Abstract
Although fructose consumption has dramatically increased and is suspected to be causally linked to metabolic abnormalities, the mechanisms involved are still only partially understood. We discuss the available data and investigate the effects of dietary fructose on risk factors associated with metabolic disorders. The evidence suggests that fructose may be a predisposing cause in the development of insulin resistance in association with the induction of hypertriglyceridemia. Experiments in animals have shown this relation when they are fed diets very high in fructose or sucrose, and human studies also show this relation, although with conflicting results due to the heterogeneity of the studies. The link between increased fructose consumption and increases in uric acid also has been confirmed as a potential risk factor for metabolic syndrome, and insulin resistance/hyperinsulinemia may be causally related to the development of hypertension. Collectively, these results suggest a link between high fructose intake and insulin resistance, although future studies must be of reasonable duration, use defined populations, and improve comparisons regarding the effects of relevant doses of nutrients on specific endpoints to fully understand the effect of fructose intake in the absence of potential confounding factors.
Keywords: antioxidants, fructose, hypertension, oxidative stress, sugar-sweetened beverages
Introduction
It has generally been assumed that a high intake of refined carbohydrates, specifically fructose, may increase the risk of insulin resistance (IR)6. This is of particular importance because over the past 3 decades there has been a shift in the amount and source of sweeteners used in developed countries (1). In the past, the intake of fructose has been between 16 and 20 g/d, largely due to the consumption of fresh fruit; however, the consumption of fructose has increased to 60–150 g/d and originates mostly from sucrose (2). In addition, with the advent of isomerization, separation, and crystallization technologies, it became possible to produce crystalline fructose and high-fructose corn syrup (HFCS), a manufactured disaccharide (3). The introduction of HFCS in the 1970s primarily resulted in the accelerated consumption of sweeteners, due to the ease with which HFCS is solubilized in processed food. Fructose is advantageous because it is at least 1.5 times sweeter than sucrose and inexpensive to produce; consequently, it has been used widely in foods during the preparation of canned fruits, jams, paste candies, cakes, powder for beverages, and soft drinks. This review outlines the effects of dietary fructose on risk factors associated with metabolic abnormalities by using observational data from rodents and humans.
Metabolism of Fructose Compared With Other Dietary Monosaccharides
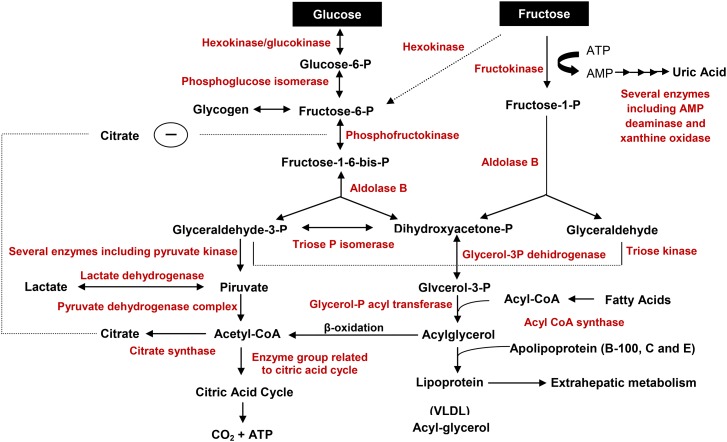
Fructose is a sugar that exists in foods as a simple sugar and as a component of the disaccharide sucrose, which consists of 1 molecule of glucose and 1 molecule of fructose that are absorbed in the small intestine and transported into the enterocytes via glucose transporter 5 (GLUT5), a specific transporter that is not insulin dependent. Once inside the enterocyte, fructose diffuses into the blood vessels through transport mediated by GLUT2 at the basolateral pole (4), where it is rapidly phosphorylated at the carbon 1 (mediated by fructokinase) or at the carbon 6 (mediated by a hexokinase) positions. Most fructose is phosphorylated at carbon 1 and accumulates rapidly in the liver, where it is hydrolyzed into 2 trioses: dihydroxyacetone and glyceraldehyde-phosphate. These 2 trioses may follow 3 different paths: 1) participate in the glycolytic pathway producing pyruvate, which is converted to lactic acid under anaerobic conditions or enters the citric acid cycle as acetyl CoA under aerobic conditions, thus releasing energy; 2) dihydroxyacetone phosphate may be reduced to glycerol-3-phosphate, which is needed for the synthesis of lipids including TGs and phospholipids; and 3) dihydroxyacetone phosphate may be condensed to form fructose-1,6-diphosphate, forming glucose or glycogen. Consequently, pyruvate, lipid, and glycogen are produced. Figure 1 presents the metabolic pathways of glucose and fructose.
FIGURE 1.
Metabolic pathways of dietary glucose and fructose in liver cells. Enzymes are shown in red. P, phosphate.
Glucose utilization can be regulated before cleavage, whereas fructose is less regulated. The control of glucose metabolism is related to a phosphorylated derivative of fructose called fructose-2,6-diphosphate (5), which plays an important role in the regulation of glycolysis and gluconeogenesis. Fructose-2,6-diphosphate affects the activity of 2 key regulatory enzymes that control the catabolism and anabolism of carbohydrates in the liver (phosphofructokinase and fructose-1,6-diphosphatase). However, high concentrations of ATP and citrate inhibit phosphofructokinase (6), and AMP stimulates fructose-2,6-diphosphatase, which forms fructose-6-phosphate, lowering the concentrations of fructose-2,6-diphosphate. This reduced concentration of fructose-2,6-diphosphate stimulates fructose-1,6-diphosphatase, favoring the conversion of fructose-1,6-diphosphate to fructose-6-phosphate, an intermediate in gluconeogenesis. In contrast, increased fructose-2,6-diphosphate stimulates 6-phosphofructokinase, which converts fructose-6-phosphate to fructose-1,6-diphosphate, promoting glycolysis. Thus, the main difference between hepatic fructose and glucose metabolism is that fructose molecules by-pass the main rate-controlling step in glycolysis involving 6-phosphofructokinase.
Fructose, Lipogenesis, and IR
Initially, fructose attracted great interest as a sweetener for patients with diabetes due to its low glycemic index compared with glucose (7). However, the consumption of fructose increases concentrations of plasma TGs, the conversion rate of lipids in the liver, and the secretion and removal of TGs (8), which suggests that lipogenesis may be the key biochemical process that is induced by fructose. An increase in the fructose intake rate generates the substrate for de novo lipogenesis (DNL). FAs incorporated into TGs or other lipids are associated with increased VLDL synthesis, which is important in nonalcoholic fatty liver disease (NAFLD) (9). Furthermore, when hepatic DNL is induced, new lipids are synthesized, nonesterified FAs are re-esterified, and hepatic lipid oxidation is downregulated. These events create an imbalance between hepatic lipid “inputs” and “exports,” leading to net intrahepatic fat accumulation. In this sense, lipid metabolism disorders are closely linked to IR, because ectopic lipid deposition may generate toxic lipid-derived metabolites, including diacylglycerol, fatty acyl CoA, and ceramides, and the intracellular accumulation of TGs leads to a higher serine/threonine phosphorylation of insulin receptor substrate 1 (IRS-1), which reduces insulin signaling (10). IR accentuates the lipolysis of TGs in the adipose tissue and inhibits FFA esterification, which increases circulating FFA concentrations that are captured by the liver (11).
