Abstract
Colorectal cancer (CRC) is the third most common cancer in both men and women in the United States. Various a priori dietary patterns that take into account diet complexity have been associated with CRC risk. This systematic review augments the evidence for an association between CRC risk and the Mediterranean Diet Score (MDS) and the Healthy Eating Index (HEI), and provides new evidence for a novel Dietary Inflammatory Index (DII). Human studies published in English after 31 December 2008 were reviewed. Five case-control studies and 7 prospective cohort studies conducted in the United States and Europe were identified. Five of the studies examined the MDS, 4 examined the HEI, and 4 examined the DII. Comparing highest to lowest score groups, higher MDSs were associated with an 8–54% lower CRC risk, and higher HEI scores were associated with a 20–56% lower CRC risk. More proinflammatory diet scores were associated with a 12–65% higher CRC risk compared with more anti-inflammatory diets in studies that used the DII. The results reported by sex suggested similar associations for men and women. This review builds upon the evidence supporting the association between higher overall diet quality and lower risk of CRC. Increasing scores of MDS and HEI and anti-inflammatory DII scores are characterized by high intake of plant-based foods and low intake of animal products. Future studies in more diverse populations and with consistent scoring calculations are recommended.
Keywords: colorectal cancer, dietary patterns, Mediterranean diet, Healthy Eating Index, Dietary Inflammatory Index, epidemiology, systematic review
Introduction
Colorectal cancer (CRC)8 was the third and second most commonly diagnosed cancer worldwide in men and women, respectively, during the year 2012 (1). There is evidence that ∼70% of CRC cases could be prevented through changes in diet and lifestyle (2). CRC is more common in developed countries, where an estimated 60% of all CRC cases occur (3). Western lifestyle behaviors characterized by high consumption of animal products but low vegetable and fruit consumption and physical inactivity have been associated with an increased risk of CRC (4, 5). Specifically, dietary factors such as a higher intake of red meat, processed meat, saturated fat, and alcohol may increase CRC risk, whereas a higher intake of fiber, calcium, vegetables, and fruits is associated with lower CRC risk (6–8), independent of BMI. Overall, these diet exposures suggest that an overall prudent diet that is consistent with dietary guidance may be most beneficial for reducing CRC risk. In fact, dietary constituents, when consumed together as an eating pattern, represent a “dose” of dietary exposure that is greater than any individual constituent and integrates alterations in metabolism and bioavailability (9). Thus, evaluating the whole diet with the use of dietary pattern analyses has emerged as an appealing alternative to single food group or nutrient analyses.
Given that diet is a complicated interaction of multiple food components and the fact that individuals consume multiple foods instead of a single nutrient or food item, investigating CRC risk with overall diet pattern rather than individual dietary factors could alleviate statistical complexity when modeling nutritional factors as exposure (10). There are typically 2 types of dietary patterns that are defined in the literature: exploratory or a posteriori dietary patterns, which define groupings of patterns analytically based on consumption patterns within a study population, and hypothesis-driven or a priori dietary patterns, which are defined based on existing evidence such as dietary guidelines (11). To date, 4 systematic reviews that included studies examining dietary patterns and CRC risk have been published (10–13). Several new studies on this topic have been reported since the last review, and a new a priori dietary index has emerged that characterizes diet based on its inflammatory potential [the Dietary Inflammatory Index (DII)] (14). The objective of the current review is to evaluate systemically the relation between 3 a priori dietary patterns [the Mediterranean Diet Score (MDS), Healthy Eating Index (HEI), and DII] and CRC. These a priori dietary patterns were chosen because they have been the most studied of the available a priori dietary patterns in relation to CRC to date. We limited the search to articles published after 2008 to avoid overlap with previous review articles on this topic.
Methods and Results
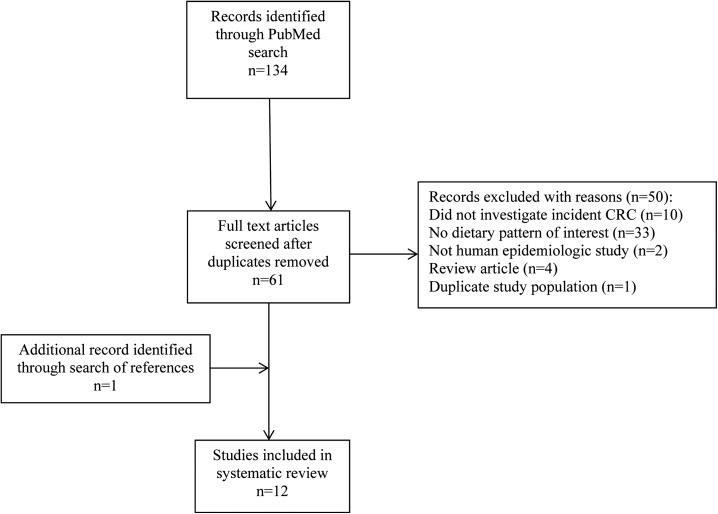
A literature search of the MEDLINE database was conducted in April 2015 with the use of the following exposure search terms: “Mediterranean diet score,” “MDS,” “modified Mediterranean diet score,” “MMDS,” “alternate Mediterranean diet score,” “aMDS,” “healthy eating index,” “HEI,” “alternate healthy eating index,” “alternative healthy eating index,” “a-HEI,” “healthy eating index 2010,” “HEI-2010,” “dietary inflammatory index,” “DII,” “dietary pattern,” and “diet score,” in combination with each of the following outcome terms: “colon cancer,” “rectal cancer,” “colorectal cancer,” “colorectal adenoma,” “colon adenoma,” “colon neoplasm,” and “colorectal neoplasm.” Search results were restricted to epidemiologic studies in human populations published in English between January 2009 and April 2015. Eligible studies included those that used a priori dietary pattern indexes including the MDS, HEI, or DII, rather than a posteriori dietary pattern analyses (e.g., principal components analyses). All reviews and survivorship studies were excluded from this systematic review. Only 1 study was identified with the use of adenomatous polyps as the outcome (15), so studies examining adenomatous polyps were not included in the review. Only studies that reported the estimated associations of interest, such as ORs, HRs, or RRs, and 95% CIs, were included. For those duplicate studies that used the same study population, we only included the most recent publication (Figure 1). Two researchers independently performed the literature search, study selection, and data extraction. Studies were only included when both researchers agreed based on the inclusion and exclusion criteria; when inconsistent opinions occurred, a third investigator made the final decision.
