Abstract
Retinol binding protein 4 (RBP4), previously called retinol binding protein (RBP), is considered a specific carrier of retinol in the blood. It is also an adipokine that has been implicated in the pathophysiology of insulin resistance. RBP4 seems to be correlated with cardiometabolic markers in inflammatory chronic diseases, including obesity, type 2 diabetes, metabolic syndrome, and cardiovascular diseases (CVDs). It has recently been suggested that inflammation produced by RBP4 induces insulin resistance and CVD. The clinical relevance of this hypothesis is discussed in this review. Knowledge concerning the association of RBP4 with inflammation markers, oxidative stress, and CVDs as well as concerning the role of diet and antioxidants in decreasing RBP4 concentrations are discussed. Special attention is given to methodologies used in previously published studies and covariates that should be controlled when planning new studies on this adipokine.
Keywords: retinol binding protein 4, cardiovascular diseases, inflammation, immunity, antioxidants, diet, adipokines
Introduction
Vitamin A is an essential nutrient that plays a key role in vision, cell growth, differentiation, and embryonic development (1, 2). The liver is the main storage site for retinol (3). Retinol-binding protein 4 (RBP4)8, also known as retinol binding protein (RBP), is a plasma retinol transporter that carries retinol from the liver to the periphery, and very little plasma RBP4 originates from adipose tissue (4). The loss of RBP4 induces vitamin A deficiency in Rbp4 knockout mice (5). Apo-RBP4 is defined as RBP4 that is not bound to retinol, whereas retinol-bound RBP4 (holo-RBP4) associates with transthyretin (TTR) in plasma to prevent the loss of RBP through kidney filtration (6). The cell surface receptor for RBP4 is known as stimulated by retinoic acid 6 (STRA6) (7, 8), which is not only a vitamin A transporter but also a surface signaling receptor (9).
RBP4 has been known as a negative acute phase inflammatory reactant. Yang et al. (10) indicated that RBP4 was a novel adipokine and that its concentrations are elevated in insulin-resistant states associated with obesity and type 2 diabetes (T2D). Several other studies observed high concentrations of RBP4 in obesity (11–15) as a chronic inflammatory state and in its complications including T2D (16–20), metabolic syndrome (21–27), and cardiovascular diseases (CVDs) (28-38).
RBP4 plays a role in progression of insulin resistance through immunity (39) and inflammatory mechanisms (40) in adipose and vascular tissues. Recently, RBP4 was implicated in cardiovascular incidents. However, there is controversy about the role of RBP4 as a marker of inflammation and CVD prediction. Therefore, a critical review of those studies is needed. To the extent of our knowledge, there are no RBP4 reviews investigating completely all of the inflammatory and immunity effects of RBP4, especially in CVD, and its association with diet and antioxidants. This article reviews recent studies of the role of RBP4 in chronic inflammation, immune response, and oxidative stress and of the relation between RBP4 and diet and antioxidants, with a special focus on CVD, to show areas of ambiguity related to this topic. The literature search was based on PubMed listings up to 10 September 2014.
RBP4 and CVD
Finding risk factors of CVD and using them as targets for drug or diet therapy has been an interesting area of research that has made it possible to prevent and treat CVD. RBP4 has recently been implicated in the pathogenesis of CVD. Higher circulating RBP4 concentrations have been observed in subjects with previous clinical arteriosclerosis (41, 42), high-grade carotid stenosis (43), inflammatory dilated cardiomyopathy (28), coronary artery disease (32), and advanced heart failure (36) compared with control subjects. However, other studies did not find significant differences in RBP4 concentrations between patients with coronary artery disease and healthy individuals (44, 45). These findings are mainly in agreement with the hypothesis that circulating RBP4 could be a possible marker of atherosclerosis.
Chronic vascular inflammation exerts a prominent role in the development of atherosclerosis (46–48). Vascular inflammation begins with endothelial secretion of proinflammatory cell surface adhesion molecules, including vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), and endothelial-leukocyte adhesion molecule 1 (E-selectin), as well as endothelial production of soluble proinflammatory factors, including monocyte chemoattractant protein 1 (MCP-1) and IL-6. MCP-1 promotes the recruitment and adherence of leukocytes to the endothelium (46).
The relation between RBP4 and oxidative stress markers is less controversial. Most of the studies reported a positive relation between RBP4 and oxidative stress markers [urinary 8-isoprostane (49), 8-iso-prostaglandin F2α (8-isoPGF2α) (50), 13-(S)-hydroxyoctadecadienoic acid (50), and malondialdehyde (51)] and a negative relation between RBP4 and antioxidant glutathione (49). Thus, RBP4 may have a role in oxidative stress, and its mechanisms need to be elucidated (Table 1).
TABLE 1.
Methodology of studies investigating the association between RBP4 and oxidative stress1
| Markers | Study design | Study population | Sex | Age,2 y | Study size, n | Cytokine measurement method | RBP4 measurement method | Analysis | Relation | RBP4 specimen | Authors, year (ref) |
| Uri-8-isoPGF2α | CC | 101 Subjects with T2D; 22 controls | 62 M; 39 F | 61.6 ± 4.5 | 123 | EIA kit | EIA | Correlation | None | Serum | Takebayashi et al., 2007 (20) |
| Urinary 8-isoprostane | CS | Healthy obese and overweight children | 45 M; 38 F | 8–15 | 83 | ELISA | Immunononenephelometry | ANCOVA | + | Serum | Codoñer-Franch et al., 2013 (49) |
| Uri-8-isoPGF2α | CS | Chinese | 910 M; 838 F | 50–70 | 1748 | ELISA | ELISA | Correlation | + | Plasma | Liu et al., 2014 (50) |
| 13-HOD | CS | Chinese | 910 M; 838 F | 50–70 | 1748 | Colorimetric EIA kit | ELISA | Correlation | + | Plasma | Liu et al., 2014 (50) |
| Glutathione | CS | Healthy obese and overweight children | 45 M; 38 F | 8–15 | 83 | ELISA | Immunononenephelometry | Multivariate regression | − | Serum | Codoñer-Franch et al., 2013 (49) |
| MDA | CC | Normotensive subjects | 111 M; 47 F | 49 | 66 | TBAR | ELISA validated by Western blot | Linear correlation | + | Plasma | Solini et al., 2012 (51) |
CC, case-control; CS, cross-sectional; EIA, enzyme immunoassay; F, female; M, male; MDA, malondialdehyde; RBP4, retinol binding protein 4; ref, reference; T2D, type 2 diabetes; Uri-8-isoPGF2α, urinary 8-iso-prostaglandin F2α 13-HOD, 13-(S)-hydroxyoctadecadienoic acid; +, positive; −, negative.
Values are ranges, means ± SDs, or the median.
Cellular oxidative stress leads to the activation of vascular inflammation (46, 47). Because RBP4 concentrations are positively related to oxidative stress markers (Table 1), RBP4 may have a role in the initiation of endothelial inflammation. Farjo et al. (40) indicated that RBP4 induces gene expression of factors that are implicated in the initiation of vascular inflammation. Despite no observed relation between RBP4 and VCAM-1 (49, 52) in human studies, a positive relation between RBP4 and ICAM-1 and between RBP4 and E-selectin was evident only in diabetic patients (54) (Table 2). Surprisingly, an inverse relation was observed between RBP4 and E-selectin and between RBP4 and ICAM-1 in obese patients with rheumatoid arthritis, with no known reason (52) (Table 2). Collectively, although RBP4 has been implicated in the initiation of vascular inflammation in vitro (40), controversial results were observed in human studies.
TABLE 2.
Methodology of studies investigating the association between RBP4 and other CVD risk markers1
| Marker | Study design | Study population | Sex | Age,2 y | Study size, n | Cytokine measurement method | RBP4 measurement method | Analysis | Relation | RBP4 specimen | Authors, year (ref) |
| MCP-1 | CS | Chinese | 910 M; 838 F | 50–70 | 1748 | Milliplex (Millipore) human cytokine/chemokine panel | Sandwich ELISA | Correlation | + | Plasma | Liu et al., 2014 (50) |
| MCP-1 | Basement analysis of an intervention | Healthy obese subjects | NM | 24–62 | 16 | Gene expression | Gene expression | Correlation | + | Adipose biopsy | Yao-Borengasser et al., 2007 (53) |
| MCP-1 | CS | Obese patients with rheumatoid arthritis | NM | NM | 217 | Solid-phase HS and RD system | Solid-phase sandwich ELISA | Mixed regression method | None | Serum | Dessein et al., 2014 (52) |
| E-selectin | CC | Hypertensive patients with endothelial dysfunction | 111 M; 47 F | 49 | 92 | ELISA | ELISA validated by Western blot | Linear correlation | None | Plasma | Solini et al., 2012 (51) |
| E-selectin | CS | Obese patients with rheumatoid arthritis | NM | NM | 217 | Solid-phase HS and RD system | Solid-phase sandwich ELISA | Mixed regression method | − | Serum | Dessein et al., 2014 (52) |
| E-selectin | CS | Patients with T2D | 32 M; 18 F | 20–80 | 50 | ELISA | ELISA | Correlation | + | Serum | Park et al., 2009 (54) |
| VCAM-1 | CS | Obese patients with rheumatoid arthritis | NM | NM | 217 | Solid-phase HS and RD system | Solid-phase sandwich ELISA | Mixed regression method | None | Serum | Dessein et al., 2014 (52) |
| VCAM-1 | CS | Healthy obese and overweight children | 45 M; 38 F | 8–15 | 83 | ELISA | Immunononenephelometry | Multivariate regression | None | Serum | Codoñer-Franch et al., 2013 (49) |
| ICAM-1 | CS | Obese patients with rheumatoid arthritis | NM | NM | 217 | Solid-phase HS and RD system | Solid-phase sandwich ELISA | Mixed regression method | None | Serum | Dessein et al., 2014 (52) |
| ICAM-1 | CS | Patients with T2D | 32 M; 18 F | 20–80 | 50 | ELISA | ELISA | Correlation | + | Serum | Park et al., 2009 (54) |
| FABP4 | CS | OD, nOD, OnD, and CVD patients and controls | 139 M; 145 F | 51–64 | 248 | Sandwich immunoassay | Monoclonal antibody–based immunoassay | Correlation | + | Serum | Alkharfy et al., 2012 (44) |
| Lipocalin 2 | CS | OD, nOD, OnD, and CVD patients and controls | 140 M; 145 F | 51–65 | 249 | Monoclonal antibody–based rapid immunoassay | Monoclonal antibody–based rapid immunoassay | Correlation | + | Serum | Alkharfy et al., 2012 (44) |
CC, case-control; CS, cross-sectional; CVD, cardiovascular disease; E-selectin, endothelial-leukocyte adhesion molecule 1; F, female; FABP4, fatty acid binding protein 4; ICAM-1, intercellular adhesion molecule 1; M, male; MCP-1, monocyte chemoattractant protein 1; NM, not mentioned; nOD, nonobese diabetic; OD, obese diabetic; OnD, obese nondiabetic; RBP4, retinol binding protein 4; ref, reference; T2D, type 2 diabetes; VCAM-1, vascular cell adhesion molecule 1; positive; −, negative.
