Abstract
Proteins from plant-based compared with animal-based food sources may have different effects on cardiovascular disease (CVD) risk factors. Numerous epidemiologic and intervention studies have evaluated their respective health benefits; however, it is difficult to isolate the role of plant or animal protein on CVD risk. This review evaluates the current evidence from observational and intervention studies, focusing on the specific protein-providing foods and populations studied. Dietary protein is derived from many food sources, and each provides a different composite of nonprotein compounds that can also affect CVD risk factors. Increasing the consumption of protein-rich foods also typically results in lower intakes of other nutrients, which may simultaneously influence outcomes. Given these complexities, blanket statements about plant or animal protein may be too general, and greater consideration of the specific protein food sources and the background diet is required. The potential mechanisms responsible for any specific effects of plant and animal protein are similarly multifaceted and include the amino acid content of particular foods, contributions from other nonprotein compounds provided concomitantly by the whole food, and interactions with the gut microbiome. Evidence to date is inconclusive, and additional studies are needed to further advance our understanding of the complexity of plant protein vs. animal protein comparisons. Nonetheless, current evidence supports the idea that CVD risk can be reduced by a dietary pattern that provides more plant sources of protein compared with the typical American diet and also includes animal-based protein foods that are unprocessed and low in saturated fat.
Keywords: amino acids, intestinal microbiota, DASH diet, cardiovascular diseases, OmniHeart
Introduction
Increasing dietary protein may benefit cardiovascular health by aiding in weight loss/maintenance, improving the lipid/lipoprotein profile, and reducing blood pressure (1–3). However, research demonstrating the health benefits of dietary protein must be interpreted with caution because increasing the consumption of protein/protein-rich foods typically results in other changes in the diet (i.e., energy, nutrients, and foods) because dietary protein is derived from many different foods. For example, increasing protein/protein-rich foods can alter the intakes of nutrients (e.g., saturated fat and refined carbohydrates) and/or foods (e.g., fruits, vegetables, and whole grains), depending on what protein food sources are increased and what they replace. Therefore, the effects of increased protein consumption are influenced by the specific protein food source and resulting macronutrient and micronutrient (and bioactives) substitutions. As such, evidence about total protein consumption must consider the source of protein, which components of the diet it is replacing, and the nutrients and bioactives that accompany the protein in the food matrix.
The evolution of dietary guidance reflects our growing understanding of how different nutrients, bioactives, and foods affect health. Carbohydrates are classified as refined or complex, and fats are differentiated as SFAs, trans FAs, MUFAs, and various PUFAs. Likewise, dietary proteins are classified on the basis of their plant or animal origin. Current dietary guidelines state that the RDA for adults is 0.8 g protein/kg body weight; however, the acceptable macronutrient distribution range of 10–35% of total calories from protein allows for more flexibility in meal planning, according to individual needs and preferences (4, 5). There is also an RDA for each essential amino acid, which can be met with a variety of different protein-providing foods. However, there are no recommendations for the relative contribution of animal- and plant-based sources of protein (4).
Specific questions about the relation between protein and health were first addressed in the 2010 Dietary Guidelines Advisory Committee’s evidence review. A primary recommendation of the 2010 Dietary Guidelines for Americans was to shift food intake patterns to a more plant-based diet (6), emphasizing beans/legumes, whole grains, nuts/seeds, and vegetables, all of which are sources of plant protein. The 2015 Dietary Guidelines Advisory Committee (DGAC)5 maintained the focus on dietary patterns higher in plant-based foods in their Scientific Report and included the recommendation that empty calories from added sugars be replaced in part with a greater variety of plant protein (7). The general perception of plant protein has similarly evolved. Although it was once perceived as less nutritious and an incomplete source of essential amino acids, plant protein is now viewed as a healthy option for meeting protein needs/recommendations.
Evidence about disparate health effects of plant compared with animal protein is mixed. Protein is not consumed in isolation but as part of a food matrix. Therefore, it is difficult to control for the potential effects of other nutrients provided by these foods and attribute any observed benefits solely to protein content. In addition, specific dietary sources of both plant and animal protein were shown to have different health effects. Thus, general statements about plant or animal protein may be too simplistic and effects may depend on the food matrix and accompanying nutrients/bioactives. The purpose of this article is to review key epidemiologic and intervention research about the potential differential effects of plant and animal protein, with particular attention given to specific protein source comparisons, population-specific variations, and the implications of heterogeneous research designs. Limitations in our current knowledge, potential mechanisms, and considerations for altering protein consumption are also discussed.
Current Status of Knowledge
Observational studies
For decades, observational evidence has suggested that plant and animal protein influence cardiovascular disease (CVD) risk differently. A health benefit of plant protein was first indicated by findings that vegetarian populations tend to have lower blood pressure and plasma cholesterol than their omnivorous counterparts (8–10). However, the Seventh-Day Adventist populations in which these associations between vegetarian diets and blood pressure were found also tend to be nonsmokers, consume less alcohol, have a lower body weight, be more physically active, and consume a generally healthier diet, all of which lower blood pressure. Dietary patterns also consist of whole foods, not individual nutrients; therefore, it is not surprising that fruit and vegetable intakes as well as nutrients in plant-based foods, such as fiber, magnesium, and potassium are correlated with plant protein intake (11, 12). Because these are all consumed in combination and are inversely correlated with blood pressure, it is not possible to attribute associations with lower blood pressure solely to plant protein.
Because these initial indications of a protein–blood pressure relation, numerous epidemiologic studies have investigated associations for plant and animal protein specifically. However, results from meta-analyses and systematic reviews consistently indicate that the role of vegetable compared with animal protein on blood pressure remains unclear (13–15). In brief, this conclusion is based primarily on findings from large cohort studies in both Western and Asian populations. Studies of industrialized populations tend to report a beneficial association between plant protein and blood pressure. In the Chicago Western Electric Study, a cohort of >1700 generally healthy middle-aged men who were followed for 8 y, vegetable protein intake was inversely related to both systolic and diastolic pressures; whereas total and animal protein intake demonstrated a positive association with blood pressure (16). Analysis of data from the PREMIER study, a multicenter intervention trial of lifestyle modifications on blood pressure in healthy US adults with above optimal blood pressure, similarly found an inverse association between blood pressure and plant protein intake, with no relation for total or animal protein (12). An inverse association between vegetable protein intake and blood pressure was also reported in the INTERMAP (International Population Study on Macronutrients and Blood Pressure) study, a study of >4500 men and women from the United Kingdom, United States, China, and Japan, although no relation between blood pressure and total or animal protein was found in this population (17). Conversely, studies of rural Japanese and Chinese populations have found the opposite relation, with blood pressure being inversely related to animal protein intake (18–20). The only systematic review, to our knowledge, designed to focus specifically on different types of protein reported that an inverse association is more commonly found for plant protein (21). However, in accordance with the preceding discussion of disparate results, the investigators similarly stated that the current evidence remains limited by inconsistent findings and potential confounders inherent to observational studies (21).
For cardiovascular disease outcomes, results for the role of plant compared with animal protein are similarly mixed, depending on the population studied and how protein groups are specified (Table 1). Of note are the somewhat disparate findings from the Nurses’ Health Study. In initial analyses, both animal and vegetable protein intakes were found to contribute to the lower ischemic heart disease risk associated with greater total protein intake (22). However, a subsequent analysis calculated low-carbohydrate scores with respective animal and plant contributions and found a differential association, with a higher vegetable low-carbohydrate score being associated with lower CVD mortality and a higher animal low-carbohydrate score increasing CVD mortality (29). More specific analyses of particular food sources of protein (e.g., red meat, poultry, fish, dairy, eggs, legumes, nuts, beans, etc.) have similarly demonstrated that each protein source is associated with a different degree of both coronary heart disease (CHD) risk (26) and CVD mortality (30). Substituting 1 standard serving of red meat [∼3 oz (85 g)] with different plant protein sources was found to reduce CHD risk by 13–30% in the Nurses’ Health Study (26) and by 7–19% in combined analyses of the Nurses’ Health Study and Health Professionals Follow-Up cohorts (30). These results demonstrate that different types of plant protein and different types of animal protein often elicit different associations. This may reflect confounding from other nonprotein dietary components that are also provided in the whole food matrix. Thus, specificity in characterizing each type of plant and/or animal protein is required to accurately assess associations with CVD risk factors, morbidity, and mortality.
TABLE 1.