Clinical studies on fructose overconsumption.
Human studies have shown that fructose intake results in higher rates of DNL than does glucose intake (12). Consequently, fructose is more lipogenic than glucose, an effect that may be exacerbated in patients with hyperlipidemia (13), IR, or type 2 diabetes (14). Furthermore, fructose does not stimulate the production of insulin and leptin, which are involved in energy homeostasis. Indeed, a low insulin concentration after ingesting fructose would be associated with lower mean leptin concentrations than would be seen after ingesting glucose. A clinical trial conducted in 12 women showed that an isocaloric diet with a high fructose content resulted in lower blood glucose and higher serum TGs than a diet with high amounts of glucose (15). Dietary fructose during 4 wk in men reduced sensitivity to insulin, increased hepatic steatosis, and increased TG circulation (16). Assy et al. (17) found that patients with NAFLD without the classic risk factors consumed more soft drinks and sweetened juices compared with the control group and showed a correlation between the severity of hepatic steatosis and the quantity of soft drink consumed. Moreover, as expected, IR levels were higher in patients with NAFLD than in controls. Another study showed that the presence of fructose as the only source of carbohydrate in a very-low-calorie diet delayed the expected improvement in plasma glucose and insulin concentrations (18). However, moderate fructose intakes by healthy subjects for 2 wk had no deleterious effects on insulin sensitivity compared with the same amount of sucrose (19). Healthy subjects who consumed up to 1.5 g fructose/kg body weight per day for 4 wk showed increased plasma TG concentrations, but IR was not induced (20).
A high-fructose diet in studies in animals.
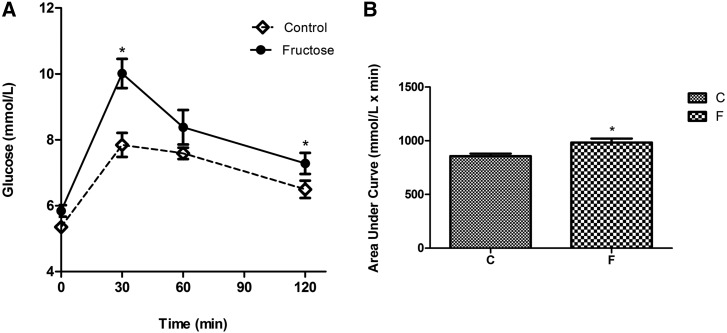
High-fructose or high-sucrose diets are clearly associated with IR development and disturbed glucose homeostasis in rodents. Rats fed a diet that substituted sucrose for starch developed several alterations in glucose and lipid metabolism over time. The earliest event observed after 1 wk was an increase in hepatic TG content (21). Between 2 and 5 wk, fasting hyperinsulinemia developed, indicating whole-body IR. In rats fed 66% fructose for 3 wk, the insulin receptor mRNA and the numbers of insulin receptors on skeletal muscle were significantly lower than those in rats fed a standard diet (22). In another study, it was found that after 28 d of fructose consumption, there was no change in the concentrations of insulin receptor, but its autophosphorylation, a necessary mechanism for its action, was reduced to 72% in the liver (23). Rats fed a diet containing 35% fructose for 4 wk showed reduced insulin sensitivity associated with impaired hepatic insulin action and glucose availability (24). The effect of a high-fructose diet corroborates its glycemic effect, which was evident in oral-glucose-tolerance tests performed in rats after they were fed 20% fructose for 18 wk (Figure 2). Compared with control rats, blood glucose concentrations were significantly higher in the fructose-fed rats at 30 wk, and the return to baseline was incomplete during the response tests (Figure 2A). Consequently, the AUCs for glucose were significantly increased in these rats (P < 0.05) (Figure 2B).
FIGURE 2.
Oral-glucose-tolerance test values (A) and the time course AUC (B) in rats fed a high-fructose diet (20% wt:vol fructose solution as a substitute for drinking water for 20 wk). The test was performed, as described in reference 25, 2 wk before the rats (n = 8) were killed. Values are means ± SEMs. *P < 0.05 compared with the control group (Student’s t test). C, control diet; F, high-fructose diet.
In addition, fructose also may cause DNL stimulation through the enhanced intrahepatic synthesis of triose phosphate precursors and an increased expression of lipogenic genes. It has been suggested that these mechanisms may involve the inhibition of PPAR-α in liver cells, the stimulation of hepatic DNL, and reduced hepatic lipid oxidation (26). In the early stages of sucrose overfeeding, rodents develop significant changes in their hepatic metabolism, with relatively few changes in their glucose homeostasis, and no significant changes in their extrahepatic insulin sensitivity. In sequence, fructose may increase the expression of key lipogenic enzymes in the liver, and it induces sterol regulatory element-binding protein 1 (SREBP-1) expression, the principal inducer of hepatic lipogenesis (27).
Relations between Fructose, Weight Gain, and Obesity
Fructose may not induce the level of satiety that is observed after a glucose-based meal. Because insulin and leptin provide key signals that convey information about energy intakes and body fat stores to the central nervous system for the long-term regulation of food intakes and energy homeostasis, reduced insulin and leptin production may contribute to increased energy intakes and weight gain in animals and humans. Moreover, ghrelin has been the focus of attention because of its potent effects on stimulating food intake. Compared with dietary glucose, pure fructose consumed with mixed meals reduced circulating insulin and leptin concentrations and attenuated the postprandial suppression of ghrelin (15), which might contribute to increased caloric intakes and ultimately to weight gain and obesity after the chronic consumption of a high-fructose diet.
No evidence of body weight changes.
Lê et al. (16) showed that a diet containing 1.5 g fructose/kg administered daily to healthy humans over 4 wk increased plasma TG and glucose concentrations without any significant changes in body weight, liver and muscle lipid content, or IR. Similarly, a 24-h study did not determine any substantial differences in plasma glucose, insulin, leptin, or ghrelin concentrations when subjects consumed meals containing sucrose or HFCS, and the TG profiles were similar (28). These responses were midway between the lower responses after pure fructose syrup consumption and the higher responses after glucose solution ingestion. No differences in the food intakes were apparent during a meal consumed 50 min later or in the components of the food-intake regulatory mechanisms. Another long-term study undertaken in overweight/obese individuals showed no body weight changes after 10-wk supplementation with glucose or fructose, indicating that the effects of fructose or glucose on food intake might not differ in the long term (29).
Lipid deposition.
