Abstract
Recently, low-residue diets were removed from the American Academy of Nutrition and Dietetics’ Nutrition Care Manual due to the lack of a scientifically accepted quantitative definition and the unavailability of a method to estimate the amount of food residue produced. This narrative review focuses on defining the similarities and/or discrepancies between low-residue and low-fiber diets and on the diagnostic and therapeutic values of these diets in gastrointestinal disease management. Diagnostically, a low-fiber/low-residue diet is used in bowel preparation. A bowel preparation is a cleansing of the intestines of fecal matter and secretions conducted before a diagnostic procedure. Therapeutically, a low-fiber/low-residue diet is part of the treatment of acute relapses in different bowel diseases. The available evidence on low-residue and low-fiber diets is summarized. The main findings showed that within human disease research, the terms “low residue” and “low fiber” are used interchangeably, and information related to the quantity of residue in the diet usually refers to the amount of fiber. Low-fiber/low-residue diets are further explored in both diagnostic and therapeutic situations. On the basis of this literature review, the authors suggest redefining a low-residue diet as a low-fiber diet and to quantitatively define a low-fiber diet as a diet with a maximum of 10 g fiber/d. A low-fiber diet instead of a low-residue diet is recommended as a diagnostic value or as specific therapy for gastrointestinal conditions.
Keywords: dietary intake, fiber, gastrointestinal disorders, low-fiber diet, low-residue diet, bowel preparation
Introduction
Digestion in the human gastrointestinal tract (GIT)7 involves a complex series of organs and glands that process food. During passage through the GIT, food digestion starts in the mouth and is completed in the small intestine. Some material passes undigested through the small intestine and is fermented in the colon. Both mechanical forces and chemical reactions break down ingested food into small molecules (1). Most digested molecules of food, as well as water and minerals, are absorbed through the small intestine. Nondigested nutrients are further metabolized in the large intestine by the intestinal microbiota and partly made available to the host (2). However, some remnants, such as indigestible food material, microorganisms, and secretions and desquamated intestinal cells, the so-called residue of digestion, remain and are discharged from the GIT as feces.
In some gastrointestinal conditions, such as Crohn disease, ulcerative colitis, bowel inflammation, irritable bowel syndrome (IBS), and diverticulitis, as well as pre- and/or postabdominal surgery, low-residue diets are prescribed to limit the amount of food waste that has to move through the large intestine. The American Academy of Nutrition and Dietetics removed the low-residue diet from the Nutrition Care Manual because there is no scientifically acceptable definition for the term “residue” or “residue-low” and the lack of qualitative studies limits scientific evidence on the effects of low-residue diets (3). In addition, the amount of residue produced during passing through the GIT cannot be estimated.
In most studies, information on the quantity of residue in the diet refers to the amount of fiber. Fiber is the part of fruit, vegetables, and grains that is not digested by the body and is proposed as a necessary component of a healthy diet and required for normal bowel movements (4). In practice, within human disease research, both the terms “low residue” and “low fiber” are used interchangeably. In this narrative review, we critically review the diagnostic and therapeutic values of low-residue and low-fiber diets in gastrointestinal disease management.
Current Status of Knowledge
Defining “residue” and “dietary fiber”
Residue.
Until now, there has been no scientifically acceptable definition of residue. The impossibility of estimating the amount of residue produced by the digestion of various foods complicates a consensus definition for residue. In the literature, residue mostly refers to any indigestible food substance that remains in the intestinal tract and contributes to stool bulk (3, 5). This means that the residue of digestion is primarily indigestible material (i.e., dietary fiber), microorganisms, and secretions and cells shed from the alimentary tract. The latter 2 increase the fecal output after digestion (3).
Dietary fiber.
Originally, Trowell (6) defined fiber as components derived from the cell wall of a plant that resist hydrolysis by digestive enzymes and absorption in the small intestine. Typical plant cell wall components are pectin, cellulose, and hemicellulose. This definition was ascertained as being too strict and other substances, such as starch and fructans, were also defined as fiber. However, until now, a universally accepted definition for dietary fiber has been unavailable. The Codex Committee on Nutrition and Foods for Special Dietary Uses defined dietary fiber as carbohydrate polymers with a degree of polymerization [DP; or monomeric (single sugar) units] not lower than 3, which are neither digested nor absorbed in the small intestine. A DP not lower than 3 is intended to exclude mono- and disaccharides. Dietary fiber consists of one or more 1) edible carbohydrate polymers naturally occurring in the food as consumed; 2) carbohydrate polymers, which have been obtained from food raw material by physical, enzymatic, or chemical means; and 3) synthetic carbohydrate polymers (7). This definition is supported by the American Association of Cereal Chemists (8). The WHO and FAO agree with the latter definition but with a slight variation. They state that dietary fiber is a polysaccharide with ≥10 monomeric units (9). A recent report from the Ninth Vahouny Fiber Symposium indicated that the scientific community agrees on maintaining a worldwide consensus with regard to the inclusion of nondigestible carbohydrates with a minimum DP of 3 as dietary fiber and on a core, nonexhaustive list of beneficial physiologic effects of dietary fibers (10). The European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies published in 2010 a scientific opinion on dietary reference values for the intake of carbohydrates, dietary fiber, fats, and water. In this opinion, dietary fiber was defined as nondigestible carbohydrates plus lignin (11). In addition, in the United States, the FDA proposed to accept this definition (12). Table 1 gives an overview of different types of carbohydrates and their common food source, average DP, and main properties (13, 14).