Values are ranges or the median.
In vitro studies indicated that RBP4 has a major role in plaque rupture by increasing vascular smooth muscle cell proliferation (55) and the induction of E-selectin (40) as a plaque formation marker (56). RBP4 is also known as an independent determinant index of plaque severity (43). Therefore, it should be expected that RBP4 is positively related to carotid artery intima media thickness as a marker of subclinical atherosclerosis (57). The relation between RBP4 concentrations and carotid artery intima media thickness and between RBP4 and E-selectin is controversial in human studies (Table 2 and Table 3). The positive relation of RBP4 with coronary artery calcification was shown in a study by Huang et al. (29) (Table 3). Additional studies are needed to confirm this observation. RBP4 concentrations are positively related to the early endothelial dysfunction marker flow-mediated dilation (60) in patients with rheumatoid arthritis (52) but not in patients with endothelial dysfunction (51). The inverse relation between RBP4 and flow-mediated vasodilatation (flow-mediated dilation) are evident in normotensive individuals and in diabetic patients (20, 51, 54). The association of RBP4 with CVD markers, including fatty acid binding protein 4 (FABP4) (44), lipocalin 2 (44), and the Framingham risk score (38), is shown in Table 2. The controversial results on the relation between RBP4 and hypertension are shown in Table 4. Although RBP4 is correlated with some CVD markers, it is unlikely to have a role in vascular dysfunction.
TABLE 3.
Methodology of studies investigating the association between RBP4 and CVD risk markers1
| Marker | Study design | Study population | Sex | Age,2 y | Study size, n | RBP4 measurement method | Analysis | Relation | RBP4 specimen | Authors, year (ref) |
| CIMT | CS | Postmenopausal women | F | 42–58 | 709 | Western blot | ANOVA or KW based on RBP4 quartile | None | Serum | Huang et al., 2012 (29) |
| CIMT | CS | NM | 50% F | 70 | 1008 | ELISA | Multivariable-adjusted analyses | − | NM | Ingelsson and Lind, 2009 (30) |
| CIMT | CS | 35 hypertensive and 35 normotensivehealthy lean subjects | F | <65 | 70 | ELISA | Overall linear regression | + | Serum | Solini et al., 2009 (42) |
| CIMT | CS | T2D | 32 M; 18 F | 20–80 | 50 | ELISA | Multiple linear regression | None | Serum | Park et al., 2009 (54) |
| CIMT | CS | T2D | 144 M; 140 F | 35–70 | 284 | ELISA | Correlation | + | Serum | Xiao et al., 2013 (58) |
| CIMT | CS | 34 Patients with T2D and 8 smokers | 44 M; 52 F | 55 ± 1.3 | 96 | ELISA | Correlation | + | Serum | Bobbert et al., 2010 (59) |
| CIMT | CC | 101 Patients with T2D and 22 controls | 62 M; 39 F | 61.6 ± 4.5 | 123 | EIA | Linear regression analysis | None | Serum | Takebayashi et al., 2007 (20) |
| CIMT | CS | Obese patients with rheumatoid arthritis | NM | NM | 217 | Solid-phase sandwich ELISA | Mixed regression method | + | Serum | Dessein et al., 2014 (52) |
| FMD | CS | Obese patients with rheumatoid arthritis | NM | NM | 218 | Solid-phase sandwich ELISA | Mixed regression method | + | Serum | Dessein et al., 2014 (52) |
| FMD | CS | Hypertensive patients with endothelial dysfunction | 111 M; 47 F | 49 | 92 | ELISA validated by Western blot | Correlation | None | Plasma | Solini et al., 2012 (51) |
| FMD | CS | Patients with T2D | 32 M; 18 F | 20–80 | 50 | ELISA | Correlation | − | Serum | Park et al., 2009 (54) |
| FMD | CC | 101 Patients with T2D and 22 controls | 62 M; 39 F | 61.6 ± 4.5 | 123 | EIA | Linear regression analysis | − | Serum | Takebayashi et al., 2007 (20) |
| FMD | CC | Normotensive subjects | 112 M; 47 F | 47 | 66 | ELISA | Correlation | − | Plasma | Solini et al., 2012 (51) |
| CAC | CS | Postmenopausal women | F | 42–58 | 709 | Western blot | Curvilinear association | + | Serum | Huang et al., 2012 (29) |
| Framingham score | CS | Healthy adults | 116 M; 175 F | 19–70 | 291 | EIA | Multiple linear regression | + | Serum | Won et al., 2012 (38) |
CAC, coronary artery calcification; CC, case-control; CIMT, carotid intima media thickness; CS, cross-sectional; CVD, cardiovascular disease; EIA, enzyme immunoassay; F, female; FMD, flow-mediated dilation; KW, Kruskal-Wallis test; M, male; NM, not mentioned; RBP4, retinol binding protein 4; ref, reference; T2D, type 2 diabetes; +, positive; −, negative.
Values are ranges, means ± SDs, or medians.
TABLE 4.
Methodology of studies investigating the association between RBP4 and blood pressure1
| Study design | Study population | Sex | Age,2 y | Study size, n | RBP4 measurement method | Analysis | Relation | RBP4 specimen | Authors, year (ref) |
| CS | Chinese | 910 M; 838 F | 50–70 | 1748 | Sandwich ELISA | Multivariable logistic regression | None | Plasma | Liu et al., 2014 (50) |
| CS | 30 Patients with MetS + HT vs. 30 NT patients with MetS | 11 M; 34 F | 38 ± 11 | 60 | NM | Unpaired Student‘s t test | + | NM | Gil et al., 2013 (61) |
| CS | 35 HT and 35 NT patients | F | <65 | 70 | ELISA | Univariate correlation | + | Plasma | Solini et al., 2009 (42) |
| CS | Obese and nonobese HT and control subjects | 225 M; 106 F | <20 | 331 | ELISA | Linear regression | + | Serum | Deng et al., 2014 (62) |
| CS | Healthy subjects | M | 59 ± 14 | 153 | ELISA | Correlation | None | Serum | Chiba et al., 2009 (63) |
| CS | Healthy subjects | F | 57 ± 14 | 224 | ELISA | Correlation | + | Serum | Chiba et al., 2009 (63) |
| CS | HT vs. NT patients | 111 M; 47 F | 49 | 92 | ELISA validated by Western blot | Student’s t test or Mann-Whitney | None | Plasma | Chiba et al., 2009 (63) |
CS, cross-sectional; F, female; HT, hypertensive; M, male; MetS, metabolic syndrome; NM, not mentioned; NT, normotensive; RBP4, retinol binding protein 4; ref, reference; +, positive; −, negative.
Values are ranges, means ± SDs, or the median.
RBP4 concentrations have been shown to be predictive of CVD in several studies. RBP4 reduction predicted ischemia events in patients with familial hypercholesterolemia (64). It was also shown that RBP4 acts as an independent predictor of macrovascular diseases in patients with T2D (65). RBP4 concentrations manifest a linear trend with visceral obesity and Framingham risk score as a coronary heart disease risk point scale independent of obesity (38). Two longitudinal studies with controversial findings show the relation of RBP4 with CVD risk factors, despite not detecting holo- or apo-RBP4 (37, 66). Using a longer follow-up time and MS assay, Sun et al. (37) showed that full-length and total RBP4 were associated with coronary heart disease risk markers. In contrast, Mallat et al. (66), studying a larger sample size, did not report the same results. Reasons behind these conflicting results may be the different methods of measuring of RBP4 concentrations and differences in sample size, sex, and ethnicity.
For many years, biomarkers have been seen as a promising tool for improvement in prevention, early diagnosis, and management of CVD. However, the relation between RBP4 and cardiovascular risk factors is still controversial. To our knowledge, few longitudinal studies using more accurate assays have investigated the relation between RBP4 and CVD risk factors. Well-designed longitudinal studies that use larger sample sizes and that consider potential confounders are needed to confirm previous observations.
RBP4 in Relation to Inflammation and Immune Responses
Obesity is identified as a low-grade chronic inflammation state and defined as an elevated expression of inflammatory cytokines and infiltration of immune cells into adipocytes (67). The development of inflammatory responses leads to the induction of insulin resistance and a higher incidence of cardiovascular events (68). Inflammation may be the crucial way through which RBP4 exerts its function in the pathogenesis of insulin resistance and CVDs (40, 69).