Although acute fructose consumption can not stimulate leptin secretion, fasting leptin concentrations increased after chronic high-fructose intakes over 1–4 wk in healthy individuals, which indicates that high-fructose feeding may suppress food intakes in the long term (16). Stanhope et al. (29) showed marked differences in the metabolic effects of fructose and glucose during an 8-wk outpatient study when subjects consumed their usual diets ad libitum and either fructose- or glucose-sweetened beverages that comprised 25% of their energy requirements. Fructose-sweetened but not glucose-sweetened beverages promoted intra-abdominal lipid deposition and hepatic lipid production, cholesterol metabolism was shifted unfavorably, and insulin sensitivity was diminished, which suggests that fructose consumption may specifically promote lipid deposition in visceral adipose tissues. DiMeglio and Mattes (30) found that 15 healthy men and women given carbohydrate loads (450 kcal/d) in the form of calorically sweetened soda for 4 wk gained significantly more weight than when the same carbohydrate load was given in a solid form as jelly beans. Ludwig et al. (31) showed that the quantity of sugar-sweetened beverages ingested by adolescents predicted their initial BMI. Furthermore, Raben et al. (32) found that moderately overweight men and women who drank calorically sweetened beverages experienced greater weight gains than those who consumed diet drinks over a 10-wk study period.
Hyperuricemia and Diets Containing Fructose
In the liver, fructose loading drastically stimulates ATP hydrolysis because of its rapid phosphorylation to fructose-1-phosphate, which increases AMP and uric acid synthesis (33). Despite its early designation as an antioxidant (34, 35), uric acid is estimated to be responsible for up to 60% of the antioxidant capacity in plasma (35), and it is now known to have pro-oxidative properties (36). These include the stimulation of vascular smooth muscle cell proliferation, release of inflammatory chemotactic substances (37), induction of monocyte chemotaxis (38), inhibition of proliferation and migration of endothelial cells (39), oxidative stress in adipocytes via the activation of NAD(P)H oxidase and a reduction in the concentration of NO (36). The latter study showed that the reactive oxygen species produced by NAD(P)H oxidase increased lipid peroxidation, highlighting the relation between uric acid, oxidative stress, and inflammation leading to metabolic syndrome (MeS). Furthermore, high concentrations of uric acid are seen in a wide variety of metabolic diseases (40, 41), which supports the hypothesis that hyperuricemia is a causal factor in the progression of MeS, as shown by Choi and Ford (42).
Fructose contribution to hyperuricemia in human research.
Previous studies (43–45) have shown that fructose can induce hyperuricemia; this supports work that has demonstrated an association between a high consumption of high-sugar drinks and an increase in serum uric acid concentrations (46, 47). However, other studies (19, 48, 49) have not found a link between fructose and serum uric acid concentrations. Studies have hypothesized that links exist between fructose intake, hyperuricemia, and IR. Insulin-induced glucose utilization involves the stimulation of key metabolic pathways in insulin-sensitive cells and increases in the blood flow and nutritive circulation to the major insulin-sensitive tissue, skeletal muscle (50). This effect of insulin is caused by its activation of endothelial NO synthase (51). The ability of insulin to produce muscle vasodilation is impaired in obese subjects, and this may contribute to altered glucose homeostasis through “prereceptor” IR (52). Because endothelial NO synthase is potently inhibited by uric acid (53), it has been proposed that the inhibition of the vascular effects of insulin by uric acid may be involved in fructose-induced IR. Moreover, it was observed that uric acid is a biomarker for fatty liver (54). A study reported that the prevalence of NAFLD in Chinese individuals was significantly higher in those individuals with hyperuricemia than in those without hyperuricemia (24.75% and 9.54%, respectively; P < 0.001) (55). In addition, uric acid concentrations are elevated in the majority of adolescents with hypertension (56), and a prospective study reported that the reduction in uric acid concentrations in patients with kidney disease and hyperuricemia resulted in significantly slower progression of cardiorenal disease (57).
Fructose feeding and hyperuricemia in animals.
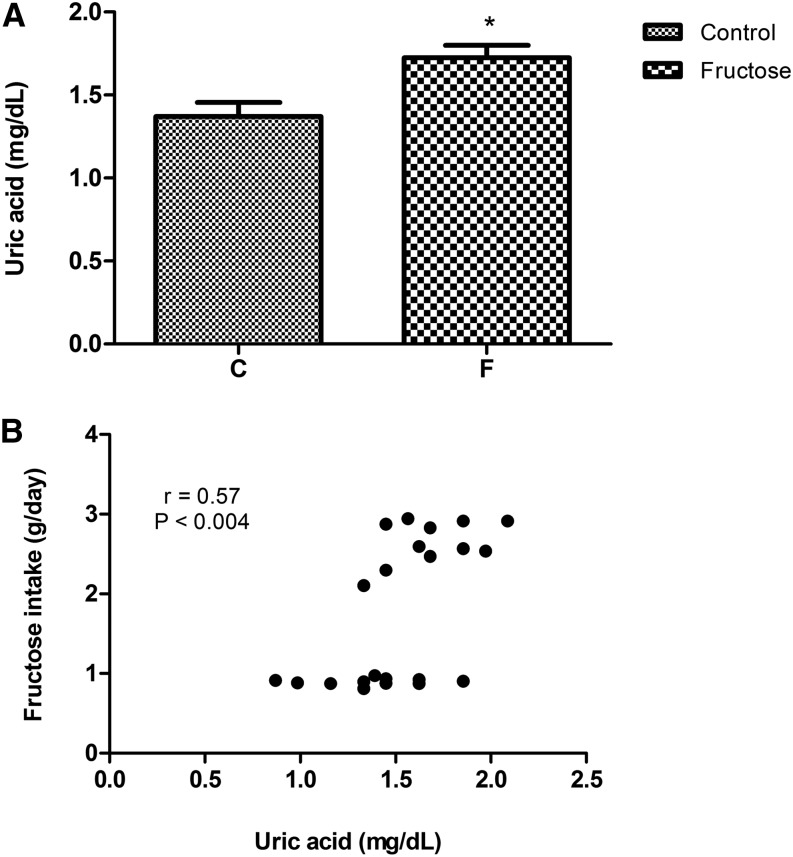
Fructose-induced increases in uric acid concentrations may contribute to adverse effects (58). Serum uric acid concentrations are elevated in rats fed a diet containing 20% wt:vol fructose in water for 20 wk, and we found a significant direct relation between hyperuricemia and fructose intake (Figure 3). Hence, excess fructose ingestion may exert a specific metabolic effect that is dose dependent. Nakagawa et al. (60) showed that lowering uric acid concentrations with a uricosuric agent in rats fed a high-fructose diet prevented IR development, and experimental evidence suggests that chronic hyperuricemia may cause steatosis and hypertriglyceridemia. Experiments with allopurinol-treated rodents showed that the lipogenic effects of fructose may be partially mediated through the direct effects of uric acid, which stimulates hepatic fat accumulation (61, 62). Lanaspa et al. (62) identified a mechanism that involves the translocation of NAD(P)H oxidase to the mitochondria accompanied by the inactivation of aconitase, accumulation of citrate, and the stimulation of fat synthesis. Previous studies showed that hyperuricemia induced by uricase (63) or oxonic acid (64) inhibition led to high serum TG concentrations and steatosis in rats. These additive effects are likely to be fully operational in humans who lack uricase expression, and they act synergistically to induce glomerular hypertension and IR and to elevate hepatic TGs and oxidative stress. Another group documented that high-fructose diets directly cause renal dysfunction, which was based on a study in which rats fed high-fructose diets developed hypertension, hypertriglyceridemia, hyperuricemia, hypertrophy of the kidneys, glomerular hypertension, and reductions in glomerular flows (65). Gersch et al. (66) showed that a diet high in fructose accelerated surgically induced chronic renal failure in rats, an effect that may have been mediated by hyperuricemia or other MeS components, including inflammation or hyperinsulinemia.