FIGURE 1.
PRISMA flow chart of the review process. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; CRC, colorectal cancer.
Guidelines set by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement were used throughout the entirety of the review (16). For each selected study, information on reference, location, study population, sample size with number of cases and follow-up time, sources of controls, dietary assessment tool employed and the period of evaluation, the dietary index investigated in the study (i.e., main exposure), main effect estimates, and confounding variables adjusted in analysis were reported in Tables 1 and 2. Because of differences in FFQ used, different food items included in each index, different cutoffs for scoring diet, and adjustment for different confounding variables, as well as the limited number of studies for each dietary index, we did not perform quantitative meta-analysis for this paper.
TABLE 1.
Characteristics of case-control studies investigating the MDS, HEI, and DII and CRC risk identified in PubMed and published after 31 December 20081
| Reference | Location | Sample size, n | Sources of controls | Dietary assessment | Dietary index | Results2 | Covariates included in model |
| Djuric et al. (2012) (17) | Detroit, MI, United States | 1163 cases and 1501 controls | Random digit dialing from same county as case; matched on age, sex, and race | FFQ Block 98.2 (110 items) | HEI-1996 | In low quercetin consumers, ORQr4 vs. Qr13: 0.78 (95% CI: 0.55, 1.12) | Age, sex, education, family history of CRC, regular NSAID use, BMI, physical activity, dietary factors |
| In high quercetin consumers, ORQr4 vs. Qr13: 0.59 (95% CI: 0.42, 0.83) | |||||||
| Grosso et al. (2014) (18) | Catania, Italy | 338 cases and 676 controls | Patients without clinical symptoms of cancer from same hospital who previously were recruited for adherence to Mediterranean diet; matched on age and sex | Standardized questionnaire designed for the study | MDS | ORHigh vs. Low3: 0.46 (95% CI: 0.28, 0.75) | Smoking status, family history of CRC, obesity, diabetes, physical activity, alcohol intake |
| Kontou et al. (2012) (19) | Athens, Greece | 250 cases and 250 controls | Volunteers from general population without clinical symptoms of cancer; matched on age, sex, and SES | FFQ (69 items) for monthly consumption | MDS | OR3,4: 0.88 (95% CI: 0.84, 0.92) | Age, sex, BMI, physical activity, smoking status, family history of CRC, presence of metabolic syndrome |
| Miller et al. (2010) (20) | Pennsylvania | Men: 225 cases and 330 controls | Random digit dialing from same region | FFQ (137 times; modified version of Diet History Questionnaire) | HEI-2005 | Men, ORQr4 vs. Qr1: 0.56 (95% CI: 0.31, 0.99) | Age, education, smoking status, BMI, total energy intake, family history of CRC, NSAID use, postmenopausal hormone use (women) |
| Women: 206 cases and 396 controls | Women, ORQr4 vs. Qr1: 0.44 (95% CI: 0.24, 0.77) | ||||||
| Zamora-Ros et al. (2014) (21) | Barcelona, Spain | 424 cases and 401 controls | Randomly selected patients from the same hospital who were admitted for a newly incident disease | Spanish dietary history questionnaire (EPIC) | DII | ORQr4 vs Qr13: 1.65 (95% CI: 1.08, 2.56) | Age, sex, total energy intake, BMI, tobacco use, physical activity, medication use, family history of CRC |
CRC, colorectal cancer; DII, Dietary Inflammatory Index; EPIC, European Prospective Investigation into Cancer and Nutrition; HEI, Healthy Eating Index; MDS, Mediterranean Diet Score; NSAID, nonsteroidal anti-inflammatory drugs; Qn, quintile; Qr, quartile; SES, socioeconomic status.
OR estimates for studies in this table were calculated for CRC risk, comparing the highest diet score group with the lowest diet score group in the respective study, except where otherwise noted. The lowest diet score group indicates the least compliance to the diet guideline for articles with MDSs or HEI as exposure, or the most anti-inflammatory potential of diet for the articles with DII as exposure.
The estimated association is for overall study population, including both men and women.
Denotes odds of CRC for every 1-unit increase in MDS.
TABLE 2.