Summary of observational studies that evaluated the effect of different dietary protein sources on cardiovascular events and cardiovascular disease risk and mortality1
| Study cohort (reference) | Location | Study population, n | Duration of follow-up, y | Dietary components evaluated | Main findings | Estimated effect of protein substitution |
| Nurses’ Health Study (Hu et al., 1999) (22) | United States | Women, 30–55 y at baseline (n = 80,082) | 14 | Total, animal, and vegetable protein intake | Total protein inversely associated with risk of IHD (both animal and vegetable protein contributed to lower risk) | NA |
| Iowa Women’s Health Study (Kelemen et al., 2005) (23) | United States | Women, 55–69 y at baseline (n = 29,017) | 15 | Increasing total protein in place of CHO, substituting animal protein with plant protein | 30% reduction in CHD mortality with isoenergetic substitution of vegetable protein for CHO (95% CI: 0.49, 0.99) and animal protein (95% CI: 0.51, 0.98) | |
| Positive association between CHD mortality and substituting refined CHO with red meat (RR: 1.44; 95% CI: 1.06, 1.94) and dairy products (RR: 1.41; 95% CI: 1.07, 1.86) | ||||||
| Nurses’ Health Study (Halton et al., 2006) (24) | United States | Women, 30–55 y at baseline (n = 82,802) | 20 | Animal and vegetable low-CHO scores (on the basis of percentage of energy from CHO, protein, and fat from animal/plant sources) | Vegetable low-CHO score associated with lower risk of CHD (RR comparing extreme deciles: 0.70; 95% CI: 0.56, 0.88) (vegetable protein inversely associated with CHD risk only in age- and smoking-adjusted analyses; became NS in multivariate analyses) | NA |
| NIH-AARP Diet and Health Study (Sinha et al., 2009) (25) | United States | Men and women, 50–71 y (n ∼500,000) | 10 | Red, white, and processed meat intakes | Red and processed meat intakes associated with modest increase in total, cancer, and CVD mortality | If red meat consumption was reduced to amount consumed by first quintile, CVD mortality would be reduced by 11% in men and 26% in women |
| Nurses’ Health Study (Bernstein et al., 2009) (26) | United States | Women, 30–55 y at baseline (n = 84,136) | 26 | Protein source groups (total meat, red meat, red meat excluding processed meat, poultry, fish, high-fat dairy, low-fat dairy, eggs, nuts, beans); effect of substituting a serving of 1 major protein source for another | Higher intakes of red meat, red meat excluding processed meat, and high-fat dairy associated with increased CHD risk | Replacing 1 serving red meat/d with 1 serving2 of: |
| Higher intakes of poultry, fish, and nuts associated with lower CHD risk | Nuts would reduce risk by 30% (95% CI: 17%, 42%) | |||||
| Fish would reduce risk 24% (95% CI: 6%, 39%) | ||||||
| Poultry would reduce risk 19% (95% CI: 3%, 33%) | ||||||
| Low-fat dairy would reduce risk 13% (95% CI: 6%, 19%) | ||||||
| Health Professionals Follow-Up Study (Preis et al., 2010) (27) | United States | Men, 40–75 y at baseline (n = 43,960) | 18 | Total, animal, and vegetable protein intakes | No association between any dietary protein category and total IHD | NA |
| Vegetable protein inversely related to risk of fatal IHD (RR: 0.66; 95% CI; 0.49, 0.88) | ||||||
| In healthy men, higher intakes of total and animal protein increased risk of IHD | ||||||
| Health Professionals Follow-Up Study (Preis et al., 2010) (28) | United States | Men, 40–75 y at baseline (n = 43,960) | 18 | Total, animal, and vegetable protein intakes | No association between any dietary protein category and risk of stroke | NA |
| Modest positive association for total protein and ischemic stroke in men with hypercholesterolemia (P-interaction: 0.05) | ||||||
| Nurses’ Health Study and Health Professionals Follow-Up Study (Fung et al., 2010) (29) | United States | Men, 40–75 y at baseline (n = 44,548) and women, 34–59 y at baseline (n = 85,168) | 20 for men; 26 for women | Animal and vegetable low-CHO scores (on the basis of percentage of energy from CHO, protein, and fat from animal/plant sources) | Animal low-CHO score associated with higher CVD mortality (HR: 1.14; 95% CI: 1.01, 1.29) | NA |
| Higher vegetable low-CHO score associated with lower CVD mortality (HR: 0.77; 95% CI: 0.68, 0.87) | ||||||
| Nurses’ Health Study and Health Professionals Follow-Up Study (Pan et al., 2012) (30) | United States | Men, 40–75 y at baseline (n = 37,698) and women, 30–55 y at baseline (n = 83,644) | Up to 22 for men; up to 28 for women | Total red meat, processed red meat, and unprocessed red meat intakes | Greater CVD mortality associated with red meat intake, both unprocessed (HR: 1.18; 95% CI: 1.13, 1.23) and processed (HR: 1.21; 95% CI: 1.13, 1.31) | For total mortality risk, replacing 1 serving red meat/d of with 1 serving3,4 of: |
| If <0.5 serving red meat consumed/d, 8.6% of CVD deaths in men and 12.2% in women could be prevented | Nuts would reduce risk by 19% (95% CI: 0.77%, 0.86%) | |||||
| Poultry would reduce risk by 14% (95% CI: 0.82%, 0.91%) | ||||||
| Whole grains would reduce risk by 14% (95% CI: 0.82%, 0.88%) | ||||||
| Legumes would reduce risk by 10% (95% CI: 0.86%, 0.94%) | ||||||
| Low-fat dairy products would reduce risk by 10% (95% CI: 0.86%, 0.94%) | ||||||
| Fish would reduce risk by 7% (95% CI: 0.90%, 0.97%) | ||||||
| EPIC (Rohrmann et al., 2013) (31) | 10 European countries | Men and women, 35-69 y at baseline (n = 448,568) | 12.7(median) | Red meat, processed meat, and white meat intakes | Only processed meat associated with higher all-cause mortality from both CVD and cancer (HR: 1.18; 95% CI: 1.11, 1.25) | NA |
| Reducing processed meat consumption to <20 g/d would prevent 3.3% of all deaths | ||||||
| Cohort of Swedish men (Kaluza et al., 2014) (32) | Sweden | Men, 45–79 y at baseline (n = 37,035) | 11.8 | Meat consumption (processed and unprocessed) | Processed, but not unprocessed, meat consumption positively associated with risk of heart failure | NA |
| For each 50-g increase in daily processed meat consumption, HF incidence increased by 8% and HF mortality increased by 38% |
CHD, cardiovascular heart disease; CHO, carbohydrate; CVD, cardiovascular disease; EPIC, European Prospective Investigation into Cancer and Nutrition; HF, heart failure; IHD, ischemic heart disease; NA, not applicable.
Refers to a commonly used unit or portion size for each food specified on the FFQ (e.g., 1 slice of processed meat or 1 hamburger patty). Over the period of 1980–2002, other portion sizes included the following: milk, 8 oz; yogurt, 1 cup; hard cheese, 1 oz; chicken, ranged from 6–8 oz (1980) to 3 oz (2002); bacon, 2 slices; beef, pork, lamb as main dish, ranged from 6–8 oz (1980) to 4–6 oz (2002); eggs, 1 egg; fish: canned tuna: ranged from 3–4 oz (1980) to 2–3 oz (2002), dark meat fish: 3–5 oz, other fish: 3–5 oz; nuts, 1 oz; beans or lentils, 1/2 cup (26).
The standard serving size for unprocessed meat was 85 g or 3 oz. Processed meat included bacon (2 slices or 13 g), hot dogs (1 hot dog or 45 g), and sausage, salami, bologna, and other processed meats (1 piece or 28 g) (30).
Serving sizes for nonmeat items were not specified, but standard serving sizes are as follows: poultry and fish, ∼3 oz; nuts, ∼1 oz; low-fat dairy: 1 cup of milk or yogurt or 1.5 oz cheese; whole grains: 1 slice of bread, 1 oz ready-to-eat-cereal, or 1/2 cup cooked cereal, rice, or pasta; legumes, 1/2 cup cooked (33).
Meta-analyses have similarly indicated that differentiating between different types of red meat is necessary to evaluate their association with the risk of heart disease. For instance, in the EPIC (European Prospective Investigation into Cancer and Nutrition) cohort, a positive association with all-cause and CVD mortality was found only for processed meat consumption and not for red meat or poultry (31). Furthermore, in a 2010 meta-analysis, Micha et al. (34) concluded that only processed red meat intake, not total red meat intake, was associated with 42% greater risk of CHD. Dietary guidelines recommend reducing high-fat red meats because they are higher in saturated fat and cholesterol. However, red meat is not among the top contributors to saturated fat intake in the US diet (35). The primary difference between unprocessed and processed meat is sodium and nonsalt preservatives. Per gram, processed meat contains ∼400% more sodium and ∼50% more nitrates than unprocessed meat (36). Thus, it was proposed that the preservatives and sodium content of processed meat may account for a large part of the strong association between CHD risk and processed meat. However, in other studies, elevated CVD risk was found with higher intake of red meat in addition to processed meat or total red meat, suggesting that the greater risk associated with red meat is not entirely explained by processed meat and its salt/preservative content (25, 26, 36). Therefore, characterizing the specific food sources is essential for interpreting the associations of dietary protein on CVD outcomes and intermediate risk factors such as blood pressure.
The discrepancies for both blood pressure and CVD outcomes may be due in part to specific characteristics of the population studied. For instance, although Japanese and US populations consume similar absolute amounts of animal protein, they often consume different foods (e.g., fish compared with red meat) (12). Therefore, the finding of a recent meta-analysis that animal protein intake contributes more to stroke reduction than vegetable protein may be because of studies in which fish rather than red meat was the primary source of animal protein (37). This difference in animal protein food sources may also be one factor responsible for the different blood pressure findings of epidemiologic studies, because industrialized Western populations tend to consume more red meat and poultry compared with rural Chinese or Japanese populations that often consume more fish (19, 28). However, differences in the absolute amount of animal protein consumed and other differences between these populations could also help to account for the discrepant associations with animal protein intake (i.e., detrimental associations in Western populations compared with beneficial relations in rural populations). Nonetheless, the consumption of different types of animal protein in different populations makes it difficult to generate conclusive statements about associations between increased animal protein intake and CVD risk. Similarly, the composition of the background diet in which dietary protein is evaluated may be population specific. For example, disparate findings for the relation between low-carbohydrate/high-protein scores and CHD risk in US (24) compared with Swedish (38) women may be due in large part to the type of carbohydrate being replaced by protein (39). Compared with US women, Swedish women consume greater amounts of cereal fiber (39). Therefore, it is possible that US women benefited from consuming more protein because they replaced a greater proportion of refined carbohydrates in their diet, whereas Swedish women were likely replacing more whole grains, which resulted in no benefit. The type of carbohydrate replaced by protein may also influence CVD risk factors such as blood pressure. For instance, refined carbohydrates, which are rapidly metabolized and thus more likely to cause spikes in glucose and insulin, may increase the risk of insulin resistance (40–42), which is linked to hypertension (43–46). Conversely, complex carbohydrates such as whole grains are metabolized more slowly and provide nutrients associated with lower blood pressure (e.g., dietary fiber, magnesium, and potassium) (47–49). Thus, replacing refined carbohydrates with protein may be more likely to reduce blood pressure than replacing complex carbohydrates.
