Abstract
Links between short sleep duration and obesity, type 2 diabetes, hypertension, and cardiovascular disease may be mediated through changes in dietary intake. This review provides an overview of recent epidemiologic studies on the relations between habitual short sleep duration and dietary intake in adults from 16 cross-sectional studies. The studies have observed consistent associations between short sleep duration and higher total energy intake and higher total fat intake, and limited evidence for lower fruit intake, and lower quality diets. Evidence also suggests that short sleepers may have irregular eating behavior deviating from the traditional 3 meals/d to fewer main meals and more frequent, smaller, energy-dense, and highly palatable snacks at night. Although the impact of short sleep duration on dietary intake tends to be small, if chronic, it may contribute to an increased risk of obesity and related chronic disease. Mechanisms mediating the associations between sleep duration and dietary intake are likely to be multifactorial and include differences in the appetite-related hormones leptin and ghrelin, hedonic pathways, extended hours for intake, and altered time of intake. Taking into account these epidemiologic relations and the evidence for causal relations between sleep loss and metabolism and cardiovascular function, health promotion strategies should emphasize improved sleep as an additional factor in health and weight management. Moreover, future sleep interventions in controlled studies and sleep extension trials in chronic short sleepers are imperative for establishing whether there is a causal relation between short sleep duration and changes in dietary intake.
Keywords: short sleep duration, dietary intake, obesity, sleep, BMI, CLOCK, time
Introduction
A sleep duration of 7–9 h for adults (26–64 y of age) and 7–8 h for older adults (≥65 y of age) is necessary for optimal health, whereas a duration of <6 h for adults and 5–6 h for older adults is insufficient, according to the National Sleep Foundation (1). The average self-reported sleep duration in adults deviates from recommended sleep duration (2). Although only 12% of US adults had reported <6 h of sleep in 1998, the prevalence of short sleep duration has steadily increased (3). The NHANES estimated that, during 2007–2010, up to 37.3% of US adults (≥20 y of age) reported sleeping 6 h or less, whereas only 60.4% reported sleeping 7–9 h (4). Consequently, short sleep duration has been an area of increasing public health concern (5, 6).
The relations between short sleep duration and anthropometric measures have been extensively investigated in observational studies and reviewed in Advances in Nutrition (7). Cross-sectional studies have found a significant association between short sleep duration and higher prevalence of weight gain or obesity in both children and adults (8–10), with a higher waist circumference (11) and percentage of body fat (12). Consistent with that, a recent meta-analysis of prospective studies observed that short sleep duration (≤6 h) was associated with a 45% increased risk of obesity compared with normal sleep duration (13).
Short sleep duration has also been investigated in the context of chronic diseases. Self-reported short sleep has been associated with hypertension (14), type 2 diabetes (15), cardiovascular disease (16), and all-cause mortality (17) independent of weight status, possibly as a result of its altering several metabolic markers that predispose individuals to cardiometabolic diseases (18, 19). Cardiometabolic markers, including higher systolic and diastolic blood pressure (21), inflammation (22), impaired glucose tolerance (15), and higher serum TGs and lower serum HDL cholesterol (23) concentrations, have also been associated with short sleep (20). Because cardiometabolic conditions have strong nutritional determinants, links between short sleep and metabolic disruption may be mediated through changes in dietary intake (24).
The link between sleep and diet was formally tested by Hicks et al. (25) in 1986 and thereafter by Lucero et al. (26) in 1990. This review provides an overview of recent epidemiologic studies on the relations between habitual short sleep duration and dietary intake in adults and explores potential mechanisms mediating these associations. Conclusions drawn from this overview may help us better understand the complex relation between sleep and obesity and other cardiometabolic traits, and—along with evidence from experimental studies—develop lifestyle recommendations for the prevention of obesity and related chronic disease.
Relations between Sleep Duration and Diet
Experimental evidence
Short-term, acute sleep restriction studies with experimental study design have been conducted to assess the impact of short sleep duration on health and dietary intake by accurately monitoring energy intake in adults and also allowing testing of causality and mechanism. Brondel et al. (27) observed increased total energy intake (∼22%) in men after 1 night of 4 h sleep restriction. Although increases in total calories have also been observed by others (28–31), this observation has not been consistent (32, 33), and instead, differences may appear in the intake of specific nutrients, including a higher intake of total fat (27, 29, 30, 34), SFAs (30), snacks (35), and carbohydrate-rich snacks—particularly between the hours of 1900 and 0700 (32); postdinner snacks rich in carbohydrates, proteins, and fiber (31); and dessert (29); as well as a lower intake of carbohydrates (34) and differences in the timings of intake (29, 36).
If the observed dietary changes after acute sleep restriction, particularly increases in total energy, total fat, and SFA intake and energy-dense late night snacks, are sustained over the long-term and are not met by an equivalent increase in energy expenditure, they may explain the associations between short sleep and obesity and chronic diseases. Because the controlled settings of clinical trials investigating small and selected subsets of adults offer limited generalizability, examining habitual sleep loss outside the laboratory and in larger cohorts is necessary to understand the risk of chronic diseases, including obesity, in real life environments (37, 38).
Epidemiologic evidence
We conducted a comprehensive PubMed literature search for publications on habitual short sleep duration (assessed subjectively or objectively) and dietary intake (total energy or macronutrients) in adults. We also crossreferenced the citations from recovered studies. Cohort studies in children and micronutrients were excluded. We identified 16 published studies investigating the relations between sleep duration and diet derived from cross-sectional studies, which have generally been conducted in national surveys and population-based observational studies (Table 1 and Table 2).
TABLE 1.
