <45 years | Smokers 93% | NA | NA | NA | 8% | NR | NS | AC 18% SQ 64% |
NR | NS | Stage I–III 92% Stage IV 8% |
Stage I–III 96% Stage IV 4% |
NS | ||
| McDuffie, 1989[3] | 187 (7%) | Saskat-chewan (CA), 1979–1986 | ≤50 years | Smokers 85% | Smokers 78% | NS | One first-degree relative with LC | 46% | 22% | P<0.001 | NR | P<0.005 | NA | |||||
| 7.5% | 6% | NS | ||||||||||||||||
| Ramalingam, 1998 [4] | 2804 (9%) | Metropolitan Detroit SEER registry, 1973–1992 | <50 years | NA | NA | 40% | 31% | P<0.001 | AC 46% SQ 27% |
AC 34% SQ 38% |
P<0.001 | Local 19% Distant 53% |
Local 25% Distant 49% |
P<0.001 | ||||
| Kreuzer, 1998 [5] | 251 | Germany, 1990–1996 | ≤45 years | Smokers 95% | Smokers 94% | NA | One first-degree relative with LC | 27% | 15% | NA | AC 42% SQ 24% |
AC 31% SQ 39% |
P=0.3 | NA | NA | NA | ||
| 10% | 7% | NA | ||||||||||||||||
| Kuo, 2000 [6] | 127 (2%) | Taiwan, 1987–1996 | <40 years | NA | NA | 52% | NR | P<0.001 | AC 61% SQ 21% |
NR | P=0.0004 | Stage I 9% Stage IV 47% |
Stage I 15% Stage IV 40% |
NS | ||||
| Radzikowska, 2001 [7] | 757 (14%) | Poland, 1995 | ≤50 years | Smokers 76% | Smokers 77% | P=0.349 | Cancer cases in family (mother and father) | 24% | 12% | P<0.001 | AC 13% SQ 35% |
AC 8% SQ 42% |
P<0.001 | Stage I 24% Stage IV 19% |
Stage I 23% Stage IV 15% |
P=0.059 | ||
| P<0.001 | ||||||||||||||||||
| Mauri, 2006 [8] | 115 (6%) | Greece, 1989–2004 | ≤45 years | Smokers 77% | Smokers 75% | P=0.326 | NA | 18% | 12% | P=0.071 | AC 49% SQ 24% |
AC 43% SQ 37% |
P=0.004 | Stage IV 12% | Stage IV 22% | P=0.016 | ||
| Subramanian, 2010 [9] | 2775 (1%) | SEER registry, 1988–2003 | ≤40 years | NA | NA | 49% | 42% | P<0.0001 | AC 58% SQ 13% |
AC 45% Q 26% |
P<0.0001 | Stage I 12% Stage IV 57% |
Stage I 21% Stage IV 43% |
P<0.0001 | ||||
| Inoue, 2014 [10] | 704 (6%) | Japan, 2004 | ≤50 years | Smokers 47% | Smokers 57% | P<0.001 | NA | 47% | 37% | P<0.001 | AC 79% SQ 7% |
AC 67% SQ 23% |
P<0.001 | Stage I 64% Stage III–IV 24% |
Stage I 65% Stage III–IV 20% |
P<0.001 | ||
| Rich, 2015 [11] | 651 (0.4%) | English National Lung Cancer Audit, 2004–2011 | 18–39 years | NA | NA | 44% | 43% | NR | AC 48% SQ 13% |
AC 33% SQ 33% |
NR | Stage I 9% Stage IIIB–IV 71% |
Stage I 14% Stage IIIB–IV 61% |
NR | ||||
Smokers (current smokers and exsmokers) versus Nonsmokers. NA – not assessed; NS ,– not significant; NR – not reported; LC – lung cancer; SEER – surveillance, epidemiology, and end results; AC – adenocarcinoma; SQ – squamous cell carcinoma
Our patient’s tumor sample tested positive for EML4-ALK rearrangement. To date, only a few studies have investigated molecular alterations in younger patients, with controversial results. Higher frequency of EML4-ALK rearrangement (11.6%) and EGFR mutation (20%) were shown in 53 non-small cell LC patients ≤50 years old compared with patients of all ages [17]. In contrast, Ye et al. showed no difference in terms of oncogenic mutations (P=0.396), but a higher prevalence of TP53 mutations (P<0.001) in 36 resected lung adenocarcinoma from patients younger than 40 years compared to their older counterparts [18]; similarly, Kim et al. did not find a statistically significant difference in EGFR and EML4-ALK status between young and old patients [19]. Further investigation is needed to address the issue of genetic derangements in young patients with LC.