FIGURE 3.
Serum uric acid concentrations (A) and Spearman correlations between fructose intakes and uric acid concentrations (B) in rats at 20 wk of treatment with the AIN93M diet and a 20% wt:vol fructose supplementation in drinking water. Values are means ± SEMs, n = 11 rats/group. *P < 0.05 compared with control group (Student’s t test). Data were derived from reference 59.
Fructose and the Risk of Developing Hypertension
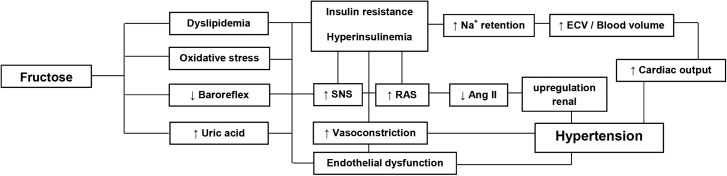
The mechanisms by which fructose causes elevated blood pressure (BP) is not fully known, but some possibilities have been suggested (Figure 4). These include the attenuation of the baroreflex and increased sympathetic nervous system activity (67), elevation of circulating catecholamines (68), an increase in the activity of the rennin-angiotensin system (69), increased sodium reabsorption (70), increased production of uric acid (60), and endothelial dysfunction (71). There is, however, little evidence that fructose per se directly increases BP.
FIGURE 4.
Proposed mechanisms for hypertension resulting from fructose in the diet. Ang II, angiotensin II; ECV, extracellular volume, RAS, renin-angiotensin system; SNS, sympathetic nervous system.
Human studies on fructose and hypertension.
Compared with individuals with normal BP, those with essential hypertension are relatively intolerant to glucose (72). Nevertheless, BP reductions in hypertensive individuals do not necessarily reduce the levels of glucose intolerance and hyperinsulinemia. Fructose supplementation in healthy, normal-weight (16) and overweight subjects (29) with doses amounting to 30% of their total energy requirements failed to significantly alter their BP. Conversely, data collected from almost 2700 persons from 10 US/United Kingdom populations showed direct associations between the consumption of sugar-sweetened beverages and BP increases, which were independent of body weight and height (73). A systematic review of 12 cross-sectional and prospective cohort studies that encompassed >400,000 participants showed that sugar-sweetened beverage intakes were significantly associated with higher BP and an increased incidence of hypertension (74). The authors concluded that intakes of >12 fluid ounces of sugar-sweetened beverages/d increased the risk of hypertension by at least 6%, and these intakes can increase mean systolic BP by at least 1.8 mm Hg over ∼18 mo. High fructose intakes may be linked to high calorie intake and weight gain and with IR, and all of these factors are themselves associated with high BP. In individuals with IR, the vasodilatory effect of insulin can be inhibited; these individuals cannot compensate for the decrease in sodium reabsorption in the proximal tubule, thus causing sodium retention (75). Furthermore, a reduction in renal excretion may occur due to various functional or pathologic changes intrinsic to the kidney or to neurohumoral factors, which then influences renal excretion. As a consequence, sodium excretion can be maintained and a change in the glomerular filtration rate or tubular reabsorption may be masked by a high BP. Nevertheless, in prolonged hypertension lasting for months or years, observed pathologic changes in glomerular capillaries result in a decrease in the glomerular filtration rate (requiring an elevated BP) and inhibition of tubular reabsorption in order to maintain sodium homeostasis (76).
Studies in rodents on fructose and hypertension.
Many studies have also shown that a high-fructose diet can induce hypertension in rats (77–80). However, there are also other reports that do not show a link between fructose and increased BP (81–83). These conflicting reports could be due to other dietary components fed to the rats (84, 85), the different strains and ages of rats used in the various studies (83), and the different techniques used to measure BP (81, 82). Hwang et al. (77) showed that an adrenergic inhibitor reduced BP, but not IR, in fructose-fed rats. Therefore, if fructose increased sympathetic nervous system activity, then this change is not the cause of the IR, hyperinsulinemia, and hypertriglyceridemia. Suzuki et al. (84) showed that pioglitazone treatment in rats with IR and hypertension resulted in a reduction in glycemic concentrations and in systolic arterial pressure and resulted in the normalization of insulin and TG concentrations. This indicated that the IR was responsible for the development of hypertension in rats fed a diet high in fructose. Chronically, insulin activates the central nervous system and causes an increase in peripheral vascular tone, leading to increased BP. Consequently, this may contribute to IR as a result of vasoconstriction. Vasoconstriction will cause a decrease in blood flow and a reduction in the supply of glucose to the tissues (86). Catena et al. (22) showed that hyperinsulinemia is determined by the number of insulin receptors in tissues, and that this receptor density is inversely related to salt intake. Therefore, the antinatriuretic effect of the insulin is significantly reduced by a high salt intake, but not in fructose-fed rats, suggesting that the mechanism that normally limits insulin-induced sodium reabsorption is absent in this model (59). Consequently, in the long term, a high salt and fructose intake results in impaired sodium homeostasis, contributing to hypertension. Although much work is focused on fructose-induced IR and cardiorenal disease, not all sources of fructose can cause a deleterious effect. Natural fruit is also rich in antioxidants that may have a beneficial effect (87). Forman et al. (88) reported that fructose intake is not correlated with a high BP when most of the intake is from fruit. Fruit contains other compounds such as vitamin C, quercetin, and resveratrol, which may alter the metabolic activities of fructose. Accordingly, fructose in natural foods exists at lower concentrations and is completely different from the predominant fructose sources in the American diet, which comprise processed products with high amounts of added sugars and high proportions of fructose; these problems should be targeted more explicitly in dietary guidelines to support cardiometabolic and general health (89, 90).
Conclusions
There is no doubt that high-fructose feeding can cause several adverse effects (Table 1), because fructose stimulates DNL more than other carbohydrate sources. However, this review’s conclusions should be viewed with caution, because it is unclear whether the effects of dietary fructose that have been observed in animals occur in humans. The relative doses of fructose consumed in animal experiments are much larger than those consumed by humans. Conversely, it has been reported that lower sucrose intakes by rodents also lead to IR development; therefore, such modest doses of fructose may require longer durations of exposure to induce effects relating to insulin sensitivity. Although some studies in humans have shown that high fructose doses can result in IR, postprandial hypertriglyceridemia, intra-abdominal fat accumulation, hyperuricemia, and elevated BP, it is important to determine the doses of sugar that alter health outcomes and thus identify populations who are particularly susceptible or protected from the adverse health effects of high-sugar diets. Investigating these important questions may provide a more complete understanding about how excess fructose consumption can cause disease and how the incidence rates of diet-related diseases can be reduced. This will help to reconcile the seemingly inconsistent results and will provide more reliable data for the design of randomized trials. We hope that this work will encourage investigators to focus on fructose and generate the data needed to address the questions in this review.