Cross-sectional studies on sleep duration and dietary intake1
| Author, year (reference) | n | Region | Age, y | F | BMI, kg/m2 | Total energy intake, kcal | Sleep duration,2 h | Key observations |
| Tu, 2012 (39) | 68,832 | China | 59.6 ± 9.0 | 100 | 24.0 ± 3.4 | 1601 ± 386 | 11.5% | Short sleep duration was associated with lower total energy, tea, and fruit intake (P < 0.05). |
| Ohida, 2001 (40) | 31,260 | Japan | ≥20 | 52 | NA | NA | 28% | Short sleep duration was associated with increased odds for irregular eating behavior, unbalanced food variety, and trying to eat less (all P < 0.05). |
| Kim, 2011 (41) | 27,983 | United States | 54.2 ± 9.0 | 100 | 27.2 ± 5.9 | 1547 ± 549 | 26.7 | Eating during conventional hours decreased, whereas snack eating (associated with higher intake of fats and sweets and lower intake of fruits and vegetables) increased with decreasing sleep duration from 7 to 8 h of sleep. |
| Kant, 2014 (42) | 15,199 | United States (NHANES) | 28.9 ± 12.1 | 50 | 33.1 ± 1.4 | NA | 39.4 | Short sleepers reported a slightly lower percentage of energy from protein (P = 0.007), and higher total sugar (P = 0.04). Total number of eating episodes and energy intake were not related to sleep duration. However, short sleepers reported lowest breakfast and dinner consumption (P < 0.04), a higher percentage of ≥50% of energy from snacks (P = 0.002), and lower percentage of energy from main meals (P < 0.0004). Whereas the number of snack episodes did not differ across sleep duration groups, percentage of total energy from all snacks reported at or after 2000 was higher and more frequent after dinner (P = 0.03), with no difference in the percentage of energy from snacks. |
| Dashti, 2015 (43) | 14,906 | United States and Europe | 53.9 ± 13.6 | 53 | 26.9 ± 4.7 | 2168 ± 831 | 7.4 ± 1.1 | Associations between sleep duration and relative macronutrient intake were evident in age- and sex-stratified analyses only. A significant association was observed between sleep duration and lower SFA intake (men: −0.11%; women: −0.10%) in younger adults (20–64 y of age), and lower carbohydrate (−0.31%), higher total fat (0.18%), and higher PUFA (0.05%) intake in older women (aged 65–80 y) (all P < 0.05). |
| Grandner, 2013 (37) | 5587 | United States (NHANES) | 46.3 ± 16.5 | 53 | 28.7 ± 6.8 | 2146 ± 1026 | 39.9 | Very short sleepers (<5 h) had the lowest total energy intake (2036 kcal), whereas short sleepers (5–6 h) had the highest (2201 kcal). Very short sleepers also had a lower intake of protein, carbohydrates, sugars, dietary fiber, and total fat than did normal sleepers (7–8 h), whereas short sleepers had a higher intake of protein, carbohydrates, sugars, and total fat, but a lower intake of dietary fiber, than normal sleepers had (all P < 0.05). Food variety was the least in very short sleepers and low in short sleepers compared with normal sleepers. |
| Shi, 2008 (44) | 2828 | China | 47.0 ± 14.5 | 54 | 23.5 ± 3.5 | 2351 ± 47 | 11.6 | Short sleepers had a 1.5% higher fat intake (P < 0.001) and a 1.8% lower carbohydrate intake (P < 0.001) per day compared with normal sleepers. |
| Imaki, 2002 (45) | 2000 | Japan | 20–59 | 0 | NA | NA | 16.0 | Short-duration sleepers consumed insufficient amounts of vegetables, ate their meals at irregular frequencies, snacked between meals, ate out, had irregular eating habits, and preferred very salty food (all P < 0.05). |
| Stamatakis, 2008 (46) | 1125 | Midwestern United States | 54.2 ± NA | 77 | 29.7 | NA | 36.2 | Short sleep duration was associated with lower fruit and vegetable intake, a high-fat diet, and often eating fast food. Associations were stronger among nonobese individuals, especially for the association between short sleep duration and a high-fat diet (all P < 0.05). |
| Stern, 2014 (47) | 769 | United States | 63.03 | 100 | 25.93 | 20213 | 33.7 | Short sleepers had a 1.0% higher total energy intake (P = 0.01) and lower diet quality (P = 0.04) as assessed by the AHEI-2005 compared with normal (7 h) sleepers. Sleep quality also was associated with intake. Average-sleep-quality sleepers consumed a lower percentage of calories from carbohydrates (P = 0.015) and those having restless or very restless sleep consumed a higher percentage of calories from fat (P = 0.016) than did those who reported sound or very sound sleep. |
| Grandner, 2010 (48) | 459 | United States | 68.0 ± 7.8 | 100 | NA | NA | NA | Actigraphic sleep duration correlated negatively with total energy intake, as well as intake of total fat, MUFAs, trans FAs, SFAs, PUFAs, cholesterol, γ-tocopherol and α-tocopherol equivalent (all P < 0.004). Self-reported sleep duration was not related to dietary intake. |
| Haghighatdoost, 2012 (49) | 410 | Iran | 20.7 ± 1.6 | 100 | 21.6 ± 2.8 | 2286 ± 765 | 35.1 | Short-duration sleepers had the highest energy intake (2406 kcal; normal sleepers, 2092 kcal) and percentage energy from carbohydrates; lowest intake of percentage energy from protein, fiber, fruits, whole grains, and beans (all P < 0.05); and lowest value of HEI and DDS, as well as diversity scores for fruits, vegetables, and whole grains (P < 0.05). |
| Patterson, 2014 (50) | 223 | United States | 38 ± 12 | 65 | 24 | 2450 ± NA | 35.4 | Sleep duration was inversely associated with energy intake in unadjusted models only. |
| Galli, 2012 (51) | 118 | United States | 40.3 ± 6.7 | 77 | 38.7 ± 6.4 | 2279 ± 689 | 6.0 ± 0.8 | Sleep duration and energy intake were inversely related (r = −0.23; P = 0.015). Severity of sleep apnea (as indicated by the respiratory disturbance index) was associated with a shift in macronutrient composition, with 0.17% lower carbohydrate intake (P = 0.03) and 0.13% higher fat intake (P = 0.05) per additional increase in index. |
| Santana, 2012 (52) | 58 | Brazil | 66.2 ± 4.1 | 66 | 33.8 ± 2.6 | 1281 ± 430 | 6.6 ± 1.6 | Sleep duration correlated negatively with protein (r = −0.43; P = 0.02), MUFA (r = −0.40; P = 0.03), and dietary cholesterol (r = −0.50; P 0.01) intake in obese men. |
| Rontoyanni, 2007 (12) | 30 | Greece | 46.5 ± 9.1 | 100 | 25.7 ± 3.38 | 1640 ± 438 | 6.7 ± 0.7 | A weak positive correlation was observed between sleep duration and SFA intake (r = 0.392; P < 0.05) only. |
Values for age and total energy intake categories are means ± SDs, values for female category are percentages, values for BMI category are means ± SDs or percentage obese, and values for sleep duration are mean h ± SD or percentage short sleep duration, except where otherwise indicated. Dietary assessment was measured via a validated FFQ (39, 43, 47, 49), a semiquantitative FFQ (48), a block FFQ (41), a 24 h dietary recall (37, 42, 52), a 3 d food record (44, 51), objectively measured doubly labeled water (50), or a lifestyle questionnaire (40, 45, 46). Statistical models used included univariate (ANOVA) (49), bivariate [chi-square test (45) or Pearson correlation (12, 52)], multivariate [linear/logistic regression model (37, 39, 40, 42–44, 46, 47, 50, 51) or partial correlation (48)], or principal component (41) analyses. Adjustments in multivariate analyses included demographic, socioeconomic, and health factors (age, sex, income, education, BMI, and physical activity), as well as dietary pattern (37); age, sex, race, and BMI (51); age, race/ethnicity, total body fat mass, physical activity, income, education level, smoking status, alcohol intake, comorbidity, and leptin (47); age, sex, race/ethnicity, education level and weight (50); education level, occupational status, history of night-shift work, family income, menopausal status, marital status, and number of live births (39); age, race/ethnicity, family income, education level, employment status, smoking status, alcohol use, any self-reported current chronic disease condition, BMI, day of recalled intake, and month of examination (42); age, sociodemographic characteristics, physical and mental health status, and other sleep-related characteristics (46); age, sex, and BMI (43); or age, sex, income, education, residence, occupation, smoking status and alcohol use (44). AHEI-2005, Alternative Healthy Eating Index–2005; DDS, Dietary Diversity Score; HEI, Healthy Eating Index; NA, not available.
Assessed via self-report, i.e., “How many hours of sleep did you get?” (12, 37, 39-47, 49, 50); a 7 d sleep diary (12); or objectively via 7 d actigraphy (48, 51). Short sleep duration is defined as <6 h (39, 40, 45, 49), ≤6 h (37, 42, 47, 50), or <7 h (41, 44, 46).
Values are medians.
TABLE 2.