Intervention studies
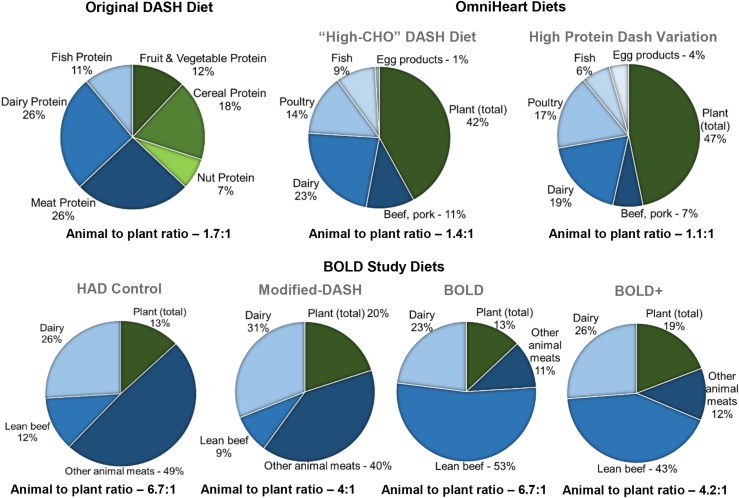
Large-scale controlled feeding studies also provide insight into the potential differential effects of plant compared with animal protein. The Dietary Approaches to Stop Hypertension (DASH) trial, a randomized, parallel-arm, multicenter study conducted in the 1990s, was designed to evaluate the effects of healthy dietary patterns in >400 adults (50, 51). The Optimal Macronutrient Intake Trial to Prevent Heart Disease (OmniHeart), a randomized crossover study designed on the basis of the results from the DASH trial was conducted in the 2000s in 164 adults (52). Findings from DASH and OmniHeart are typically referenced as evidence of the beneficial effects of greater plant protein consumption. Conversely, the Beef in an Optimal Lean Diet (BOLD) study, a smaller-scale intervention study, was designed to evaluate the effects of a dietary pattern higher in animal protein from lean beef (53). Results from the BOLD study were interpreted to suggest that animal and plant protein consumptions exert similar effects on the CVD risk factors evaluated. Additional characteristics (sample size, duration, dietary interventions, results, etc.) of these studies are presented in Table 2. The controlled feeding study design provided the additional benefit of maintaining participants’ body weight, thus preventing weight loss from being a potential confounder in the interpretation of results. Adherence to the dietary interventions was monitored by daily self-reporting. Compliance was ≥93% in each study, indicating that the macronutrient targets were successfully achieved (51–53). Detailed discussion of the dietary interventions used in these 3 studies and implications about their results are presented in the next sections. The relative plant and animal contributions to the total protein content of the relevant diets used in DASH, OmniHeart, and BOLD are shown in Figure 1. A comparison of the macronutrient profiles and percentage of calories provided by plant and animal protein in each diet is provided in Table 3.
TABLE 2.
Controlled feeding intervention studies that compared the effects of different amounts of plant- and animal-based protein1
| Relative contribution of animal vs. plant protein |
|||||||||
| Diet (reference) | Study design | Duration of diet periods, wk | Study population, n | Intervention diet(s) | Total protein,% kcal | Plant-based, % total protein | Animal-based, % total protein | Control and/or comparator diet(s) | Effect of intervention diet 2 |
| DASH (50, 51, 54) | Parallel | 8 | Adults with SBP < 160 mm Hg and DBP 80 – 95 mm Hg [n = 436 for (50)][n = 459 for (51)] | Increased fruits, vegetables, low-fat dairy | 15 | 37 | 63 | Average American diet control | BP reduction: 5.5/3 mm Hg |
| Reduced SFA and total fat | Fruits and Vegetables Diet comparator | ↓ TC by 13.7 mg/dL | |||||||
| ↓ LDL-C by 10.7 mg/dL | |||||||||
| ↓ HDL-C by 3.7 mg/dL | |||||||||
| ↔ TG | |||||||||
| OmniHeart (52) | Crossover | 6 | Adults with prehypertension and hypertension (n = 164) | High-protein DASH diet variation replaced 10% of CHO with protein, emphasizing protein from plant sources | 25 | 47 | 54 | Carbohydrate-rich DASH diet control | SBP reduction: 1.4 mm Hg |
| Unsaturated-fat DASH diet variation (10% CHO replaced by unsaturated fat) | ↓ LDL-C by 3.3 mg/dL | ||||||||
| ↓ HDL-C by 1.3 mg/dL | |||||||||
| ↓ TG by 15.7 mg/dL | |||||||||
| BOLD (53, 55) | Crossover | 5 | Normotensive adults (n = 36) | BOLD, modified-DASH diet rich in animal protein, particularly lean beef | 19 | 13 | 87 | HAD control | BOLD ↓ AI by 4.1% |
| BOLD+, moderate protein diet rich in animal protein | 27 | 19 | 80 | M-DASH | BOLD+ ↓ SBP | ||||
| M-DASH, BOLD, and BOLD+ similarly ↓ TC, LDL-C, HDL-C, and non-HDL-C | |||||||||
AI, augmentation index; BOLD, Beef in an Optimal Lean Diet; BP, blood pressure; CHO, carbohydrate; DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; HAD, Healthy American Diet; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; M-DASH, DASH-like diet comparator; SBP, systolic blood pressure; TC, total cholesterol.
Results reported for DASH and BOLD studies are relative to control diet; results from OmniHeart Study represent changes relative to the standard DASH diet (i.e., additional reductions beyond those achieved with the carbohydrate-rich DASH diet control).
FIGURE 1.
Relative plant and animal protein contributions to the total protein content of the diets used in the DASH, OmniHeart, and BOLD studies. All plant-based protein sources are indicated by green; all animal-based protein sources are indicated by shades of blue. BOLD, Beef in an Optimal Lean Diet; CHO, carbohydrate; DASH, Dietary Approaches to Stop Hypertension; HAD, Healthy American Diet; OmniHeart, Optimal Macronutrient Intake Trial to Prevent Heart Disease.
TABLE 3.
Macronutrient profiles and percentage of total calories provided by plant and animal protein sources in DASH, OmniHeart, and BOLD diets1
| OmniHeart |
BOLD |
||||||
| Macronutrient | DASH | Carbohydrate-rich DASH | High-protein DASH | HAD | M-DASH | BOLD | BOLD+ |
| Carbohydrate, % kcal | 55 | 58 | 48 | 50 | 55 | 54 | 45 |
| Fiber, g/d | 31 | 30 | 30 | 24 | 36 | 32 | 38 |
| Fat, % kcal | 27 | 27 | 27 | 33 | 27 | 28 | 28 |
| Cholesterol, mg/d | 150 | 150 | 150 | 287 | 188 | 168 | 193 |
| SFA, % kcal | 6 | 6 | 6 | 12 | 6 | 6 | 6 |
| MUFA, % kcal | 13 | 13 | 13 | 11 | 9 | 11 | 12 |
| PUFA, % kcal | 8 | 8 | 8 | 7 | 8 | 7 | 7 |
| Protein, % kcal | 18 | 15 | 25 | 17 | 18 | 19 | 27 |
| Plant2 | 6.6 | 5.5 | 12 | 2.2 | 3.6 | 2.5 | 5.1 |
| Meat3 | 6.7 | 5.5 | 9 | 10.4 | 8.8 | 12.2 | 14.6 |
| Dairy | 4.8 | 4.0 | 4 | 4.4 | 5.6 | 4.4 | 7.0 |
All values are based on a 2100-kcal diet. BOLD, Beef in an Optimal Lean Diet; DASH, Dietary Approaches to Stop Hypertension; HAD, Healthy American Diet; M-DASH, modified-DASH; OmniHeart, Optimal Macronutrient Intake Trial to Prevent Heart Disease.
Contributors to plant protein category, DASH: fruits and vegetables, cereals, nuts.
Contributors to meat protein category, DASH: poultry, mammals, and fish; OmniHeart diets: beef, pork, poultry, fish, and egg products; BOLD diets: lean beef and other animal sources.
Many smaller-scale intervention studies have been conducted with specific types of protein, particularly soy protein. Especially in studies designed to lower lipids/lipoproteins, soy is typically the primary or sole source of plant protein. This focus on soy protein emanates from the initial finding that animal proteins (e.g., casein and beef) cause hypercholesterolemia and atherosclerosis in rabbits (56). In subsequent clinical studies, replacing animal protein almost entirely with soy protein significantly reduced plasma cholesterol in both healthy young women (57) and people with hypercholesterolemia (58). Further investigations of the cholesterol-lowering effects of soy protein culminated in the seminal 1995 review that provided the basis for the Food and Drug Administration-qualified health claim that stated “including 25 g soy protein/d in a diet that is low in saturated fat and cholesterol may reduce the risk of heart disease” (59). However, substantially weaker effects were found in other meta-analyses (60, 61), including the AHA Science Advisory, which reported an average LDL cholesterol reduction of 3% (62). Additional LDL cholesterol reductions of ∼3–6% can also be achieved if soy is used to replace foods high in saturated fat and cholesterol (63). For blood pressure, there is observational (64) and preliminary intervention evidence from smaller-scale trials (65) for a benefit of soy protein, although the AHA Science Advisory concluded that it has no effect (62). However, soy is unique in its isoflavone content, and its effects on lipids may be due to a combination of its protein and nonprotein compounds. As such, soy protein interventions may not be representative of all sources of isolated plant protein. Reviews of these studies suggest no clear effects of specific protein sources (13, 21). In general, the smaller-scale studies discussed above are typically designed to supplement the background diet with a particular type of protein, whereas the focus of this review is to evaluate the effect of plant and animal proteins in the context of an overall dietary pattern. Therefore, the following discussion focuses on DASH, OmniHeart, BOLD, and other evidence-based plant-based dietary patterns (i.e., Portfolio and Ornish diets).
DASH.
The DASH dietary pattern was designed to incorporate multiple nutrients for which there is observational evidence of a blood pressure-lowering effect. Specifically, this diet incorporates low-fat dairy products, fish, chicken, and lean meats to reduce total and saturated fat while increasing protein and calcium (54). DASH also includes higher quantities of fruits, vegetables, whole grains, nuts, legumes, and seeds, all of which provide potassium, magnesium, and fiber in addition to being plant sources of protein. The DASH trial also included a second intervention diet, the fruits and vegetables (FV) diet, which only increased the fruit and vegetable content of the typical American diet, replacing mainly carbohydrate-rich sweet desserts and beverages. Compared with the typical American diet, consuming the DASH diet for 8 wk significantly reduced both systolic and diastolic blood pressures (66) and total cholesterol and LDL cholesterol (50) (Table 2). Although the FV diet significantly reduced systolic blood pressure (−2.8 mm Hg) compared with the control diet, additional benefits were achieved with the DASH diet, which further reduced systolic and diastolic blood pressures by 2.7 mm Hg and 1.9 mm Hg, respectively, compared with the FV diet (51). This additive effect on blood pressure reduction indicates that the combination of dietary changes characterizing the DASH dietary pattern, including a higher plant protein intake, resulted in greater health benefits than the single modification of replacing added sugars with fruits and vegetables.
Compared with the typical American diet, the DASH diet is higher in nuts, legumes, and other plant-based foods; therefore, it is higher in plant protein. For instance, the typical American control diet provided no nuts or legumes, whereas the DASH diet included 25 g nuts/d and 11 g legumes/d (67). Because of this, it is often presented as a high plant protein diet, with its beneficial effects partially attributed to this aspect of the diet. However, only ∼37% of the total protein provided in the standard DASH diet is derived from plant sources [including fruit and vegetables (12%), cereal (18%), and nuts (7%)]. The remaining ∼63% is provided by animal sources [including dairy (26%), fish (11%), and animal (26%)] (CM Champagne, Pennington Biomedical Research Center, personal communication, 2014). Therefore, although the DASH diet has a higher ratio of plant to animal protein than the typical American diet, it still provides ∼1.7 times more animal protein than plant protein (Figure 1).
