Abstract
Patient: Male, 62
Final Diagnosis: Auto-immune pancreatitis
Symptoms: Jaundice • lymfadenopathy
Medication: —
Clinical Procedure: Laboratory • imaging
Specialty: Gastroenterology and Hepatology
Objective:
Unusual clinical course
Background:
Autoimmune pancreatitis (AIP) is an important clinical pathologic concept of IgG-4-related disease. AIP is a rare cause of chronic pancreatitis, characterized by a fibroinflammatory process by lymphoplasmacytic infiltrates, storiform fibrosis, obliterative phlebitis, and increased IgG4+ plasma cells, leading to dysfunction of the pancreas. Affected patients with AIP frequently have disease affecting other organs or sites with similar histologic changes, elevated IgG4+ plasma cell infiltrate, and good response to corticosteroid therapy. These diseases often are not limited to the pancreas and the pancreas may not be involved at all.
Case Report:
We report a 62-year-old man with obstructive jaundice with pre-existent submandibular lymphadenopathy. Diagnosis of AIP was based on diagnostic criteria by the HISORT-criteria in combination with elevated IgG-4 serum levels. CT revealed a focal enlargement of the head of the pancreas, as well as mesenteric peripancreatic and mediastinal lymphadenopathy. He was treated with high-dose steroid in combination with azathioprine and showed good clinical response.
Conclusions:
We report a case with pre-existent submandibular lymphadenopathy and obstructive jaundice based on AIP type 1, both in the context of IgG4-related disease.
MeSH Keywords: Digestive System Abnormalities; Immunoglobulin G; Pancreatitis, Chronic
Background
Autoimmune pancreatitis (AIP) is a rare benign disorder of presumed autoimmune etiology, associated with characteristic clinical, histologic, imaging, and serum marker findings [1]. AIP may occur as a primary pancreatic disorder, but is often associated with extra-pancreatic manifestations. AIP is describes as part of a clinicopathological entity of IgG-4 related disease. In IgG-4-related disease, sclerosing cholangitis, Sjögren’s disease, retroperitoneal fibrosis, mediastinal or hilar lymphadenopathy, interstitial nephritis, thyroiditis, or inflammatory bowel disease are the more common features [2,3]. The diagnosis of AIP is most often made in overt pancreatic disease, with less awareness of associative systemic manifestation. We describe a patient with AIP with extra-pancreatic lymphadenopathy as the first extra-pancreatic manifestations and presentation of an IgG-4-related disease.
Case Report
A 62-year-old man was analyzed for painless obstructive jaundice. His medical history revealed the past three years persistent submandibular lymphadenopathy with histological findings of reactive inflammatory changes with elevated IgG-4 plasma cells without evidence of malignancy or auto-immune disease. CT showed no other localization of lymphadenopathy and IgG4-levels were not measured. Three years after he was diagnosed with submandibular lymphadenopathy he was analyzed for painless obstructive jaundice. Physical examination showed jaundice and known submandibular lymph nodes. Blood chemistry revealed a bilirubin 130 umol/L (n: 0–17 umol/L), alkaline phosphatase 542 U/L (n: 40–125 U/L), Gamma-glutamyl transpeptidase (GGT) 910 U/L (n: 0–55 U/L), Alanine-Amino-Transferase (ALAT) 840 U/L (n: 0–45 U/L) and Aspartate aminotransferase (ASAT) 467 U/L (n: 0–35 U/L). CT revealed a focal enlargement in the pancreatic caput with loss of pancreatic cleft, mesenteric peripancreatic and mediastinal lymphadenopathy(Figure 1). Additionally, an endoscopic retrograde cholangiopancreatography (ERCP) was performed showing distal stenosis of the common bile duct. An endoprosthesis was placed. Brush cytology of the stenosis showed no malignancy. Subsequent endoscopic ultrasound revealed a diffusely edematous pancreas most prominent in head of the pancreas t, with no abnormalities of the main pancreatic duct. Fine needle aspiration showed lymphocytic infiltrates and was again negative for malignancy, but IgG-4 staining could not be performed. Autoimmune serology, such as antinuclear antibody (ANA) and Anti-neutrophil cytoplasmic antibody (ANCA), were negative. IgG4 was markedly elevated (20.7 g/L, n: 0.08–1.4 g/L), highly suggestive of IgG4-related autoimmune pancreatitis (AIP). Retrospective revision of histological analysis of submandibular lymphadenopathy showed follicular hyperplasia and elevated IgG4+ plasma cells with an total ratio more than 0.9 (Figure 2). Treatment with 20 mg prednisolone was started. The patient showed good clinical, biochemical and radiological response. Complete resolution of pancreatic swelling and disappearance of mediastinal and cervical lymphadenopathy was observed by follow-up CT (Figure 2) after two weeks and previous inserted endoprosthesis was able to be removed after three months. Subsequently, Azathioprine 50 mg was started and prednisolone tapered slowly. Resolution of submandibular lymphadenopathy was also observed during physical examination during visits at our clinic at 2 weeks and 3 months. Biochemical parameter of IgG4 level also decreased without normalization after one year follow-up. Evidently, the patient is still in remission, with low-dose prednisolone of 5 mg in combination with azathioprine.