TABLE 1.
Classification of carbohydrates according to average DP and their main properties1
| Effects |
|||||||
| Carbohydrate | Example | Average DP | Digestion | Fermentation | Upper GIT | Colon | Food source |
| Monosaccharides | Glucose, fructose, galactose | 1 | Complete | Fruit (juice) | |||
| Disaccharides | Sucrose, lactose, maltose | 2 | Complete | Cane and root beet sugar, candy, soft drinks, milk, beer | |||
| Lactulose | Not digested | Good | Delayed GE | F | |||
| Oligosaccharides | Raffinose, stachyose, kestose, verbascose, nystose | 3–9 | Good | Legumes, beans (stachyose), sweet potatoes (raffinose), wheat | |||
| Maltodextrin | 3–9 | Good | Potatoes, wheat | ||||
| Fructo-oligosaccharides | 3–9 | Not digested | Good | No | F | Leeks, onions, soybeans | |
| Galacto-oligosaccharides | 3–9 | Not digested | Good | No | F | Fruit | |
| Arabino-xylo-oligosaccharides | 3–9 | Not digested | Good | No | F | Wheat | |
| Polydextrose | 3–9 | Not digested | Good | No | F | ||
| Polysaccharides | Inulin | >9 | Not digested | Very good | No | F | Chicory, onion |
| Starch: freshly cooked | >9 | Complete | Warm potatoes | ||||
| Raw cereals | >9 | Complete | Grains | ||||
| Resistant starch2 | >9 | Not digested | Variable | Variable | F + stool bulk | Green bananas, cold potatoes | |
| Cellulose, hemicelluloses | >9 | Not digested | No | Small | Stool bulk | Vegetables | |
| Pectin | >9 | Variable | Variable | F + stool bulk | Vegetables | ||
| Gums | >9 | Good | Viscosity ↑ | F | Vegetables | ||
| Related compounds | Lignin | >9 | Not digested | No | Stool bulk | Wheat, vegetables | |
The classification of carbohydrates and their main properties was adapted from the European Food Safety Authority Panel on Dietetic Products Nutrition Allergies (11) and Jones (12). DP, degree of polymerization; F, fermentation; GE, gastric emptying; GIT, gastrointestinal tract; ↑, increase.
Resistant starch includes physically inaccessible starch (RS1), resistant granules (RS2), and retrograded amylose (RS3).
Different types of fibers can also be distinguished on the basis of their chemical or physical properties (Table 1 and Table 2). Chemically, fibers are divided into carbohydrates and noncarbohydrates, such as lignin. Physically, dietary fiber is differentiated in 2 groups on the basis of its solubility: insoluble in water and nonfermentable and nonviscous fibers compared with soluble and fermentable fibers.
TABLE 2.
Classification of fibers based on chemical and physical properties1
| Dietary fiber type | Chemical classification | Physical classification |
| Lignin | Noncarbohydrate | Insoluble, nonfermentable, nonviscous |
| Cellulose | NPS: cellulose | Insoluble, nonfermentable, nonviscous |
| Hemicellulose | NPS: noncellulose polysaccharide | (In)soluble, (non)fermentable, (non)viscous |
| Pectin | NPS: noncellulose polysaccharide | Soluble, fermentable, (non)viscous |
| Gum | NPS: noncellulose polysaccharide | Soluble, fermentable, (non)viscous |
| Fructo-oligosaccharides | Nondigestible oligosaccharide | Soluble, fermentable, (non)viscous |
| Galacto-oligosaccharides | Nondigestible oligosaccharide | Soluble, fermentable, (non)viscous |
| Resistant starch (RS1, RS2, and RS3) | Resistant starch | Soluble, fermentable, (non)viscous |
NPS, nonstarch polysaccharide; RS1, resistant starch with physically inaccessible starch; RS2, resistant starch with resistant granules; RS3, resistant starch with retrograded amylose.
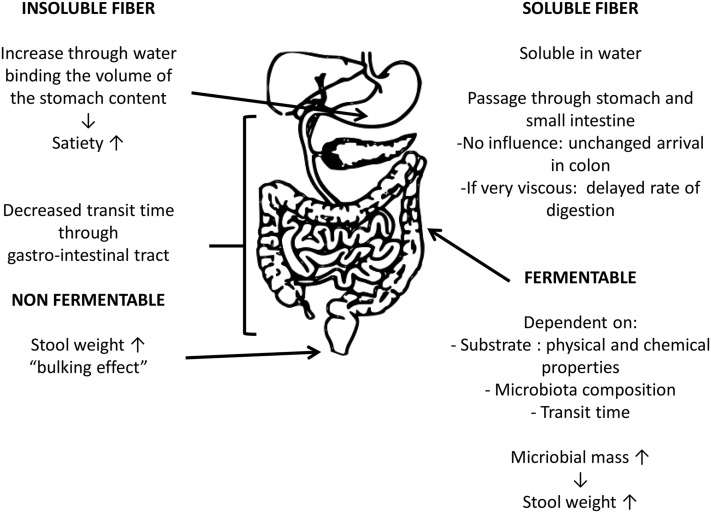
Nonfermentable fibers are very slowly or not fermented by the bacteria in the colon, they retain water, and they will cause a laxative effect because of their direct contribution to stool bulk as undigested material without affecting fermentation or microbial growth, a so-called bulking effect (15, 16). Fermentable fibers are fermented by the colonic microbiota. Because bacteria comprise ∼55% of fecal dry weight, an increase in microbial mass causes an increase in fecal output, thereby inducing a possible laxative effect (15–17) (Figure 1).