TABLE 1.
Additional intervention studies with fructose administration1
| Route and species | Amount of fructose , % | Time | Effect | Authors (Reference) |
| Drink | ||||
| Mouse | 10 | 3 wk | ↑ Fasting glucose (∼95%) and ↑ nonfasting insulin (∼60%) concentrations | Huang et al. (91) |
| 2 mo | ↑ Glucose (26%), ↑ TG (22%), ↑ insulin (95%) concentrations, and ↑ SBP (22%) values | De Angelis et al. (67) | ||
| 30 | 8 wk | ↑ Hepatic TG concentrations (∼500%) | Kanuri et al. (92) | |
| Rat | 4 | 14 wk | ↑ SBP (∼27%) | Vasdev et al. (80) |
| 10 | 8 wk | ↑ Glucose (8%), ↑ TG (43%) concentrations, and ↑ IR index (20%) | Jalal et al. (93) | |
| 5 wk | ↑ TG (162%), ↑ insulin (176%) concentrations, and ↑ IR index (238%) | Xu et al. (94) | ||
| 6 wk | ↑ TG (149%), ↑ insulin (75%) concentrations, and ↑ SBP (10%) values | Suzuki et al. (84) | ||
| 2 and 5 wk | ↑ Insulin (28%), TG (108%) concentrations, ↑ IR index (48%), and ↑ SBP (12%) values | Zhao et al. (79) | ||
| 20 | 20 wk | ↑ Glucose (48%), ↑ insulin (62%) concentrations, and ↑ IR index (128%) | Dornas et al. (59) | |
| 30 d | ↑ TG concentrations (∼55%) | Motoyama et al. (95) | ||
| Guinea pig | 10 | 19 d | ↑ TG concentrations in relation to glucose treatment (155%) | Bar-On and Stein (8) |
| Food | ||||
| Rat | 56.8 | 6 wk | ↑ TG (∼500%) and ↑ insulin (∼23%) concentrations | Faure et al. (96) |
| 60 | 4 wk | ↑ Insulin (45%) and ↑ uric acid (84%) concentrations | Nakagawa et al. (60) | |
| 8 and 9 wk | ↑ TG (∼140%) concentrations and ↑ SBP (∼14%) values | |||
| 30 d | ↑ Glucose (19%), ↑ insulin (67%) concentrations, and ↑ IR index (72%) | Nandhini et al. (97) | ||
| 6 wk | ↑ Glucose (45%), ↑ TG (233%), ↑ insulin (130%) concentrations, and ↑ IR index (240%) | Liu et al. (25) | ||
| 35 d | ↑ Glucose (20%), ↑ TG (277%), ↑ insulin (239%) concentrations, and ↑ IR index (307%) | El Mesallamy et al. (98) | ||
| 65 | 2 wk | ↑ Glucose (54%), ↑ TG (441%) concentrations, and ↑ SBP (12%) values | Kim et al. (78) | |
| 4 wk | ↑ TG (112%), ↑ hepatic TG (75%), and ↑ insulin (63%) concentrations | Busserolles et al. (99) | ||
| 66 | 2 wk | ↑ Glucose (∼30%), ↑ TG (∼146%), ↑ insulin (∼118%) concentrations, and ↑ SBP (∼16%) values | Hwang et al. (77) | |
| 66 | 8 wk | ↑ Glucose (42%), ↑ TG (30%), and ↑ insulin (157%) concentrations | D’Angelo et al. (81) | |
| Hamster | 60 | 2 wk | ↑ TG (∼215%) and ↑ insulin (∼146%) concentrations | Taghibiglou et al. (100) |
| Dog | 60 | 20–28 d | ↑ TG (160%), ↑ insulin (210%) concentrations, and ↑ 22% MBP values | Martinez et al. (101) |
| Human | 15 | 5 wk | ↑ Insulin concentrations (14%) in hyperinsulinemic and glucose-intolerant subjects (235%) | Hallfrisch et al. (102) |
| 20 | 5 wk | ↑ TG (47%) in hyperinsulinemic and (20%) nonhyperinsulinemic subjects | Reiser et al. (103) |
IR, insulin resistance; MBP, mean blood pressure; SBP, systolic blood pressure; ↑, increase.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: BP, blood pressure; DNL, de novo lipogenesis; GLUT, glucose transporters; HFCS, high-fructose corn syrup; IR, insulin resistance; IRS-1, insulin receptor substrate 1; MeS, metabolic syndrome; NAFLD, nonalcoholic fatty liver disease; SREBP-1, sterol regulatory element-binding protein 1.
References
- 1.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 2004;79:537–43. [DOI] [PubMed] [Google Scholar]
- 2.Park YK, Yetley EA. Intakes of food sources of fructose in the United States. Am J Clin Nutr 1993;58:737S–47S. [DOI] [PubMed] [Google Scholar]
- 3.Hanover LM, White JS. Manufacturing, composition, and applications of fructose. Am J Clin Nutr 1993;58:724S–32S. [DOI] [PubMed] [Google Scholar]
- 4.Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr 1993;58: Suppl:754S–65S. [DOI] [PubMed] [Google Scholar]
- 5.Van Schaftingen E. The discovery and role of fructose-2,6-bisphosphatate. Acta Gastroenterol Belg 1988;51:141–6. [PubMed] [Google Scholar]
- 6.Tornheim K, Lowenstein JM. Control of phosphofructokinase from rat skeletal muscle: effects of fructose diphosphate, AMP, ATP, and citrate. J Biol Chem 1976;251:7322–8. [PubMed] [Google Scholar]
- 7.Uusitupa MIJ. Fructose in the diabetic diet. Am J Clin Nutr 1994;59:753S–7S. [DOI] [PubMed] [Google Scholar]
- 8.Bar-On H, Stein Y. Effect of glucose and fructose administration on lipid metabolism in the rat. J Nutr 1968;94:95–105. [DOI] [PubMed] [Google Scholar]
- 9.Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF. Fructose consumption as a risk factor for nonalcoholic fatty liver disease. J Hepatol 2008;48:993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest 2000;106:171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab 2000;11:351–6. [DOI] [PubMed] [Google Scholar]
- 12.Lê KA, Ith M, Kreis R, Faeh D, Bortolotti M, Tran C, Boesch C, Tappy L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr 2009;89:1760–5. [DOI] [PubMed] [Google Scholar]
- 13.Jeppesen J, Chen YI, Zhou MY, Schaaf P, Coulston A, Reaven GM. Postprandial triglyceride and retinyl ester responses to oral fat: effects of fructose. Am J Clin Nutr 1995;61:787–91. [DOI] [PubMed] [Google Scholar]
- 14.Abraha A, Humphreys SM, Clark ML, Matthews DR, Frayn KN. Acute effect of fructose on postprandial lipaemia in diabetic and nondiabetic subjects. Br J Nutr 1998;80:169–75. [PubMed] [Google Scholar]
- 15.Teff KL, Elliott SS, Tschöp M, Kieffer TJ, Rader D, Heiman M, Townsend RR, Keim NL, D’Alessio D, Havel PJ. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab 2004;89:2963–72. [DOI] [PubMed] [Google Scholar]
- 16.Lê KA, Faeh D, Stettler R, Ith M, Kreis R, Vermathen P, Boesch C, Ravussin E, Tappy L. A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am J Clin Nutr 2006;84:1374–9. [DOI] [PubMed] [Google Scholar]
- 17.Assy N, Nasser G, Kamayse I, Nseir W, Beniashvili Z, Djibre A, Grosovski M. Soft drink consumption linked with fatty liver in the absence of traditional risk factors. Can J Gastroenterol 2008;22:811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizkalla SW, Lebouc Y, Serog P, Apfelbaum M. Carbohydrate intake affects insulin binding to human erythrocytes in normal weight subjects but not in subjects with family obesity. Metabolism 1981;30:900–7. [DOI] [PubMed] [Google Scholar]