When interpreting the beneficial effects of the DASH diet, it is important to appreciate that it incorporates multiple dietary changes, including having less total fat, SFAs, cholesterol, added sugars, and sodium; an improved PUFA:SFA ratio; and more total protein, fiber, potassium, phosphorous, and calcium. Any of these factors and the dietary components that they provide may have influenced study outcomes. For instance, although dairy products were the primary source of the increased calcium, other dairy product components may also have played a role in lowering blood pressure (CM Champagne, Pennington Biomedical Research Center, personal communication, 2015). Therefore, it is not possible to determine the contribution of any one dietary component, and the reductions in blood pressure and blood lipids cannot be attributed solely to greater plant protein intake.
OmniHeart.
OmniHeart was designed to test the effect of 2 macronutrient variations of the standard DASH diet. This was achieved by replacing 10% of the carbohydrates in a higher carbohydrate DASH diet (58% carbohydrate, 15% protein, and 27% fat) with either protein or unsaturated fat. The carbohydrate-rich DASH diet was modified from the standard DASH diet (55% carbohydrate, 18% protein, and 27% fat) to provide a 10% kcal comparison with the high-protein DASH diet variation (48% carbohydrate, 25% protein, and 27% fat) (52). The resulting high-protein DASH diet variation provided 25% of calories from protein, an amount substantially higher than the typical American intake of ∼15%. This 10% increase in protein emphasized plant protein but also modestly increased nonplant protein sources to maintain similar micronutrient profiles among the 3 intervention diets (68). Compared with the carbohydrate-rich DASH diet, the high-protein DASH diet variation provided 72% more plant protein. This was achieved by using larger portion sizes of plant protein food sources (i.e., nuts, seeds, and legumes) and adding higher protein vegetables, whole grains, beans, legumes, nuts, seeds, seitan, and soy products to the diet. Soy-based foods (i.e., tofu, soy nuts, and soy breakfast sausage) comprised 21% of the plant protein content of the high-protein DASH diet variation. Because these additions tend to be high-fiber foods, more refined breads and cereals were occasionally used to maintain a similar fiber content among the 3 intervention diets (68).
Despite the emphasis on plant protein, the high-protein DASH diet variation also provided 54% more animal protein compared with the carbohydrate-rich DASH diet, primarily from skinless poultry and egg white products. In all 3 diets, skinless poultry was the main contributor to meat protein intake (41–49%), followed by fish (tuna and scrod only) and shellfish (29–34%), and lean beef (11–19%). Dairy protein (low-fat or fat-free milk, yogurt, cheese, and pudding) also increased by 27% in the high-protein DASH diet variation. Therefore, the OmniHeart high-protein DASH diet variation consisted of approximately equal percentages of plant and animal protein [12% plant and 13% animal (9% meat, 4% dairy)], as opposed to the higher animal protein content of the carbohydrate-rich DASH diet [5.5% plant and 9.5% animal (5.5% meat, 4% dairy)] (52) (Table 3).
All 3 diet interventions, the carbohydrate-rich DASH diet, the high-protein DASH diet variation, and the high-unsaturated fat DASH diet variation, had favorable effects on blood pressure, blood lipids, and estimated 10-y CHD risk (52). However, compared with the carbohydrate-rich DASH diet, the high-protein DASH diet variation achieved significantly greater reductions in systolic and diastolic blood pressures and LDL cholesterol (52). It also significantly lowered TGs, a benefit that is not typically achieved by the standard DASH diet (52). However, because both the total protein content and the relative contribution of plant protein were increased in the high-protein DASH diet variation, it cannot be concluded that these results are an indication of effects specific to plant protein rather than a broader effect of replacing carbohydrates with protein.
BOLD.
The DASH diet and OmniHeart high-protein DASH diet variation provide evidence for the benefits of plant protein intake, whereas the BOLD study was designed to test the effects of DASH variations that emphasize animal protein, primarily from lean beef. This study used a modified-DASH diet, BOLD, and BOLD+ diet. The nutritional profiles of these diets are presented in Table 3. The modified-DASH and BOLD diets were matched for macronutrient composition and provided 18% and 19% of total calories from protein, respectively, but differed in their primary protein source (Figure 1, Table 3). Although the modified-DASH diet provided 20% plant protein, the BOLD diet contained only 13% plant protein and much greater amounts of lean beef (113 g/d compared with 28 g/d) (55). Rather than lean beef, the primary source of animal protein in the modified-DASH diet was other animal sources (40%), such as poultry, pork, and fish (53) (Figure 1). Therefore, the diets differed in both their total plant protein content and the specific food sources of animal protein. The BOLD+ diet was a moderate protein diet (27% protein) but provided a similar relative amount of plant protein as the modified-DASH diet (19% and 20%, respectively) (Figure 1) (55). However, lean beef was the primary contributor to animal protein (43%) in the BOLD+ diet compared with the other animal sources used in the modified-DASH diet.
Compared with the Healthy American Diet control (17% protein), the 3 intervention diets similarly lowered total cholesterol, LDL cholesterol, and non-HDL cholesterol (53). Only the BOLD diet significantly reduced the augmentation index (55), a measure of arterial stiffness that is defined as the percentage of the central pulse pressure attributed to the reflected arterial pulse wave (69). In stiff arteries, reflection of the incident arterial pulse wave by peripheral impedance occurs faster, augmenting the central blood pressure (69). The augmentation index is associated with CVD risk (70) and can be used as a risk marker for coronary artery disease (71, 72). The systolic blood pressure reductions achieved with the BOLD diet were similar to those observed for the modified-DASH diet. A significantly greater reduction in systolic blood pressure was achieved only with the higher protein BOLD+ diet, which had a comparable total protein content with the OmniHeart high-protein DASH diet variation (55). On the basis of these findings, the investigators concluded that this was indicative of no differential effect of plant or animal protein and that the type or source of protein (animal compared with plant) was secondary to the effect of increasing total protein in place of carbohydrates. However, as discussed in the sections above, the intervention diets differed in both the absolute amount of plant and animal protein content and the specific food sources of animal protein. In addition, the control diet not only provided different relative amounts of plant and animal proteins, but was also higher in saturated fat, cholesterol, and sodium and lower in fiber, potassium, calcium, and magnesium. Therefore, the vascular and lipid/lipoprotein improvements achieved cannot be attributed solely to the protein content of the intervention diets. Furthermore, the relative plant and animal protein contributions of the intervention diets cannot be directly compared without regard to the specific food sources of these proteins.
Exclusively plant-based dietary patterns.
The health-promoting effects of plant-based diets, such as the Portfolio and Ornish diets, are well established and provide evidence for the benefit of plant-based foods as a whole. However, they cannot be used as a direct comparison with animal protein consumption because these dietary patterns differ in multiple aspects, not just the source or type of protein. The Portfolio Diet is low in saturated fat and cholesterol and is characterized by its combination of plant sterols, viscous fiber, soy protein, and almonds, the 4 functional foods recommended by the AHA and National Cholesterol Education Panel Adult Treatment Panel III for their cholesterol-lowering capacity. Portfolio Diet interventions can achieve LDL cholesterol reductions that are not significantly different from those produced by first-generation statin therapy (73). In short-term intervention studies, LDL cholesterol reductions of 28–35% were achieved (73–75). Under free-living conditions, hyperlipidemic participants have also achieved LDL cholesterol reductions of >20% over a 1-y period (76). Interestingly, these improvements were achieved without complete adherence to dietary recommendations. After 1 y, only 2 of 55 participants were following a vegan diet, with 5 following a lacto-ovo-vegetarian diet. The remaining participants returned to an omnivorous diet. Despite this, a shift in the ratio of plant to animal protein intake was achieved because the total protein intake of the entire study population remained similar to prestudy values, whereas the percentage of animal protein was considerably reduced (76). The Portfolio Diet was also shown to lower the inflammatory marker C-reactive protein to a similar extent as statin therapy, an effect that has not been achieved by conventional cholesterol-lowering diets (73, 77).
The Ornish diet is also a plant-based diet that reduces CVD risk. It advocates intensive lifestyle changes and incorporates aerobic exercise, meditation, and smoking cessation in addition to dietary modifications. The diet is characterized by the exclusion of animal products (except for minimal amounts of nonfat yogurt), salt, alcohol, sugar, and caffeine; being very low fat (<10% of total calories); and emphasizing fresh fruits and vegetables, whole grains, legumes, tubers, and soybean products. Under short-term inpatient conditions, the Ornish diet improved left ventricular response to exercise, reduced total cholesterol by 20.5%, and reduced anginal episodes by 91% in patients with ischemic heart disease (78). These reductions in LDL cholesterol and the frequency of anginal episodes were also maintained under free-living conditions (79). Patients also achieved a significant regression in coronary artery stenosis, with the degree of regression being correlated to the degree of lifestyle change (79). After 5 y, these intensive lifestyle changes produced continued improvements and regression of coronary atherosclerosis compared with the standard-care control group (80). The LDL cholesterol reductions of 40% at 1 y and 20% after 5 y were also comparable with the effects of statin therapy.
Although greater plant protein intake is a primary feature of both diets, the resulting cardiovascular benefits cannot be ascribed solely to the replacement of animal protein with plant protein. Both dietary patterns consist of multiple components, including being low in fat and cholesterol. For the Portfolio Diet, each of the 4 key dietary components (i.e., plant sterols, viscous fiber, soy protein, and almonds) are well recognized for their cholesterol-lowering properties, and each likely contributes 4–7% to the overall observed LDL cholesterol reduction (81). The Ornish diet emphasizes complex carbohydrates and whole foods, avoidance of simple sugars, and lifestyle changes, such as aerobic exercise, stress management, smoking cessation, and group psychosocial support (80). It is likely that each of these factors contribute to the benefits derived from adopting these dietary patterns/lifestyle changes, and it is not possible to determine the relative contribution of each component. Furthermore, neither of these diets provide a direct comparison with animal protein because the control and intervention diets differ in multiple characteristics other than their relative plant and animal protein content.