Characteristics of cohort studies investigating MDS, HEI, and DII and CRC risk identified in PubMed and published after 31 December 20081
| Reference | Location and study name | Sample size and follow-up information | Dietary assessment | Dietary index investigated | Results2 | Covariates included in model |
| Bamia et al. (2013) (22) | Europe, EPIC | 480,308 people (4355 cases); average of 11.6 y of follow-up | Center- specific FFQ; previous year | MMDS and CSMMDS | MMDS, HRHigh vs. Low3: 0.89 (95% CI: 0.80, 0.99) | Age at enrollment, sex, BMI, physical activity, educational level, smoking status at enrollment, and energy intake |
| CSMMDS, HRHigh vs. Low3: 0.92 (95% CI: 0.84, 1.00) | ||||||
| Fung et al. (2010) (23) | United States, NHS and HPFS | 87,256 women (1432 cases) 45,490 men (1032 cases); follow-up ≤26 y (average follow-up year not available) | FFQ (140 items) for HPFS FFQ (61 items in 1980 and 116 items since 1986) for NHS; previous year | Alternate Mediterranean Diet score | HRQn5 vs Qn13: 0.89 (95% CI: 0.77, 1.01) | Age, BMI, physical activity, pack-years of smoking, alcohol intake, family history, aspirin use, colonoscopy, history of polyps, multivitamin use, and energy intake |
| Jarvandi et al. (2013) (24) | United States, NIH-AARP Diet and Health Study | 484,020 people (7598 cases); average of 9.2 y of follow-up | FFQ (124 items); previous year | HEI-2005 | HRQr4 vs Qr13: 1.35 (95% CI: 1.26, 1.44) | Age, sex, race/ethnicity, diabetes, educational level, BMI, physical activity, smoking, hormone replacement therapy in women, family history of colon cancer, vitamin and mineral supplements, and total energy |
| Reedy et al. (2010) (25) | United States, NIH-AARP Diet and Health Study | 492,306 people (3110 cases); 5 y of follow-up (average follow-up years not available) | FFQ (124 items); previous year | 1.HEI-2005 | HEI-2005, men, HRQn5 vs. Qn1: 0.72 (95% CI: 0.62, 0.83) Women, HRQn5 vs. Qn1: 0.80 (95% CI: 0.64, 0.98) | Age, ethnicity, education, BMI, smoking, physical activity, energy, and menopausal hormone therapy (women only) |
| 2. AHEI | AHEI, men, HRQn5 vs. Qn1: 0.70 (95% CI: 0.61, 0.81) Women, HRQn5 vs. Qn1: 0.80 (95% CI: 0.64, 1.00) | |||||
| 3. MDS | MDS, men: HRQn5 vs. Qn1: 0.72 (95% CI: 0.63, 0.83) Women, HRQn5 vs. Qn1: 0.89 (95% CI: 0.72, 1.11) | |||||
| Shivappa et al. (2014) (26) | United States, Iowa Women’s Health Study | 34,703 women (1,636 cases); average of 19.6 y of follow-up | FFQ (121 items); previous year | DII | HRQn5 vs. Qn1: 1.20 (95% CI: 1.01, 1.43) | Age, BMI, smoking status, pack-years of smoking, hormone replacement therapy, education, diabetes, and total energy intake |
| Tabung et al. (2014) (27) | United States, Women’s Health Initiative | 152,536 women (1920 cases); average of 11.3 y of follow-up | FFQ (122 items); previous 3 mo | DII | HRQn5 vs. Qn1: 1.22 (95% CI: 1.05, 1.43) | Age, total energy intake, BMI, race/ethnicity, physical activity, educational level, smoking status, family history of CRC, hypertension, diabetes, arthritis, history of colonoscopy, history of occult blood tests, NSAID use, category and duration of estrogen use, category and duration of estrogen and progesterone use, dietary modification trial arm, hormone therapy trial arm, and calcium and vitamin arm |
| Wirth et al. 2015 (28) | United States, NIH-AARP Diet and Health Study | 292,118 men and 197,324 women (6225 cases); mean follow-up of 9.1 y | FFQ (124 items); previous year | DII | HRQr4 vs. Qr13: 1.40 (95% CI: 1.28, 1.53) | Age, smoking status, BMI, diabetes, energy intake, physical activity, marital status, and education |
AHEI, Alternate Healthy Eating Index; CRC, colorectal cancer; CSMMDS, Center-Specific Modified Mediterranean Diet Score; DII, Dietary Inflammatory Index; EPIC, European Prospective Investigation into Cancer and Nutrition; HEI, Healthy Eating Index; HPFS, Health Professionals Follow-Up Study; MDS, Mediterranean Diet Score; MMDS, Modified Mediterranean Diet Score; NHS, Nurses’ Health Study; NIH-AARP, NIH-American Association of Retired Persons; NSAID, nonsteroidal anti-inflammatory drugs; SES, socioeconomic status; Qn, quintile; Qr, quartile.
HR estimates for studies in this table were calculated for CRC risk, comparing the highest diet score group with the lowest diet score group in the respective study, except for the study conducted by Jarvandi et al. (24), in which the reference group is the highest HEI-2005 score group. The lowest diet score group indicates the least compliance to the diet guideline for articles with MDS or HEI as exposure, or the most anti-inflammatory potential of diet for the articles with DII as exposure.
The estimated association is for overall study population, including both men and women.
The initial search yielded 134 articles, which were reduced to 61 after the removal of duplicates. After a full-text review was conducted, 50 studies were excluded, with detailed reasons explained in Figure 1. One additional study was identified based on a review of study reference lists, leaving a total of 12 studies included in this review (17–28). Five case-control studies were conducted across 4 different countries: United States (n = 2) (17, 20), Greece (n = 1) (19), Italy (n = 1) (18), and Spain (n = 1) (21) (Table 1). Among the 7 cohort studies, 6 were conducted in the United States (23–28), and 1 was conducted in Europe (22) (Table 2). Two cohort studies that investigated the DII and CRC association included only female participants (26, 27), whereas all other studies included both male and female participants. Two other studies (20, 25) reported only sex-specific associations, which are shown in Figure 2A and B, whereas overall associations combined for both sexes, when available, are reported in Tables 1 and 2 for the remaining 8 studies.