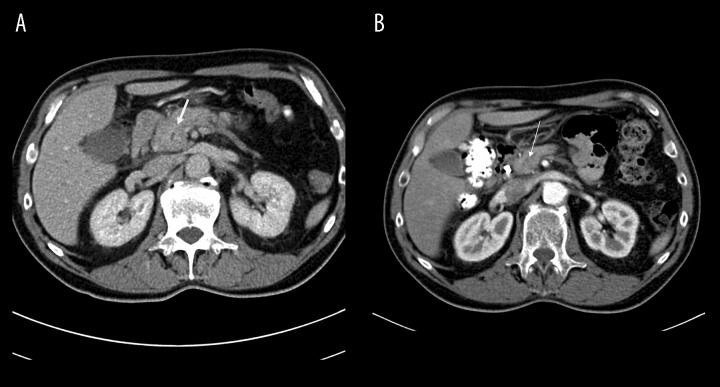
Figure 1.
(A) Focal diffuse enlargement of the head of the pancreas with loss of pancreatic clefts. (B) Resolution of pancreatic swelling and disappearance enlargement of the head of the pancreas after steroid treatment.
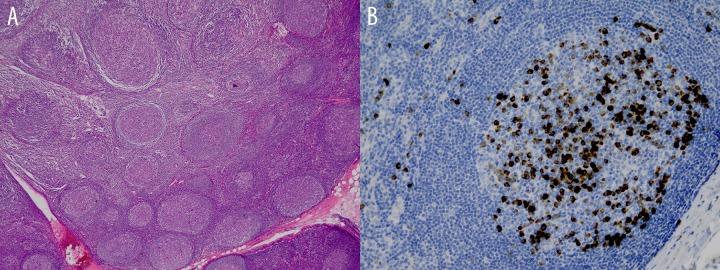
Figure 2.
Follicular hyperplasia (A) and immunostaining (B) for IgG4 in IgG4-related disease with average IgG4-positive cells of 3 high power field and total ratio >0.9.
Discussion
AIP-type 1 is a rare cause of chronic pancreatitis characterized by a fibroinflammatory process leading to dysfunction of the pancreas. AIP-type 1 is a clinicopathologic entity with unknown etiology and shows good clinical outcome after steroid therapy. Patients with AIP type 1 can have extrapancreatic manifestations and can be considered merely a component of a systemic disease known as IgG4-autoimmune disease. IgG4 related disease is a systemic fibro-inflammatory condition with tumor-like infiltrations and manifestations in almost all parts of the human body [4]. To diagnose AIP-type there is no single diagnostic test. Because of its diffuse and aspecific manifestation, diagnostic criteria such as the “HISORt”-criteria, Japanese diagnostic criteria 2011 and the International Consensus Diagnostic Criteria are proposed. These criteria are composed of specific combinations of radiological, histological, serological findings, other organ involvement and response to steroid therapy [5–9]. Several diagnostic criteria are used for AIP type 1, however, the Mayo Clinic HISOrt criteria are most often used. The diagnosis is based on a combination of imaging (computed tomography), histologic (lymphoplasmacytic infiltrate, sclerosis, and obliterative phlebitis), and serologic criteria (elevated serum IgG4 level) [10,11]. The extrapancreatic lesions may precede or follow the pancreatitis for many years prior as shown in the patient we described [12]. In the patient the combination radiological pancreatic finding, marked elevation of IgG4 and in combination with histological findings the diagnosis of AIP- type 1 was diagnosed. Furthermore, response to steroids supported this diagnosis. In retrospect, histological analysis of the patient submandibular lymphadenopathy showed elevated IgG4-plasma cells with an average IgG4 level of three high-power field and a total ratio of more than 0.9 conclusively by the Boston consensus for IgG4-related disease [13]. The exact pathogenesis mechanism of IgG4 related disease and in this specific cause the AIP type1 is unknown [14]. Most possibly this disease is triggered by a certain type of antigen which activates the immune system resulting in damage to the tissue. A T-cel response along with B-cel activation and production of several kinds of cytokines and transforming growth factor (TGF) lead to fibrosis and clinical manifestation of this disease [15–18]. In AIP, a benign clinical course is common. However, it is essential to exclude pancreatic carcinoma, cholangiocarcinoma, lymphoproliferative malignancy or other auto-immune disorders. This can lead to unnecessary invasive therapy like Whipple resection or chemotherapy. In general, the disease is highly responsive to steroids and this response may be used as a diagnostic tool. We do suggest histological analysis is crucial, however sample error and inconclusive histological findings can delay early recognition and treatment. Treatment with steroids is solely based on observational