Holo-RBP4 binds to STRA6 and directly induces insulin resistance in adipocytes through c-Jun N-terminal kinases (9). Subsequently, RBP4 suppresses insulin signaling like other cytokines by inducing suppressor of cytokine signaling 3 (SOCS3) (70). Either holo- or apo-RBP4 indirectly inhibits insulin signaling by inducing cytokine secretion in macrophages cocultured with adipocytes independent of retinol and STRA6 through the c-Jun N-terminal kinases pathway and Toll-like receptor 4 (69, 70). RBP’s role in inducing endothelial cell inflammation through NAD(P)H and NF-κB pathways occurs independently of STRA6 and retinol (40). It is unclear whether holo- or apo-RBP4 or both are involved in the induction of insulin resistance and endothelial inflammation.
A number of studies have investigated the association between RBP4 and inflammatory markers, including C-reactive protein (CRP) (Table 5), IL-6 (Table 6), other cytokines (Table 7), and TNF (Table 8). Biological studies have shown that holo- or apo-RBP4 induces the secretion of certain inflammatory and/or cardiovascular risk markers, including TNF-α, IL-6, MCP-1, IFN-γ, IL-1β, IL-2, IL-12, IL-10, and IL-8 in macrophages (69), as well as ICAM-1, VCAM-1, E-selectin, IL-6, and MCP-1 in endothelial cells (40). The many conflicting results in human studies make it difficult to reach a definitive conclusion on the relation between cytokines and either circulating concentrations or adipose tissue gene expression of Rbp4.
TABLE 5.
Methodology of studies investigating the association between RBP4 and CRP1
| Study design | Study population | Sex | Age,2 y | Study size, n | Cytokine measurement method | RBP4 measurement method | Analysis | Relation | RBP4 specimen | Authors, year (ref) |
| CS | Normal, obese, and overweight children | 42 M; 37 F | 14–6 | 79 | Chemiluminescent immunometric assay | ELISA | Correlation | None | Serum | Aeberli et al., 2007 (12) |
| In | Obese adolescents | NM | 14–18 | 15 | Particle-enhanced immunonephlometry assay | Particle-enhanced immunonephlometry assay | Multivariant regression | + | Serum | Balagopal et al., 2007 (71) |
| CC | 102 Patients with T2D and 22 controls | 63 M; 39 F | 61.6 ± 4.5 | 123 | High-Sensitivity CRP assay | EIA | Correlation | None | Serum | Takebayashi et al., 2007 (20) |
| CS | Postmenopausal women | F | 42–58 | 709 | DPC Immulite 2000 (Diagnostic Products Corporation) | Western blot | ANOVA or KW based on RBP4 quartile | None | Serum | Huang et al., 2012 (24) |
| CS | Healthy Chinese | 910 M; 838 F | 50–70 | 1748 | Particle-enhanced immunonephlometry assay | ELISA | Correlation | + | Plasma | Liu et al., 2014 (50) |
| CS | Healthy Chinese | 1458 M; 1831 F | 50–70 | 3289 | Particle-enhanced immunoturbidimetric assay | Sandwich ELISA | Correlation | None | Plasma | Qi et al., 2007 (24) |
| CS | Nonobese, nondiabetic patients with CKD | 28 M; 18 F | 44–80 | 51 | ELISA | Adipose expression | Correlation | None | Adipose biopsy | Barazzoni et al., 2011 (72) |
| CS | Nonobese, nondiabetic patients with CKD | 29 M; 18 F | 44–80 | 51 | ELISA | ELISA | Linear and multiple regression | + | Plasma | Barazzoni et al., 2011 (72) |
| CC | Children with T1D or controls | 51 M; 35 F | 14.2 | 40 T1D,41 controls | ELISA | ELISA | Mann-Whitney | None | Serum | Espe et al., 2007 (73) |
| CC | Children with T1D or controls | 51 M; 35 F | 14.2 | 40 T1D,41 controls | ELISA | HPLC | Mann-Whitney | None | Retinol | Espe et al., 200 (73) |
| CC | Children with T1D or controls | 51 M; 35 F | 14.2 | 40 T1D,41 controls | ELISA | HPLC | Mann-Whitney | None | Retinol/RBP4 | Espe et al., 200 (73) |
| CS | Healthy obese subjects | F | 45 ± 9.3 | 63 | Nephelometry | Nephelometry | NM | None | Serum | Broch et al., 2010 (74) |
| CS | Healthy Swedish men | M | 58 | 100 | ELISA | Western blot | Correlation | None | Serum | Wallenius et al., 2011 (75) |
| CS | Healthy obese and overweight children | 45 M; 38 F | 15–8 | 83 | Immunonephelometry | Immunononenephelometry | ANCOVA | None | Adipose biopsy | Codoner-Franch et al., 2013 (49) |
| CS | Obese patients with rheumatoid arthritis | NM | NM | 217 | Immunoturbidimetric method | Solid-phase sandwich ELISA | Mixed regression method | None | Serum | Dessein et al., 2014 (52) |
| CS | Patients with ovarian cancer | F | 59.2 | 41 | NM | Latex agglutination immunoassay | Correlation | − | Serum | Watanabe et al., 2014 (76) |
| CC | 1036 CAD patients and 1889 controls | 1846 M; 1079 F | 45–79 | 2925 | Sandwich ELISA | Particle-enhanced immunonephlometry assay | Correlation | − | Serum | Mallat et al., 2009 (66) |
| CS | Patients with T2D | 32 M; 18 F | 20–80 | 50 | NM | ELISA | Correlation | None | Serum | Park et al., 2009 (54) |
| CS | Healthy subjects | M | 59 ± 14 | 153 | Nepherometry | ELISA | Correlation | None | Serum | Chiba et al., 2009 (63) |
| CS | Healthy subjects | F | 57 ± 14 | 224 | Nepherometry | ELISA | Correlation | + | Serum | Chiba et al., 2009 (63) |
| CC | Healthy subjects | F | 59.5 ± 6.6 | 472 | NM | MS immunoassay | Correlation | None | Plasma | Sun et al., 2013 (37) |
CAD, coronary artery disease; CC, case-control; CKD, chronic kidney disease; CRP, C-reactive protein; CS, cross-sectional; F, female; In, intervention; M, male; NM, not mentioned; RBP4, retinol binding protein 4; ref, reference; T1D, type 1 diabetes; T2D, type 2 diabetes; +, positive; −, negative.
Values are ranges, means ± SDs, or medians.
TABLE 6.
Methodology of studies investigating the association between RBP4 and IL-61
| Study design | Study population | Sex | Age,2 y | Study size, n | Cytokine measurement method | RBP4 measurement method | Analysis | Relation | RBP4 specimen | Authors, year (ref) |
| In | Obese adolescents | NM | 14–18 | 15 | ELISA | Particle-enhanced immunonephlometry assay | Correlation | + | Serum | Balagopal et al., 2007 (71) |
| CS | Normal and impaired glucose-tolerant subjects and obese, nonobese patients with and without T2D | 164 M; 69 F | 48.3 ± 1.8 to 63.2 ± 3.1 | 233 | ELISA | Gene expression | Multivariate regression | − | Adipose biopsy | Erikstrup et al., 2009 (77) |
| CS | Normal and impaired glucose-tolerant subjects and obese, nonobese patients with and without T2D | 164 M; 69 F | 48.3 ± 1.8 to 63.2 ± 3.1 | 233 | Muscle or adipose gene expression | Gene expression | Multivariate regression | None | Adipose biopsy | Erikstrup et al., 2009 (77) |
| CS | Chinese | 910 M; 838 F | 50–70 | 1748 | Milliplex (Millipore) human cytokine/chemokine panel | ELISA | Correlation | + | Plasma | Liu et al., 2014 (50) |
| CS | Healthy Swedish men | M | 58 | 100 | ELISA | Western blot | Correlation | None | Serum | Wallenius et al., 2011 (75) |
| Basement analysis of an intervention | Healthy men | M | 19–29 | 65 | Access immunoassay system | RIA kit | Partial correlation for age and BMI | None | Serum | Shea et al., 2007 (78) |
| CC | Patients with psoriasis | 19 M; 11 F | 48.6 ± 10.7 | 30 | ELISA | ELISA | Correlation | − | Serum | Nakajima et al., 2013 (79) |
| CS | Healthy obese and overweight children | 45 M; 38 F | 15–8 | 83 | ELISA | Immunonenephelometry | Multivariant regression | None | Serum | Codoñer-Franch et al., 2013 (49) |
| CS | Obese patients with rheumatoid arthritis | NM | NM | 217 | Solid-phase sandwich ELISA | Solid-phase sandwich ELISA | Mixed regression method | None | Serum | Dessein et al., 2014 (52) |
CC, case-control; CS, cross-sectional; In, intervention; F, female; M, male; NM, not mentioned; RBP4, retinol binding protein 4; ref, reference; T2D, type 2 diabetes; +, positive; −, negative.
Values are ranges, means ± SDs, or medians.
TABLE 7.
Association between RBP4 and other cytokines1
| Cytokine | Study design | Study population | Sex | Age,2 y | Study size, n | Cytokine measurement method | RBP4 measurement method | Analysis | Relation | RBP4 specimen | Authors, year (ref) |
| IL-8 | CC | Patients with dilated inflammatory cardiomyopathy | 48 M; 6 F | 41.84 ± 9.77 to 49.54 ± 13.97 | 54 | Multiplex ELISA (Biorad) | ELISA | Correlation | + | Plasma | Bobbert et al., 2009 (28) |
| IL-10 | CS | Nonobese, nondiabetic CKD patients undergoing MHD treatment | 29 M; 18 F | 44–80 | 15 | mRNA level | ELISA | Correlation | None | Plasma | Barazzoni et al., 2011 (72) |
| IL-18 | CS | Healthy obese subjects | F | 45 ± 9.3 | 63 | Solid-phase enzyme immunoassay | Nephelometry | NM | None | Serum | Broch et al., 2010 (74) |
| CD68 | Basement analysis of an intervention | Healthy obese subjects | NM | 24–62 | 16 | Gene expression | Gene expression | Correlation | + | Adipose biopsy | Yao-Borengasser et al., 2007 (53) |
CC, case-control; CKD, chronic kidney disease; CS, cross-sectional; F, female; M, male. MHD, maintenance hemodialysis; NM, not mentioned; RBP4, retinol binding protein 4; ref, reference; +, positive.