Summary of relations (references) between short sleep duration and dietary intake1
| Intake | Higher | Lower |
| Total energy intake, kcal | (37, 47–51) | (39) |
| Absolute intake of nutrients, g | ||
| Total fat | (37, 48) | |
| MUFAs | (48, 52) | |
| PUFAs | (48) | |
| SFAs | (48) | (12) |
| Trans fats | (48) | |
| Cholesterol | (48, 52) | |
| Total protein | (37, 52) | |
| Total carbohydrates | (37) | |
| Sugars | (37, 42) | |
| Dietary fiber | (37, 49) | |
| Relative intake of nutrients, % energy | ||
| Total fat | (43, 44) | |
| PUFAs | (43) | |
| SFAs | (43) | |
| Total protein | (42, 49) | |
| Total carbohydrates | (43, 49) | (44) |
| Foods, servings | ||
| Fruits | (39, 46, 49) | |
| Vegetables | (45, 46) | |
| Tea | (39) | |
| Whole grains | (49) | |
| Beans | (49) | |
| Diet quality2 | (47, 49) | |
| Dietary behavior | ||
| Irregular eating3 | (40, 45) | |
| Food variety | (37, 40) | |
| Trying to eat less | (40) | |
| Irregular meal times | (45) | |
| Main meals | (42, 41) | |
| Snacking | (41, 42, 45) | |
| Eating out | (45) |
Sleep duration was assessed via self-report, i.e., “How many hours of sleep did you get?” (12, 37, 39-47, 49, 50), a 7 d sleep diary (12), or objectively via 7 d actigraphy (48, 51). Short sleep duration is defined as <6 h (39, 40, 45, 49), ≤6 h (37, 42, 47, 50), or <7 h (41, 44, 46). Dietary assessment was measured via a validated FFQ (39, 43, 47, 49), a semiquantitative FFQ (48), a block FFQ (41), a 24 h dietary recall (37, 42, 52), a 3 d food record (44, 51), objectively measured doubly labeled water (50), or a lifestyle questionnaire (40, 45, 46).
Based on Healthy Eating Index or Alternative Healthy Eating Index-2005.
Defined as not eating 3 times at determined times.
Total energy intake.
Because energy intake is a major contributor to higher BMI, numerous cross-sectional analyses of observational studies examined the association between sleep duration and total energy intake. Consistent with the findings of experimental sleep restriction studies, observational studies of habitual sleep duration and dietary intake have generally found an association between short sleep and higher total calories (37, 47–51). Stern et al. (47) reported a 1% higher energy intake in postmenopausal women in the Women Health Initiative (WHI)3 study who self-reported sleep duration ≤6 h compared with normal sleep duration (7 h). A subset of the WHI participants with 7 d wrist actigraphic sleep recordings further support this relation, indicating a significant negative correlation (r = −0.162) between objectively estimated sleep duration and total energy intake (48). This negative correlation appears to be of a larger magnitude (r = −0.23) in a population of obese adults with prevalent sleep apnea (51). In NHANES participants, Grandner et al. (37) found that short sleepers (5–6 h) reported the highest total energy intake (2201 kcal), whereas despite having the highest BMIs, very short sleepers (<5 h) reported the lowest intake (2036 kcal)—possibly because of the underreporting of intake—relative to normal sleepers (7–8 h; 2151 kcal). Finally, studies that used objective measures of energy intake further support an inverse link between sleep and energy intake, indicating that adults reporting ≤6 h of sleep consumed 178 kcal/d more than those reporting ≥9 h (50). Inconsistent findings from other studies (12, 39) might be due to differences in the prevalence of short sleep duration in the respective populations.
In summary, evidence from epidemiologic studies is consistent with that from experimental studies, suggesting that sleep loss promotes increases in daily caloric intake. Although some studies also observe modest increases in energy expenditure in short-duration sleepers (18, 31, 50), as a result of extended hours of wakefulness, this appears to be often overridden by considerably higher increases in intake, emphasizing the importance of alterations in intake.
Nutrients, dietary composition, and foods.
Thus far, epidemiologic studies examining sleep duration in relation to diet are widely variable in their focus and findings. These cross-sectional investigations assess the impact of sleep duration on either the absolute (37, 42, 48, 52) or relative (42–44, 49) intake of nutrients or foods (39, 45, 46, 53).
Starting with nutrients, in the NHANES, Grandner et al. (37) found that short sleepers (5–6 h) reported higher absolute protein, carbohydrate, sugar, and total fat intake, but lower intake of dietary fiber than did normal sleepers (7–8 h), whereas very short sleepers (<5 h) reported lower intake of protein, carbohydrates, sugars, dietary fiber, and total fats than did normal sleepers. In a subset of postmenopausal women (n = 459) in the WHI study with actigraphic sleep duration, Grandner et al. (48) identified negative correlations between actigraphic sleep duration and absolute intake of total fat, MUFAs, PUFAs, SFAs, trans FAs, and cholesterol, whereas self-reported sleep duration from the same WHI participants did not correlate significantly with dietary nutrients. General disagreements between subjective and actigraphic sleep measures may contribute to the observed differences in the correlations between sleep duration and dietary intake (54). A study of elderly obese patients also reported negative correlations between sleep duration and MUFA and dietary cholesterol intake among men only, as well as a negative correlation with total protein intake (52). In contrast, a small study in Greek women reported a weak positive correlation between sleep duration and SFAs (12). Finally, Kant et al. (42) identified in a recent NHANES analysis including up to 15,199 adults that short sleepers (≤6 h) reported higher total sugar intake. In summary, although results from studies assessing the relation between absolute nutrient intake and sleep duration are inconsistent, current evidence suggests a trend toward higher absolute intake of fat in short sleep duration.
Because changes in dietary composition independent of total intake increase obesity risk (55), other investigations have assessed whether sleep duration influences dietary composition. Accordingly, studies have adjusted for total energy intake or expressed nutrients as percentages of total energy intake in their analyses, thus controlling for variation in total calories consumed (42–44, 49). In a recent meta-analysis including up to 14,906 participants of European descent from 9 US and European cohort studies of the Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium, we observed associations between sleep duration and macronutrient composition in age- and sex-stratified groups (43). We identified a significant association between shorter sleep duration and higher relative SFA intake in younger (20–64 y of age) men and women, and with higher relative carbohydrate, lower relative total fat, and lower relative PUFA intake in older (65–80 y of age) women. An analysis of 2828 Chinese adults residing in Jiangsu province observed that individuals with a self-reported short sleep duration of <7 h had 1.8% lower carbohydrate and 1.5% higher fat intake/d than did normal duration sleepers (7–9 h) (44). Kant et al. (42) also observed in the NHANES that both short and long sleepers reported a lower percentage of energy from protein than did normal-duration sleepers. Finally, a study of younger adult women found that those with a self-reported short sleep duration (<6 h) had the highest percentage energy intake from carbohydrates and lowest energy intake from protein relative to those with normal sleep duration (6–8 h) (49).
Collectively, these studies suggest that short sleepers may consume diets of higher fat and lower protein composition; however, results for carbohydrate composition remain inconsistent.
Other studies have focused primarily on the effects of sleep loss on food intake. Reports from a large study of 68,832 Chinese women in the Shanghai Women’s Health Study identified associations between self-reported short sleep duration (<6 h) and lower tea and fruit intake (39). Meanwhile, an investigation of 2000 Japanese factory workers observed that short sleepers (<6 h) reported consuming fewer vegetables than did normal sleepers (6–9 h), as assessed with the use of a dietary habits questionnaire (45). Similar results were found in a cohort of 1125 US adults residing in the Midwest, which reported an association between short sleep duration (<7 h) and 75% increased odds of low (1–2 servings/d) fruit and vegetable consumption (46). A cross-sectional analysis of 410 young women further support the associations between short sleep duration (<6 h) and a lower intake of fruits in addition to lower dietary fiber, whole grains, and beans (49). In summary, results from a few studies investigating the link between sleep duration and foods support the relation between short sleep duration and lower intakes of healthy foods; however, these investigations remain sparse.
Diet quality.