Potential mechanisms explaining any differential benefits
If there is a differential effect of plant or animal protein on cardiovascular health, it may be due to the context in which it is consumed (e.g., the background diet and component of the diet it is replacing) and many other contributing factors, including nonprotein compounds provided in the food matrix, the amino acid profile of specific foods, and interactions with the gut microbiome.
Whole food package.
In addition to providing protein, plant- and animal-based foods provide a unique profile of amino acids, FAs, micronutrients, carbohydrates, and bioactives, all of which may have beneficial or adverse effects. A brief overview of the different dietary components provided by the major food sources of plant and animal protein is presented in Table 4. Each of these major food sources likely has different effects on CVD risk factors; however, these effects may be due to other components of their nutritional profile, not necessarily their protein content. For instance, cooking red meat at high temperatures can create heterocyclic amines, which are associated with higher rates of certain cancers (25), and advanced glycation end-products that can raise blood pressure via vasoconstriction and oxidative stress (98). Many components of red meat, including heme iron, cholesterol, and advanced glycation and lipoxidation end-products, were proposed as underlying factors potentially responsible for the relation between red meat consumption and type 2 diabetes, a significant CVD risk factor (99). In support of this, a recent analysis of the EPIC-Potsdam cohort identified specific metabolites that were associated with red meat consumption (i.e., high ferritin, low glycine, and altered hepatic-derived lipid concentrations) and acted as significant mediators of the association between total red meat consumption and diabetes risk (100). Although fish is also considered an animal protein, it provides a different package of nutrients, such as omega-3 FAs, and greater fish consumption is associated with reduced CVD risk and mortality (94). Similarly, the various food groups that provide plant protein contain a spectrum of dietary compounds and bioactives that affect human health differently. Therefore, the broad classification of animal and plant protein may be overly simplistic. These differences in bioactives also make direct dietary comparisons particularly challenging. Moreover, attempting to compare protein isolates from plant and animal sources does not reflect how these foods are consumed in the dietary patterns of free-living individuals. As a result, to better account for the total nutritional package, it may be preferable and more accurate to specify the particular food that provides the plant or animal protein.
TABLE 4.
Examples of other compounds and potential confounding factors found in major food sources of plant and animal protein1
| Source | Protein source | AAs | Lipid content | Micronutrients | Plant bioactives and other compounds |
| Plant | Legumes/beans (82–84) | Limiting AA: Met (generally low in SAA and Trp) | Generally low in fat but relatively high in PUFA | B vitamins (riboflavin, thiamin, niacin, B-6, B-12), vitamin E (soybeans) | Dietary fiber, primarily insoluble (e.g., lignin); amylose/resistant starch; nonstarch polysaccharides; oligosaccharides (e. g. raffinose) |
| Greater Lys content than cereals; also high in Ile | Fe (lentils, beans), Ca, K, Zn (lentils, beans), Se (lentils), Mg | Polyphenols (phenolic acids, flavonoids, tannins, ferulic acid, anthocyanins) (lentils, red kidney beans, black beans) | |||
| Phytosterols, phytoestrogens [e.g., lignans, isoflavones (soybeans)] | |||||
| Antinutrients: phytic acid, saponins, enzyme inhibitors (e. g. protease inhibitors), lectins | |||||
| Whole grain cereals (85) | Limiting AA: Lys (also low in Ile, Thr, Leu, and His) | Unsaturated FAs, primarily oleic acid and LA | B vitamins; vitamin E; carotenoids (lutein, zeaxanthin, β-carotene) | Dietary fiber, soluble (e.g., β-glucan) and insoluble; resistant starch; oligosaccharides (e.g., inulin) | |
| High in Met; good source of Phe, Trp, Val, and Iso | Mg, Zn, Se | Polyphenols [phenolic acids, ferulic acid, flavonoids, avenanthramides (oats), alkylresorcinols, γ-oryzanols (rice)] | |||
| Phytosterols, phytoestrogens (e.g., lignans) | |||||
| Methyl donors and lipotropes (betaine, choline) | |||||
| Antinutrients: phytic acid (inositol), tannins, enzyme inhibitors | |||||
| Tree nuts (and peanuts)/seeds (86, 87) | Relatively high in total protein (peanuts, walnuts, almonds) | Relatively high in total fat but low in SFA | Vitamin E; α-tocopherols (almonds, hazelnuts); γ-tocopherols (pecans, pistachios, walnuts); B vitamins; carotenoids (pistachios) | Dietary fiber (almonds) | |
| High in Arg and acidic AAs (Asp, Glu) | Good source of PUFAs [ALA (walnuts) and LA (Brazil nuts)] and MUFAs (most nuts) | K, Ca, Mg (Brazil nuts, pumpkin seeds), Se (Brazil nuts), Zn, Cu, Fe, Mn, P | Polyphenols (phenolic acids, flavonoids, stilbenes, proanthocyanins, lignans) (walnuts, pecans) | ||
| Limiting AA: Thr, Trp | Phytosterols (pistachios), squalene (Brazil nuts) and terpenoids, sphingolipids, phytoestrogens | ||||
| Low Arg-to-Lys ratio; low in Iso, Lys, and SAA | Melatonin (walnuts) | ||||
| Vegetables/fruits (88–90) | Generally lower total protein content (especially fruits) | Low | Vitamin C (citrus fruits, strawberries); carotenoids (orange-colored fruits/vegetables, spinach); vitamin K; folate; vitamin E | Dietary fiber, primarily insoluble (except cooked potatoes, oranges, grapefruit); pectin (fruits) | |
| Limiting AAs: Met; [some also low in Lys (starchy vegetables), Leu, Thr, and SAA (fruits)] | K (broccoli, banana), Mg, Fe, Ca | Polyphenols (flavonoids, resveratrol, isothiocyanates, phenolics, capsaicin, anthocyanins, tannins) | |||
| Phytosterols, phytoestrogens | |||||
| Organosulfur compounds, saponins, terpenes | |||||
| Animal | Red meat (26, 91, 92) | Complete protein | May be high in SFAs and cholesterol if not lean; moderate source of ω-6 FAs; may contain moderate amounts of the ω-3 FA DPA if grass-fed; CLA from ruminants | B vitamins (B-6, B-12 and niacin) | Choline, carnitine, carnosine, ubiquinone, glutathione |
| Fe (heme), Zn, P, K | Nitrite | ||||
| Carcinogenic compounds (heterocyclic amines, advanced glycation end-products) if cooked at high temperature (i.e., charred/blackened) | |||||
| Fish/seafood (93, 94) | Complete protein | High in long-chain ω-3 PUFAs (EPA, DPA, and DHA) (oily fish) | Vitamin D, vitamin B-12, vitamin A, vitamin E | Ubiquinone/coenzyme Q10 | |
| Low in SFAs | Se and I; also Fe, Zn, Mg, Ca | Taurine | |||
| Mercury (and other contaminants) if from contaminated waters (large fish, e.g., swordfish, shark, mackerel) | |||||
| Eggs (95) | Complete protein | Cholesterol | Vitamin D, vitamin A and carotenoids (lutein and zeaxanthin), vitamin B-12 | Lecithin, choline | |
| Particularly high in Leu | ω-3 PUFAs if free range | S, Fe, P, Ca, Cu, I, Mg, | |||
| Poultry | Complete protein | Higher in MUFAs; can contain more PUFAs if fed enriched meal | B vitamins, vitamin E | ||
| Relatively low in SFAs but can be high fat if dark meat | Zn, Fe, Mg, Se | ||||
| Dairy (96, 97) | Complete protein | Fat content varies on the basis of type of product (full-fat vs. low-fat) | Vitamin A, B vitamins, vitamin D (if fortified) | ||
| Contains whey and casein (particularly good source of BCAAs) | Ca, P, K, Mg, Zn, Se | ||||
| Can be high in Na [salted cheeses] | |||||
| Processed meat2 (34) | Modestly lower | Modestly higher total fat content (similar SFA content, similar or lower cholesterol) | ∼4-fold higher Na content | ∼50% more nonsalt additives/preservatives (nitrates, nitrites, nitrosamines) | |
| Less Fe | Compounds formed by high-temperature commercial cooking (heterocyclic amines) |
Examples of specific foods that contain particularly high amounts of a particular compound are provided in square brackets. AA, amino acid; ALA, α-linolenic acid; DPA, docosapentaenoic acid; LA, linoleic acid; SAA, sulfur-containing amino acid (Met and Cys).
Compared with nutritional content of red (unprocessed) meat.
Amino acid content.
In general, plant-based proteins are lower in essential amino acids (particularly methionine, lysine, and tryptophan) but provide greater amounts of the nonessential amino acids arginine, glycine, alanine, and serine (101). Essential and nonessential amino acids, and particular amino acids within these categories, have unique physiologic effects. In animal studies, the essential amino acids lysine and methionine can produce a marked hypercholesterolemic response (101, 102). Conversely, the essential BCAAs (leucine, isoleucine, and valine), which are particularly high in whey protein from dairy products, promote protein synthesis and decrease exercise-induced muscle damage (103, 104). Leucine in particular may play a unique role in stimulating muscle protein synthesis (105). Whey protein was also extensively investigated for lipid-lowering properties, although consistent results were only observed for short-term studies (96). Supplementation with BCAAs was studied intensively for potential benefits in liver disease and other catabolic states with inconclusive results (106). In terms of nonessential amino acids, arginine may be particularly beneficial for blood pressure because it is the precursor of the vasodilatory nitric oxide (5, 15). Therefore, it is possible that the differing concentrations of these amino acids in plant and animal proteins could be partly responsible for any differential effects.
Greater intake of certain amino acids was associated with lower blood pressure. In the INTERMAP study, individuals with high vegetable protein and low animal protein intakes consumed significantly greater proportions of glutamate, cysteine, proline, phenylalanine, and serine (17). The systolic and diastolic blood pressures of these individuals was also significantly lower (−4.15 mm Hg and −2.15 mm Hg, respectively) than those of individuals in the bottom quartiles of vegetable protein intake and top quartiles of animal protein intake (17). However, an analysis of amino acid intake and blood pressure in the Heart Institute of Spokane-Dietary Intervention and Evaluation Trial found somewhat discrepant results. In this population, blood pressure was positively associated with methionine and alanine but inversely related to threonine and histidine (98). Methionine is a precursor of homocysteine, a recognized CVD risk factor, and oral histidine administration in rats was shown to reduce blood pressure (98). However, for alanine and threonine, potential mechanisms for these associations are not known.
Gut microbiome.