Risk factors for the onset of LC specifically in young adults are still unknown. The increased frequency of adenocarcinoma and the long latency time between smoking exposure and cancer appearance suggest that LC among young people does not require as much carcinogen exposure, but rather genetic derangements. However, the smoking status is similar between younger and older patients in a few series collecting these data, with approximately 75–95% of young patients reporting smoking sometime. Whether the number of cigarettes smoked per day or the age at which smoking began lead to an early onset of LC is still unclear. These patients might have genetic susceptibility to develop LC or inherited sensitivity to smoking-related carcinogenesis. Recently, case-control studies have showed that gene mutations or polymorphisms involving xenobiotic metabolizing enzymes and DNA repair pathways are associated with increased risk of early-onset LC [20–23].
Our patient was a never-smoker female, and she did not report previous exposure to carcinogens; thus, when we investigated possible risk factors for LC, we focused on genetic, immunological, and/or infective predisposition. The hypothesis of a genetic component in the early onset of LC is also supported by a few series showing an increased family history among young patients. In a case-control study, the authors demonstrated the greatest contribution to LC risk (7-fold increase) among 40- to 59-year-old non-smoker subjects with a first-degree relative affected by LC [24]. Our patient did not report family history of LC among her first-degree relatives. Her father died because of a kidney cancer, which is not included in any genetic syndrome involving LC; moreover, germ-line mutations of TP53 gene or constitutional karyotype alterations were not observed. Hereditary LC syndromes are rare, and, while germline EGFR T790M mutation has been reported as a predisposing genetic feature, especially in non-smoker patients [25], no evidence of germline EML4-ALK rearrangement has been reported in LC.
The medical history of the patient included the diagnosis of celiac disease, which is associated with an increased risk of lymphoma. In a study from a large Swedish cohort, the authors found a neutral risk of LC in celiac disease, with a hazard ratio of 1.00 beyond the first year of follow-up after celiac diagnosis [26]. More recently, a large-population cohort study in Finland showed a decreased risk of LC among 32 439 celiac patients [27].
Additional significant findings in our case were the recent diagnosis of autoimmune hepatitis, anti-cytomegalovirus (CMV), immunoglobulin (Ig) G, and anti-Epstein-Barr virus (EBV) IgG and IgM positivity. Subsequent examinations excluded the positivity of CMV and EBV DNA and of all hepatitis viruses. The association between solid cancer and autoimmune systemic disease is uncommon and especially involves scleroderma and LC [28], even though increased risk for LC has also been reported in systemic lupus erythematosus (SLE) patients [29]. In a recent paper, a 4-fold risk of LC in patients with systemic sclerosis, discoid lupus erythematosus, and polymyositis/dermatomyositis has been described [30]. However, there are no data about a possible link between autoimmune hepatitis and risk of LC. Similarly, EBV and CMV infections seem to have a role in the pathogenesis of solid tumors, such as lymphoepithelioma-like carcinoma and glioblastoma, respectively, but no involvement in LC risk has been reported. Indeed, microRNA studies in LC did not support any role of EBV in LC [31]. To date, the only virus infections associated with LC are human immunodeficiency virus (HIV) and human papilloma virus (HPV) infections [32,33]. Thus, we have no data to support the hypothesis that LC risk in our patient had a genetic, immunological, or infective basis.