FIGURE 1.
Overview of possible effects of different fibers on gastrointestinal digestion and fermentation.
A low-residue compared with a low-fiber diet
In the literature, there have been discrepancies among diet recommendations as to the actual composition of low-fiber and/or low-residue diets. Until now, no clear definition has been proposed for a low-residue diet. Cunningham (3) described that all foods produce some gastrointestinal residue, as does normal gastrointestinal function, and fiber-containing foods produce the bulk of the gastrointestinal residue.
In addition, no clear definition has been provided for a low-fiber diet. Lijoi et al. (18) described a low-fiber diet as a diet with a total daily fiber intake <10 g. Similarly, in a clinical study on the effects of a low-fiber diet in IBS, fiber intake was also set at ∼10 g fiber/d (19). Although these studies defined a low-fiber diet in a quantitative way, no reference was made to the type of dietary fiber. Other studies only defined examples of foods that were and were not allowed in a low-fiber diet (20).
Low-residue or low-fiber diets in gastrointestinal disease management
Diagnostic: bowel preparation protocols.
Bowel preparation is often conducted before a colonoscopy, colonography, or gynecological surgery. Currently, mechanical bowel preparation with oral lavage solutions such as polyethylene glycol, sodium phosphate, sodium picosulfate, or magnesium citrate, often preceded by 1 or 2 d of a clear-liquid diet, are commonly used. In addition, stimulant laxatives and/or prokinetic agents can be combined with these cathartic agents. However, patients often find this preparation to be unpleasant and time-consuming. The most common problems that lead to less than adequate colon cleansing include lack of compliance with the clear-liquid diet and difficulty taking the preparation (21). In addition, cathartic agents are not without reported side effects such as nausea, vomiting, bloating, abdominal cramping, sleep discomfort, headaches, dizziness, and absence from school or work (22).
A number of studies investigated the intake of a low-fiber or low-residue diet as an adjuvant to cathartic regimens with regard to colon-cleansing efficacy and patient compliance. Dietary restriction of fiber or residue may result in a reduction in the size and number of stools for the patients, resulting in less discomfort for the patient (23, 24). An overview of the studies in humans is presented in Table 3.
TABLE 3.
Overview of studies investigating the effect of a low-residue or low-fiber diet on bowel preparation in humans1
| Authors (reference) | Study group, n | Design | Investigation | Results |
| Delegge and Kaplan (21) | 506 (64% F) | RCT | Comparison of the efficacy of 2 bowel-cleansing regimens before colonoscopy | The low-residue regimen resulted in significantly better colon cleansing and better patient tolerance and willingness to repeat the cathartic bowel preparation |
| Clear liquid = 222 | ||||
| Low-residue = 284 | ||||
| Park et al. (25) | 214 (44% F) | RCT | Efficacy and tolerability of bowel preparation protocols with a low-residue diet compared with the traditional clear-liquid diet | The low residue 1-d diet provided cleansing efficacy similar to that of a clear liquid diet and offered the benefit of improved tolerability |
| Clear liquid = 106 | ||||
| Low-residue = 108 | ||||
| Sipe et al. (26) | 230 | PRTC | To study whether a low-residue diet affects bowel preparation with oral sulfate solution | A low-residue diet did not impair the quality of bowel preparation achieved with oral sulfate solution |
| Clear liquid = 114 | Improvement of patient satisfaction with low-residue diet | |||
| Low-residue = 116 | ||||
| Kim et al. (27) | 184 (53.8% F) | PRTC | Success rate of the bowel preparation, adverse events, tolerability, cecal intubation time, polyp and adenoma detection rate | The adverse events, including abdominal distension, pain, nausea, vomiting, and abdominal discomfort, were significantly lower in the picosulfate + low-residue group than in the PEG group |
| Sodium picosulfate + low-residue = 94 | ||||
| PEG-ES = 90 | ||||
| Soweid et al. (28) | 200 (47.8% F) | PCS | Efficacy and tolerability of PEG-ES given with a clear-liquid diet compared with a fiber-free diet for colonoscopy preparation | Fiber-free diet given with PEG-ES on the day before colonoscopy is a more effective regimen than the standard clear-liquid diet regimen and is better tolerated by patients |
| Clear liquid = 98 | ||||
| Fiber-free = 102 | ||||
| Melicharkova et al. (29) | 248 (56% F) | PRCT | Efficacy and tolerability of a low-residue breakfast ingested on the day before colonoscopy as compared with a clear-fluid diet | A low-residue breakfast improved patient tolerance without affecting quality of low-volume colon cleansing before colonoscopy |
| CFs = 122 | ||||
| Low-residue + CFs = 126 | ||||
| Stolpman et al. (30) | 201 (43.28% F) | PRTC | To examine whether a change in precolonoscopy dietary restriction leads to better patient tolerance without compromising colonoscopy examination quality | Patients allowed to have a limited low-residue diet before colonoscopy achieve a bowel preparation quality that is noninferior to patients on a strict clear-liquid diet limitation |
| Clear liquids = 101 | ||||
| Low-residue = 100 | ||||
| Wu et al. (31) | 804 (43.6% F) | PCS | Impact of a 2-d low-residue diet on the quality of bowel preparation before colonoscopy | A low-residue diet before colonoscopy provided better bowel cleansing than an unrestricted diet |
| Liedenbaum et al. (20) | 50 (42% F) | RCT | Comparison of bowel preparations with and without a low-fiber diet to determine the image quality and accuracy in polyp detection at CT colonography | The use of a low-fiber diet in bowel preparation for CT colonography results in significantly less untagged feces and shows a trend toward better residue homogeneity |
| No diet = 25 | ||||
| Low-fiber = 25 | ||||
| Lijoi et al. (18) | 83 (100% F) | RCT | Comparison of a 7-d minimal-residue (low fiber intake) preoperative diet with a mechanical bowel preparation before laparoscopic gynecological surgery | The low-fiber diet and mechanical bowel preparation provided similar quality of surgical field exposure |
| Fiber group = 42 | The low-fiber diet was better tolerated by the patients (increased compliance) | |||
| Controls = 41 |
CF, clear fluid; CT, computed tomographic; PCS, prospective cohort study; PEG, polyethylene glycol; PEG-ES, polyethylene glycol electrolyte solution; PRCT, prospective randomized controlled trial; RCT, randomized controlled trial.