studies, since there have been no randomized controlled trials. Time till response is variable, usually occurring within two weeks to four months and exacerbations and spontaneous remissions may occur. [19–21]. The patient showed complete resolution of pancreatic mass, submandibular lymphadenopathy, jaundice and IgG4 levels within two weeks following initiation of a fairly low dose of 20 mg corticosteroids. A recent survey on treatment of AIP showed that 98% of patients responded to corticosteroids within a few weeks. A small group however may require maintenance therapy [22]. Long-term prognosis of AIP is still unclear. If untreated, both pancreatic and extra pancreatic complications may cause substantial morbidity and mortality (pancreatic atrophy, liver cirrhosis). Furthermore, azathioprine can be used in patients with relapse after steroid therapy. Because the patient presented with prolonged and extensive extra pancreatic disease, azathioprine was started early. For evaluation of prolonged disease clinical, IgG-4 plasma levels and serial CT was performed. The patient showed good clinical, radiological response. Biochemical parameter of IgG4 level also decreased without normalization during after one year follow-up. Complete resolution of pancreatic swelling and disappearance of mediastinal and cervical lymphadenopathy was observed by follow-up CT after two weeks and previous inserted endoprosthesis was able to be removed after three months. Also, in the patient resolution of submandibular lymphadenopathy was also observed and prevailed at two weeks and three months during visits at our clinic. Evidently, the patient is still in remission with low dose prednisolone of 5 mg in combination with azathioprine.
Conclusions
The importance of recognition of AIP type 1 disease lies in the remarkable response to steroid therapy. AIP type 1 should be considered in patients based on a combination of diagnostic criteria, including histology, imaging, and serological evaluation. AIP type 1 is an important clinical entity of IgG-4 related disease, and other organ involvement should raise awareness.
Footnotes
Conflicts of Interest
None declared.
References:
- 1.Finkelberg DL, Sahani D, Deshpande V, Brugge WR. Autoimmune pancreatitis. N Engl J Med. 2006;355(25):2670–76. doi: 10.1056/NEJMra061200. [DOI] [PubMed] [Google Scholar]
- 2.Kamisawa T, Takuma K, Egawa N, et al. Autoimmune pancreatitis and IgG4-related sclerosing disease. Nat Rev Gastroenterol Hepatol. 2010;7(7):401–9. doi: 10.1038/nrgastro.2010.81. [DOI] [PubMed] [Google Scholar]
- 3.Hirano K, Shiratori Y, Komatsu Y, et al. Involvement of the biliary system in autoimmune pancreatitis: a follow-up study. Clin Gastroenterol Hepatol. 2003;1(6):453–64. doi: 10.1016/s1542-3565(03)00221-0. [DOI] [PubMed] [Google Scholar]
- 4.Tan TJ, Ng YL, Tan D, et al. Extrapancreatic findings of IgG4-related disease. Clin Radiol. 2014;69(2):209–18. doi: 10.1016/j.crad.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 5.Chari ST, Takahashi N, Levy MJ, et al. A diagnostic strategy to distinguish autoimmune pancreatitis from pancreatic cancer. Clin Gastroenterol Hepatol. 2009;7(10):1097–103. doi: 10.1016/j.cgh.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Sah RP, Chari ST, Pannala R, et al. Differences in clinical profile and relapse rate of type 1 versus type 2 autoimmune pancreatitis. Gastroenterology. 2010;139(1):140–48. doi: 10.1053/j.gastro.2010.03.054. quiz e12–13. [DOI] [PubMed] [Google Scholar]