Recent findings have also demonstrated a potential role of the gut microbiome in mediating the effect of certain animal proteins on CVD. The recent review by Tang and Hazen (107) summarizes the results and proposed mechanisms underlying this interaction between the diet, gut microbiota, and CVD. Briefly, hypothesis-generating metabolomics studies first identified that plasma concentrations of trimethylamine N-oxide (TMAO) correlated with CVD risk (108). It was subsequently established that TMAO is formed via microbial and hepatic metabolism of the dietary compounds phosphatidylcholine (lecithin), choline, and carnitine. These compounds are first metabolized to trimethylamine by trimethylamine-lyase enzymes, which are unique to gut microbiota, making this step dependent on microbial colonization of the gastrointestinal tract. Trimethylamine is then oxidized in the liver by flavin monooxygenase 3, forming TMAO that enters circulation. Elevated plasma TMAO was consistently shown to be a strong predictor of major adverse cardiac events, including sudden death and nonfatal myocardial infarction and stroke (109). In apoE−/− mouse models, TMAO supplementation enhances macrophage foam cell formation in the artery wall (108), promotes aortic root atherosclerotic plaque development (108), reduces reverse cholesterol transport (110), modifies bile acid pool size and composition (110), and alters cholesterol and sterol metabolic pathways in the artery wall, liver, and intestines (110).
However, despite the consistent prognostic value of TMAO in humans and the mechanistic insights provided by mouse models, the direct molecular target of TMAO responsible for mediating these effects remains unknown. Because animal-based foods such as red meat and eggs are the primary dietary sources of l-carnitine and choline, these types of food were the focus of proposed dietary causes of TMAO formation and heightened atherosclerotic risk. However, choline is an essential nutrient required for metabolic processes such as DNA methylation, and is also found in fish/seafood and in plant-based foods, including soy. It has yet to be evaluated whether choline from plant-based sources produces the same TMAO-generating effect. Irrespective of whether choline is obtained from a plant- or animal-based food, it contains the same quaternary ammonium cation that is the active site for trimethylamine-lyase enzymes. Therefore, it seems unlikely that choline from different dietary sources would be differentially recognized and metabolized by gut microbiota. Thus, it may be that the adverse effects and TMAO generation attributed to animal-based sources of l-carnitine and choline may instead be due to other compounds in the protein source (Table 4) or associated lifestyle factors that increase CVD risk. It is also possible that compounds in these foods or the lifestyle factors associated with their consumption may shift the composition of the gut microbiota toward microbial species with greater capacity for producing trimethylamine, ultimately increasing TMAO concentrations.
Conclusion
The 2010 DGAC’s evidence review stated that there is only limited and inconsistent evidence for a differential effect of plant-based compared with animal-based protein, and the 2015 DGAC has not updated this statement. However, despite its limitations, the current evidence base has identified key factors that require consideration when evaluating comparisons of plant and animal protein, designing future studies, and updating dietary guidelines. It is likely that cultural and individual preference variations in the background diet, the specific food source and concomitant compounds ingested with the protein, and how the food is prepared all play a key role in determining whether that type of protein has beneficial cardiovascular effects. For both intermediate risk factors (e.g., blood pressure) and CVD risk outcomes, inverse associations with plant or animal protein may particularly depend on the type of carbohydrate (i.e., refined compared with complex) being replaced by greater protein consumption or the type of animal protein consumed (i.e., red meat or fish). Specific plant protein sources such as soy were also shown to lower cholesterol, but there is not similar evidence for other plant-based sources of protein and this effect of soy may be due to components other than protein. Even with greater specificity and characterization of plant and animal protein sources, the unique nutrition profiles and bioactives provided in the whole-food matrix make direct comparisons particularly challenging. Although the DASH diet and OmniHeart high-protein DASH diet variation demonstrate the beneficial effects of increasing plant-based food consumption, these benefits cannot be attributed solely to plant protein because these dietary patterns include multiple components. These studies also were not designed specifically to compare plant and animal protein and did not include a matched comparator diet that differed only in the source of protein. Similarly, although the BOLD study demonstrated that a DASH diet variation that incorporated lean beef can elicit similar cardiovascular benefits, the intervention diets contained different plant and animal sources of protein (e.g., lean beef compared with other animal sources) and cannot conclusively address the question of differential effects.
If the current interest in plant protein continues and more conclusive evidence of a differential health effect is found, it will be important to consider how an increase in plant protein intake can be best achieved. As previously noted, an increase in one dietary macronutrient typically results in the replacement of another; thus, achieving a beneficial effect will likely depend on how plant protein is incorporated in the diet. If the recommendation is that plant protein be consumed in place of carbohydrates, this should be achieved by replacing refined carbohydrates and added sugars rather than fiber-rich complex carbohydrates such as whole grains. If plant protein is recommended in place of dietary lipids, saturated fat and trans fat should be the focus of replacement, not unsaturated fats. If plant protein is recommended as a substitute for animal protein, differences in energy, protein density, and the amino acid and nutrient profile of plant-based compared with animal-based foods must be kept in mind. Because many plant-based foods are typically less energy dense, shifting the plant-to-animal protein ratio may also result in fewer calories consumed and weight loss, which would provide further CVD risk reduction. Greater quantities of plant-based sources of protein would need to be consumed, however, to achieve the same total protein intake because plant-based foods provide less protein per gram of food. For nuts/peanuts, which are relatively more energy dense than other plant-based foods, this may need to be taken into consideration to ensure that this does not translate into the consumption of excess calories to achieve the same protein intake. This increased consumption of plant sources of protein would also provide the benefit of greater intakes of particular nutrients and bioactives (although animal-based sources of protein can serve a similar role in their provision of important dietary compounds, depending on the particular food source; Table 4). Approximately 70% of total protein consumed in the United States is from animal products, primarily meat, fish, and poultry, followed by dairy (88, 111). This preference for animal protein may make it challenging to achieve a greater plant protein intake by substituting animal protein. Although plant protein could be increased in other ways, such as substituting refined carbohydrates and/or solid fats, this may be similarly challenging, given Americans’ penchant for sweets and other foods that contain solid fats. Regardless, there is ample room in the current US diet for shifting the type/source of dietary protein, fat, and/or carbohydrate toward evidence-based dietary patterns that emphasize whole foods and include protein from both plant and animal sources.
In conclusion, numerous observational and intervention studies have sought to address the question of whether plant and animal protein differ for reducing CVD risk factors. However, evidence to date is inconclusive and is likely to remain so, because it is difficult to isolate the independent effects of specific proteins. The contribution of other components in the plant or animal food source and the background diet are important considerations. To minimize CVD risk, evidence supports plant-based dietary patterns that emphasize protein-rich plant foods and include some animal-based sources of protein (e.g., fish/seafood, eggs, low-fat dairy, poultry, and lean meats) in place of refined carbohydrates and processed meats.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: BOLD, Beef in an Optimal Lean Diet; CHD, coronary heart disease; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; DGAC, Dietary Guidelines Advisory Committee; FV diet, fruit and vegetables diet; OmniHeart, Optimal Macronutrient Intake Trial to Prevent Heart Disease; TMAO, trimethylamine N-oxide.
References
- 1.Hu FB. Protein, body weight, and cardiovascular health. Am J Clin Nutr 2005;82(1 Suppl):242S–7S. [DOI] [PubMed] [Google Scholar]
- 2.Leidy HJ, Clifton PM, Astrup A, Wycherley TP, Westerterp-Plantenga MS, Luscombe-Marsh ND, Woods SC, Mattes RD. The role of protein in weight loss and maintenance. Am J Clin Nutr 2015;101:1320S–9S. [DOI] [PubMed] [Google Scholar]
- 3.Vasdev S, Stuckless J. Antihypertensive effects of dietary protein and its mechanism. Int J Angiol 2010;19:e7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington (DC): Institute of Medicine; 2005. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez NR, Miller SL. Effective translation of current dietary guidance: understanding and communicating the concepts of minimal and optimal levels of dietary protein. Am J Clin Nutr 2015;101:1353S–8S. [DOI] [PubMed] [Google Scholar]
- 6.US Department of Agriculture and US Department of Health and Human Services. Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans, 2010. Washington (DC): USDA and US Department of Health and Human Services; 2010. [Google Scholar]
- 7.Office of Disease Prevention and Health Promotion, 2015 Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. Washington (DC): Office of Disease Prevention and Health Promotion; 2015.