Values are ranges or means ± SDs.
TABLE 8.
Methodology of studies investigating the association between RBP4 and TNF-α1
| Cytokine | Study design | Study population | Sex | Age,2 y | Study size, n | Cytokine measurement method | RBP4 measurement method | Analysis | Relation | RBP4 specimen | Authors, year (ref) |
| TNF-α | CC | Patients with T2D | 62 M; 75 F | 30–60 | 137 | ELISA | ELISA | Correlation | + | Serum | Al-Daghri et al., 2009 (35) |
| TNF-α | CS | Obese and nonobese T2D patients with NIGT | 164 M; 69 F | 48.3 ± 1.8 to 63.2 ± 3.1 | 233 | ELISA | Gene expression | Multivariant regression | + | Adipose biopsy | Erikstrup et al., 2009 (71) |
| TNF-α | CS | Obese and nonobese T2D patients with NIGT | 164 M; 69 F | 48.3 ± 1.8 to 63.2 ± 3.1 | 233 | Muscle gene expression | Gene expression | Multivariant regression | + | Adipose biopsy | Erikstrup et al., 2009 (71) |
| TNF-α | CS | Obese and nonobese T2D patients with NIGT | 164 M; 69 F | 48.3 ± 1.8 to 63.2 ± 3.1 | 233 | Adipose gene expression | Gene expression | Multivariant regression | None | Adipose biopsy | Erikstrup et al., 2009 (71) |
| TNF-α | CS | Obese and nonobese T2D patients with NIGT | 164 M; 69 F | 48.3 ± 1.8 to 63.2 ± 3.1 | 233 | Muscle gene expression | RBP or retinol or the RBP-to-retinol ratio (ELISA) | Multivariant regression | None | Plasma | Erikstrup et al., 2009(71) |
| TNF-α | CS | Chinese | 910 M; 838 F | 50–70 | 1748 | Milliplex (Millipore) human cytokine panel | ELISA | Correlation | + | Plasma | Liu et al., 2014 (50) |
| TNF-α | CS | Nonobese, nondiabetic patients with CKD | 165 M; 69 F | 44–80 | 15 | mRNA level | ELISA | Correlation | + | Plasma | Barazzoni et al., 2011 (66) |
| TNF-α/IL-10 | CS | Nonobese nondiabetic CKD patients undergoing MHD treatment | 910 M; 838 F | 44–80 | 15 | mRNA level | ELISA | Correlation | + | Plasma | Barazzoni et al., 2011 (66) |
| sTNFR1,2 | CS | Healthy obese subjects | 29 M; 18 F | 45 ± 9.3 | 63 | Sandwich ELISA | Nephelometry | NM | None | Serum | Broch et al., 2010 (67) |
| TNF-α | CS | Healthy Swedish men | M | 58 | 100 | ELISA | Western blot | Correlation | − | Serum | Wallenius et al., 2011 (69) |
| sTNFR1,2 | CS | Healthy Swedish men | M | 58 | 100 | ELISA | Western blot | Correlation | None | Serum | Wallenius et al., 2011 (69) |
| TNF-α | CC | 66 NT or 92 HT patients | 111 M; 47 F | 49 | 66 | ELISA | ELISA validated by Western blot | Correlation | None | Plasma | Solini et al., 2012 (51) |
CC, case-control; CKD, chronic kidney disease; CS, cross-sectional; F, female; HT, hypertensive; M, male; MHD, maintenance hemodialysis; NIGT, normal or impaired glucose tolerance; NM, not mentioned; NT, normotensive; RBP, retinol binding protein; RBP4, retinol binding protein 4; ref, reference; sTNFR1,2, soluble TNF-α receptors 1 and 2; T2D, type 2 diabetes; +, positive; −, negative.
Values are ranges, means ± SDs, or medians.
With no known molecular mechanism, few human studies have shown a positive relation between RBP4 and CRP (50, 71, 72). Most studies observed no association between RBP4 and CRP (12, 20, 24, 31, 49, 52, 72–75) (Table 5). When macrophages were incubated with IL-6, the secretion and expression of Rbp4 were not affected by IL-6 (80). Although RBP4 induces IL-6 secretion in macrophages (69) and endothelial cells (40), few human studies observed a positive relation between RBP4 and IL-6 (50, 71), whereas other studies found a negative (77) or no (49, 52, 75, 77, 78) relation (Table 6).
Studies that investigated RBP4 in relation to other inflammatory markers are shown in Table 7. Plasma RBP4 concentrations are positively correlated with plasma IL-8 as a neutrophil chemoattractant factor in patients with inflammatory dilated cardiomyopathy (28) (Table 7). The in vitro part of that same study revealed that IL-8 intensifies the expression of Rbp4 by adipocytes dose-dependently (28). Furthermore, a positive correlation was observed between the adipose tissue expression of Rbp4 and the inflammatory marker CD68 (53) (Table 7). Although these results implicated the role of RBP4 in adipose tissue inflammation, additional studies are needed to confirm these findings.
In vitro RBP4 induces TNF-α secretion in T cells (39) and macrophages (69), whereas TNF-α inhibits Rbp4 gene expression in adipocytes (81) and macrophages (80). These results indicate that RBP4 might be a positive regulator of TNF-α and that RBP4 may be inhibited by TNF-α. However, conflicting results were observed in clinical studies of the relation between RBP4 and TNF-α (Table 8). Several in vivo studies showed a positive relation between RBP4 and TNF-α (35, 50, 72, 77) but not between RBP4 and soluble TNF-α receptor 1 and soluble TNF-α receptor 2 (75). Other studies did not show any relation between RBP4 and TNF-α (51, 74, 75, 77) (Table 8). These discrepancies may be due to variations in study design and methodologies.
The binding of RBP4 to retinol and TTR is affected by several factors, including serum retinol, vitamin A intake, the acute phase response, protein-energy malnutrition, and liver and renal diseases (82–85). In addition, age, sex, BMI, body fat percentage, lipid variables, fasting blood glucose, physical activity, TTR, waist circumference, waist-to-hip ratio, ethnicity, insulin concentration, intakes of SFAs and TGs, and blood pressure are potential covariates that should be adjusted to find the precise correlation between RBP4 and cytokines (86-89). For studies to succeed, all of these covariates must be considered and adjustments made.
Circulating RBP4 concentration depends on vitamin A status (90).Therefore, serum retinol concentration and vitamin A intake should be included as covariates in association analysis involving RBP. To avoid false associations, apo- and holo-RBP4 should be analyzed separately in relation to cytokines (12, 91). Several studies found a positive correlation between CRP and RBP4 (50, 63, 71, 72) when serum retinol was not considered as a covariate. Furthermore, a few studies considered vitamin A status (retinol/RBP) (12, 91) and dietary vitamin A intake (12) as confounders. By considering these covariates, no significant correlations were observed between RBP and CRP or IL-6 (12, 91).
The use of ELISA kits, with limited dynamic range, could result in conflicting results. Commercial ELISA kits do not differentiate between holo- and apo-RBP4 and evaluate the whole RBP4 concentration. Therefore, it is not clear whether holo- or apo-RBP4 is related to inflammation or CVD. According to in vitro studies, apo-RBP4 induces inflammatory cascades (40, 69). Clinical studies have consistently shown that apo-RBP4 is secreted by adipose tissue (91) and is elevated in obese individuals (91) and patients with T2D (77). Apo-RBP4 should be incorporated in association analysis. (69). Thus, apo-RBP4 should be involved in association analysis with inflammatory and cardiovascular risk markers.
Variability in findings may emerge due to a lack of high-affinity and reliable methods of RBP4 measurement (92). Previous studies used commercial ELISA kits, which underestimate RBP4 concentrations due to assay saturation (93). They are unable to distinguish between the full-length and the truncated forms of RBP4 that may affect metabolic risk factors (92). Western blot measures the full length of RBP4 and is considered the gold-standard method (93). MS immunoassay quantitates the full-length and truncated isoforms of RBP4 (92). Although these 2 assays may analyze RBP4 more accurately and precisely than ELISA, only a few studies used Western blot (31, 75) or MS (37, 92, 94) to measure RBP4 concentrations. Another reason behind the conflicting results may be the type of specimen used to evaluate RBP4 concentrations because the use of plasma anticoagulants may cause spurious results (93).
Different populations, sample sizes, geographical regions, health status, BMI, and ethnicity may justify the conflicting results observed in studies. In one study, the role of single nucleotide polymorphisms in Rbp4 was not considered during the evaluation of the relation between RBP4 and biomarkers (95). RBP4 concentrations differ according to acute inflammatory states, obesity, insulin resistance, glucose intolerance, CVDs, and the use of certain drugs (86, 87). Most of the case-control studies analyzed RBP4 in correlation with cytokines in whole study populations in which the effects of diseases and drugs may have been ignored. It is better to make correlations in patients and healthy subjects separately because inflammatory states and drugs directly affect RBP4 associations. To evaluate the impact of RBP4 on chronic inflammation in human subjects, there is an emerging need to design studies with large sample sizes in which all of the confounders can be controlled.