Data regarding clinical outcome of young LC patients have been presented in only a few retrospective studies [2,4,6–11] (Table 3). According to Roviaro GC et al., no significant statistical differences were observed in terms of survival between patients younger or older than 45 years, in the whole population and according to type of treatment or disease [2]. This was also confirmed by another series in which the majority of examined patients were included in clinical trials, thus making the 2 groups well balanced in comorbidity patterns and treatment modalities [8]. On the other hand, other studies showed a longer survival in the young group, despite the higher frequency of advanced disease. This finding could be explained by the higher chance of receiving more aggressive treatments, including multimodality treatments and further lines of chemotherapy, due to the lower prevalence of comorbidities [4,6,7,9–11].
Table 3.
Studies comparing young lung cancer patients to older patients with sample size of more of 100 patients: data about patients’ outcome.
| 1-year OS rate | 5-year OS rate | OS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Young | Old | P | Young | Old | P | Young | Middle-age | Old | P | |
| Roviaro, 1985 [2] | NA | 21% | 25% | NS | NA | |||||
| Ramalingam, 1998 [4] | NA | 16% | 13% | P<0.001 | NA | |||||
| Kuo, 2000 [6] | NA | NA | 9 months | 8 months | 4 months | P<0.0001 | ||||
| Radzikowska, 2001 [7] | 33% | 29% | P<0.049 | NA | NR | P=0.01107 | ||||
| Mauri, 2006 [8] | NA | NA | 12 months | NA | 11.5 months | P=0.277 | ||||
| Subramanian, 2010 [9] | NA | NA | Younger patients had better stage-wise overall and disease-specific survival than older patients | P<0.0001 | ||||||
| Inoue, 2014 [10] | NA | 79% | 69% | P<0.001 | NA | |||||
| Rich, 2015 [11] | NA | NA | Young patients had a lower overall mortality than older patients (62% vs. 86%, respectively) | P<0.001 | ||||||
OS – overall survival; NA – not assessed; NS – not significant; NR – not reported.
In case of disease relapse, our patient could benefit from a first-generation ALK inhibitor, which has demonstrated remarkable clinical outcomes including better response rate and prolonged survival compared to standard chemotherapy [34,35]. However, despite an initial improvement, ALK-positive tumors inevitably develop several resistance mechanisms to the targeted drug, resulting in progression of the disease. Other strategies, including the development of new-generation ALK inhibitors, are currently under clinical evaluation to overcome the acquired resistance in these patients [36].
Conclusions
Despite a comprehensive review of the patient’s medical and family history, we did not identify any underlying risk factor for LC. Larger prospective studies are needed to define the molecular signature and clinical behavior of LC in young patients. To collect evidence of no-smoking related pathways involved in cancer risk, a wide clinical overview should be performed when LC diagnosis occurs in a young patient. Given the rarity of the disease in this setting, only an international multi-center study could address this issue.
Abbreviations:
- SEER
Surveillance, Epidemiology, and End Results Program;
- LC
lung cancer;
- CT
computed tomography;
- PET/CT
positron emission tomography/computerized tomography;
- VATS
video-assisted thoracic surgery;
- FISH
fluorescent in situ hybridization;
- ECOG
eastern cooperative oncology group;
- GGT
gamma-glutamyl-transpeptidase;
- CMV
cytomegalovirus;
- Ig
immunoglobulin;
- EBV
Epstein-Barr virus;
- SLE
systemic lupus erythematosus;
- HIV
Human Immunodeficiency Virus;
- HPV
Human papilloma virus
References:
- 1.SEER Stat Fact Sheets: Lung and Bronchus Cancer. Available at http://seer.cancer.gov/statfacts/html/lungb.html. Accessed March 03, 2015.