Two cross-sectional studies have examined the relation between sleep duration and diet quality (based on validated healthy eating indexes). In postmenopausal women participating in the WHI study, Stern et al. (47) observed that women sleeping ≤6 h had diets of lower quality than women sleeping 7 h, according to their scores from the Alternative Healthy Eating Index–2005, which assessed diet quality based on foods and nutrients related to chronic disease risk. Similarly, Haghighatdoost et al. (49) reported in an analysis of young women that short sleep duration of <6 h was significantly associated with lower diet quality according to their Healthy Eating Index scores tabulated from 10 various dietary components. Further investigation in men is necessary to determine the consistency of the identified relation between short sleep duration and lower diet quality. The link between short sleep duration and lower diet quality may also result from suboptimal nutrition resulting from food insecurity (56, 57), with studies from NHANES having shown that food insecurity is also associated with short sleep duration (58).
Dietary behavior.
Dietary behavior (i.e., all food-related behaviors independent of intake) also appears to be related to sleep duration, based on cross-sectional studies. In a large investigation of over 30,000 Japanese individuals, Ohida et al. (40) observed that sleep loss was associated with increased odds of self-reported irregular eating behavior (defined as not eating 3 times at determined times), unbalanced food variety, and trying to eat less. This is supported by another study of Japanese factory workers that observed that self-reported short sleep duration was associated with irregular meal times, snacking between meals, eating out, and other self-reported irregular eating habits (45).
The shift from meals to snacks with short sleep was first observed in a study of college students in 1986 (25), and later supported by recent studies of adolescents (59) and adults (41). Results from principal component analysis indicated that conventional eating (i.e., eating during conventional eating hours from breakfast to dinner) decreased with short sleep, whereas snacking or irregular eating (i.e., dominance of snacks over meals) increased with short sleep (41). Likewise in the NHANES, Kant et al. (42) observed that although the total number of eating episodes and snacking was not related to sleep duration, short sleepers reported less breakfast and dinner consumption, and more frequent snacking at or after 2000, specifically, after dinner. Not surprisingly, food variety in short (5–6 h) and very short (<5 h) sleepers also tends to be lower than in normal sleepers (7–8 h) (37).
In summary, these studies suggest that short sleepers tend to deviate from traditional and daytime eating to unconventional and irregular nighttime eating.
Mechanisms Relating Sleep Duration and Diet
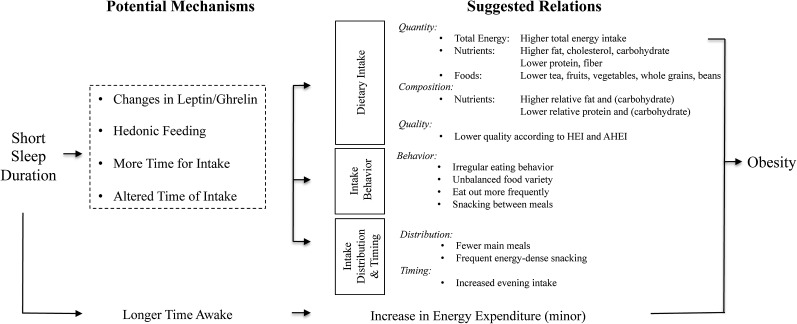
The impact of sleep on dietary intake is likely to be multifactorial, and numerous mechanisms have been proposed and examined (Figure 1) (57, 60–65).
FIGURE 1.
Schematic diagram of the potential dietary and nondietary pathways leading from short sleep duration to obesity. AHEI, Alternative Healthy Eating Index; HEI, Healthy Eating Index.
Leptin and ghrelin (energy homeostatic control)
Among the most investigated mechanisms explaining the link between sleep and diet is a homeostatic control hypothesized to increase hunger during sleep deprivation through changes in the appetite-related hormones leptin, an adipocyte-derived hormone that suppresses appetite (66), and ghrelin, a stomach-derived peptide that stimulates appetite (67). A study by Spiegel et al. (68) showed that 2 consecutive nights of 4 h sleep restriction induced an 18% decrease in leptin and 28% increase in ghrelin; however, these findings have not been consistently reported in sleep restriction experimental studies, and this may be due to inadequate adjustment for total caloric intake, which influences the concentrations of these hormones (28, 33). Meanwhile, some epidemiologic studies observed significantly lower leptin (47, 69) and higher ghrelin concentrations, or both (70), among short sleepers after adjustments for BMI (70) or total body fat mass (47). A shift to increase hunger via these hormones may potentiate an increase in total energy intake in short sleepers, and—if unmet by an equivalent increase in energy expenditure, as observed in experimental studies (31) and suggested by low physical activity levels among short sleepers in epidemiologic studies (46)—would lead to energy imbalance.
Hedonic factor
Emerging hedonic pathways provide an additional potential mechanism by which sleep loss leads to changes in dietary intake (38). St-Onge et al. (71) observed enhanced activity of the brain reward and food-sensitive centers in response to unhealthy food stimuli in 25 normal-weight adults after a 4 h sleep restriction protocol lasting 5 d compared with those getting 9 h of sleep. These neuroimaging experiments suggest that sleep restriction enhances hedonic stimulus processing in the brain and alters brain connectivity, providing greater reward of food with sleep loss (34, 71–73); it may also blunt the activity of appetitive evaluation regions during food craving, affecting food decisions (74). This enhanced reward may mediate the increased intake of total energy and highly palatable, energy-dense foods and snacks in short sleepers.
More time for intake
Short sleep results in extended hours of wakefulness that presents additional opportunities to increase food intake. These hours are typically uncommon for conventional meals (late night and early morning). Therefore, additional eating events typically appear to be in the form of convenient carbohydrate-rich and energy-dense snacks (42), favoring snacks over meals. The snacking behavior observed in short-sleeping children (59) and adults (32) may contribute to higher total energy intake and affect dietary composition and quality, resulting in obesity and other chronic conditions.
Altered time of intake
Emerging evidence suggests that the effect of calories on metabolism and health depends on the timing of intake. Late-time eating, particularly between 2200 and 0500 (after dinner and before breakfast), is common among short sleepers as a result of being awake at altered times (42, 53). Baron et al. (53) observed that calories consumed after 2000 significantly predict higher BMI, independent of age, sleep timing, and sleep duration. Garaulet et al. (75) observed among overweight and obese individuals on a 20 wk weight loss intervention that late eaters are less successful at weight loss than are early eaters, independent of 24 h caloric intake, suggesting that timing of food intake is an independent predictor of weight loss success. Additional investigations provide further evidence for the role of timing of food intake on BMI, weight loss maintenance, glucose tolerance, and metabolic health (76–80), and such timing may be related to differences in hormonal profiles, expression of key metabolic genes, and functionality of organs involved in digestion (81).
Limitations, Research Needs, and Health Implications
Limitations of existing literature on sleep and diet
Several limitations hamper the generalizability and consistency of the identified findings. First, all of the epidemiologic evidence to date is derived from cross-sectional analyses, which cannot inform temporal relations or causal pathways, and it is conceivable that dietary intake has an impact on sleep (82) or that there are shared underlying interindividual differences that affect both sleep and diet in parallel. Furthermore, the studies described include populations of varying age groups, sex, and geographic regions (US, European, and others) with different lifestyle habits, dietary patterns, and cardiometabolic disease risk, which may contribute to inconsistent findings.
Inconsistency in the observed relations between sleep and dietary intake in part may be due to differences in measurement methods for both sleep and diet. Sleep duration was most commonly assessed subjectively through self-report (12, 37, 39–47, 49, 50), sleep logs (53), or a 7 d sleep diary (12), whereas in a few studies, 7 d actigraphy was used instead (48, 51, 53). Although the concordance between self-reported and actigraphic sleep duration tend to be significant, self-reported sleep duration tends to overestimate actigraphic sleep duration (51, 83, 84). It is conceivable that self-reported and actigraphic sleep durations may reflect other sleep measures related to dietary intake (53), and that self-reported sleep also accounts for sleepiness or fatigue as well, which may further influence energy balance (38, 48).