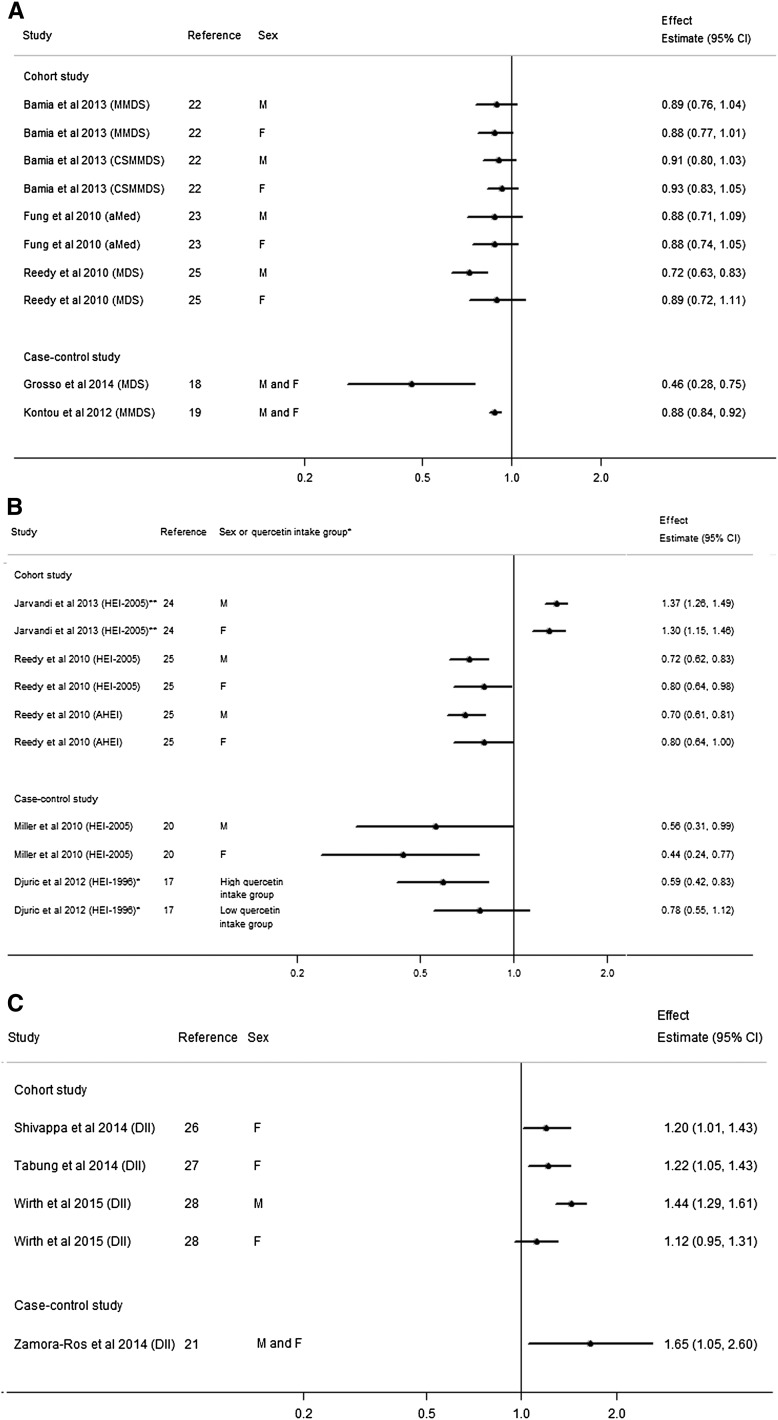
FIGURE 2.
Forest plot of the associations between the MDS (A), HEI, and AHEI (B), and DII (C) and colorectal cancer risk in cohort and case-control studies stratified by sex (where available). *The studies in this figure were all stratified by sex except the study by Djuric et al. (17), which stratified analysis by high and low quercetin intake group. **The highest HEI index group was treated as referent group in the study by Jarvandi et al. (24), whereas the other studies in this figure used lowest HEI index group as the referent group. AHEI, Alternate Healthy Eating Index; aMed, alternate Mediterranean Diet index; CSMMDS, Center-Specific Modified Mediterranean Diet Score; DII, Dietary Inflammatory Index; HEI, Healthy Eating Index; MDS, Mediterranean Diet Score; MMDS, Modified Mediterranean Diet Score.
All studies used an FFQ for dietary assessment with the number of items ranging from 61 to 137. In the case-control studies, participants were asked to report diet from 1 wk to 2 y before the interview (17–21). Participants reported dietary intake 3 mo before baseline in one cohort study (27) and for the year before baseline in the other cohort studies (22–26, 28). In prospective cohort studies, follow-up time ranged from 5 y to almost 26 y. The characteristics of each study are described in Tables 1 and 2.
In the case-control studies, multivariate logistic regression modeling was used to calculate ORs in all 5 studies, with inclusion of differing covariates, although there was much overlap in the variables included in adjusted models. For cohort studies, Cox proportional hazards regression was used to calculate HRs and 95% CIs with adjustment for multiple covariates.
MDS and CRC risk
Several versions of the MDS have been used in recent studies of CRC risk: the original MDS, the Modified Mediterranean Diet Score, the Center-Specific Modified Mediterranean Diet Score [in the European Prospective Investigation into Cancer and Nutrition (EPIC) study], the Alternate Mediterranean Diet Index, and a version based on the Mediterranean pyramid. The scoring components and methods were similar between the various studies. In brief, the scores included 9 components in which one point was assigned when intake was above sex-specific median value for components considered to be healthy, such as non-refined cereal, vegetables, and fruit; otherwise, zero points were assigned. For unhealthy components, such as meat, one point was assigned when intake was below sex-specific medians; otherwise, 0 was assigned. The scores had different scoring standards for alcohol intake. Values were energy-adjusted, and the range of each MDS was from 0 to 9, with increasing scores representing greater conformity to the Mediterranean diet.