RBP4 in Relation to Diet and Antioxidants
Despite the conflicting results in studies on the relation between RBP4 and cytokines and CVD risk factors, lifestyle intervention studies (low-calorie diet and exercise) were less controversial in cases of lowering RBP4 concentrations. Previously published studies indicate that several drugs (20, 22, 53, 65, 96) and antioxidants (97–101) may decrease Rbp4 gene expression. RBP4 concentrations were not influenced by the consumption of fruit and vegetable juice concentrates (100), but they were decreased by vitamin D– or vitamin D plus calcium–fortified yogurt beverages (102). However, none of the studies evaluated changes in serum retinol after a decrease in RBP4 concentrations. Despite having sufficient dietary vitamin A intake in Rbp4 knockout mice, serum vitamin A concentrations decreased to levels similar to those seen in later stages of human vitamin A deficiency (103, 104). Because vitamin A circulates mainly in the form of holo-RBP4 in blood (3, 105), pharmacologic doses of antioxidants and anti-inflammatory drugs may possibly reduce retinol and RBP4 concentrations. However, because vitamin A is provided to peripheral tissues in the form of retinyl esters and β-carotene in chylomicrons (106), it is not clear whether a decrease in RBP4 affects human vitamin A status. The possibility of a decrease in the ratio of RBP to retinol by antioxidants, drugs, and lifestyle-induced weight loss should be investigated. Additional studies are needed to evaluate the effect of antioxidants and anti-inflammatory drugs on vitamin A utilization and alteration of liver reserves.
Conflicting results were found in studies of the relation between vitamin A intake and RBP4 concentrations. Hermsdorff et al. (88) found a positive association between vitamin A intake and RBP4 concentrations in healthy, nonobese Spanish women. Consistently, RBP deficiency in mice embryos increased the vulnerability of the embryos to alterations in maternal vitamin A intake (107). However, lower concentrations of RBP4 caused by dichlorodiphenyltrichloroethane (DDT) in patients with malaria were not associated with lower vitamin A intake (108). Similarly, Rbp4 promoter polymorphism in mice with T2D was not associated with retinol intake (109). The relation between vitamin A intake and concentrations of RBP4 is not clear, and additional studies are needed.
Lifestyle intervention seems to be the best way of alleviating RBP4 concentrations in patients with obesity, T2D, and CVD. Studies have shown that a low-calorie diet considerably decreased RBP4 concentrations (13, 71, 110–116). However, the effect was dependent on the amount of weight loss as well as the quality and diversity of the diets (111, 116). In a few studies, lifestyle intervention caused a decrease in RBP4 concentrations independent of weight loss (71, 116). Because RBP4 is related to coronary risk factors (37, 38, 65) and can induce insulin resistance in lean subjects (69), weight loss is not enough to decrease RBP4 concentrations. To design a CVD prevention diet for healthy individuals, more studies are needed that will show the relations of dietary patterns, diet diversity, and different food groups with RBP4 concentrations.
Conclusions
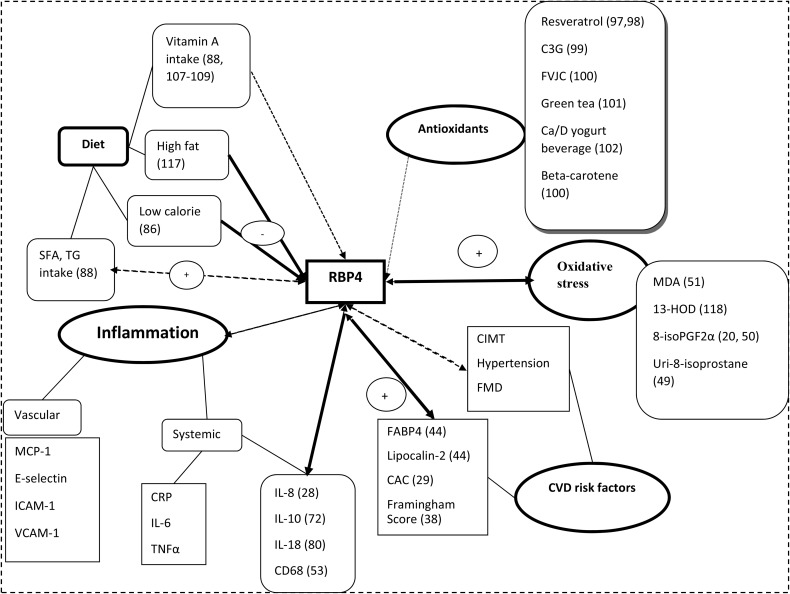
The relation of RBP4 to diet, antioxidants, oxidative stress, inflammation, and CVDs is summarized in Figure 1. Although in vitro studies indicated that RBP4 directly induces insulin resistance and CVDs through inflammatory pathways, the clinical relevance of this claim is unclear. A number of human studies confirmed that higher RBP4 concentrations are positively related to inflammatory factors and CVD risks, whereas other studies showed negative or null associations. These discordances emerge from different methodologies in the measurement of RBP4 and cytokine concentrations as well as differences in characteristics of study participants. RBP4 concentrations are affected by many covariates, which need to be controlled for in studies. More longitudinal studies with larger sample sizes are needed to investigate the relation of RBP4 to inflammation, diet, and CVD risks and its role in CVD risk prediction. It is not clear in clinical studies whether apo- or holo-RBP4 is related to inflammatory markers and/or CVDs. There is an emerging need for biological research to evaluate the possible mechanisms that RBP4 exerts in oxidative stress, inflammation, and insulin resistance. Further studies are needed to show the effect of RBP4 on indexes of CVD progression and the molecular mechanisms of RBP4 on the initiation and progression of CVD.
FIGURE 1.
RBP4 in relation to diet, antioxidants, oxidative stress, inflammation, and CVD risk factors. C3G, cyanidin 3-glucoside; CAC, coronary artery calcification; Ca/D, calcium and vitamin D; CIMT, carotid intima media thickness; CRP, C-reactive protein; CVD, cardiovascular disease; E-selectin, endothelial-leukocyte adhesion molecule 1; FABP4, fatty acid binding protein 4; FMD, flow-mediated dilation; FVJC, fruit and vegetable juice concentrate; ICAM-1, intercellular adhesion molecule 1; MCP-1, monocyte chemoattractant protein 1; MDA, malondialdehyde; RBP4, retinol binding protein 4; Uri-8-isoprostane, urinary 8-isoprostane; VCAM-1, vascular cell adhesion molecule 1; 8-isoPGF2α, 8-iso-prostaglandin F2α 13-HOD, 13-(S)-hydroxyoctadecadienoic acid.
Acknowledgments
All authors read and approved the final version of the manuscript.
Footnotes
Abbreviations used: CVD, cardiovascular disease; E-selectin, endothelial-leukocyte adhesion molecule 1; FABP, fatty acid binding protein 4; ICAM-1, intercellular adhesion molecule 1; MCP-1, monocyte chemoattractant protein1; RBP, retinol binding protein; RBP4, retinol binding protein 4; SOCS3, suppressor of cytokine signaling 3; STRA6, stimulated by retinoic acid 6; T2D, type 2 diabetes; TTR, transthyretin; VCAM-1, vascular cell adhesion molecule 1; 8-isoPGF2α, 8-iso-prostaglandin F2α.