- 8.Sacks FM, Rosner B, Kass EH. Blood pressure in vegetarians. Am J Epidemiol 1974;100:390–8. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong B, van Merwyk AJ, Coates H. Blood pressure in Seventh-day Adventist vegetarians. Am J Epidemiol 1977;105:444–9. [DOI] [PubMed] [Google Scholar]
- 10.Sacks FM, Castelli WP, Donner A, Kass EH. Plasma lipids and lipoproteins in vegetarians and controls. N Engl J Med 1975;292:1148–51. [DOI] [PubMed] [Google Scholar]
- 11.Beilin LJ. Vegetarian and other complex diets, fats, fiber, and hypertension. Am J Clin Nutr 1994;59(5 Suppl):1130S–5S. [DOI] [PubMed] [Google Scholar]
- 12.Wang YF, Yancy WS Jr, Yu D, Champagne C, Appel LJ, Lin PH. The relationship between dietary protein intake and blood pressure: results from the PREMIER study. J Hum Hypertens 2008;22:745–54. [DOI] [PubMed] [Google Scholar]
- 13.Obarzanek E, Velletri PA, Cutler JA. Dietary protein and blood pressure. JAMA 1996;275:1598–603. [DOI] [PubMed] [Google Scholar]
- 14.Teunissen-Beekman KF, van Baak MA. The role of dietary protein in blood pressure regulation. Curr Opin Lipidol 2013;24:65–70. [DOI] [PubMed] [Google Scholar]
- 15.Appel LJ. The effects of protein intake on blood pressure and cardiovascular disease. Curr Opin Lipidol 2003;14:55–9. [DOI] [PubMed] [Google Scholar]
- 16.Stamler J, Liu K, Ruth KJ, Pryer J, Greenland P. Eight-year blood pressure change in middle-aged men: relationship to multiple nutrients. Hypertension 2002;39:1000–6. [DOI] [PubMed] [Google Scholar]
- 17.Elliott P, Stamler J, Dyer AR, Appel L, Dennis B, Kesteloot H, Ueshima H, Okayama A, Chan Q, Garside DB, et al. . Association between protein intake and blood pressure: the INTERMAP Study. Arch Intern Med 2006;166:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kihara M, Fujikawa J, Ohtaka M, Mano M, Nara Y, Horie R, Tsunematsu T, Note S, Fukase M, Yamori Y. Interrelationships between blood pressure, sodium, potassium, serum cholesterol, and protein intake in Japanese. Hypertension 1984;6:736–42. [DOI] [PubMed] [Google Scholar]
- 19.Zhou B, Zhang X, Zhu A, Zhao L, Zhu S, Ruan L, Zhu L, Liang S. The relationship of dietary animal protein and electrolytes to blood pressure: a study on three Chinese populations. Int J Epidemiol 1994;23:716–22. [DOI] [PubMed] [Google Scholar]
- 20.Zhou BF, Wu XG, Tao SQ, Yang J, Cao TX, Zheng RP, Tian XZ, Lu CQ, Miao HY, Ye FM, et al. . Dietary patterns in 10 groups and the relationship with blood pressure. Collaborative Study Group for Cardiovascular Diseases and Their Risk Factors. Chin Med J (Engl) 1989;102:257–61. [PubMed] [Google Scholar]
- 21.Altorf-van der Kuil W, Engberink MF, Brink EJ, van Baak MA, Bakker SJ, Navis G, van ’t Veer P, Geleijnse JM. Dietary protein and blood pressure: a systematic review. PLoS One 2010;5:e12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Speizer FE, Hennekens CH, Willett WC. Dietary protein and risk of ischemic heart disease in women. Am J Clin Nutr 1999;70:221–7. [DOI] [PubMed] [Google Scholar]
- 23.Kelemen LE, Kushi LH, Jacobs DR Jr, Cerhan JR. Associations of dietary protein with disease and mortality in a prospective study of postmenopausal women. Am J Epidemiol 2005;161:239–49. [DOI] [PubMed] [Google Scholar]
- 24.Halton TL, Willett WC, Liu S, Manson JE, Albert CM, Rexrode K, Hu FB. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med 2006;355:1991–2002. [DOI] [PubMed] [Google Scholar]
- 25.Sinha R, Cross AJ, Graubard BI, Leitzmann MF, Schatzkin A. Meat intake and mortality: a prospective study of over half a million people. Arch Intern Med 2009;169:562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation 2010;122:876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Preis SR, Stampfer MJ, Spiegelman D, Willett WC, Rimm EB. Dietary protein and risk of ischemic heart disease in middle-aged men. Am J Clin Nutr 2010;92:1265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Preis SR, Stampfer MJ, Spiegelman D, Willett WC, Rimm EB. Lack of association between dietary protein intake and risk of stroke among middle-aged men. Am J Clin Nutr 2010;91:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fung TT, van Dam RM, Hankinson SE, Stampfer M, Willett WC, Hu FB. Low-carbohydrate diets and all-cause and cause-specific mortality: two cohort studies. Ann Intern Med 2010;153:289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, Willett WC, Hu FB. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med 2012;172:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rohrmann S, Overvad K, Bueno-de-Mesquita HB, Jakobsen MU, Egeberg R, Tjonneland A, Nailler L, Boutron-Ruault MC, Clavel-Chapelon F, Krogh V, et al. . Meat consumption and mortality–results from the European Prospective Investigation into Cancer and Nutrition. BMC Med 2013;11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaluza J, Akesson A, Wolk A. Processed and unprocessed red meat consumption and risk of heart failure: prospective study of men. Circ Heart Fail 2014;7:552–7. [DOI] [PubMed] [Google Scholar]
- 33.American Heart Association [Internet]. Dallas: The Association. What is a serving? [cited 2015 Jul 22]. Available from: http://www.heart.org/HEARTORG/Caregiver/Replenish/WhatisaServing/What-is-a-Serving_UCM_301838_Article.jsp.
- 34.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation 2010;121:2271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Cancer Institute Applied Research Program [Internet]. Bethesda (MD): National Cancer Institute Applied Research Program [updated 2013 Oct 18; cited 2015 Jan 3]. Available from: http://appliedresearch.cancer.gov/diet/foodsources/sat_fat/sf.html.
- 36.Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes–an updated review of the evidence. Curr Atheroscler Rep 2012;14:515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z, Xu G, Yang F, Zhu W, Liu X. Quantitative analysis of dietary protein intake and stroke risk. Neurology 2014;83:19–25. [DOI] [PubMed] [Google Scholar]
- 38.Lagiou P, Sandin S, Weiderpass E, Lagiou A, Mucci L, Trichopoulos D, Adami HO. Low carbohydrate-high protein diet and mortality in a cohort of Swedish women. J Intern Med 2007;261:366–74. [DOI] [PubMed] [Google Scholar]
- 39.Willett WC. Low-carbohydrate diets: a place in health promotion? J Intern Med 2007;261:363–5. [DOI] [PubMed] [Google Scholar]
- 40.Gross LS, Li L, Ford ES, Liu S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am J Clin Nutr 2004;79:774–9. [DOI] [PubMed] [Google Scholar]
- 41.Liu S, Manson JE. Dietary carbohydrates, physical inactivity, obesity, and the ‘metabolic syndrome’ as predictors of coronary heart disease. Curr Opin Lipidol 2001;12:395–404. [DOI] [PubMed] [Google Scholar]
- 42.Hu FB, van Dam RM, Liu S. Diet and risk of type II diabetes: the role of types of fat and carbohydrate. Diabetologia 2001;44:805–17. [DOI] [PubMed] [Google Scholar]
- 43.Reaven GM. Relationships among insulin resistance, type 2 diabetes, essential hypertension, and cardiovascular disease: similarities and differences. J Clin Hypertens (Greenwich) 2011;13:238–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991;14:173–94. [DOI] [PubMed] [Google Scholar]
- 45.Pollare T, Lithell H, Berne C. Insulin resistance is a characteristic feature of primary hypertension independent of obesity. Metabolism 1990;39:167–74. [DOI] [PubMed] [Google Scholar]
- 46.Kopp W. Pathogenesis and etiology of essential hypertension: role of dietary carbohydrate. Med Hypotheses 2005;64:782–7. [DOI] [PubMed] [Google Scholar]
- 47.Anderson JW, Baird P, Davis RH Jr, Ferreri S, Knudtson M, Koraym A, Waters V, Williams CL. Health benefits of dietary fiber. Nutr Rev 2009;67:188–205. [DOI] [PubMed] [Google Scholar]
- 48.Champagne CM. Magnesium in hypertension, cardiovascular disease, metabolic syndrome, and other conditions: a review. Nutr Clin Pract 2008;23:142–51. [DOI] [PubMed] [Google Scholar]
- 49.D’Elia L, Barba G, Cappuccio FP, Strazzullo P. Potassium intake, stroke, and cardiovascular disease a meta-analysis of prospective studies. J Am Coll Cardiol 2011;57:1210–9. [DOI] [PubMed] [Google Scholar]
- 50.Obarzanek E, Sacks FM, Vollmer WM, Bray GA, Miller ER 3rd, Lin PH, Karanja NM, Most-Windhauser MM, Moore TJ, Swain JF, et al. . Effects on blood lipids of a blood pressure-lowering diet: the Dietary Approaches to Stop Hypertension (DASH) Trial. Am J Clin Nutr 2001;74:80–9. [DOI] [PubMed] [Google Scholar]
- 51.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, et al. . A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997;336:1117–24. [DOI] [PubMed] [Google Scholar]
- 52.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER 3rd, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, et al. . Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA 2005;294:2455–64. [DOI] [PubMed] [Google Scholar]
- 53.Roussell MA, Hill AM, Gaugler TL, West SG, Heuvel JP, Alaupovic P, Gillies PJ, Kris-Etherton PM. Beef in an Optimal Lean Diet study: effects on lipids, lipoproteins, and apolipoproteins. Am J Clin Nutr 2012;95:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sacks FM, Obarzanek E, Windhauser MM, Svetkey LP, Vollmer WM, McCullough M, Karanja N, Lin PH, Steele P, Proschan MA, et al. . Rationale and design of the Dietary Approaches to Stop Hypertension trial (DASH). A multicenter controlled-feeding study of dietary patterns to lower blood pressure. Ann Epidemiol 1995;5:108–18. [DOI] [PubMed] [Google Scholar]
- 55.Roussell MA, Hill AM, Gaugler TL, West SG, Ulbrecht JS, Vanden Heuvel JP, Gillies PJ, Kris-Etherton PM. Effects of a DASH-like diet containing lean beef on vascular health. J Hum Hypertens 2014;28:600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carroll KK. Dietary protein in relation to plasma cholesterol levels and atherosclerosis. Nutr Rev 1978;36:1–5. [DOI] [PubMed] [Google Scholar]
- 57.Carroll KK, Giovannetti PM, Huff MW, Moase O, Roberts DC, Wolfe BM. Hypocholesterolemic effect of substituting soybean protein for animal protein in the diet of healthy young women. Am J Clin Nutr 1978;31:1312–21. [DOI] [PubMed] [Google Scholar]
- 58.Sirtori CR, Agradi E, Conti F, Mantero O, Gatti E. Soybean-protein diet in the treatment of type-II hyperlipoproteinaemia. Lancet 1977;1:275–7. [DOI] [PubMed] [Google Scholar]
- 59.Anderson JW, Johnstone BM, Cook-Newell ME. Meta-analysis of the effects of soy protein intake on serum lipids. N Engl J Med 1995;333:276–82. [DOI] [PubMed] [Google Scholar]