Furthermore, the dietary assessment methodologies also present important limitations. Studies of sleep and diet have used a variety of dietary assessment tools, including FFQs (39, 41, 43, 47–49,), 24 h dietary recall (37, 42, 52, 53), 3 d food records (44, 51), and a 7 d food log (53), whereas others have used less-reliable lifestyle questionnaires with the use of undefined loose cutoffs, such as “infrequent intake” (40, 45, 46). The FFQ methodology is often limited in its ability to assess total energy intake, and it does not usually allow the characterization of daily intake pattern (e.g., timing or frequency of eating occasions and snacking patterns, etc.), whereas the 24 h dietary recall may not provide a good representative measure of typical daily intake. In addition, studies investigating snacking have loosely defined snacking as an eating episode between meals, and the lack of precise definition may result in large variations in the classification of intake. In addition, assessment of sleep and diet in large cohort studies tends not to be done concurrently, possibly introducing random misclassification (40).
Inconsistencies in data analyses are also evident. There is great variability in the cutoffs used to define short sleep duration, and the most common cutoffs are <6 h (39, 40, 45, 49), ≤6 h (37, 42, 47, 50), and <7 h (41, 44, 46), which often represents similar groups, because sleep is often assessed in 1 h increments. Further subdividing short sleepers into short and very short sleepers may also result in conflicting findings among studies (37). Whereas categorizing sleep duration in data analysis may reduce the misclassification resulting from recall bias by grouping individuals with similar perceived sleep duration, collapsing sleep duration into categories may also reduce the power necessary to detect associations. However, linear associations between sleep and dietary intake could impede the detection of nonlinear associations (16, 17, 70). Furthermore, data analysis methodologies vary between univariate (ANOVA) (49), bivariate [chi square test (45), Pearson correlation (12, 52), or t test (53)], multivariate [linear/logistic regression model (37, 39, 40, 42–44, 46, 47, 50, 51) or partial correlation (48)], or principal component (41) analyses. Moreover, adjustments for health status and other potential confounders in multivariate analyses varied and were not consistent across studies. Studies commonly included age, sex, gender/ethnicity, and BMI (37, 42–44, 47, 50, 51), and others further accounted for additional demographics and socioeconomic and health factors (37, 39, 42, 44, 46, 47, 50), possibly leading to contradictory findings.
Evidence gaps and research needs
Investigations of the link between sleep and diet are scarce and several factors should be taken into account moving forward in order to address the large uncertainties still present (Table 3). Greater emphasis should be placed on accurate assessment of sleep duration via polysomnography and habitual diet through multiple food records or dietary recalls and further focusing on the timing and frequency of intake and snacking. Defined cutoffs for short sleep duration, such as those presented by the National Sleep Foundation, should be adopted in order to maintain consistency among studies. However, sleep duration is only one of several sleep variables. Preliminary evidence suggests that insufficient, disruptive, and late sleep is also associated with a higher intake of fat, lower intake of carbohydrates, and lower dietary quality (47, 52, 53, 85, 86), and further investigations are necessary to consider the impact of sleep fragmentation, efficiency, and quality on dietary intake. Because napping in the United States is highly prevalent (∼51%) (3), and objectively measured napping duration has previously been associated with diet (48), napping also requires consideration in future analyses. Furthermore, longitudinal studies of sleep duration and changes in intake are lacking and necessary to examine how change in sleep could affect dietary behavior (87). Seasonal differences in sleep duration have been shown in children (88) and adults (89, 90) in whom objectively measured sleep duration was significantly longer in the fall than in the spring. Whether seasonality needs to be accounted for in future investigations is yet to be determined.
TABLE 3.
Recommendations for future studies investigating the relation between sleep duration and dietary intake
| Recommendations |
| Study design |
| 1) Use an objective assessment of sleep variables, including duration |
| 2) Collect data related to sleep and diet simultaneously |
| 3) Include daytime sleep (i.e., napping) in addition to nighttime (nocturnal) sleep |
| 4) Assess for effect modification by relevant genetic variants |
| 5) Conduct intervention studies and sleep extension clinical trials |
| 6) Conduct longitudinal studies assessing the impact of changes in sleep duration on dietary intake |
| 7) Use an accurate assessment of dietary intake through validated questionnaires or objectively measured intake, taking into account timing of intake, frequency of consumption, and snacking |
| Data analysis |
| 1) Use common cutoffs for defining short sleep duration |
| 2) Test for nonlinear (U-shaped) associations between sleep and investigated outcomes |
| 3) Account for seasonality of data collection and timing of assessment |
| 4) Test for potential effect modifiers, such as age, sex, BMI, race/ethnicity, or disinhibition |
| 5) Account for potential confounders in multivariate analyses |
| 6) Confirm findings from small studies in larger population-based cohort studies and test causality and mechanisms in controlled experimental studies |
The heritability of sleep duration is estimated to be 40% (91), and population-based association studies (92) and recent genome-wide association studies (93) have identified genetic variants associated with habitual sleep duration, including loci flanking the thyroid-specific transcription factor paired box 8 (PAX8) and flanking immediate early response 3 (IER3) and flotillin 1 (FLOT1). Future investigations should assess whether genetic variants associated with sleep duration or known to influence the metabolic mediators linking sleep, metabolism, and obesity, such as CLOCK circadian locomotor output cycles kaput (CLOCK) (94) and tribbles pseudokinase 1 (TRIB1) (95), can modify the links between short sleep duration and dietary intake in gene × environment interaction investigations in order to pinpoint personalized sleep recommendations necessary to achieve healthy intake profiles (43).
Furthermore, studies reporting the association between sleep duration and diet tend to be gender-specific [women (12, 39, 41, 47–49); men (45)] and include a narrow age-group [younger (49, 53); older (39, 41, 47, 48, 52)] by design, whereas those that include both genders identify more associations in sex-specific strata (37, 43). The mechanisms underlying these sex-specific associations are unclear, but could include sex-specific hormonal differences, differences in self-reporting behaviors (96), or differences in sleep duration between older adult men and women (1, 47, 52). Exploration of effect modification by age and gender, as well as BMI and race/ethnicity, in nationally representative samples, is required.
Finally, causal relations cannot be inferred from epidemiologic studies, so it is imperative that we integrate information from epidemiologic studies along with controlled sleep intervention studies and sleep extension trials in chronic short sleepers to establish the causal relation between short sleep and changes in dietary intake. In an experimental sleep restriction study, having healthy adults transition from sleep restriction to adequate sleep opportunity led to reduced food intake, including lower fat and carbohydrate intake, and prevented weight gain (31), whereas sleep extension trials in chronically sleep-deprived (<6.5 h) young adults at risk of obesity observed that sleep extended to 8.5 h for 2 wk was associated with lower overall appetite and desire for sweet and salty food, which encompassed energy-dense and highly palatable snacks (97). Additional studies are currently underway in adults (registered at clinicaltrials.gov as NCT00261898) and infants (registered at clinicaltrials.gov as NCT00125580).