- 19.Crapo PA, Kolterman OG. The metabolic effects of 2-week fructose feeding in normal subjects. Am J Clin Nutr 1984;39:525–34. [DOI] [PubMed] [Google Scholar]
- 20.Lê KA, Faeh D, Stettler R, Debard C, Loizon E, Vidal H, Boesch C, Ravussin E, Tappy L. Effects of four-week high-fructose diet on gene expression in skeletal muscle of healthy men. Diabetes Metab 2008;34:82–5. [DOI] [PubMed] [Google Scholar]
- 21.Pagliassotti MJ, Prach PA, Koppenhafer TA, Pan DA. Changes in insulin action, triglycerides, and lipid composition during sucrose feeding in rats. Am J Physiol 1996;271:R1319–26. [DOI] [PubMed] [Google Scholar]
- 22.Catena C, Cavarape A, Novello M, Giacchetti G, Sechi LA. Insulin receptors and renal sodium handling in hypertensive fructose-fed rats. Kidney Int 2003;64:2163–71. [DOI] [PubMed] [Google Scholar]
- 23.Ueno M, Bezerra RM, Silva MS, Tavares DQ, Carvalho CR, Saad MJ. A high-fructose diet induces changes in pp185 phosphorylation in muscle and liver of rats. Braz J Med Biol Res 2000;33:1421–7. [DOI] [PubMed] [Google Scholar]
- 24.Thorburn AW, Storlien LH, Jenkins AB, Khouri S, Kraegen EW. Fructose-induced in vivo insulin resistance and elevated plasma triglyceride levels in rats. Am J Clin Nutr 1989;49:1155–63. [DOI] [PubMed] [Google Scholar]
- 25.Liu IM, Tzeng TF, Liou SS. A Chinese herbal decoction, Dang Gui Bu Xue Tang, prepared from Radix astragali and Radix angelicae sinensis, ameliorates insulin resistance induced by a high-fructose diet in rats. Evid Based Complement Alternat Med 2011;248231:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roglans N, Vila L, Farre M, Alegret M, Sanchez RM, Vazquez-Carrera M, Laguna JC. Impairment of hepatic Stat-3 activation and reduction of PPARalpha activity in fructose-fed rats. Hepatology 2007;45:778–88. [DOI] [PubMed] [Google Scholar]
- 27.Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem 1999;274:30028–32. [DOI] [PubMed] [Google Scholar]
- 28.Stanhope KL, Griffen SC, Bair BR, Swarbrick MM, Keim NL, Havel PJ. Twenty-four-hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals. Am J Clin Nutr 2008;87:1194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, et al. Consuming fructose sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 2009;119:1322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes Relat Metab Disord 2000;24:794–800. [DOI] [PubMed] [Google Scholar]
- 31.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet 2001;357:505–8. [DOI] [PubMed] [Google Scholar]
- 32.Raben A, Vasilaras TH, Moller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr 2002;76:721–9. [DOI] [PubMed] [Google Scholar]
- 33.Kurtz TW, Kabra PM, Booth BE, Al-Bander HA, Portale AA, Serena BG, Tsai HC, Morris RC Jr. Liquid-chromatographic measurements of inosine, hypoxanthine, and xanthine in studies of fructose-induced degradation of adenine nucleotides in humans and rats. Clin Chem 1986;32:782–6. [PubMed] [Google Scholar]
- 34.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA 1981;78:6858–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nieto FJ, Iribarren C, Gross MD, Comstock GW, Cutler RG. Uric acid and serum antioxidant capacity: a reaction to atherosclerosis? Atherosclerosis 2000;148:131–9. [DOI] [PubMed] [Google Scholar]
- 36.Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol 2007;293:C584–96. [DOI] [PubMed] [Google Scholar]
- 37.Rao GN, Corson MA, Berk BC. Uric acid stimulates vascular smooth muscle cell proliferation by increasing platelet-derived growth factor A-chain expression. J Biol Chem 1991;266:8604–8. [PubMed] [Google Scholar]
- 38.Zare F, Magnusson M, Bergström T, Brisslert M, Josefsson E, Karlsson A, Tarkowski A. Uric acid, a nucleic acid degradation product, down-regulates dsRNA-triggered arthritis. J Leukoc Biol 2006;79:482–8. [DOI] [PubMed] [Google Scholar]
- 39.Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol 2005;16:3553–62. [DOI] [PubMed] [Google Scholar]
- 40.Nakanishi N, Okamoto M, Yoshida H, Matsuo Y, Suzuki K, Tatara K. Serum uric acid and risk for development of hypertension and impaired fasting glucose or type II diabetes in Japanese male office workers. Eur J Epidemiol 2003;18:523–30. [DOI] [PubMed] [Google Scholar]
- 41.Mellen PB, Bleyer AJ, Erlinger TP, Evans GW, Nieto FJ, Wagenknecht LE, Wofford MR, Herrington DM. Serum uric acid predicts incident hypertension in a biethnic cohort: the atherosclerosis risk in communities study. Hypertension 2006;48:1037–42. [DOI] [PubMed] [Google Scholar]
- 42.Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med 2007;120:442–7. [DOI] [PubMed] [Google Scholar]
- 43.Fox IH, Kelley WN. Studies on the mechanism of fructose-induced hyperuricemia in man. Metabolism 1972;21:713–21. [DOI] [PubMed] [Google Scholar]
- 44.Emmerson BT. Effect of oral fructose on urate production. Ann Rheum Dis 1974;33:276–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macdonald I, Keyser A, Pacy D. Some effects, in man, of varying the load of glucose, sucrose, fructose, or sorbitol on various metabolites in blood. Am J Clin Nutr 1978;31:1305–11. [DOI] [PubMed] [Google Scholar]
- 46.Turner JL, Bierman EL, Brunzell JD, Chait A. Effect of dietary fructose on triglyceride transport and glucoregulatory hormones in hypertriglyceridemic men. Am J Clin Nutr 1979;32:1043–50. [DOI] [PubMed] [Google Scholar]
- 47.Choi JW, Ford ES, Gao X, Choi HK. Sugar sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum 2008;59:109–16. [DOI] [PubMed] [Google Scholar]