Case-control studies.
Two case-control studies reported inverse associations between the MDS and CRC (18, 19). Grosso et al. (18) enrolled 338 CRC cases visiting an urban hospital in Italy and 676 controls from the same hospital without clinical symptoms of cancer matched on age and sex. Participants with a high MDS had 54% lower odds (OR: 0.46; 95% CI: 0.28, 0.75), and those with a medium MDS had 47% lower odds of CRC (OR: 0.53; 95% CI: 0.39, 0.74) than did participants with a low MDS.
Kontou et al. (19) used a modified version of the MDS, assigning individuals’ ratings based on their positions in the Mediterranean diet pyramid. The primary aim of the analysis was to examine a potential interaction between the MDS and presence of metabolic syndrome (MetS) in relation to CRC. The study population included 250 cases of CRC admitted to a hospital in Athens, Greece, and 250 volunteer controls from the general population matched on age, sex, and sociodemographic characteristics. MDS was inversely associated with CRC (OR: 0.88; 95% CI: 0.84, 0.92, for every 1-unit increase in MDS). The significant association remained after stratification by MetS status.
Cohort studies.
Three cohort studies evaluated the association of MDS and CRC with the use of slightly different versions of the MDS (22, 23, 25). Bamia et al. (22) examined the association with CRC risk in 143,752 men and 336,556 women enrolled from 10 European countries in the EPIC study, and demonstrated a reduction in risk of 11% in the highest MDS group compared with the lowest (HR: 0.89; 95% CI: 0.80, 0.99). Similarly, Fung et al. (23) used data from the Nurses’ Health Study and the Health Professionals’ Follow-Up Study (n = 87,256 women and 45,490 men) to demonstrate a nonsignificant 11% lower CRC risk in the highest quartile of Alternate Mediterranean Diet Index score compared with the lowest (OR: 0.89; 95% CI: 0.77, 1.01). With the use of a sample of 293,576 men and 198,730 women from the NIH-American Association of Retired Persons (NIH-AARP) Diet and Health Study, Reedy et al. (25) showed a 28% lower risk of CRC in men in the highest quintile of MDS compared with those in the lowest (OR: 0.72; 95% CI: 0.63, 0.83).
In general, better conformity to a Mediterranean diet was associated with a lower CRC risk, with risk estimates ranging from 0.72 to 0.93 comparing highest to lowest score group, which were statistically significant in the EPIC study, as well as in the NIH-AARP study in men but not women (Figure 2A).
HEI and CRC risk
The HEI (1995) and HEI-2005 are based on recommendations from MyPyramid and the US Dietary Guidelines for Americans (29). The HEI-2005, which replaced the original HEI (1995) (30), was designed based on the 2005 Dietary Guidelines for Americans (31); an energy-density method was used to calculate consumption of each food component. The HEI-2005 includes 12 components, with total score ranging from 0 to 100 points. Six components were scored in a 0–5 point scale, 5 components were worth 0–10 points, and 1 component—calories from solid fat, alcohol, and added sugar—was scored in a 0–20 point scale. Scores were assigned to individuals on an evenly prorated approach, except for saturated fat and sodium.
The Alternate Healthy Eating Index (AHEI) incorporates some components of the HEI, but was calculated for use in the Nurses’ Health Study. It includes 9 components, with total points ranging from 0 to 87.5 (32). The scores are assigned in an evenly prorated approach, except for a dichotomous score assigned to multivitamins. The AHEI differs qualitatively from the HEI-2005 in that it includes scoring for dietary fat quality, inclusion of alcohol intake, cereal fiber, ratio of white- to red meat, and duration of multivitamin use. For all 3 scores, HEI (1995), HEI-2005, and AHEI, a higher score reflects better diet quality.
Case-control studies.
Two case-control studies investigated the association between diet and CRC with the use of the HEI (17, 20). The 1995 version of the HEI was used by Djuric et al. (17) in a sample of residents from the Metropolitan Detroit Tricounty area. Researchers identified 1163 CRC cases diagnosed between the ages of 45 and 80 y with the use of a rapid case-reporting system, an original member of the National Cancer Institute’s Surveillance, Epidemiology, and End Result cancer registry. Random digit dialing was used in the same geographical area to recruit 1501 controls matched on age, sex, and race. The primary aim of the analysis was to examine dietary quercetin intake with risk of CRC; therefore, effect estimates were only reported when stratified by high and low quercetin intake. The highest quartile of HEI score was associated with 41% lower odds of CRC compared with the lowest quartile in the participants with high quercetin intake. A nonsignificant reduction in risk for the highest HEI quartile was observed in the group with low quercetin intake (OR: 0.78; 95% CI: 0.55, 1.12) (results corrected from the original paper; Z Djuric, University of Michigan, personal communication, 2015).
The other case-control study evaluating diet quality with HEI was conducted by Miller et al. (20) with the use of the HEI-2005 in participants from a contiguous 19-county area in Pennsylvania (20). Identified via the Pennsylvania State Cancer Registry, the analysis included 225 male and 206 female cases of CRC. A total of 330 men and 396 women were recruited as controls by random-digit dialing from the same region. On average, cases had lower HEI-2005 scores than controls (mean ± SD scores: 60.7 ± 11.6 and 63.9 ± 11.7 for male and female cases, respectively, and 63.3 ± 12.7 and 67.7 ± 11.0 for male and female controls, respectively). The authors reported risk estimates for each sex separately. With the use of the lowest HEI-2005 quartile as the referent, the highest quartile of HEI-2005 was associated with lower odds of CRC in men (OR: 0.56; 95% CI: 0.31, 0.99). In women, the significant inverse association was evident in both the highest quartile (OR: 0.44; 95% CI: 0.24, 0.77) and third quartile (OR: 0.59; 95% CI: 0.35, 0.99).