Delegge and Kaplan (21) first compared the tolerability and efficacy of a low-residue diet with a clear-liquid diet 1 d before a routine colonoscopy. In addition, Park et al. (25) and Sipe et al. (26) compared a prepackaged low-residue diet with a clear-fluid regimen. All diets were combined with cathartic agents. In a recent study by Kim et al. (27), the clinical efficacy of reduced-volume sodium picosulfate and a prepackaged low-residue diet was compared with that of the standard bowel preparation by using 4 L polyethylene glycol solution. In all 4 former studies, the low-residue regimen did not impair the quality of the bowel preparation and a better colon cleansing was achieved. Furthermore, they confirmed that the low-residue diet was better tolerated and an improvement in patient satisfaction was observed. In another study, the efficacy of a fiber-free diet was compared with a clear-liquid diet for colonoscopy preparation. Similar to the results of the low-residue diets, the fiber-free diet was better tolerated by patients (28). In addition, a recent randomized controlled trial showed that the quality of bowel preparation was not compromised by a low-residue breakfast on the day preceding the colonoscopy. In addition, this regimen was better accepted by patients and there was an increased compliance with the low-residue diet than with a clear-fluid diet (29). In contrast to the previous studies, Stolpman et al. (30) found that bowel preparation quality, polyp detection rates, and patient tolerance and acceptance were similar between a low-residue and a clear-liquid diet and proposed a low-residue diet as an alternative approach for bowel preparation.
Wu et al. (31) determined in a prospective study whether the residue content of the diet before a colonoscopy independently predicts inadequate bowel preparation. Therefore, they scored the residue content (1 = high residue, 2 = normal residue, 3 = low residue, and 4 = no residue) of all meals consumed during the 2 d preceding the colonoscopy. Immediately after the procedure, the quality of the bowel cleansing was rated by use of the Ottawa bowel preparation scale (32). A good correlation between the dietary residue content score and the Ottawa bowel preparation score was found (r = −0.475, P = 0.001), indicating that a low-residue diet resulted in better bowel cleansing than did a regular diet.
Computed tomographic colonography is an alternative to colonoscopy in the detection of polyps and carcinoma of the colon and rectum. Liedenbaum et al. (20) demonstrated that limited bowel preparation in combination with a low-fiber diet for computed tomographic colonography resulted in improved subjective tagging quality of residual feces and showed a trend toward better residue homogeneity.
In addition, in laparoscopic gynecological surgery a bowel preparation is conducted to decrease peritoneal contamination in case of bowel injury and to empty the bowel of its contents in order to improve the access to the surgical field and to facilitate the handling of the bowel itself (33). Lijoi et al. (18) first compared the effects of a low-fiber preoperative diet with a typical mechanical bowel preparation in laparoscopic gynecological surgery on the exposure of the surgical field. The low-fiber diet and mechanical bowel preparation provided similar quality of surgical field exposure. However, increased compliance was noted by the patients consuming the low-fiber diet.
Recently, the European Society of Gastrointestinal Endoscopy stated in their guidelines that they recommend a low-fiber diet on the day preceding colonoscopy. The guideline was based on a targeted literature review to evaluate the evidence supporting the use of bowel preparation for colonoscopy. A Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system was adopted to define the strength of the recommendation and the quality of evidence. The recommendation for a low-fiber diet on the day preceding colonoscopy had only a moderate quality of evidence because the potential benefits of a restricted diet before colonoscopy are not well studied. In addition, the European Society of Gastrointestinal Endoscopy does not make any recommendations regarding the use of a low-fiber diet for >24 h before the examination. Some endoscopists routinely prescribe a low-fiber diet during the 3 d preceding colonoscopy rather than on a single day because of the slow transit time in some patients. However, no study has so far compared the use of a 1-d regimen with a 3-d regimen (34).
Treatment in bowel disorders.