- 7.Deheragoda MG, Church NI, Rodriguez-Justo M, et al. The use of immunoglobulin g4 immunostaining in diagnosing pancreatic and extrapancreatic involvement in autoimmune pancreatitis. Clin Gastroenterol Hepatol. 2007;5(10):1229–34. doi: 10.1016/j.cgh.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 8.Deshpande V, Chicano S, Finkelberg D, et al. Autoimmune pancreatitis: a systemic immune complex mediated disease. Am J Surg Pathol. 2006;30(12):1537–45. doi: 10.1097/01.pas.0000213331.09864.2c. [DOI] [PubMed] [Google Scholar]
- 9.Kwon S, Kim MH, Choi EK. The diagnostic criteria for autoimmune chronic pancreatitis – It is time to make a consensus. Pancreas. 2007;34(3):279–86. doi: 10.1097/MPA.0b013e31802eff5f. [DOI] [PubMed] [Google Scholar]
- 10.Deshpande V, Mino-Kenudson M, Brugge W, Lauwers GY. Autoimmune pancreatitis – More than just a pancreatic disease? A contemporary review of its pathology. Arch Pathol Lab Med. 2005;129(9):1148–54. doi: 10.5858/2005-129-1148-APMTJA. [DOI] [PubMed] [Google Scholar]
- 11.Iwasaki S, Kamisawa T, Koizumi S, et al. Characteristic findings of endoscopic retrograde cholangiopancreatography in autoimmune pancreatitis. Gut Liver. 2015;9(1):113–17. doi: 10.5009/gnl13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamisawa T, Okamoto A. IgG4-related sclerosing disease. World J Gastroenterol. 2008;14(25):3948–55. doi: 10.3748/wjg.14.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25(9):1181–92. doi: 10.1038/modpathol.2012.72. [DOI] [PubMed] [Google Scholar]
- 14.Kamisawa T, Funata N, Hayashi Y. Lymphoplasmacytic sclerosing pancreatitis is a pancreatic lesion of IgG4-related systemic disease. Am J Surg Pathol. 2004;28(8):1114. doi: 10.1097/01.pas.0000126634.43301.45. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Notohara K, Levy MJ, et al. IgG4-positive plasma cell infiltration in the diagnosis of autoimmune pancreatitis. Mod Pathol. 2007;20(1):23–28. doi: 10.1038/modpathol.3800689. [DOI] [PubMed] [Google Scholar]
- 16.Chari ST, Smyrk TC, Levy MJ, et al. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006;4(8):1010–16. doi: 10.1016/j.cgh.2006.05.017. quiz 934. [DOI] [PubMed] [Google Scholar]
- 17.Ghazale A, Chari ST, Smyrk TC, et al. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol. 2007;102(8):1646–53. doi: 10.1111/j.1572-0241.2007.01264.x. [DOI] [PubMed] [Google Scholar]
- 18.Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344(10):732–38. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 19.Sugumar A, Kloppel G, Chari ST. Autoimmune pancreatitis: pathologic sub-types and their implications for its diagnosis. Am J Gastroenterol. 2009;104(9):2308–10. doi: 10.1038/ajg.2009.336. quiz 2311. [DOI] [PubMed] [Google Scholar]
- 20.Hirano K, Tada M, Isayama H, et al. Long-term prognosis of autoimmune pancreatitis with and without corticosteroid treatment. Gut. 2007;56(12):1719–24. doi: 10.1136/gut.2006.115246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito T, Nakano I, Koyanagi S, et al. Autoimmune pancreatitis as a new clinical entity. Three cases of autoimmune pancreatitis with effective steroid therapy. Dig Dis Sci. 1997;42(7):1458–68. doi: 10.1023/a:1018862626221. [DOI] [PubMed] [Google Scholar]
- 22.Ito T, Nishimori I, Inoue N, et al. Treatment for autoimmune pancreatitis: consensus on the treatment for patients with autoimmune pancreatitis in Japan. J Gastroenterol. 2007;42(Suppl.18):50–58. doi: 10.1007/s00535-007-2051-y. [DOI] [PubMed] [Google Scholar]