Translating evidence on sleep and diet for health and weight-loss interventions
Sleep loss undermines health and wellness and is becoming extremely widespread. Although the existing evidence on sleep duration and diet has significant limitations, the overall evidence on sleep and health would suggest that accounting for sleep might benefit health interventions. In addition to diet and physical activity, health guidelines, obesity prevention campaigns, and weight-loss programs should include improved sleep and sleep hygiene as an additional behavioral component to target the widespread prevalence of obesity and chronic disease (18). Short sleepers engage in pro-obesogenic eating behaviors, which contributes to the obesity epidemic. In addition, short sleep duration also compromises the efficacy and success of weight-loss interventions, resulting in the loss of fat-free body mass during periods of reduced caloric intake (98). Our results also suggest that longer sleep duration could attenuate genetic predisposition to obesity (43), and additional studies suggest that sleep could further attenuate genetic risk of various chronic diseases (91, 99). Taking into account the causal evidence and epidemiologic relations between sleep loss and metabolism and cardiovascular function, health promotion strategies should emphasize improved sleep as an additional factor in health and weight management.
Addressing the importance of sleep for health requires emphasis on minimizing behaviors and environmental conditions that could be disruptive to sleep. Diet-related recommendations include consuming foods that promote sleep while avoiding others that might disrupt sleep, and limiting food intake within 2–3 h before bedtime. Additional general recommendations can be found in a previous review from Advances in Nutrition (7), the National Sleep Foundation (100), the American Academy of Sleep Medicine and Sleep Research Society (101), and Healthy People 2020 (102). Adopting these recommendations may improve dietary profiles through improvements in sleep.
Conclusions
Sleep exerts a wide range of physiologic functions, and, in this review, we have provided evidence that short sleep duration is associated with higher total caloric intake, higher absolute intake of fat, and diets with relatively higher fat and lower protein composition, and limited evidence that short sleep duration is associated with lower intake of fruits and vegetables and diets of lower quality. It is also possible that sleep has an impact on intake behaviors and the timing of caloric intake. Specifically, evidence points to the fact that eating behaviors deviate from the traditional 3 meals/d to fewer main meals and smaller, more frequent energy-dense and highly palatable snacks throughout the day and primarily concentrated at night in short sleepers. Mechanisms mediating the associations between sleep duration and dietary intake are likely to be multifactorial, and include differences in appetite-related hormones leptin and ghrelin, hedonic pathways, extended hours for intake, and altered time of intake; however, additional mechanisms may exist, and epidemiologic studies along with controlled sleep intervention studies and sleep extension trials in chronic short sleepers are imperative to establish these causal relations. These critical nutritional nuances contribute to an unhealthy diet that predisposes people to obesity and various chronic diseases.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CLOCK, circadian locomotor output cycles kaput; FLOT1, flotillin 1; IER3, immediate early response 3; PAX8, paired box 8; TRIB1, tribbes pseudokinase 1; WHI, Women Health Initiative.
References
- 1.Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, Hazen N, Herman J, Katz ES, Kheirandish-Gozal L, et al. . National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health 2015;1:40–3. [DOI] [PubMed] [Google Scholar]
- 2.National Sleep Foundation. International bedroom poll: summary of findings. Arlington (VA): National Sleep Foundation; 2013. [Google Scholar]
- 3.National Sleep Foundation. 2002 Adult sleep habits. Washington (DC): National Sleep Foundation; 2002. [Google Scholar]
- 4.CDC. National Health and Nutrition Examination Survey [Internet]. Hyattsville, MD: US Department of Health and Human Services, CDC, National Center for Health Statistics; 2007–2010 [cited 2015 July 2]. Available from: http://www.cdc.gov/nchs/nhanes.htm.
- 5.Zee PC, Badr MS, Kushida C, Mullington JM, Pack AI, Parthasarathy S, Redline S, Szymusiak RS, Walsh JK, Watson NF. Strategic opportunities in sleep and circadian research: report of the Joint Task Force of the Sleep Research Society and American Academy of Sleep Medicine. 2014. pp. 219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Institute of Medicine (US) Committee on Sleep Medicine and Research. Colten HR, Altevogt BM. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington (DC): National Academies Press (US); 2006. [PubMed] [Google Scholar]
- 7.Golem DL, Martin-Biggers JT, Koenings MM, Davis KF, Byrd-Bredbenner C. An integrative review of sleep for nutrition professionals. Adv Nutr 2014;5:742–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cappuccio FP, Taggart FM, Kandala N-B, Currie A, Peile E, Stranges S, Miller MA. Meta-analysis of short sleep duration and obesity in children and adults. Sleep 2008;31:619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall NS, Glozier N, Grunstein RR. Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep Med Rev 2008;12:289–98. [DOI] [PubMed] [Google Scholar]
- 11.Stranges S, Cappuccio FP, Kandala N-B, Miller MA, Taggart FM, Kumari M, Ferrie JE, Shipley MJ, Brunner EJ, Marmot MG. Cross-sectional versus prospective associations of sleep duration with changes in relative weight and body fat distribution: the Whitehall II Study. Am J Epidemiol 2008;167:321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rontoyanni VG, Baic S, Cooper AR. Association between nocturnal sleep duration, body fatness, and dietary intake in Greek women. Nutrition 2007;23:773–7. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y, Zhai L, Zhang D. Sleep duration and obesity among adults: a meta-analysis of prospective studies. Sleep Med 2014;15:1456–62. [DOI] [PubMed] [Google Scholar]
- 14.Stranges S, Dorn JM, Cappuccio FP, Donahue RP, Rafalson LB, Hovey KM, Freudenheim JL, Kandala N-B, Miller MA, Trevisan M. A population-based study of reduced sleep duration and hypertension: the strongest association may be in premenopausal women. J Hypertens 2010;28:896–902. [DOI] [PubMed] [Google Scholar]
- 15.Chaput J-P, Després J-P, Bouchard C, Tremblay A. Association of sleep duration with type 2 diabetes and impaired glucose tolerance. Diabetologia 2007;50:2298–304. [DOI] [PubMed] [Google Scholar]
- 16.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J 2011;32:1484–92. [DOI] [PubMed] [Google Scholar]
- 17.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep 2010;33:585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nedeltcheva AV, Scheer FAJL. Metabolic effects of sleep disruption, links to obesity and diabetes. Curr Opin Endocrinol Diabetes Obes 2014;21:293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol 2005;99:2008–19. [DOI] [PubMed] [Google Scholar]
- 20.Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Pract Res Clin Endocrinol Metab 2010;24:731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knutson KL, Van Cauter E, Rathouz PJ, Yan LL, Hulley SB, Liu K, Lauderdale DS. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Arch Intern Med 2009;169:1055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab 2010;24:775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrov MER, Kim Y, Lauderdale D, Lewis CE, Reis JP, Carnethon MR, Knutson K, Glasser SJ. Longitudinal associations between objective sleep and lipids: the CARDIA study. Sleep 2013;36:1587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shechter A, Grandner MA, St-Onge MP. The role of sleep in the control of food intake. Am J Lifestyle Med 2014;8:371–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hicks RA, McTighe S, Juarez M. Sleep duration and eating behaviors of college students. Percept Mot Skills 1986;62:25–6. [DOI] [PubMed] [Google Scholar]