Low-residue or low-fiber diets may also be of value in specifically indicated clinical situations. Gastrointestinal diseases such as Crohn disease, ulcerative colitis, bowel obstruction, diverticulitis, pre- and/or postabdominal surgery, and other gastrointestinal or inflammatory disorders such as in patients with infectious gastrointestinal disease or neoplastic disease are conditions that may require a low-residue or low-fiber diet. In case of a flare-up, the diet can reduce the frequency and volume of stools and induce a primary remission in disease. In some countries, such as Japan, enteral nutrition (i.e., liquid diets consisting of nutrients broken down into their smaller units) is used for remission induction in patients (35). In children with persistent diarrhea, a diet with green banana and pectin reduced the amounts of stool and diarrheal duration (36). Currently, a number of human studies, still preliminary, are investigating the influence of low-fiber intake on the symptom signature of several gastrointestinal disorders.
IBS is a multifactorial functional disorder of the GIT that affects ∼10–15% of the adult population. The exact etiology has not been identified. Yet, environmental, psychosocial, physiologic, and genetic factors are believed to play a role. A high-fiber diet, or the addition of supplementary bran to the diet, has long been the most common first-line treatment for IBS. However, different studies have shown that patients with IBS reported a worsening of symptoms upon consuming a high-fiber diet (37–39). A by-product of fiber fermentation is gas, which can lead to unpleasant side effects such as abdominal pain, cramps, and distension. Woolner and Kirby (19) determined in a controlled trial in 204 patients with IBS whether a low-fiber diet (10 g fiber/d for 4 wk) could be an effective treatment for IBS. Before and after the low-fiber diet, patients’ symptoms were assessed by using a questionnaire. Their results indicated that approximately half of the patients (49%) treated with a low-fiber diet and bulking agents, if required, reported a substantial improvement in symptoms (60–100% improvement) after 4 wk of the dietary regimen. An alternating bowel habit and diarrhea were most likely to be helped by the diet, followed by bloating, urgency, pain, and flatulence. Currently, a fermentable oligosaccharide, disaccharide, monosaccharide, and polyol (FODMAP)–restricted diet is increasingly recommended as the first-line therapy in the management of IBS (40).
Diverticulosis in the large intestine is a condition resulting from herniation of the mucosa through defects in the colonic muscle layer. In Western and industrialized countries, ∼60% of adults >60 y will develop colonic diverticula (41). In the majority of cases, the condition is asymptomatic, with only 10–25% of affected individuals developing symptoms (38). Asymptomatic diverticulosis is commonly attributed to constipation caused by a low-fiber diet, although evidence for this mechanism is limited. Peery et al. (42) examined the associations between constipation and low dietary fiber intake with the risk of asymptomatic diverticulosis in 539 patients. Participants underwent colonoscopy and assessment of diet, physical activity, and bowel habits. The results of this study indicated that neither constipation nor a low-fiber diet was associated with an increased risk of diverticulosis. The same authors previously showed that a high-fiber diet does not protect against asymptomatic diverticulosis (43). In another study, Wick (44) suggested that patients with diverticular disease experience fewer symptoms with the consumption of low-fiber, bland diets during symptomatic periods but recommended that once the acute episode or highly symptomatic period is resolved or chronic disease is managed that patients should gradually increase dietary fiber to 20–30 g daily.
High-fiber diets are often used in the management of constipation. The amount of fiber consumed by patients in the treatment of constipation was at least 10 g/d (45). The addition of dietary fiber increases fecal mass and colonic transit time. However, some studies reported a worsening of symptoms (i.e., increased bloatedness and abdominal discomfort) in patients with chronic constipation when dietary fiber intake was increased (46, 47). Ho et al. (48) investigated in a prospective case-control study the effect of a no-fiber diet for 2 wk in 63 patients with idiopathic constipation. Patients were then instructed to completely stop their intake of dietary fiber, including vegetables, cereals, fruit, whole-meal bread, and brown rice for 2 wk. After 2 wk, patients were asked to reduce dietary fiber intake to an amount that they found acceptable for the long term. Patients were followed up at 1-mo intervals and final results were analyzed after 6 mo. The study showed that constipation and its associated symptoms can be effectively reduced by stopping or even lowering the intake of dietary fiber.
Recently, Lau et al. (49) compared for the first time the feasibility and patient tolerance of a clear-fluid (n = 54) and low-residue (n = 50) diet started on postoperative day 1 after elective colorectal surgery. They showed that a low-residue diet, rather than a clear-fluid diet, on postoperative day 1 after colorectal surgery was associated with less nausea, faster return of bowel function, and a shorter hospital stay without increasing postoperative morbidity.
Conclusions
The use of low-fiber/low-residue diets was explored in both diagnostic and therapeutic situations. The major concern with any bowel preparation, before colonoscopy, colonography, or laparoscopic gynecological surgery, is the ability to provide a clean bowel. Previous studies investigating the efficiency of bowel preparation protocols mostly focused on the comparison of effectiveness among different types of cathartics and quantities of solutions as well as dosing intervals (50–52). However, dietary adaptations may provide an alternative way of bowel preparation. The comparison of the applicability of a low-residue/low-fiber regimen (alone or in combination with cathartic agents) with traditional bowel-cleansing methods showed that the low-residue/low-fiber regimen did not impair the quality of the bowel preparation. In addition, the patients tolerated the low-residue/low-fiber diet better and their satisfaction improved. Furthermore, the compliance with the dietary regimen was better compared with a liquid diet or a normal diet without restrictions.