- 2.Roviaro GC, Varoli F, Zannini P, et al. Lung cancer in the young. Chest. 1985;87:456–59. doi: 10.1378/chest.87.4.456. [DOI] [PubMed] [Google Scholar]
- 3.McDuffie HH, Klaassen DJ, Dosman JA. Characteristics of patients with primary lung cancer diagnosed at age of 50 years or younger. Chest. 1989;96:1298–301. doi: 10.1378/chest.96.6.1298. [DOI] [PubMed] [Google Scholar]
- 4.Ramalingam S, Pawlish K, Gadgeel S, et al. Lung cancer in young patients: analysis of a surveillance, epidemiology, and end results database. J Clin Oncol. 1998;16:651–57. doi: 10.1200/JCO.1998.16.2.651. [DOI] [PubMed] [Google Scholar]
- 5.Kreuzer M, Kreienbrock L, Gerken M, et al. Risk factors for lung cancer in young adults. Am J Epidemiol. 1998;147:1028–37. doi: 10.1093/oxfordjournals.aje.a009396. [DOI] [PubMed] [Google Scholar]
- 6.Kuo CW, Chen YM, Chao JY, et al. Non-small cell lung cancer in very young and very old patients. Chest. 2000;117:354–57. doi: 10.1378/chest.117.2.354. [DOI] [PubMed] [Google Scholar]
- 7.Radzikowska E, Roszkowski K, Glaz P. Lung cancer in patients under 50 years old. Lung Cancer. 2001;33:203–11. doi: 10.1016/s0169-5002(01)00199-4. [DOI] [PubMed] [Google Scholar]
- 8.Mauri D, Pentheroudakis G, Bafaloukos D, et al. Non-small cell lung cancer in the young: a retrospective analysis of diagnosis, management and outcome data. Anticancer Res. 2006;26:3175–81. [PubMed] [Google Scholar]
- 9.Subramanian J, Morgensztern D, Goodgame B, et al. Distinctive characteristics of non-small cell lung cancer (NSCLC) in the young: a surveillance, epidemiology, and end results (SEER) analysis. J Thorac Oncol. 2010;5:23–28. doi: 10.1097/JTO.0b013e3181c41e8d. [DOI] [PubMed] [Google Scholar]
- 10.Inoue M, Okumura M, Sawabata N, et al. Clinicopathological characteristics and surgical results of lung cancer patients aged up to 50 years: the Japanese Lung Cancer Registry Study 2004. Lung Cancer. 2014;83:246–51. doi: 10.1016/j.lungcan.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Rich AL, Khakwani A, Free CM, et al. Non-small cell lung cancer in young adults: presentation and survival in the English National Lung Cancer Audit. QJM. 2015 doi: 10.1093/qjmed/hcv052. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Zheng YL, Loffredo CA, Alberg AJ, et al. Less efficient g2-m checkpoint is associated with an increased risk of lung cancer in African Americans. Cancer Res. 2005;65:9566–73. doi: 10.1158/0008-5472.CAN-05-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raz DJ, Gomez SL, Chang ET, et al. Epidemiology of non-small cell lung cancer in Asian Americans: incidence patterns among six subgroups by nativity. J Thorac Oncol. 2008;3:1391–97. doi: 10.1097/JTO.0b013e31818ddff7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emori Y, Kiura K, Yoshino T, et al. Very young patient with peculiar squamous cell carcinoma of the lung. Intern Med. 1999;38:979–83. doi: 10.2169/internalmedicine.38.979. [DOI] [PubMed] [Google Scholar]
- 15.Katsuragi N, Shiraishi Y, Kita H, et al. 21-year-old man with squamous cell carcinoma of the lung. Kyobu Geka. 2007;60:529–32. [PubMed] [Google Scholar]
- 16.Tajiri T, Suita S, Shono K, et al. Lung cancer in a child with a substantial family history of cancer. Eur J Pediatr Surg. 1999;9:409–12. doi: 10.1055/s-2008-1072294. [DOI] [PubMed] [Google Scholar]
- 17.Vanden Bussche CJ, Illei PB, Lin MT, et al. Molecular alterations in non-small cell lung carcinomas of the young. Hum Pathol. 2014;45:2379–87. doi: 10.1016/j.humpath.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Ye T, Pan Y, Wang R, et al. Analysis of the molecular and clinicopathologic features of surgically resected lung adenocarcinoma in patients under 40 years old. J Thorac Dis. 2014;6:1396–402. doi: 10.3978/j.issn.2072-1439.2014.08.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim L, Kim KH, Yoon YH, et al. Clinicopathologic and molecular characteristics of lung adenocarcinoma arising in young patients. J Korean Med Sci. 2012;27:1027–36. doi: 10.3346/jkms.2012.27.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller DP, Liu G, De Vivo I, et al. Combinations of the variant genotypes of GSTP1, GSTM1, and p53 are associated with an increased lung cancer risk. Cancer Res. 2002;62:2819–23. [PubMed] [Google Scholar]