- 48.Sun SZ, Flickinger BD, Williamson-Hughes PS, Empie MW. Lack of association between dietary fructose and hyperuricemia risk in adults. Nutr Metab (Lond) 2010;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang DD, Sievenpiper JL, de Souza RJ, Chiavaroli L, Ha V, Cozma AI, Mirrahimi A, Yu ME, Carleton AJ, Di Buono M, et al. The effects of fructose intake on serum uric acid vary among controlled dietary trials. J Nutr 2012;142:916–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baron AD, Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G. Insulin-mediated skeletal muscle vasodilation contributes to both insulin sensitivity and responsiveness in lean humans. J Clin Invest 1995;96:786–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent: a novel action of insulin to increase nitric oxide release. J Clin Invest 1994;94:1172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction: implications for the syndrome of insulin resistance. J Clin Invest 1996;97:2601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi YJ, Yoon Y, Lee KY, Hien TT, Kang KW, Kim KC, Lee J, Lee MY, Lee SM, Kang DH, et al. Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. FASEB J 2014;28:3197–204. [DOI] [PubMed] [Google Scholar]
- 54.Sartorio A, Del Col A, Agosti F, Mazzilli G, Bellentani S, Tiribelli C, Bedogni G. Predictors of nonalcoholic fatty liver disease in obese children. Eur J Clin Nutr 2007;61:877–83. [DOI] [PubMed] [Google Scholar]
- 55.Li Y, Xu C, Yu C, Xu L, Miao M. Association of serum uric acid level with non-alcoholic fatty liver disease: a cross-sectional study. J Hepatol 2009;50:1029–34. [DOI] [PubMed] [Google Scholar]
- 56.Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension 2003;42:247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis 2006;47:51–9. [DOI] [PubMed] [Google Scholar]
- 58.Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, Gersch MS, Benner S, Sánchez-Lozada LG. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr 2007;86:899–906. [DOI] [PubMed] [Google Scholar]
- 59.Dornas WC, de Lima WG, dos Santos RC, Guerra JF, de Souza MO, Silva M, Souza e Silva L, Diniz MF, Silva ME. High dietary salt decreases antioxidant defenses in the liver of fructose-fed insulin-resistant rats. J Nutr Biochem 2013;24:2016–22. [DOI] [PubMed] [Google Scholar]
- 60.Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, Ouyang X, Feig DI, Block ER, Herrera-Acosta J, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol 2006;290:F625–31. [DOI] [PubMed] [Google Scholar]
- 61.Sánchez-Lozada LG, Tapia E, Bautista-García P, Soto V, Avila-Casado C, Vega-Campos IP, Nakagawa T, Zhao L, Franco M, Johnson RJ. Effects of febuxostat on metabolic and renal alterations in rats with fructose-induced metabolic syndrome. Am J Physiol Renal Physiol 2008;294:F710–8. [DOI] [PubMed] [Google Scholar]
- 62.Lanaspa MA, Sanchez-Lozada LG, Choi YJ, Cicerchi C, Kanbay M, Roncal-Jimenez CA, Ishimoto T, Li N, Marek G, Duranay M, et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J Biol Chem 2012;287:40732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wexler BC. Allantoxan amide-induced myocardial necrosis in Sprague-Dawley vs spontaneously hypertensive rats. Proc Soc Exp Biol Med 1982;170:476–85. [DOI] [PubMed] [Google Scholar]
- 64.Wexler BC, Greenberg BP. Effect of increased serum urate levels on virgin rats with no arteriosclerosis versus breeder rats with preexistent arteriosclerosis. Metabolism 1977;26:1309–20. [DOI] [PubMed] [Google Scholar]
- 65.Sánchez-Lozada LG, Tapia E, Jiménez A, Bautista P, Cristóbal M, Nepomuceno T, Soto V, Avila-Casado C, Nakagawa T, Johnson RJ, et al. Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am J Physiol Renal Physiol 2007;292:F423–9. [DOI] [PubMed] [Google Scholar]
- 66.Gersch MS, Mu W, Cirillo P, Reungjui S, Zhang L, Roncal C, Sautin YY, Johnson RJ, Nakagawa T. Fructose but not dextrose accelerates the progression of chronic kidney disease. Am J Physiol Renal Physiol 2007;293:F1256–61. [DOI] [PubMed] [Google Scholar]
- 67.De Angelis K, Senador DD, Mostarda C, Irigoyen MC, Morris M. Sympathetic overactivity precedes metabolic dysfunction in a fructose model of glucose intolerance in mice. Am J Physiol Regul Integr Comp Physiol 2012;302:R950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tran LT, MacLeod KK, McNeill JH. Chronic etanercept treatment prevents the development of hypertension in fructose-fed rats. Mol Cell Biochem 2009;330:219–28. [DOI] [PubMed] [Google Scholar]
- 69.Kobayashi R, Nagano M, Nakamura F, Higaki J, Fujioka Y, Ikegami H, Mikami H, Kawaguchi N, Onishi S, Ogihara T. Role of angiotensin II in high fructose induced left ventricular hypertrophy in rats. Hypertension 1993;21:1051–5. [DOI] [PubMed] [Google Scholar]
- 70.Baum M. Insulin stimulates volume absorption in the rabbit proximal convoluted tubule. J Clin Invest 1987;79:1104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takagawa Y, Berger ME, Tuck ML, Golub MS. Impaired endothelial alpha-2 adrenergic receptor-mediated vascular relaxation in the fructose-fed rat. Hypertens Res 2002;25:197–202. [DOI] [PubMed] [Google Scholar]
- 72.DeFronzo RA. Insulin resistance, hyperinsulinemia, and coronary artery disease: a complex metabolic web. J Cardiovasc Pharmacol 1992;20:S1–16. [DOI] [PubMed] [Google Scholar]
- 73.Brown IJ, Stamler J, Van Horn L, Robertson CE, Chan Q, Dyer AR, Huang CC, Rodriguez BL, Zhao L, Daviglus ML, et al. ; International Study of Macro/Micronutrients and Blood Pressure Research Group Sugar-sweetened beverage, sugar intake of individuals, and their blood pressure: international study of macro/micronutrients and blood pressure. Hypertension 2011;57:695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Malik AH, Akram Y, Shetty S, Malik SS, Yanchou Njike V. Impact of sugar-sweetened beverages on blood pressure. Am J Cardiol 2014;113:1574–80. [DOI] [PubMed] [Google Scholar]
- 75.Stenvinkel P, Ottosson-Seeberger A, Alvestrand A. Renal hemodynamics and sodium handling in moderate renal insufficiency: the role of insulin resistance and dyslipidemia. J Am Soc Nephrol 1995;5:1751–60. [DOI] [PubMed] [Google Scholar]
- 76.Hall JE, Mizelle HL, Hildebrandt DA, Brands MW. Abnormal pressure natriuresis: a cause or a consequence of hypertension? Hypertension 1990;15:547–59. [DOI] [PubMed] [Google Scholar]
- 77.Hwang IS, Ho H, Hoffman BB, Reaven GM. Fructose-induced insulin resistance and hypertension in rats. Hypertension 1987;10:512–6. [DOI] [PubMed] [Google Scholar]