In addition to a diagnostic value, low-fiber/low-residue diets have therapeutic potential in the treatment of different gastrointestinal disorders. The amount of fiber in the diet differs between acute phases of disease and periods of remission. In a situation of relapse, the same advice is given as for the preparation for a colonoscopy (i.e., a low-fiber diet or a maximum of 10 g fiber/d). After the induction of remission, the amount of fiber in the diet is gradually increased to the amount of fiber in a healthy diet. Surprisingly and contrary to strongly held beliefs, a recent study showed that stopping or reducing dietary fiber intake improves constipation and its associated symptoms (44). More studies are warranted to investigate the effect of reducing fiber intake in the treatment of constipation.
Because of the lack of a scientifically accepted quantitative definition and the unavailability of a method to estimate the amount of food residues produced while passing through the GIT, low-residue diets were removed from the Nutrition Care Manual in the United States after 2011. Additional arguments for this removal included that food components are not the only source of residue giving volume to stools and no criteria have been defined to differentiate low-, medium-, and high-residue diets (17, 20, 26). In practice, information related to the quantity of residue in the diet usually refers to the amount of fiber. Often, low-fiber diets are considered as an alternative for a low-residue diet and both terms are used interchangeably (3, 5). A low-residue diet currently is no longer used for bowel resection, ileostomy, Crohn disease, and ulcerative colitis, but low-fiber therapy is recommended for these acute-phase conditions.
Although several authors mentioned the importance of the amount of fiber in the diet, only Lijoi et al. (18) and Woolner and Kirby (19) quantitatively defined a low-fiber diet as a diet with <10 g fiber/d. The latter definition of a low-fiber diet is a good recommendation to be used in clinical practice. In addition to the amount of fiber, the type of fiber may also be of importance. In almost all studies, no distinction was made between the different types of fiber. As mentioned above, fibers can be classified as insoluble fibers, which do not dissolve in water, and as soluble fibers, which dissolve in water (15). Both soluble and insoluble fibers can increase the volume of stool. For a colonoscopy, it is important that the colon cleansing is sufficient and that there are no more fiber residues present.
In general, the use of low-fiber diets for gastrointestinal disease may have previously been overlooked due to the general belief that a high fiber intake is associated with specific health benefits. For instance, SCFAs (acetate, propionate, and butyrate) are end products of colonic fiber fermentation that have been shown to contribute to colonic health. SCFAs provide energy to colonic epithelial cells, decrease luminal pH, and improve mineral absorption (2). However, the use of low-fiber diets in bowel preparation is restricted to 1 d, so there will be no concern about lack of minerals or vitamins. In the case of acute disease relapse, the low-residue/low-fiber diet is maintained several days, but, if supervised by a dietician, nutritional or energy deficiencies are unlikely to occur.
In conclusion, there is insufficient evidence to further justify the clinical use of a low-residue diet. On the basis of this literature review, we suggest redefining a low-residue diet as a low-fiber diet and to quantitatively define a low-fiber diet as a diet with a maximum of 10 g fiber/d. A low-fiber diet can be applied in both diagnostic and therapeutic situations. Diagnostically, a low-fiber diet is used in the preparation for a colonoscopy. Therapeutically, a low-fiber diet is part of the treatment in acute relapses of IBS, inflammatory bowel diseases, or diverticulitis. Upon achieving remission, the amount of fiber should be systematically increased until achieving the recommended amount of fiber in a healthy diet.
Acknowledgments
All authors read and approved the final version of the manuscript.
Footnotes
Abbreviations used: DP, degree of polymerization; GIT, gastrointestinal tract; IBS, irritable bowel syndrome.
References
- 1.Kong F, Singh RP. Disintegration of solid foods in human stomach. J Food Sci 2008;73:R67–80. [DOI] [PubMed] [Google Scholar]
- 2.Hamer HM, De Preter V, Windey K, Verbeke K. Functional analysis of colonic bacterial metabolism: relevant to health? Am J Physiol Gastrointest Liver Physiol 2012;302:G1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham E. Are low-residue diets still applicable? J Acad Nutr Diet 2012;112:960. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JW, Baird P, Davis RH, Ferreri S, Knudtson M, Koraym A, Waters V, Williams CL. Health benefits of dietary fiber. Nutr Rev 2009;67:188–205. [DOI] [PubMed] [Google Scholar]
- 5.Tarleton S, DiBaise JK. Low-residue diet in diverticular disease: putting an end to a myth. Nutr Clin Pract 2011;26:137. [DOI] [PubMed] [Google Scholar]
- 6.Trowell H. Definition of dietary fiber and hypotheses that it is a protective factor in certain diseases. Am J Clin Nutr 1976;29:417–27. [DOI] [PubMed] [Google Scholar]
- 7.Codex Alimentarius 2008. Report of the 30th session of the Codex Committee on Nutrition and Foods for Special Dietary Uses. Cape Town, South Africa, 3–7 November 2008, ALINORM 09/32/26. [cited 2013 Aug 20]. Available from: http://www.codexalimentarius.net.
- 8.American Association of Cereal Chemists. AACC Intl. provides comments on the proposed FAO/WHO definition of dietary fiber, March 2007 [cited 2013 Aug 20]. Available from: http://www.aaccnet.org/initiatives/definitions/Documents/DietaryFiber/AACCIntlCODEXDietaryFibreMarch07.pdf.
- 9.FAO/WHO Codex Alimentarius Commission. [cited 2013 Aug 20]. Available from: http://www.codexalimentarius.net/download/standards/34/CXG_002e.pdf.