Cohort studies.
Two cohort studies evaluated the association between the HEI-2005 or the AHEI and CRC risk (24, 25). Jarvandi et al. (24) categorized the HEI-2005 into quartiles in analysis and treated the highest quartile group as the referent (i.e., comparing an unhealthy diet to a healthy diet), whereas Reedy et al. (25) categorized the HEI-2005 and AHEI into quintiles in analysis and treated the lowest quartile group as the referent (i.e., comparing a healthy diet to an unhealthy diet). Results were consistent from both studies, showing that higher HEI-2005 and AHEI scores were associated with significantly lower CRC risk in men and women. Jarvandi et al. (24) reported a 35% higher risk of CRC for those who were least adherent to the HEI-2005 compared with those who were most adherent [multivariate HRQuartile1 vs. Quartile4: 1.35; 95% CI: 1.26, 1.44]. Reedy et al. (25) reported similar associations; men and women who scored highest on the HEI-2005 or AHEI showed a 20–30% lower risk of CRC (Figure 2B).
DII and CRC risk
The final a priori dietary pattern included in the review was the DII, which was developed (14) and construct-validated by researchers at the University of South Carolina (33, 34). Briefly, the 45 “food parameter–specific inflammatory effect scores” were derived from extensive review and scoring of the literature from 1950 to 2010, examining the effect of food and dietary constituents on 6 inflammatory biomarkers. In order to adjust for the arbitrariness of individuals’ dietary consumption, a centered percentile of z scores was assigned to each participant after linking the individual’s intake to a world database of mean intake and SD for each food variable (14). The final overall DII score was obtained by multiplying the respective “food parameter–specific inflammatory effect scores” with a centered percentile for each variable and summing up all the values for each food variable to calculate a total DII score for each participant, which ranged from approximately −10 to +10 (14). In contrast with the previous indexes, a higher DII score is representative of a proinflammatory diet and is thus generally representative of less healthy diets.
Case-control studies.
In a case-control study conducted in Barcelona, Spain, a total of 424 CRC cases were recruited from a hospital, along with 401 controls who were admitted to the same hospital for a noncancerous disease that had been previously undiagnosed (21). CRC cases had higher median DII scores than controls [median (25–75% range): 1.44 (−0.88, 3.18) and 1.06 (−0.73, 3.05), respectively]. With the use of the first quartile as the referent, individuals in the highest quartile, or most proinflammatory, diet had significantly higher odds of CRC (OR: 1.65; 95% CI: 1.05, 2.60; P-trend = 0.011).
Cohort studies.
Three prospective cohort studies examined the relation between DII and CRC risk (26–28). Tabung et al. (27) examined the association in 152,536 postmenopausal women in the Women’s Health Initiative with 1920 CRC cases identified after an 11.3 y follow-up, Shivappa et al. (26) evaluated the association in 34,703 women in the Iowa Women’s Health Study, and Wirth et al. (28) examined the association in both men and women in the NIH-AARP Diet and Health Study with an average of 9.1 y of follow-up.
Higher DII was consistently associated with higher overall CRC risk (Figure 2C). Significant associations were found by Shivappa et al. (26) (multivariate HRQuintile5 vs. Quintile1: 1.20; 95% CI: 1.01, 1.43, P-trend = 0.03), Tabung et al. (27) (multivariate HRQuintile5 vs. Quintile1: 1.22; 95% CI: 1.05, 1.43; P-trend = 0.02) in female-only population-based studies. Wirth et al. (28) found a higher risk of CRC in the highest DII quartile compared with the lowest in the NIH-AARP cohort of men and women (multivariate HRQuartile4 vs. Quartile1: 1.40; 95% CI: 1.28, 1.53, P-trend < 0.01).
Sex-specific associations
Given earlier data suggesting that dietary exposures may be associated with CRC risk differentially in men and women, we also evaluated sex-specific results when available (Figure 2A–C). Overall, the associations shown were consistent for both men and women, with the exception of the MDS and the DII in the NIH-AARP study (24), in which associations for women tended not to reach significance and HRs were not as low as for men. In the studies by Bamia et al. and Fung et al. (22, 23) the pooled analysis suggested a statistically significant or borderline-significant lower risk of CRC with higher index scores, which were no longer statistically significant when stratified by sex.
Discussion
This systematic review serves to augment previous reviews of the association between CRC and MDS and HEI, while summarizing new evidence related to the DII and CRC risk. Our findings are consistent with previous reviews showing that a higher HEI score and MDS were associated with a lower risk of CRC (10–13). In addition, newer evidence demonstrated that a diet balanced toward more proinflammatory factors (e.g., low in anti-inflammatory dietary factors and/or high in proinflammatory dietary factors) was associated with a higher risk of CRC.