References
- 1.Evans RM. The molecular basis of signaling by vitamin A and its metabolites. Harvey Lect 1994;90:105–17. [PubMed] [Google Scholar]
- 2.Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol 2006;46:451–80. [DOI] [PubMed] [Google Scholar]
- 3.Sporn MB, Roberts AB, Goodman DS. The retinoids: biology, chemistry, and medicine. New York: Raven Press; 1994. [Google Scholar]
- 4.Tsutsumi C, Okuno M, Tannous L, Piantedosi R, Allan M, Goodman D, Blaner W. Retinoids and retinoid-binding protein expression in rat adipocytes. J Biol Chem 1992;267:1805–10. [PubMed] [Google Scholar]
- 5.Quadro L, Hamberger L, Colantuoni V, Gottesman ME, Blaner WS. Understanding the physiological role of retinol-binding protein in vitamin A metabolism using transgenic and knockout mouse models. Mol Aspects Med 2003;24:421–30. [DOI] [PubMed] [Google Scholar]
- 6.Prapunpoj P. Evolutionary changes to transthyretin. FEBS J 2009;276:5329. [DOI] [PubMed] [Google Scholar]
- 7.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 2007;315:820–5. [DOI] [PubMed] [Google Scholar]
- 8.Wolf G. Identification of a membrane receptor for retinol-binding protein functioning in the cellular uptake of retinal. Nutr Rev 2007;65:385–8. [DOI] [PubMed] [Google Scholar]
- 9.Berry DC, Jin H, Majumdar A, Noy N. Signaling by vitamin A and retinol-binding protein regulates gene expression to inhibit insulin responses. Proc Natl Acad Sci USA 2011;108:4340–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005;436:356–62. [DOI] [PubMed] [Google Scholar]
- 11.Lee D-C, Lee J-W, Im J-A. Association of serum retinol binding protein 4 and insulin resistance in apparently healthy adolescents. Metabolism 2007;56:327–31. [DOI] [PubMed] [Google Scholar]
- 12.Aeberli I, Biebinger R, Lehmann R, l’Allemand D, Spinas GA, Zimmermann MB. Serum retinol-binding protein 4 concentration and its ratio to serum retinol are associated with obesity and metabolic syndrome components in children. J Clin Endocrinol Metab 2007;92:4359–65. [DOI] [PubMed] [Google Scholar]
- 13.Reinehr T, Stoffel-Wagner B, Roth CL. Retinol-binding protein 4 and its relation to insulin resistance in obese children before and after weight loss. J Clin Endocrinol Metab 2008;93:2287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu HY, Li X, Mu P, Jiang W, Zeng L. [Depot-specific expression of retinol-binding protein 4 in human adipose tissue and their relationship with obesity and insulin resistance.] Zhonghua Yi Xue Za Zhi 2010;90:3395–8 (in Chinese). [PubMed] [Google Scholar]
- 15.Friebe D, Neef M, Erbs S, Dittrich K, Kratzsch J, Kovacs P, Blüher M, Kiess W, Körner A. Retinol binding protein 4 (RBP4) is primarily associated with adipose tissue mass in children. Int J Pediatr Obes 2011;6:e345–52. [DOI] [PubMed] [Google Scholar]
- 16.Cho YM, Youn B-S, Lee H, Lee N, Min S-S, Kwak SH, Lee HK, Park KS. Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care 2006;29:2457–61. [DOI] [PubMed] [Google Scholar]
- 17.Sun L, Qi Q, Zong G, Ye X, Li H, Liu X, Zheng H, Hu FB, Liu Y, Lin X. Elevated plasma retinol-binding protein 4 is associated with increased risk of type 2 diabetes in middle-aged and elderly Chinese Adults. J Nutr 2014;144:722–8. [DOI] [PubMed] [Google Scholar]
- 18.Pullakhandam R, Palika R, Ghosh S, Reddy GB. Contrasting effects of type 2 and type 1 diabetes on plasma RBP4 levels: the significance of transthyretin. IUBMB Life 2012;64:975–82. [DOI] [PubMed] [Google Scholar]
- 19.Wang MN, Han Y, Li Q, Guo L, Yang Y, Wang W, Zhang J. Higher serum retinol binding protein 4 may be a predictor of weak metabolic control in Chinese patients with type 2 diabetes mellitus. J Int Med Res 2012;40:1317–24. [DOI] [PubMed] [Google Scholar]
- 20.Takebayashi K, Suetsugu M, Wakabayashi S, Aso Y, Inukai T. Retinol binding protein-4 levels and clinical features of type 2 diabetes patients. J Clin Endocrinol Metab 2007;92:2712–9. [DOI] [PubMed] [Google Scholar]
- 21.Graham TE, Yang Q, Blüher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson P-A, Smith U. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med 2006;354:2552–63. [DOI] [PubMed] [Google Scholar]
- 22.Jia W, Wu H, Bao Y, Wang C, Lu J, Zhu J, Xiang K. Association of serum retinol-binding protein 4 and visceral adiposity in Chinese subjects with and without type 2 diabetes. J Clin Endocrinol Metab 2007;92:3224–9. [DOI] [PubMed] [Google Scholar]
- 23.Gavi S, Qurashi S, Stuart LM, Lau R, Melendez MM, Mynarcik DC, McNurlan MA, Gelato MC. Influence of age on the association of retinol‐binding protein 4 with metabolic syndrome. Obesity (Silver Spring) 2008;16:893–5. [DOI] [PubMed] [Google Scholar]
- 24.Qi Q, Yu Z, Ye X, Zhao F, Huang P, Hu FB, Franco OH, Wang J, Li H, Liu Y. Elevated retinol-binding protein 4 levels are associated with metabolic syndrome in Chinese people. J Clin Endocrinol Metab 2007;92:4827–34. [DOI] [PubMed] [Google Scholar]
- 25.von Eynatten M, Lepper P, Liu D, Lang K, Baumann M, Nawroth P, Bierhaus A, Dugi K, Heemann U, Allolio B. Retinol-binding protein 4 is associated with components of the metabolic syndrome, but not with insulin resistance, in men with type 2 diabetes or coronary artery disease. Diabetologia 2007;50:1930–7. [DOI] [PubMed] [Google Scholar]
- 26.Lim S, Yoon JW, Choi SH, Park YJ, Lee JJ, Park JH, Lee SB, Kim KW, Lim JY, Kim YB. Combined impact of adiponectin and retinol‐binding protein 4 on metabolic syndrome in elderly people: the Korean Longitudinal Study on Health and Aging. Obesity (Silver Spring) 2010;18:826–32. [DOI] [PubMed] [Google Scholar]
- 27.Lin C-C, Lai M-M, Li T-C, Li C-I, Liu C-S, Chen C-C, Wu M-T. Relationship between serum retinol-binding protein 4 and visfatin and the metabolic syndrome. Diabetes Res Clin Pract 2009;85:24–9. [DOI] [PubMed] [Google Scholar]
- 28.Bobbert P, Weithäuser A, Andres J, Bobbert T, Kühl U, Schultheiss HP, Rauch U, Skurk C. Increased plasma retinol binding protein 4 levels in patients with inflammatory cardiomyopathy. Eur J Heart Fail 2009;11:1163–8. [DOI] [PubMed] [Google Scholar]
- 29.Huang G, Wang D, Khan UI, Zeb I, Manson JE, Miller V, Hodis HN, Budoff MJ, Merriam GR, Harman MS. Associations between retinol-binding protein 4 and cardiometabolic risk factors and subclinical atherosclerosis in recently postmenopausal women: cross-sectional analyses from the KEEPS study. Cardiovasc Diabetol 2012;11:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ingelsson E, Lind L. Circulating retinol-binding protein 4 and subclinical cardiovascular disease in the elderly. Diabetes Care 2009;32:733–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ingelsson E, Sundström J, Melhus H, Michaëlsson K, Berne C, Vasan RS, Risérus U, Blomhoff R, Lind L, Ärnlöv J. Circulating retinol-binding protein 4, cardiovascular risk factors and prevalent cardiovascular disease in elderly. Atherosclerosis 2009;206:239–44. [DOI] [PubMed] [Google Scholar]
- 32.Lambadiari V, Kadoglou NP, Stasinos V, Maratou E, Antoniadis A, Kolokathis F, Parissis J, Hatziagelaki E, Iliodromitis EK, Dimitriadis G. Serum levels of retinol-binding protein-4 are associated with the presence and severity of coronary artery disease. Cardiovasc Diabetol 2014;13:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pala L, Monami M, Ciani S, Dicembrini I, Pasqua A, Pezzatini A, Francesconi P, Cresci B, Mannucci E, Rotella CM. Adipokines as possible new predictors of cardiovascular diseases: a case control study. J Nutr Metab 2012:253428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salgado‐Somoza A, Teijeira‐Fernández E, Rubio J, Couso E, González‐Juanatey JR, Eiras S. Coronary artery disease is associated with higher epicardial retinol‐binding protein 4 (RBP4) and lower glucose transporter (GLUT) 4 levels in epicardial and subcutaneous adipose tissue. Clin Endocrinol (Oxf) 2012;76:51–8. [DOI] [PubMed] [Google Scholar]
- 35.Al-Daghri NM, Al-Attas OS, Alokail M, Draz HM, Bamakhramah A, Sabico S. Retinol binding protein-4 is associated with TNF-α and not insulin resistance in subjects with type 2 diabetes mellitus and coronary heart disease. Dis Markers 2009;26:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chavarria N, Kato TS, Khan R, Chokshi A, Collado E, Akashi H, Takayama H, Naka Y, Farr M, Mancini D. Increased levels of retinol binding protein 4 in patients with advanced heart failure correct after hemodynamic improvement through ventricular assist device placement. Circulation 2011;76:2148–52. [DOI] [PubMed] [Google Scholar]
- 37.Sun Q, Kiernan UA, Shi L, Phillips DA, Kahn BB, Hu FB, Manson JE, Albert CM, Rexrode KM. Plasma retinol-binding protein 4 (RBP4) levels and risk of coronary heart disease: a prospective analysis among women in the Nurses’ Health Study. Circulation 2013;127:1938–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Won JC, Park CY, Oh SW, Park SW. Increased plasma levels of retinol‐binding protein 4 with visceral obesity is associated with cardiovascular risk factors. J Diabetes Investig 2012;3:457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moraes-Vieira PM, Yore MM, Dwyer PM, Syed I, Aryal P, Kahn BB. RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metab 2014;19:512–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farjo KM, Farjo RA, Halsey S, Moiseyev G, Ma J-x. Retinol-binding protein 4 induces inflammation in human endothelial cells by an NADPH oxidase-and nuclear factor kappa B-dependent and retinol-independent mechanism. Mol Cell Biol 2012;32:5103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cabré A, Lazaro I, Girona J, Manzanares J, Marimon F, Plana N, Heras M, Masana L. Retinol‐binding protein 4 as a plasma biomarker of renal dysfunction and cardiovascular disease in type 2 diabetes. J Intern Med 2007;262:496–503. [DOI] [PubMed] [Google Scholar]