- 10.Howlett JF, Betteridge VA, Champ M, Craig SA, Meheust A, Jones JM. The definition of dietary fiber—discussions at the Ninth Vahouny Fiber Symposium: building scientific agreement. Food Nutr Res 2010;54:5750–5. [DOI] [PMC free article] [PubMed]
- 11.EFSA Panel on Dietetic Products Nutrition Allergies. Scientific opinion on dietary reference values for carbohydrates and dietary fibre. EFSA J 2010;8:1462–1539.
- 12.Jones JM. CODEX-aligned dietary fiber definitions help to bridge the ‘fiber gap’. Nutr J 2014;13:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients 2013;5:1417–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Preter V, Hamer HM, Windey K, Verbeke K. The impact of pre- and/or probiotics on human colonic metabolism: does it affect human health? Mol Nutr Food Res 2011;55:46–57. [DOI] [PubMed] [Google Scholar]
- 15.Schneeman BO. Fiber, inulin and oligofructose: similarities and differences. J Nutr 1999;129:1424S–7S. [DOI] [PubMed] [Google Scholar]
- 16.Stephen AM, Cummings JH. Mechanisms of dietary action of fibre in the human colon. Nature 1980;284:283–4. [DOI] [PubMed] [Google Scholar]
- 17.Stephen AM, Cummings JH. The microbial contribution to human faecal mass. J Med Microbiol 1980;13:45–56. [DOI] [PubMed] [Google Scholar]
- 18.Lijoi D, Ferrero S, Mistrangelo E, Casa ID, Crosa M, Remorgida V, Alessandri F. Bowel preparation before laparoscopic gynaecological surgery in benign conditions using a 1-week low fibre diet: a surgeon blind, randomized and controlled trial. Arch Gynecol Obstet 2009;280:713–8. [DOI] [PubMed] [Google Scholar]
- 19.Woolner JT, Kirby G. Clinical audit of the effects of low-fibre diet on irritable bowel syndrome. J Hum Nutr Diet 2000;13:249–53. [Google Scholar]
- 20.Liedenbaum MH, Denters M, De Vries A, Van Ravesteijn V, Bipat S, Vos F, Dekker E, Stoker J. Low-fiber diet in limited bowel preparation for CT colonography: influence on image quality and patient acceptance. AJR 2010;195:W31–7. [DOI] [PubMed] [Google Scholar]
- 21.Delegge M, Kaplan R. Efficacy of bowel preparation with the use of a prepackaged, low fibre diet with a low sodium, magnesium citrate cathartic vs. a clear liquid diet with a standard sodium phosphate cathartic. Aliment Pharmacol Ther 2005;21:1491–5. [DOI] [PubMed] [Google Scholar]
- 22.Hookey LC, Depew W, Vanner S. The safety profile of oral sodium phosphate for colonic cleansing before colonoscopy in adults. Gastrointest Endosc 2002;56:895–902. [DOI] [PubMed] [Google Scholar]
- 23.Dachman AH, Dawson DO, Lefere P, Yoshida H, Khan NU, Cipriani N, Rubin DT. Comparison of routine and unprepped CT colonography augmented by low fiber diet and stool tagging: a pilot study. Abdom Imaging 2007;32:96–104. [DOI] [PubMed] [Google Scholar]
- 24.Virkki R, Mäkelä P. Low residual diet and hydration improving double contract examination of the colon. Eur J Radiol 1983;3:212–4. [PubMed] [Google Scholar]
- 25.Park DI, Park SH, Lee SK, Baek YH, Han DS, Eun CS, Kim WH, Byeon JS, Yang SK. Efficacy of prepackaged, low residual test meals with 4L polyethylene glycol versus a clear liquid diet with 4L polyethylene glycol bowel preparation: a randomized trial. J Gastroenterol Hepatol 2009;24:988–91. [DOI] [PubMed] [Google Scholar]
- 26.Sipe BW, Fischer M, Baluyut AR, Bishop RH, Born LJ, Daugherty DF, Lybik MJ, Shatara TJ, Scheidler MD, Wilson SA, et al. . A low-residue diet improved patient satisfaction with split-dose oral sulfate solution without impairing colonic preparation. Gastrointest Endosc 2013;77:932–6. [DOI] [PubMed] [Google Scholar]
- 27.Kim YS, Hong CW, Kim BC, Han KS, Park JW, Seong Choi H, Joo J, Sohn DK. Randomized clinical trial comparing reduced-volume oral picosulfate and a prepackaged low-residue diet with 4-liter PEG solution for bowel preparation. Dis Colon Rectum 2014;57:522–8. [DOI] [PubMed] [Google Scholar]
- 28.Soweid AM, Kobeissy AA, Jamali FR, El-Tarchichi M, Skoury A, Abdul-Baki H, El-Zahabi L, El-Sayyed A, Barada KA, Sharara AI, et al. . A randomized single-blind trial of standard diet versus fiber-free diet with polyethylene glycol electrolyte solution for colonoscopy preparation. Endoscopy 2010;42:633–8. [DOI] [PubMed] [Google Scholar]
- 29.Melicharkova A, Flemming J, Vanner S, Hookey L. A low-residue breakfast improves patient tolerance without impacting quality of low-volume colon cleansing prior to colonoscopy: a randomized trial. Am J Gastroenterol 2013;108:1551–5. [DOI] [PubMed] [Google Scholar]