- 78.Kim HY, Okubo T, Juneja LR, Yokozawa T. The protective role of amla (Emblica officinalis Gaertn.) against fructose-induced metabolic syndrome in a rat model. Br J Nutr 2010;103:502–12. [DOI] [PubMed] [Google Scholar]
- 79.Zhao CX, Xu X, Cui Y, Wang P, Wei X, Yang S, Edin ML, Zeldin DC, Wang DW. Increased endothelial nitric-oxide synthase expression reduces hypertension and hyperinsulinemia in fructose-treated rats. J Pharmacol Exp Ther 2009;328:610–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vasdev S, Gill V, Parai S, Longerich L, Gadag V. Dietary vitamin E and C supplementation prevents fructose induced hypertension in rats. Mol Cell Biochem 2002;241:107–14. [DOI] [PubMed] [Google Scholar]
- 81.D’Angelo G, Elmarakby AA, Pollock DM, Stepp DW. Fructose feeding increases insulin resistance but not blood pressure in Sprague-Dawley rats. Hypertension 2005;46:806–11. [DOI] [PubMed] [Google Scholar]
- 82.Brands MW, Garrity CA, Holman MG, Keen HL, Alonso-Galicia M, Hall JE. High-fructose diet does not raise 24-hour mean arterial pressure in rats. Am J Hypertens 1994;7:104–9. [DOI] [PubMed] [Google Scholar]
- 83.Bezerra RM, Ueno M, Silva MS, Tavares DQ, Carvalho CR, Saad MJ, Gontijo JA. A high-fructose diet induces insulin resistance but not blood pressure changes in normotensive rats. Braz J Med Biol Res 2001;34:1155–60. [DOI] [PubMed] [Google Scholar]
- 84.Suzuki M, Nomura C, Odaka H, Ikeda H. Effect of an insulin sensitizer, pioglitazone, on hypertension in fructose-drinking rats. Jpn J Pharmacol 1997;74:297–302. [DOI] [PubMed] [Google Scholar]
- 85.Nishimoto Y, Tomida T, Matsui H, Ito T, Okumura K. Decrease in renal medullary endothelial nitric oxide synthase of fructose-fed, salt-sensitive hypertensive rats. Hypertension 2002;40:190–4. [DOI] [PubMed] [Google Scholar]
- 86.Rattigan S, Clark MG, Barrett EJ. Acute vasoconstriction-induced insulin resistance in rat muscle in vivo. Diabetes 1999;48:564–9. [DOI] [PubMed] [Google Scholar]
- 87.Jalal DI, Smits G, Johnson RJ, Chonchol M. Increased fructose associates with elevated blood pressure. J Am Soc Nephrol 2010;21:1543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Forman JP, Choi H, Curhan GC. Fructose and vitamin C intake do not influence risk for developing hypertension. J Am Soc Nephrol 2009;20:863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.DiNicolantonio JJ, Lucan SC. The wrong white crystals: not salt but sugar as aetiological in hypertension and cardiometabolic disease. Open Heart 2014;1:e000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.DiNicolantonio JJ, O’Keefe JH, Lucan SC. Added fructose: a principal driver of type 2 diabetes mellitus and its consequences. Mayo Clin Proc 2015;90:372–81. [DOI] [PubMed] [Google Scholar]
- 91.Huang DY, Boini KM, Friedrich B, Metzger M, Just L, Oswald H, Wulff P, Kuhl D, Vallon V, Lang F. Blunted hypertensive effect of combined fructose and high-salt diet in gene targeted mice lacking functional serum- and glucocorticoid-inducible kinase SGK1. Am J Physiol Regul Integr Comp Physiol 2006;290:R935–44. [DOI] [PubMed] [Google Scholar]
- 92.Kanuri G, Spruss A, Wagnerberger S, Bischoff SC, Bergheim I. Fructose-induced steatosis in mice:role of plasminogen activator inhibitor-1, microsomal triglyceride transfer protein and NKT cells. Lab Invest 2011;91:885–95. [DOI] [PubMed] [Google Scholar]
- 93.Jalal R, Bagheri SM, Moghimi A, Rasuli MB. Hypoglycemic effect of aqueous shallot and garlic extracts in rats with fructose-induced insulin resistance. J Clin Biochem Nutr 2007;41:218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu X, Zhao CX, Wang L, Tu L, Fang X, Zheng C, Edin ML, Zeldin DC, Wang DW. Increased CYP2J3 expression reduces insulin resistance in fructose-treated rats and db/db mice. Diabetes 2010;59:997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Motoyama CS, Pinto MJ, Lira FS, Ribeiro EB, do Nascimento CM, Oyama LM. Guar fiber associated with fructose reduces serum triacylglycerol but did not improve the glucose tolerance in rats. Diabetol Metab Syndr 2010;2:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Faure P, Rossini E, Lafond JL, Richard MJ, Favier A, Halimi S. Vitamin E improves the free radical defense system potential and insulin sensitivity of rats fed high fructose diets. J Nutr 1997;127:103–7. [DOI] [PubMed] [Google Scholar]
- 97.Nandhini AT, Thirunavukkarasu V, Ravichandran MK, Anuradha CV. Effect of taurine on biomarkers of oxidative stress in tissues of fructose-fed insulin-resistant rats. Singapore Med J 2005;46:82–7. [PubMed] [Google Scholar]
- 98.El Mesallamy HO, El-Demerdash E, Hammad LN, El Magdoub HM. Effect of taurine supplementation on hyperhomocysteinemia and markers of oxidative stress in high fructose diet induced insulin resistance. Diabetol Metab Syndr 2010;2:1–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Busserolles J, Gueux E, Rock E, Demigné C, Mazur A, Rayssiguier Y. Oligofructose protects against the hypertriglyceridemic and pro-oxidative effects of a high fructose diet in rats. J Nutr 2003;133:1903–8. [DOI] [PubMed] [Google Scholar]
- 100.Taghibiglou C, Carpentier A, Van Iderstine SC, Chen B, Rudy D, Aiton A, Lewis GF, Adeli K. Mechanisms of hepatic very low density lipoprotein overproduction in insulin resistance: evidence for enhanced lipoprotein assembly, reduced intracellular ApoB degradation, and increased microsomal triglyceride transfer protein in a fructose-fed hamster model. J Biol Chem 2000;275:8416–25. [DOI] [PubMed] [Google Scholar]
- 101.Martinez FJ, Rizza RA, Romero JC. High-fructose feeding elicits insulin resistance, hyperinsulinism, and hypertension in normal mongrel dogs. Hypertension 1994;23:456–63. [DOI] [PubMed] [Google Scholar]
- 102.Hallfrisch J, Ellwood KC, Michaelis OE. Effects of dietary fructose on plasma glucose and hormone responses in normal and hyperinsulinemic men. J Nutr 1983;113:1819–26. [DOI] [PubMed] [Google Scholar]
- 103.Reiser S, Powell AS, Scholfield DJ, Panda P, Ellwood KC, Canary JJ. Blood lipids, lipoproteins, apoproteins, and uric acid in men fed diets containing fructose or high-amylose cornstarch. Am J Clin Nutr 1989;49:832–9. [DOI] [PubMed] [Google Scholar]