- 26.Lucero K, Hicks RA. Relationship between habitual sleep duration and diet. Percept Mot Skills 1990;71:1377–8. [DOI] [PubMed] [Google Scholar]
- 27.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr 2010;91:1550–9. [DOI] [PubMed] [Google Scholar]
- 28.Calvin AD, Carter RE, Adachi T, Macedo PG, Albuquerque FN, van der Walt C, Bukartyk J, Davison DE, Levine JA, Somers VK. Effects of experimental sleep restriction on caloric intake and activity energy expenditure. Chest 2013;144:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spaeth AM, Dinges DF, Goel N. Sex and race differences in caloric intake during sleep restriction in healthy adults. Am J Clin Nutr 2014;100:559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.St-Onge M-P, Roberts AL, Chen J, Kelleman M, O’Keeffe M, Roy Choudhury A, Jones PJH. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr 2011;94:410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, Wright KP. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci USA 2013;110:5695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr 2009;89:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Benedict C, Lehnert H, Born J, Schultes B. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr 2009;90:1476–82. [DOI] [PubMed] [Google Scholar]
- 34.Fang Z, Spaeth AM, Ma N, Zhu S, Hu S, Goel N, Detre JA, Dinges DF, Rao H. Altered salience network connectivity predicts macronutrient intake after sleep deprivation. Sci Rep 2015;5:8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heath G, Roach GD, Dorrian J, Ferguson SA, Darwent D, Sargent C. The effect of sleep restriction on snacking behaviour during a week of simulated shiftwork. Accid Anal Prev 2012;45: Suppl:62–7. [DOI] [PubMed] [Google Scholar]
- 36.Spaeth AM, Dinges DF, Goel N. Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. Sleep 2013;36:981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grandner MA, Jackson N, Gerstner JR, Knutson KL. Dietary nutrients associated with short and long sleep duration. Data from a nationally representative sample. Appetite 2013;64:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaput J-P. Sleep patterns, diet quality and energy balance. Physiol Behav 2014;134:86–91. [DOI] [PubMed] [Google Scholar]
- 39.Tu X, Cai H, Gao Y-T, Wu X, Ji B-T, Yang G, Li H, Zheng W, Shu XO. Sleep duration and its correlates in middle-aged and elderly Chinese women: the Shanghai Women’s Health Study. Sleep Med 2012;13:1138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohida T, Kamal AM, Uchiyama M, Kim K, Takemura S, Sone T, Ishii T. The influence of lifestyle and health status factors on sleep loss among the Japanese general population. Sleep 2001;24:333–8. [DOI] [PubMed] [Google Scholar]
- 41.Kim S, DeRoo LA, Sandler DP. Eating patterns and nutritional characteristics associated with sleep duration. Public Health Nutr 2011;14:889–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kant AK, Graubard BI. Association of self-reported sleep duration with eating behaviors of American adults: NHANES 2005–2010. Am J Clin Nutr 2014;100:938–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dashti HS, Follis JL, Smith CE, Tanaka T, Cade BE, Gottlieb DJ, Hruby A, Jacques PF, Lamon-Fava S, Richardson K, et al. . Habitual sleep duration is associated with BMI and macronutrient intake and may be modified by CLOCK genetic variants. Am J Clin Nutr 2015;101:135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi Z, McEvoy M, Luu J, Attia J. Dietary fat and sleep duration in Chinese men and women. Int J Obes (Lond) 2008;32:1835–40. [DOI] [PubMed] [Google Scholar]
- 45.Imaki M, Hatanaka Y, Ogawa Y, Yoshida Y, Tanada S. An epidemiological study on relationship between the hours of sleep and life style factors in Japanese factory workers. J Physiol Anthropol Appl Human Sci 2002;21:115–20. [DOI] [PubMed] [Google Scholar]
- 46.Stamatakis KA, Brownson RC. Sleep duration and obesity-related risk factors in the rural Midwest. Prev Med 2008;46:439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stern JH, Grant AS, Thomson CA, Tinker L, Hale L, Brennan KM, Woods NF, Chen Z. Short sleep duration is associated with decreased serum leptin, increased energy intake and decreased diet quality in postmenopausal women. Obesity (Silver Spring) 2014;22:E55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grandner MA, Kripke DF, Naidoo N, Langer RD. Relationships among dietary nutrients and subjective sleep, objective sleep, and napping in women. Sleep Med 2010;11:180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haghighatdoost F, Karimi G, Esmaillzadeh A, Azadbakht L. Sleep deprivation is associated with lower diet quality indices and higher rate of general and central obesity among young female students in Iran. Nutrition 2012;28:1146–50. [DOI] [PubMed] [Google Scholar]
- 50.Patterson RE, Emond JA, Natarajan L, Wesseling-Perry K, Kolonel LN, Jardack P, Ancoli-Israel S, Arab L. Short sleep duration is associated with higher energy intake and expenditure among African-American and Non-Hispanic white adults. J Nutr 2014;144:461–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galli G, Piaggi P, Mattingly MS, de Jonge L, Courville AB, Pinchera A, Santini F, Csako G, Cizza G. Inverse relationship of food and alcohol intake to sleep measures in obesity. Nutr Diabetes 2013;3:e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santana AA, Pimentel GD, Romualdo M, Oyama LM, Santos RVT, Pinho RA, de Souza CT, Rodrigues B, Caperuto EC, Lira FS. Sleep duration in elderly obese patients correlated negatively with intake fatty. Lipids Health Dis 2012;11:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring) 2011;19:1374–81. [DOI] [PubMed] [Google Scholar]
- 54.Van Den Berg JF, van Rooij FJA, Vos H, Tulen JHM, Hofman A, Miedema HME, Neven AK, Tiemeier H. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res 2008;17:295–302. [DOI] [PubMed] [Google Scholar]
- 55.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee JS, Gundersen C, Cook J, Laraia B, Johnson MA. Food insecurity and health across the lifespan. Adv Nutr 2012;3:744–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lundahl A, Nelson TD. Sleep and food intake: A multisystem review of mechanisms in children and adults. J Health Psychol 2015;20:794–805. [DOI] [PubMed] [Google Scholar]
- 58.Ding M, Keiley MK, Garza KB, Duffy PA, Zizza CA. Food insecurity is associated with poor sleep outcomes among US adults. J Nutr 2015;145:615–21. [DOI] [PubMed] [Google Scholar]
- 59.Weiss A, Xu F, Storfer-Isser A, Thomas A, Ievers-Landis CE, Redline S. The association of sleep duration with adolescents’ fat and carbohydrate consumption. Sleep 2010;33:1201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nishiura C, Noguchi J, Hashimoto H. Dietary patterns only partially explain the effect of short sleep duration on the incidence of obesity. Sleep 2010;33:753–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garaulet M, Ortega FB, Ruiz JR, Rey-López JP, Béghin L, Manios Y, Cuenca-García M, Plada M, Diethelm K, Kafatos A, et al. . Short sleep duration is associated with increased obesity markers in European adolescents: effect of physical activity and dietary habits. The HELENA study. Int J Obes (Lond) 2011;35:1308–17. [DOI] [PubMed] [Google Scholar]
- 62.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol 2006;164:947–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.St-Onge M-P. The role of sleep duration in the regulation of energy balance: effects on energy intakes and expenditure. J Clin Sleep Med 2013;9:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Penev PD. Update on energy homeostasis and insufficient sleep. J Clin Endocrinol Metab 2012;97:1792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci 2008;1129:287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leibel RL. The role of leptin in the control of body weight. Nutr Rev 2002;60(10 Pt 2):S15–9–discussionS68–84–85–7. [DOI] [PubMed] [Google Scholar]