- 42.Solini A, Santini E, Madec S, Rossi C, Muscelli E. Retinol-binding protein-4 in women with untreated essential hypertension. Am J Hypertens 2009;22:1001–6. [DOI] [PubMed] [Google Scholar]
- 43.Kadoglou NP, Lambadiari V, Gastounioti A, Gkekas C, Giannakopoulos TG, Koulia K, Maratou E, Alepaki M, Kakisis J, Karakitsos P. The relationship of novel adipokines, RBP4 and omentin-1, with carotid atherosclerosis severity and vulnerability. Atherosclerosis 2014;235:606–12. [DOI] [PubMed] [Google Scholar]
- 44.Alkharfy KM, Al-Daghri NM, Vanhoutte PM, Krishnaswamy S, Xu A. Serum retinol-binding protein 4 as a marker for cardiovascular disease in women. PLoS One 2012;7:e48612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahmoudi MJ, Mahmoudi M, Siassi F, Hedayat M, Pasalar P, Chamari M, Abolhassani H, Rezaei N, Saboor-Yaraghi A-A. Circulating retinol-binding protein 4 concentrations in patients with coronary artery disease and patients with type 2 diabetes mellitus. Int J Diabetes Dev Ctries 2012;32:105–10. [Google Scholar]
- 46.Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol 2004;15:1983–92. [DOI] [PubMed] [Google Scholar]
- 47.Frey RS, Ushio–Fukai M, Malik AB. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal 2009;11:791–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. J Diabetes Res 2007;2007:95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Codoñer-Franch P, Mora-Herranz A, Simó-Jordá R, Pérez-Rambla C, Boix-García L, Faus-Pérez A.. Retinol-binding protein 4 levels are associated with measures of liver and renal function and oxidant/antioxidant status in obese children. J Pediatr 2013;163(2):593–5. [DOI] [PubMed] [Google Scholar]
- 50.Liu Y, Wang D, Li D, Sun R, Xia M. Associations of retinol-binding protein 4 with oxidative stress, inflammatory markers, and metabolic syndrome in a middle-aged and elderly Chinese population. Diabetol Metab Syndr 2014;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solini A, Stea F, Santini E, Bruno RM, Duranti E, Taddei S, Ghiadoni L. Adipocytokine levels mark endothelial function in normotensive individuals. Cardiovasc Diabetol 2012;11:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dessein PH, Tsang L, Norton GR, Woodiwiss AJ, Solomon A. Retinol binding protein 4 concentrations relate to enhanced atherosclerosis in obese patients with rheumatoid arthritis. PLoS One 2014;9:e92739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yao-Borengasser A, Varma V, Bodles AM, Rasouli N, Phanavanh B, Lee M-J, Starks T, Kern LM, Spencer HJ III, Rashidi AA. Retinol binding protein 4 expression in humans: relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin Endocrinol Metab 2007;92:2590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park SE, Kim DH, Lee JH, Kang ES, Ahn CW, Lee HC, Cha BS. Retinol-binding protein-4 is associated with endothelial dysfunction in adults with newly diagnosed type 2 diabetes mellitus. Atherosclerosis 2009;204:23–5. [DOI] [PubMed] [Google Scholar]
- 55.Li F, Xia K, Sheikh MSA, Cheng J, Li C, Yang T. Involvement of RBP4 in hyperinsulinism-induced vascular smooth muscle cell proliferation. Endocrine 2015;472–82. [DOI] [PubMed] [Google Scholar]
- 56.De Caterina R, Ghiadoni L, Taddei S, Virdis A, Almerigogna F, Basta G, Lazzerini G, Bernini W, Salvetti A. Soluble E-selectin in essential hypertension: a correlate of vascular structural changes. Am J Hypertens 2001;14:259–66. [DOI] [PubMed] [Google Scholar]
- 57.Crouse JR, Craven TE, Hagaman AP, Bond MG. Association of coronary disease with segment-specific intimal-medial thickening of the extracranial carotid artery. Circulation 1995;92:1141–7. [DOI] [PubMed] [Google Scholar]
- 58.Xiao Y, Xu A, Hui X, Zhou P, Li X, Zhong H, Tang W, Huang G, Zhou Z. Circulating lipocalin-2 and retinol-binding protein 4 are associated with intima-media thickness and subclinical atherosclerosis in patients with type 2 diabetes. PLoS One 2013;8:e66607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bobbert T, Raila J, Schwarz F, Mai K, Henze A, Pfeiffer AF, Schweigert FJ, Spranger J. Relation between retinol, retinol-binding protein 4, transthyretin and carotid intima media thickness. Atherosclerosis 2010;213:549–51. [DOI] [PubMed] [Google Scholar]
- 60.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002;39:257–65. [DOI] [PubMed] [Google Scholar]
- 61.Gil JS, Drager LF, Guerra-Riccio GM, Mostarda C, Irigoyen MC, Costa-Hong V, Bortolotto LA, Egan BM, Lopes HF. The impact of metabolic syndrome on metabolic, pro-inflammatory and prothrombotic markers according to the presence of high blood pressure criterion. Clinics 2013;68:1495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deng W, Zhang Y, Zheng Y, Jiang Y, Wu Q, Liang Z, Yang G, Chen B. Serum retinol-binding protein 4 levels are elevated but do not contribute to insulin resistance in newly diagnosed Chinese hypertensive patients. Diabetol Metab Syndr 2014;6:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiba M, Saitoh S, Ohnishi H, Akasaka H, Mitsumata K, Furukawa T, Shimamoto K. Associations of metabolic factors, especially serum retinol-binding protein 4 (RBP4), with blood pressure in Japanese—the Tanno and Sobetsu Study. Endocr J 2010;57:811–7. [DOI] [PubMed] [Google Scholar]
- 64.Cubedo J, Padró T, Cinca J, Mata P, Alonso R, Badimon L. Retinol‐binding protein 4 levels and susceptibility to ischaemic events in men. Eur J Clin Invest 2014;44:266–75. [DOI] [PubMed] [Google Scholar]
- 65.Zhang M, Chen P, Chen S, Sun Q, Zeng Q, Chen J, Liu Y, Cao X, Ren M, Wang J. The association of new inflammatory markers with type 2 diabetes mellitus and macrovascular complications: a preliminary study. Eur Rev Med Pharmacol Sci 2014;18:1567–72. [PubMed] [Google Scholar]
- 66.Mallat Z, Simon T, Benessiano J, Clément K, Taleb S, Wareham NJ, Luben R, Khaw K-T, Tedgui A, Boekholdt SM. Retinol-binding protein 4 and prediction of incident coronary events in healthy men and women. J Clin Endocrinol Metab 2009;94:255–60. [DOI] [PubMed] [Google Scholar]
- 67.Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol 2012;8:709–16. [DOI] [PubMed] [Google Scholar]
- 68.Romeo GR, Lee J, Shoelson SE. Metabolic syndrome, insulin resistance, and roles of inflammation—mechanisms and therapeutic targets. Arterioscler Thromb Vasc Biol 2012;32:1771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Norseen J, Hosooka T, Hammarstedt A, Yore MM, Kant S, Aryal P, Kiernan UA, Phillips DA, Maruyama H, Kraus BJ. Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase-and Toll-like receptor 4-dependent and retinol-independent mechanism. Mol Cell Biol 2012;32:2010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deng ZB, Poliakov A, Hardy RW, Clements R, Liu C, Liu Y, Wang J, Xiang X, Zhang S, Zhuang X. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes 2009;58:2498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Balagopal P, Graham TE, Kahn BB, Altomare A, Funanage V, George D. Reduction of elevated serum retinol binding protein in obese children by lifestyle intervention: association with subclinical inflammation. J Clin Endocrinol Metab 2007;92:1971–4. [DOI] [PubMed] [Google Scholar]
- 72.Barazzoni R, Zanetti M, Semolic A, Pirulli A, Cattin MR, Biolo G, Bosutti A, Panzetta G, Bernardi A, Guarnieri G. High plasma retinol binding protein 4 (RBP4) is associated with systemic inflammation independently of low RBP4 adipose expression and is normalized by transplantation in nonobese, nondiabetic patients with chronic kidney disease. Clin Endocrinol (Oxf) 2011;75:56–63. [DOI] [PubMed] [Google Scholar]
- 73.Espe K, Galler A, Raila J, Kiess W, Schweigert FJ. High-normal C-reactive protein levels do not affect the vitamin A transport complex in serum of children and adolescents with type 1 diabetes. Pediatr Res 2007;62:741–5. [DOI] [PubMed] [Google Scholar]
- 74.Broch M, Gómez JM, Auguet MT, Vilarrasa N, Pastor R, Elio I, Olona M, García-España A, Richart C. Association of retinol-binding protein-4 (RBP4) with lipid parameters in obese women. Obes Surg 2010;20:1258–64. [DOI] [PubMed] [Google Scholar]
- 75.Wallenius V, Elias E, Bergstrom G, Zetterberg H, Behre CJ. The lipocalins retinol-binding protein-4, lipocalin-2 and lipocalin-type prostaglandin D2-synthase correlate with markers of inflammatory activity, alcohol intake and blood lipids, but not with insulin sensitivity in metabolically healthy 58-year-old Swedish men. Exp Clin Endocrinol Diabetes 2011;119:75–80. [DOI] [PubMed] [Google Scholar]
- 76.Watanabe T, Shibata M, Nishiyama H, Soeda S, Furukawa S, Gonda K, Takenoshita S, Fujimori K. Serum levels of rapid turnover proteins are decreased and related to systemic inflammation in patients with ovarian cancer. Oncol Lett 2014;7:373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Erikstrup C, Mortensen OH, Nielsen A, Fischer C, Plomgaard P, Petersen A, Krogh‐Madsen R, Lindegaard B, Erhardt J, Ullum H. RBP‐to‐retinol ratio, but not total RBP, is elevated in patients with type 2 diabetes. Diabetes Obes Metab 2009;11:204–12. [DOI] [PubMed] [Google Scholar]
- 78.Shea J, Randell E, Vasdev S, Wang PP, Roebothan B, Sun G. Serum retinol-binding protein 4 concentrations in response to short-term overfeeding in normal-weight, overweight, and obese men. Am J Clin Nutr 2007;86:1310–5. [DOI] [PubMed] [Google Scholar]
- 79.Nakajima H, Nakajima K, Tarutani M, Sano S. Clear association between serum levels of adipokines and T‐helper 17‐related cytokines in patients with psoriasis. Clin Exp Dermatol 2013;38:66–70. [DOI] [PubMed] [Google Scholar]