- 30.Stolpman DR, Solem CA, Eastlick D, Adlis S, Shaw MJ. A randomized controlled trial comparing a low-residue diet versus clear liquids for colonoscopy preparation: impact on tolerance, procedure time, and adenoma detection rate. J Clin Gastroenterol 2014;48:851–5. [DOI] [PubMed] [Google Scholar]
- 31.Wu K-L, Rayner K, Chuah S-K, Chiu K-W, Chiu Y-C. Impact of a low-residue diet on bowel preparation for colonoscopy. Dis Colon Rectum 2011;54:107–12. [DOI] [PubMed] [Google Scholar]
- 32.Rostom A, Jolicoeur E. Validation of a new scale for the assessment of bowel preparation quality. Gastrointest Endosc 2004;59:482–6. [DOI] [PubMed] [Google Scholar]
- 33.Muzii L, Angioli R, Zullo MA, Calcagno M, Panici PB. Bowel preparation for gynecological surgery. Crit Rev Oncol Hematol 2003;48:311–5. [DOI] [PubMed] [Google Scholar]
- 34.Hassan C, Bretthauer M, Kaminski MF, Polkowski M, Rembacken B, Saunders B, Benamouzig R, Holme O, Green S, Kuiper T, et al. . Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2013;45:142–50. [DOI] [PubMed] [Google Scholar]
- 35.Brown AC, Rampertab SD, Mullin GE. Existing dietary guidelines for Crohn’s disease and ulcerative colitis. Expert Rev Gastroenterol Hepatol 2011;5:411–25. [DOI] [PubMed] [Google Scholar]
- 36.Rabbani GH, Teka T, Zaman B, Majid N, Khatun M, Fuchs GJ. Clinical studies in persistent diarrhea: dietary management with green banana or pectin in Bangladeshi children. Gastroenterology 2001;121:554–60. [DOI] [PubMed] [Google Scholar]
- 37.Francis CY, Whorwell PJ. Bran and irritable bowel syndrome: time for reappraisal. Lancet 1994;344:39–40. [DOI] [PubMed] [Google Scholar]
- 38.Parker TJ, Naylor SJ, Riordan AM, Hunter JO. Management of patients with food intolerance in irritable bowel syndrome: the development and use of an exclusion diet. J Hum Nutr Diet 1995;8:159–66. [Google Scholar]
- 39.Hammonds R, Whorwell PJ. The role of fibre in IBS. Ital J Gastroenterol 1997;2:9–12. [Google Scholar]
- 40.Tuck CJ, Muir JG, Barrett JS, Gibson PR. Fermentable oligosaccharides, disaccharides, monosaccharides and polyols: role in irritable bowel syndrome. Expert Rev Gastroenterol Hepatol 2014;8:819–34. [DOI] [PubMed] [Google Scholar]
- 41.Floch MH, Bina I. The natural history of diverticulitis: fact and theory. J Clin Gastroenterol 2004;38:S2–7. [DOI] [PubMed] [Google Scholar]
- 42.Peery AF, Sandler RS, Ahnen DJ, Galanko JA, Holm AN, Shaukat A, Mott LA, Barry EL, Fried DA, Baron JA. Constipation and a low-fiber diet are not associated with diverticulosis. Clin Gastroenterol Hepatol. 2013;11:1622–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peery AF, Barrett PR, Park D, Rogers AJ, Galanko JA, Martin CF, Sandler RS. A high-fiber diet does not protect against asymptomatic diverticulosis. Gastroenterology 2012;142:266–72.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wick JY. Diverticular disease: eat your fiber! Consult Pharm 2012;27:613–8. [DOI] [PubMed] [Google Scholar]
- 45.Suares NC, Ford AC. Systematic review: the effects of fibre in the management of chronic idiopathic constipation. Aliment Pharmacol Ther 2011;33:895–901. [DOI] [PubMed] [Google Scholar]
- 46.Müller-Lissner SA, Kamm MA, Scarpignato C, Wald A. Myths and misconceptions about chronic constipation. Am J Gastroenterol 2005;100:232–42. [DOI] [PubMed] [Google Scholar]
- 47.Johanson JF. Review of the treatment options for chronic constipation. MedGenMed 2007;9:25. [PMC free article] [PubMed] [Google Scholar]
- 48.Ho KS, Tan CY, Mohd Daud MA, Seow-Choen F. Stopping or reducing dietary fiber intake reduces constipation and its associated symptoms. World J Gastroenterol 2012;18:4593–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lau C, Phillips E, Bresee C, Fleshner P. Early use of low residue diets is superior to clear liquid diet after elective colorectal surgery: a randomized controlled trial. Ann Surg 2014;260:641–7. [DOI] [PubMed] [Google Scholar]
- 50.Park JS, Sohn CI, Hwang SJ, Choi HS, Park JH, Kim HJ, Park DI, Cho YK, Jeon WK, Kim BI. Quality and effect of single dose versus split dose of polyethylene glycol bowel preparation for early-morning colonoscopy. Endoscopy 2007;39:616–9. [DOI] [PubMed] [Google Scholar]
- 51.Chen CC, Ng WW, Chang FY, Lee SD. Magnesium citrate-bisacodyl regimen proves better than castor oil for colonoscopic preparation. J Gastroenterol Hepatol 1999;14:1219–22. [DOI] [PubMed] [Google Scholar]
- 52.Gupta T, Mandot A, Desai D, Abraham P, Joshi A, Shah S. Comparison of two schedules (previous evening versus same morning) of bowel preparation for colonoscopy. Endoscopy 2007;39:706–9. [DOI] [PubMed] [Google Scholar]