- 67.van der Lely AJ, Tschöp M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev 2004;25:426–57. [DOI] [PubMed] [Google Scholar]
- 68.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004;141:846–50. [DOI] [PubMed] [Google Scholar]
- 69.Chaput J-P, Després J-P, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: Results from the Quebec family study. Obesity (Silver Spring) 2007;15:253–61. [DOI] [PubMed] [Google Scholar]
- 70.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med 2004;1:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.St-Onge M-P, Wolfe S, Sy M, Shechter A, Hirsch J. Sleep restriction increases the neuronal response to unhealthy food in normal-weight individuals. Int J Obes (Lond) 2014;38:411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Benedict C, Brooks SJ, O’Daly OG, Almèn MS, Morell A, Åberg K, Gingnell M, Schultes B, Hallschmid M, Broman J-E, et al. . Acute sleep deprivation enhances the brain’s response to hedonic food stimuli: an fMRI study. J Clin Endocrinol Metab 2012;97:E443–7. [DOI] [PubMed] [Google Scholar]
- 73.St-Onge M-P, McReynolds A, Trivedi ZB, Roberts AL, Sy M, Hirsch J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am J Clin Nutr 2012;95:818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Greer SM, Goldstein AN, Walker MP. The impact of sleep deprivation on food desire in the human brain. Nat Commun 2013;4:2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee Y-C, Ordovás JM, Scheer FAJL. Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond) 2013;37:604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aljuraiban GS, Chan Q, Griep LMO, Brown IJ, Daviglus ML, Stamler J, Van Horn L, Elliott P, Frost GS. The impact of eating frequency and time of intake on nutrient quality and body mass index: The INTERMAP Study, a population-based study. J Acad Nutr Diet 2015;115:528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jakubowicz D, Froy O, Wainstein J, Boaz M. Meal timing and composition influence ghrelin levels, appetite scores and weight loss maintenance in overweight and obese adults. Steroids 2012;77:323–31. [DOI] [PubMed] [Google Scholar]
- 78.Bandín C, Scheer FAJL, Luque AJ, Avila-Gandía V, Zamora S, Madrid JA, Gómez-Abellán P, Garaulet M. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. Int J Obes (Lond) 2015;39:828–33. [DOI] [PubMed] [Google Scholar]
- 79.Wang JB, Patterson RE, Ang A, Emond JA, Shetty N, Arab L. Timing of energy intake during the day is associated with the risk of obesity in adults. J Hum Nutr Diet 2014;27: Suppl 2:255–62. [DOI] [PubMed] [Google Scholar]
- 80.Mattson MP, Allison DB, Fontana L, Harvie M, Longo VD, Malaisse WJ, Mosley M, Notterpek L, Ravussin E, Scheer FA, et al. . Meal frequency and timing in health and disease. Proc Natl Acad Sci USA 2014;111:16647–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garaulet M, Gómez-Abellán P. Timing of food intake and obesity: a novel association. Physiol Behav 2014;134:44–50. [DOI] [PubMed] [Google Scholar]
- 82.Peuhkuri K, Sihvola N, Korpela R. Diet promotes sleep duration and quality. Nutr Res 2012;32:309–19. [DOI] [PubMed] [Google Scholar]
- 83.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology 2008;19:838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baker FC, Maloney S, Driver HS. A comparison of subjective estimates of sleep with objective polysomnographic data in healthy men and women. J Psychosom Res 1999;47:335–41. [DOI] [PubMed] [Google Scholar]
- 85.Sato-Mito N, Shibata S, Sasaki S, Sato K. Dietary intake is associated with human chronotype as assessed by both morningness-eveningness score and preferred midpoint of sleep in young Japanese women. Int J Food Sci Nutr 2011;62:525–32. [DOI] [PubMed] [Google Scholar]
- 86.Kanerva N, Kronholm E, Partonen T, Ovaskainen M-L, Kaartinen NE, Konttinen H, Broms U, Männistö S. Tendency toward eveningness is associated with unhealthy dietary habits. Chronobiol Int 2012;29:920–7. [DOI] [PubMed] [Google Scholar]
- 87.Tatone-Tokuda F, Dubois L, Ramsay T, Girard M, Touchette E, Petit D, Montplaisir JY. Sex differences in the association between sleep duration, diet and body mass index: a birth cohort study. J Sleep Res 2012;21:448–60. [DOI] [PubMed] [Google Scholar]
- 88.Hjorth MF, Chaput J-P, Michaelsen K, Astrup A, Tetens I, Sjödin A. Seasonal variation in objectively measured physical activity, sedentary time, cardio-respiratory fitness and sleep duration among 8–11 year-old Danish children: a repeated-measures study. BMC Public Health 2013;13:808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lehnkering H, Siegmund R. Influence of chronotype, season, and sex of subject on sleep behavior of young adults. Chronobiol Int 2007;24:875–88. [DOI] [PubMed] [Google Scholar]
- 90.Allebrandt KV, Teder-Laving M, Kantermann T, Peters A, Campbell H, Rudan I, Wilson JF, Metspalu A, Roenneberg T. Chronotype and sleep duration: the influence of season of assessment. Chronobiol Int 2014;31:731–40. [DOI] [PubMed] [Google Scholar]
- 91.Watson NF, Harden KP, Buchwald D, Vitiello MV, Pack AI, Weigle DS, Goldberg J. Sleep duration and body mass index in twins: a gene-environment interaction. Sleep 2012;35:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Allebrandt KV, Teder-Laving M, Akyol M, Pichler I, Müller-Myhsok B, Pramstaller P, Merrow M, Meitinger T, Metspalu A, Roenneberg T. CLOCK gene variants associate with sleep duration in two independent populations. Biol Psychiatry 2010;67:1040–7. [DOI] [PubMed] [Google Scholar]
- 93.Gottlieb DJ, Hek K, Chen T-H, Watson NF, Eiriksdottir G, Byrne EM, Cornelis M, Warby SC, Bandinelli S, Cherkas L, et al. . Novel loci associated with usual sleep duration: the CHARGE Consortium Genome-Wide Association Study. Mol Psychiatry 2014. Dec 2 (Epub ahead of print;DOI: 10.1038/mp.2014.133). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garaulet M, Sánchez-Moreno C, Smith CE, Lee Y-C, Nicolás F, Ordovas JM. Ghrelin, Sleep Reduction and Evening Preference: Relationships to CLOCK 3111 T/C SNP and Weight Loss. PLoS ONE 2011;6:e17435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ollila HM, Utge S, Kronholm E, Aho V, Van Leeuwen W, Silander K, Partonen T, Perola M, Kaprio J, Salomaa V, et al. . TRIB1 constitutes a molecular link between regulation of sleep and lipid metabolism in humans. Transl Psychiatry 2012;2:e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baldwin CM, Kapur VK, Holberg CJ, Rosen C, Nieto FJ, Sleep Heart Health Study Group. Associations between gender and measures of daytime somnolence in the Sleep Heart Health Study. Sleep 2004;27:305–11. [DOI] [PubMed] [Google Scholar]
- 97.Tasali E, Chapotot F, Wroblewski K, Schoeller D. The effects of extended bedtimes on sleep duration and food desire in overweight young adults: a home-based intervention. Appetite 2014;80:220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med 2010;153:435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tare A, Lane JM, Cade BE, Grant SFA, Chen T-H, Punjabi NM, Lauderdale DS, Zee PC, Gharib SA, Gottlieb DJ, et al. . Sleep duration does not mediate or modify association of common genetic variants with type 2 diabetes. Diabetologia 2014;57:339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.National Sleep Foundation. Healthy Sleep Tips [Internet]. Washington (DC): National Sleep Foundation; 2002. [cited 2015 July 2.] Available from: http://sleepfoundation.org/sleep-tools-tips/healthy-sleep-tips. [Google Scholar]
- 101.Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, Dinges DF, Gangwisch J, Grandner MA, Kushida C, et al. . Recommended amount of sleep for a healthy adult: a joint consensus statement of the american academy of sleep medicine and sleep research society. Sleep 2015;38:843–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.US Department of Health and Human Services. Office of Disease Prevention and Health Promotion. Healthy People 2020. Washington (DC) [cited 2015 Jul 2]. Available from: [http://www.healthypeople.gov/2020/topics-objectives/topic/sleep-health].