- 60.Weggemans RM, Trautwein EA. Relation between soy-associated isoflavones and LDL and HDL cholesterol concentrations in humans: a meta-analysis. Eur J Clin Nutr 2003;57:940–6. [DOI] [PubMed] [Google Scholar]
- 61.Zhan S, Ho SC. Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am J Clin Nutr 2005;81:397–408. [DOI] [PubMed] [Google Scholar]
- 62.Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris-Etherton P, Winston M. Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation 2006;113:1034–44. [DOI] [PubMed] [Google Scholar]
- 63.Jenkins DJ, Mirrahimi A, Srichaikul K, Berryman CE, Wang L, Carleton A, Abdulnour S, Sievenpiper JL, Kendall CW, Kris-Etherton PM. Soy protein reduces serum cholesterol by both intrinsic and food displacement mechanisms. J Nutr 2010;140:2302S–11S. [DOI] [PubMed] [Google Scholar]
- 64.Nagata C, Shimizu H, Takami R, Hayashi M, Takeda N, Yasuda K. Association of blood pressure with intake of soy products and other food groups in Japanese men and women. Prev Med 2003;36:692–7. [DOI] [PubMed] [Google Scholar]
- 65.Liu XX, Li SH, Chen JZ, Sun K, Wang XJ, Wang XG, Hui RT. Effect of soy isoflavones on blood pressure: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis 2012;22:463–70. [DOI] [PubMed] [Google Scholar]
- 66.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER 3rd, Simons-Morton DG, et al. . Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 67.Karanja NM, Obarzanek E, Lin PH, McCullough ML, Phillips KM, Swain JF, Champagne CM, Hoben KP. Descriptive characteristics of the dietary patterns used in the Dietary Approaches to Stop Hypertension Trial. DASH Collaborative Research Group. J Am Diet Assoc 1999;99(8 Suppl):S19–27. [DOI] [PubMed] [Google Scholar]
- 68.Swain JF, McCarron PB, Hamilton EF, Sacks FM, Appel LJ. Characteristics of the diet patterns tested in the optimal macronutrient intake trial to prevent heart disease (OmniHeart): options for a heart-healthy diet. J Am Diet Assoc 2008;108:257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patvardhan E, Heffernan KS, Ruan J, Hession M, Warner P, Karas RH, Kuvin JT. Augmentation index derived from peripheral arterial tonometry correlates with cardiovascular risk factors. Cardiol Res Pract 2011;2011:253758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nürnberger J, Keflioglu-Scheiber A, Opazo Saez AM, Wenzel RR, Philipp T, Schäfers RF. Augmentation index is associated with cardiovascular risk. J Hypertens 2002;20:2407–14. [DOI] [PubMed] [Google Scholar]
- 71.Weber T, Auer J, O’Rourke MF, Kvas E, Lassnig E, Berent R, Eber B. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation 2004;109:184–9. [DOI] [PubMed] [Google Scholar]
- 72.Hayashi T, Nakayama Y, Tsumura K, Yoshimaru K, Ueda H. Reflection in the arterial system and the risk of coronary heart disease. Am J Hypertens 2002;15:405–9. [DOI] [PubMed] [Google Scholar]
- 73.Jenkins DJ, Kendall CW, Marchie A, Faulkner DA, Wong JM, de Souza R, Emam A, Parker TL, Vidgen E, Lapsley KG, et al. . Effects of a dietary portfolio of cholesterol-lowering foods vs lovastatin on serum lipids and C-reactive protein. JAMA 2003;290:502–10. [DOI] [PubMed] [Google Scholar]
- 74.Jenkins DJ, Kendall CW, Faulkner D, Vidgen E, Trautwein EA, Parker TL, Marchie A, Koumbridis G, Lapsley KG, Josse RG, et al. . A dietary portfolio approach to cholesterol reduction: combined effects of plant sterols, vegetable proteins, and viscous fibers in hypercholesterolemia. Metabolism 2002;51:1596–604. [DOI] [PubMed] [Google Scholar]
- 75.Jenkins DJ, Kendall CW, Marchie A, Faulkner D, Vidgen E, Lapsley KG, Trautwein EA, Parker TL, Josse RG, Leiter LA, et al. . The effect of combining plant sterols, soy protein, viscous fibers, and almonds in treating hypercholesterolemia. Metabolism 2003;52:1478–83. [DOI] [PubMed] [Google Scholar]
- 76.Jenkins DJ, Kendall CW, Faulkner DA, Nguyen T, Kemp T, Marchie A, Wong JM, de Souza R, Emam A, Vidgen E, et al. . Assessment of the longer-term effects of a dietary portfolio of cholesterol-lowering foods in hypercholesterolemia. Am J Clin Nutr 2006;83:582–91. [DOI] [PubMed] [Google Scholar]
- 77.Jenkins DJ, Kendall CW, Marchie A, Faulkner DA, Josse AR, Wong JM, de Souza R, Emam A, Parker TL, Li TJ, et al. . Direct comparison of dietary portfolio vs statin on C-reactive protein. Eur J Clin Nutr 2005;59:851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ornish D, Scherwitz LW, Doody RS, Kesten D, McLanahan SM, Brown SE, DePuey E, Sonnemaker R, Haynes C, Lester J, et al. . Effects of stress management training and dietary changes in treating ischemic heart disease. JAMA 1983;249:54–9. [PubMed] [Google Scholar]
- 79.Ornish D, Brown SE, Scherwitz LW, Billings JH, Armstrong WT, Ports TA, McLanahan SM, Kirkeeide RL, Brand RJ, Gould KL. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet 1990;336:129–33. [DOI] [PubMed] [Google Scholar]
- 80.Ornish D, Scherwitz LW, Billings JH, Brown SE, Gould KL, Merritt TA, Sparler S, Armstrong WT, Ports TA, Kirkeeide RL, et al. . Intensive lifestyle changes for reversal of coronary heart disease. JAMA 1998;280:2001–7. [DOI] [PubMed] [Google Scholar]
- 81.Jenkins DJ, Kendall CW, Marchie A, Faulkner DA, Wong JM, de Souza R, Emam A, Parker TL, Vidgen E, Trautwein EA, et al. . Direct comparison of a dietary portfolio of cholesterol-lowering foods with a statin in hypercholesterolemic participants. Am J Clin Nutr 2005;81:380–7. [DOI] [PubMed] [Google Scholar]
- 82.Rebello CJ, Greenway FL, Finley JW. A review of the nutritional value of legumes and their effects on obesity and its related co-morbidities. Obes Rev 2014;15:392–407. [DOI] [PubMed] [Google Scholar]
- 83.Bouchenak M, Lamri-Senhadji M. Nutritional quality of legumes, and their role in cardiometabolic risk prevention: a review. J Med Food 2013;16:185–98. [DOI] [PubMed] [Google Scholar]
- 84.Campos-Vega R, Loarca-Piña G, Oomah BD. Minor components of pulses and their potential impact on human health. Food Res Int 2010;43:461–82. [Google Scholar]
- 85.Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev 2010;23:65–134. [DOI] [PubMed] [Google Scholar]
- 86.Alasalvar C, Shahidi F. Tree nuts: composition, phytochemicals, and health effects. Boca Raton: CRC Press, 2009. [Google Scholar]
- 87.Sabaté J, Wien M. Consumption of nuts in the prevention of cardiovascular disease. Curr Nutr Rep 2013;2:258–66. [Google Scholar]
- 88.Young VR, Pellett PL. Plant proteins in relation to human protein and amino acid nutrition. Am J Clin Nutr 1994;59(5 Suppl):1203S–12S. [DOI] [PubMed] [Google Scholar]
- 89.Slavin JL, Lloyd B. Health benefits of fruits and vegetables. Adv Nutr 2012;3:506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu RH. Health-promoting components of fruits and vegetables in the diet. Adv Nutr 2013;4:384S–92S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McAfee AJ, McSorley EM, Cuskelly GJ, Moss BW, Wallace JM, Bonham MP, Fearon AM. Red meat consumption: an overview of the risks and benefits. Meat Sci 2010;84:1–13. [DOI] [PubMed] [Google Scholar]
- 92.Williams P. Nutritional composition of red meat. Nutr Diet 2007;64:S113–9. [Google Scholar]
- 93.Lund EK. Health benefits of seafood; is it just the fatty acids? Food Chem 2013;140:413–20. [DOI] [PubMed] [Google Scholar]
- 94.Larsen R, Eilertsen KE, Elvevoll EO. Health benefits of marine foods and ingredients. Biotechnol Adv 2011;29:508–18. [DOI] [PubMed] [Google Scholar]
- 95.Layman DK, Rodriguez NR. Egg protein as a source of power, strength, and energy. Nutr Today 2009;44:43–8. [Google Scholar]
- 96.El Khoury D, Anderson GH. Recent advances in dietary proteins and lipid metabolism. Curr Opin Lipidol 2013;24:207–13. [DOI] [PubMed] [Google Scholar]
- 97.Weaver CM. How sound is the science behind the dietary recommendations for dairy? Am J Clin Nutr 2014;99(5 Suppl):1217S–22S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tuttle KR, Milton JE, Packard DP, Shuler LA, Short RA. Dietary amino acids and blood pressure: a cohort study of patients with cardiovascular disease. Am J Kidney Dis 2012;59:803–9. [DOI] [PubMed] [Google Scholar]
- 99.Feskens EJ, Sluik D, van Woudenbergh GJ. Meat consumption, diabetes, and its complications. Curr Diab Rep 2013;13:298–306. [DOI] [PubMed] [Google Scholar]
- 100.Wittenbecher C, Muhlenbruch K, Kroger J, Jacobs S, Kuxhaus O, Floegel A, Fritsche A, Pischon T, Prehn C, Adamski J, et al. . Amino acids, lipid metabolites, and ferritin as potential mediators linking red meat consumption to type 2 diabetes. Am J Clin Nutr 2015;101:1241–50. [DOI] [PubMed] [Google Scholar]
- 101.Krajcovicova-Kudlackova M, Babinska K, Valachovicova M. Health benefits and risks of plant proteins. Bratisl Lek Listy 2005;106:231–4. [PubMed] [Google Scholar]
- 102.Giroux I, Kurowska EM, Carroll KK. Role of dietary lysine, methionine, and arginine in the regulation of hypercholesterolemia in rabbits. J Nutr Biochem 1999;10:166–71. [DOI] [PubMed] [Google Scholar]
- 103.Blomstrand E, Eliasson J, Karlsson HK, Kohnke R. Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. J Nutr 2006;136(1 Suppl):269S–73S. [DOI] [PubMed] [Google Scholar]
- 104.Negro M, Giardina S, Marzani B, Marzatico F. Branched-chain amino acid supplementation does not enhance athletic performance but affects muscle recovery and the immune system. J Sports Med Phys Fitness 2008;48:347–51. [PubMed] [Google Scholar]
- 105.Layman DK, Anthony TG, Rasmussen BB, Adams SH, Lynch CJ, Brinkworth GD, Davis TA. Defining meal requirements for protein to optimize metabolic roles of amino acids. Am J Clin Nutr 2015;101:1330S–8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Platell C, Kong SE, McCauley R, Hall JC. Branched-chain amino acids. J Gastroenterol Hepatol 2000;15:706–17. [DOI] [PubMed] [Google Scholar]
- 107.Tang WH, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J Clin Invest 2014;124:4204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, et al. . Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368:1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, et al. . Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Smit E, Nieto FJ, Crespo CJ, Mitchell P. Estimates of animal and plant protein intake in US adults: results from the Third National Health and Nutrition Examination Survey, 1988–1991. J Am Diet Assoc 1999;99:813–20. [DOI] [PubMed] [Google Scholar]