When data were available by sex, the association between these diet scores generally held, although associations tended not to be quite as strong in women compared with men. Another review including studies that explored dietary patterns and CRC risk association published before 2009 also concluded that the Mediterranean diet had an inverse association with CRC in men, but results were less conclusive for women (10). A systematic review by Yusof et al. (11) of only cohort studies concluded that the MDS was inversely associated with CRC, although only one study had reported on this association in that review. In the time since those reviews were published, 3 cohort studies (22, 35, 36) and 2 case-control studies (18, 19) reported inverse associations between the Mediterranean diet and CRC risk by using different versions of the MDS. The Mediterranean diet is considered to have a protective effect on many chronic diseases, including cancer (37). The biological mechanism may be explained by the beneficial effect of several food components: less red meat, but more vegetables, fruits, and fiber, as well as whole grains, a healthy ratio of unsaturated- to saturated fat, and a moderate amount of alcohol intake. These features were found to be related to reduced concentrations of inflammatory biomarkers and endothelial dysfunction, which could lead to lower CRC risk (38).
Our review of the literature on HEI and CRC is generally consistent with previously published reviews. In reviews by Miller et al. (10) and Yusof et al. (11), higher HEI scores were associated with lower CRC risk in both men and women. In our review, 2 cohort studies (24, 25) and 2 case-control studies (17, 20) reported associations between the HEI and CRC risk by using 3 different indexes: HEI-1996, HEI-2005, and AHEI. Evidence from all studies was generally consistent: adherence to any version of the HEI was significantly associated with decreased overall CRC risk by 20–56%, for both men and women. Fung et al. (38) found that high scores from the AHEI were associated with lower plasma concentrations of markers of inflammation, which could lead to a lower risk of CRC.
This is the first review of studies, to our knowledge, examining the DII and CRC. The DII represents a new diet quality index that characterizes diet by its inflammatory potential. To date, several studies have investigated the association between the DII and various cancer outcomes (39–41), with the most consistent association being observed for CRC risk, as reviewed in the current paper. Three cohort studies (26–28) and one case-control study (21) all reported a higher risk of CRC for participants consuming more proinflammatory diets than for those consuming more anti-inflammatory diets. For comparison, we converted the effect estimates for the DII to the same direction as other dietary indexes included in this review, and the range of effect estimates are between 0.60 and 0.89, comparing anti-inflammatory dietary potential to proinflammatory potential in the 4 studies. Chronic inflammation is known to be a central process for carcinogenesis, especially for CRC, which is supported by evidence showing that regular use of nonsteroidal anti-inflammatory drugs (NSAIDs) can lower the risk of colon cancer (42, 43). A proinflammatory diet could increase CRC risk through increased insulin resistance or glucose intolerance and increased TG concentrations (44, 45).
Case-control studies in this review tended to report stronger effect estimates than cohort studies, as has been reported in other reviews of dietary factors and cancer (46). This could be related to recall bias in case-control studies, which may skew results away from the null if disease status affects recall of cases differently from controls, or loss to follow-up or homogeneity of the study population in cohort studies, which could attenuate results toward the null.
Studies included in this review tended to account for appropriate confounders through either statistical adjustment or study design methods such as matching. All studies controlled for age, sex (when appropriate), and BMI or obesity status. The majority of studies also controlled for education, family history of CRC, physical activity, total energy intake, and smoking status. Only one of the DII studies controlled for NSAID use, although results did not differ substantially from those that did not adjust for NSAID use.
Limitations of this review include factors that affect comparability of the studies and generalizability of the results, as well as measurement error in dietary assessment. There was inconsistent reporting of mean or median dietary index scores, particularly by case status, making it difficult to compare exposures across populations. In 2 of the studies (17, 19), dietary pattern assessment was a secondary exposure; therefore, risk estimates were stratified by variables such as quercetin intake and presence of MetS, which limits our ability to compare across studies. Further limitations include relatively homogenous populations with regard to race. The vast majority of study participants were white or of European descent. Lastly, all of the studies used FFQs, which are prone to measurement error. Many foods, especially those that are plant-based, have nutrient contents that vary considerably depending on the growing conditions and preparation methods. This may distort scores from certain indexes, such as the DII, which uses nutrient intake to determine the overall DII score (14). Inconsistencies in results across studies may be due to missing food components of the scores from the FFQs in the various studies, which could lead to misclassification of exposure and attenuation of results.
Overall, diet quality has been associated not only with cancer risk and mortality, and specifically CRC, but also with other diet-associated chronic diseases, including diabetes, cardiovascular disease, and hypertension (47, 48). Although each index assesses overall diet quality with varied components, the consistency of the associations with CRC risk suggests that there is more than one way to consume a healthy diet with regard to CRC prevention. Common across the 3 scores, the healthiest overall diets tend to be high in plant-based foods and low in animal products or trans fat (14, 30, 49). These diets are likely to be nutrient-dense, high in many compounds that have anticarcinogenic properties, including antioxidants, flavonoids, dietary fiber, and anti-inflammatory phytochemicals, and low in food components that may induce oxidative stress or promote chronic inflammation.
A hypothesis-driven, or a priori, dietary pattern assessment was required for inclusion in this review. Because individual foods are not consumed in isolation, whole dietary patterns are a more comprehensive way to evaluate dietary effects on CRC. Other data-driven dietary pattern analytic methods, such as cluster analysis or principal components analyses, may limit the ability for comparisons across populations because dietary patterns are established specific to the population under study. Other a priori dietary patterns are emerging, such as a new Nordic Diet index and Paleolithic Diet score, which have been examined in relation to CRC (50, 51) and adenomatous polyps (15), respectively, although the literature was not abundant enough at this time to include in the current review.