- 21.Cote ML, Kardia SL, Wenzlaff AS, et al. Combination of glutathione S-transferase genotypes and risk of early-onset lung cancer in Caucasians and African Americans: a population-based study. Carcinogenesis. 2005;26:811–19. doi: 10.1093/carcin/bgi023. [DOI] [PubMed] [Google Scholar]
- 22.Landi S, Gemignani F, Canzian F, et al. DNA repair and cell cycle control genes and the risk of young-onset lung cancer. Cancer Res. 2006;66:11062–69. doi: 10.1158/0008-5472.CAN-06-1039. [DOI] [PubMed] [Google Scholar]
- 23.Gemignani F, Landi S, Szeszenia-Dabrowska N, et al. Development of lung cancer before the age of 50: the role of xenobiotic metabolizing genes. Carcinogenesis. 2007;28:1287–93. doi: 10.1093/carcin/bgm021. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz AG, Yang P, Swanson GM. Familial risk of lung cancer among non-smokers and their relatives. Am J Epidemiol. 1996;144:554–62. doi: 10.1093/oxfordjournals.aje.a008965. [DOI] [PubMed] [Google Scholar]
- 25.Gazdar A, Robinson L, Oliver D, et al. Hereditary lung cancer syndrome targets never smokers with germline EGFR gene T790M mutations. J Thorac Oncol. 2014;9:456–63. doi: 10.1097/JTO.0000000000000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ludvigsson JF, West J, Hubbard R, Card T. Neutral risk of lung cancer in adults with celiac disease - nationwide cohort study. Lung Cancer. 2012;78:179–84. doi: 10.1016/j.lungcan.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Ilus T, Kaukinen K, Virta LJ, et al. Incidence of malignancies in diagnosed celiac patients: a population-based estimate. Am J Gastroenterol. 2014;109:1471–77. doi: 10.1038/ajg.2014.194. [DOI] [PubMed] [Google Scholar]
- 28.Zhang JQ, Wan YN, Peng WJ, et al. The risk of cancer development in systemic sclerosis: a meta-analysis. Cancer Epidemiol. 2013;37:523–27. doi: 10.1016/j.canep.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Ni J, Qiu LJ, Hu LF, et al. Lung, liver, prostate, bladder malignancies risk in systemic lupus erythematosus: evidence from a meta-analysis. Lupus. 2014;23:284–92. doi: 10.1177/0961203313520060. [DOI] [PubMed] [Google Scholar]
- 30.Hemminki K, Liu X, Ji J, et al. Subsequent COPD and lung cancer in patients with autoimmune disease. Eur Respir J. 2011;37:463–65. doi: 10.1183/09031936.00070410. [DOI] [PubMed] [Google Scholar]
- 31.Koshiol J, Gulley ML, Zhao Y, et al. Epstein-Barr virus microRNAs and lung cancer. Br J Cancer. 2011;105:320–26. doi: 10.1038/bjc.2011.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasegawa Y, Ando M, Kubo A, et al. Human papilloma virus in non-small cell lung cancer in never smokers: a systematic review of the literature. Lung Cancer. 2014;83:8–13. doi: 10.1016/j.lungcan.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Okuma Y, Hosomi Y, Imamura A. Lung cancer patients harbouring epidermal growth factor receptor mutation among those infected by human immunodeficiency virus. Onco Targets Ther. 2014;8:111–15. doi: 10.2147/OTT.S76712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 35.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–77. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 36.Katayama R, Lovly CM, Shaw AT. Therapeutic targeting of anaplastic lymphoma kinase in lung cancer: a paradigm for precision cancer medicine. Clin Cancer Res. 2015;21:2227–35. doi: 10.1158/1078-0432.CCR-14-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]