- 80.Broch M, Ramirez R, Auguet M, Alcaide M, Aguilar C, Garcia-Espana A, Richart C. Macrophages are novel sites of expression and regulation of retinol binding protein-4 (RBP4). Physiol Res 2010;59:299–303. [DOI] [PubMed] [Google Scholar]
- 81.Sell H, Eckel J. Regulation of retinol binding protein 4 production in primary human adipocytes by adiponectin, troglitazone and TNF-α. Diabetologia 2007;50:2221–3. [DOI] [PubMed] [Google Scholar]
- 82.de Pee S, Dary O. Biochemical indicators of vitamin A deficiency: serum retinol and serum retinol binding protein. J Nutr 2002;132: Suppl:2895S–901S. [DOI] [PubMed] [Google Scholar]
- 83.Lespine A, Periquet B, Jaconi S, Alexandre M, Garcia J, Ghisolfi J, Thouvenot J, Siegenthaler G. Decreases in retinol and retinol-binding protein during total parenteral nutrition in rats are not due to a vitamin A deficiency. J Lipid Res 1996;37:2492–501. [PubMed] [Google Scholar]
- 84.Smith FR, Goodman DS. The effects of diseases of the liver, thyroid, and kidneys on the transport of vitamin A in human plasma. J Clin Invest 1971;50:2426–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wagnerberger S, Schäfer C, Bode C, Parlesak A. Saturation of retinol-binding protein correlates closely to the severity of alcohol-induced liver disease. Alcohol 2006;38:37–43. [DOI] [PubMed] [Google Scholar]
- 86.Christou GA, Tselepis A, Kiortsis D. The metabolic role of retinol binding protein 4: an update. Horm Metab Res 2012;44:6–14. [DOI] [PubMed] [Google Scholar]
- 87.Kotnik P, Fischer-Posovszky P, Wabitsch M. RBP4: a controversial adipokine. Eur J Endocrinol 2011;165:703–11. [DOI] [PubMed] [Google Scholar]
- 88.Hermsdorff HHM, Zulet MN, Puchau B, Bressan J, Martínez JA. Association of retinol-binding protein-4 with dietary selenium intake and other lifestyle features in young healthy women. Nutrition 2009;25:392–9. [DOI] [PubMed] [Google Scholar]
- 89.Berry DC, Croniger CM, Ghyselinck NB, Noy N. Transthyretin blocks retinol uptake and cell signaling by the holo-retinol-binding protein receptor STRA6. Mol Cell Biol 2012;32:3851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Muto Y, Smith JE, Milch PO, Goodman DS. Regulation of retinol-binding protein metabolism by vitamin A status in the rat. J Biol Chem 1972;247:2542–50. [PubMed] [Google Scholar]
- 91.Aigner E, Bachofner N, Klein K, De Geyter C, Hohla F, Patsch W, Datz C. Retinol-binding protein 4 in polycystic ovary syndrome—association with steroid hormones and response to pioglitazone treatment. J Clin Endocrinol Metab 2009;94:1229–35. [DOI] [PubMed] [Google Scholar]
- 92.Mills JP, Furr HC, Tanumihardjo SA. Retinol to retinol-binding protein (RBP) is low in obese adults due to elevated apo-RBP. Exp Biol Med (Maywood) 2008;233:1255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kiernan UA, Phillips DA, Trenchevska O, Nedelkov D. Quantitative mass spectrometry evaluation of human retinol binding protein 4 and related variants. PLoS One 2011;6:e17282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Graham TE, Wason CJ, Blüher M, Kahn BB. Shortcomings in methodology complicate measurements of serum retinol binding protein (RBP4) in insulin-resistant human subjects. Diabetologia 2007;50:814–23. [DOI] [PubMed] [Google Scholar]
- 95.Yang Q, Eskurza I, Kiernan UA, Phillips DA, Blüher M, Graham TE, Kahn BB. Quantitative measurement of full-length and C-terminal proteolyzed RBP4 in serum of normal and insulin-resistant humans using a novel mass spectrometry immunoassay. Endocrinology 2012;153:1519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Munkhtulga L, Nakayama K, Utsumi N, Yanagisawa Y, Gotoh T, Omi T, Kumada M, Erdenebulgan B, Zolzaya K, Lkhagvasuren T. Identification of a regulatory SNP in the retinol binding protein 4 gene associated with type 2 diabetes in Mongolia. Hum Genet 2007;120:879–88. [DOI] [PubMed] [Google Scholar]
- 97.Mercader J, Palou A, Bonet ML. Resveratrol enhances fatty acid oxidation capacity and reduces resistin and retinol-binding protein 4 expression in white adipocytes. J Nutr Biochem 2011;22:828–34. [DOI] [PubMed] [Google Scholar]
- 98.Robich MP, Osipov RM, Chu LM, Han Y, Feng J, Nezafat R, Clements RT, Manning WJ, Sellke FW. Resveratrol modifies risk factors for coronary artery disease in swine with metabolic syndrome and myocardial ischemia. Eur J Pharmacol 2011;664:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sasaki R, Nishimura N, Hoshino H, Isa Y, Kadowaki M, Ichi T, Tanaka A, Nishiumi S, Fukuda I, Ashida H. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochem Pharmacol 2007;74:1619–27. [DOI] [PubMed] [Google Scholar]
- 100.Canas JA, Damaso L, Altomare A, Killen K, Hossain J, Balagopal PB. Insulin resistance and adiposity in relation to serum β-carotene levels. J Pediatr 2012;161(1):58–64, e2. [DOI] [PubMed] [Google Scholar]
- 101.Qin B, Polansky MM, Harry D, Anderson RA. Green tea polyphenols improve cardiac muscle mRNA and protein levels of signal pathways related to insulin and lipid metabolism and inflammation in insulin‐resistant rats. Mol Nutr Food Res 2010;54: Suppl 1:S14–23. [DOI] [PubMed] [Google Scholar]
- 102.Neyestani TR, Nikooyeh B, Alavi-Majd H, Shariatzadeh N, Kalayi A, Tayebinejad N, Heravifard S, Salekzamani S, Zahedirad M. Improvement of vitamin D status via daily intake of fortified yogurt drink either with or without extra calcium ameliorates systemic inflammatory biomarkers, including adipokines, in subjects with type 2 diabetes. J Clin Endocrinol Metab 2012;97:2005–11. [DOI] [PubMed] [Google Scholar]
- 103.Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME. Impaired retinal function and vitamin A availability in mice lacking retinol‐binding protein. EMBO J 1999;18:4633–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Quadro L, Hamberger L, Gottesman ME, Wang F, Colantuoni V, Blaner WS, Mendelsohn CL. Pathways of vitamin A delivery to the embryo: insights from a new tunable model of embryonic vitamin A deficiency. Endocrinology 2005;146:4479–90. [DOI] [PubMed] [Google Scholar]
- 105.Krasinski SD, Cohn JS, Russell RM, Schaefer EJ. Postprandial plasma vitamin A metabolism in humans: a reassessment of the use of plasma retinyl esters as markers for intestinally derived chylomicrons and their remnants. Metabolism 1990;39:357–65. [DOI] [PubMed] [Google Scholar]
- 106.D’Ambrosio DN, Clugston RD, Blaner WS. Vitamin A metabolism: an update. Nutrients 2011;3:63–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim Y-K, Wassef L, Hamberger L, Piantedosi R, Palczewski K, Blaner WS, Quadro L. Retinyl ester formation by lecithin: retinol acyltransferase is a key regulator of retinoid homeostasis in mouse embryogenesis. J Biol Chem 2008;283:5611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Delport R, Bornman R, MacIntyre UE, Oosthuizen NM, Becker PJ, Aneck-Hahn NH, De Jager C. Changes in retinol-binding protein concentrations and thyroid homeostasis with nonoccupational exposure to DDT. Environ Health Perspect 2011;119:647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van Hoek M, Dehghan A, Zillikens M, Hofman A, Witteman J, Sijbrands E. An RBP4 promoter polymorphism increases risk of type 2 diabetes. Diabetologia 2008;51:1423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Schleicher E, Fritsche A, Häring H-U. High circulating retinol-binding protein 4 is associated with elevated liver fat but not with total, subcutaneous, visceral, or intramyocellular fat in humans. Diabetes Care 2007;30:1173–8. [DOI] [PubMed] [Google Scholar]
- 111.Volek JS, Phinney SD, Forsythe CE, Quann EE, Wood RJ, Puglisi MJ, Kraemer WJ, Bibus DM, Fernandez ML, Feinman RD. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids 2009;44:297–309. [DOI] [PubMed] [Google Scholar]
- 112.Christou GA, Tellis CC, Elisaf MS, Tselepis AD, Kiortsis DN. The changes in plasma retinol-binding protein 4 levels are associated with those of the apolipoprotein B-containing lipoproteins during dietary and drug treatment. Angiology 2012;67. [DOI] [PubMed] [Google Scholar]
- 113.Chan DC, Watts G, Ng T, Yamashita S, Barrett P. Effect of weight loss on markers of triglyceride‐rich lipoprotein metabolism in the metabolic syndrome. Eur J Clin Invest 2008;38:743–51. [DOI] [PubMed] [Google Scholar]
- 114.Ng TW, Watts GF, Barrett PHR, Rye K-A, Chan DC. Effect of weight loss on LDL and HDL kinetics in the metabolic syndrome associations with changes in plasma retinol-binding protein-4 and adiponectin levels. Diabetes Care 2007;30:2945–50. [DOI] [PubMed] [Google Scholar]
- 115.Mitterberger MC, Mattesich M, Klaver E, Lechner S, Engelhardt T, Larcher L, Pierer G, Piza-Katzer H, Zwerschke W. Adipokine profile and insulin sensitivity in formerly obese women subjected to bariatric surgery or diet-induced long-term caloric restriction. J Gerontol A Biol Sci Med Sci 2010;65:915–23. [DOI] [PubMed] [Google Scholar]
- 116.Hermsdorff HHM, Zulet MÁ, Abete I, Martínez JA. Discriminated benefits of a Mediterranean dietary pattern within a hypocaloric diet program on plasma RBP4 concentrations and other inflammatory markers in obese subjects. Endocrine 2009;36:445–51. [DOI] [PubMed] [Google Scholar]
- 117.Mohapatra J, Sharma M, Acharya A, Pandya G, Chatterjee A, Balaraman R, Jain MR. Retinol‐binding protein 4: a possible role in cardiovascular complications. Br J Pharmacol 2011;164:1939–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Praticò D. 2-Isoprostanes: sensitive and specific non-invasive indices of lipid peroxidation in vivo. Atherosclerosis 1999;147:1–10. [DOI] [PubMed] [Google Scholar]