Considering 3 different a priori dietary patterns, the findings from the studies reported in this review bolster previously established evidence that overall diet quality is associated with CRC risk (10–13). Although different versions of the 3 dietary indexes were used across studies, overall, the results in populations from the United States and Europe were quite consistent. Challenges to synthesizing the literature in this area include the underlying differences in score calculation procedures across studies, e.g., different versions of indices used, different scoring methods with varying cutoffs between studies, different FFQs with a varying number of relevant components for each index, and different score categorization methods (e.g., quartiles, quintiles, or continuous variables). The Dietary Patterns Methods Project has recently attempted to mitigate some of these challenges by examining the association between mortality and various dietary indices in pooled analyses of 3 different study populations (52). Further work in this area as it relates to specific cancer types would help to provide consistent conclusions.
Our review supports the idea that higher overall diet quality, whether assessed by adherence to a healthy cultural way of eating, dietary guidelines, or inflammatory potential, is associated with lower risk of CRC. Commonalities among the dietary patterns investigated include high intake of plant-based foods and low intake of animal products for the scores considered most healthy. Future research should focus on analyzing the relation between CRC and diet in diverse populations to increase generalizability and ensure a wide variability in dietary intake. Prospective cohort studies with repeated dietary measurements to incorporate fluctuations in dietary habit are recommended. Finally, for the sake of comparison, future epidemiologic studies could conduct analysis with the use of the same score and with consistent definitions and application of cutoffs. Pooled data from multiple studies would allow for larger sample size and would enable examination of specific subsites or anatomic locations of CRC.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AHEI, Alternate Healthy Eating Index; CRC, colorectal cancer; DII, Dietary Inflammatory Index; EPIC, European Prospective Investigation into Cancer and Nutrition; HEI, Healthy Eating Index; MDS, Mediterranean Diet Score; MetS, metabolic syndrome; NIH-AARP, NIH-American Association of Retired Persons; NSAID, nonsteroidal anti-inflammatory drug.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterol Clin North Am 2002;31:925–43. [DOI] [PubMed] [Google Scholar]
- 3.Binefa G, Rodriguez-Moranta F, Teule A, Medina-Hayas M. Colorectal cancer: from prevention to personalized medicine. World J Gastroenterol 2014;20:6786–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomson CA, McCullough ML, Wertheim BC, Chlebowski RT, Martinez ME, Stefanick ML, Rohan TE, Manson JE, Tindle HA, Ockene J, et al. . Nutrition and physical activity cancer prevention guidelines, cancer risk, and mortality in the Women’s Health Initiative. Cancer Prev Res (Phila) 2014;7:42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Cancer Research Fund/American Institute for Cancer Research [Internet]. Continuous Update Project Report. Food, nutrition, physical activity and the prevention of colorectal cancer 2011. [cited 2015 May 30]. Available from : http://www.aicr.org/continuous-update-project/reports/Colorectal-Cancer-2011-Report.pdf.
- 6.Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology 2010;138(6):2029–43 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogtmann E, Xiang YB, Li HL, Levitan EB, Yang G, Waterbor JW, Gao J, Cai H, Xie L, Wu QJ, et al. . Fruit and vegetable intake and the risk of colorectal cancer: Results from the Shanghai Men’s Health Study. Cancer Causes Control 2013;24:1935–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradbury KE, Appleby PN, Key TJ. Fruit, vegetable, and fiber intake in relation to cancer risk: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC). Am J Clin Nutr 2014;100 Suppl 1:394S–8S. [DOI] [PubMed] [Google Scholar]
- 9.Schulze MB, Hoffmann K. Methodological approaches to study dietary patterns in relation to risk of coronary heart disease and stroke. Br J Nutr 2006;95:860–9. [DOI] [PubMed] [Google Scholar]
- 10.Miller PE, Lesko SM, Muscat JE, Lazarus P, Hartman TJ. Dietary patterns and colorectal adenoma and cancer risk: A review of the epidemiological evidence. Nutr Cancer 2010;62:413–24. [DOI] [PubMed] [Google Scholar]
- 11.Yusof AS, Isa ZM, Shah SA. Dietary patterns and risk of colorectal cancer: a systematic review of cohort studies (2000–2011). Asian Pac J Cancer Prev 2012;13:4713–7. [DOI] [PubMed] [Google Scholar]
- 12.Fung TT, Brown LS. Dietary Patterns and the Risk of Colorectal Cancer. Curr Nutr Rep 2013;2:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Randi G, Edefonti V, Ferraroni M, La Vecchia C, Decarli A. Dietary patterns and the risk of colorectal cancer and adenomas. Nutr Rev 2010;68:389–408. [DOI] [PubMed] [Google Scholar]
- 14.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 2014;17:1689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whalen KA, McCullough M, Flanders WD, Hartman TJ, Judd S, Bostick RM. Paleolithic and Mediterranean diet pattern scores and risk of incident, sporadic colorectal adenomas. Am J Epidemiol 2014;180:1088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Djuric Z, Severson RK, Kato I. Association of dietary quercetin with reduced risk of proximal colon cancer. Nutr Cancer 2012;64:351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grosso G, Biondi A, Galvano F, Mistretta A, Marventano S, Buscemi S, Drago F, Basile F. Factors associated with colorectal cancer in the context of the Mediterranean diet: A case-control study. Nutr Cancer 2014;66:558–65. [DOI] [PubMed] [Google Scholar]
- 19.Kontou N, Psaltopoulou T, Soupos N, Polychronopoulos E, Xinopoulos D, Linos A, Panagiotakos DB. Metabolic syndrome and colorectal cancer: The protective role of Mediterranean diet–a case-control study. Angiology 2012;63:390–6. [DOI] [PubMed] [Google Scholar]
- 20.Miller PE, Lazarus P, Lesko SM, Muscat JE, Harper G, Cross AJ, Sinha R, Ryczak K, Escobar G, Mauger DT, et al. . Diet index-based and empirically derived dietary patterns are associated with colorectal cancer risk. J Nutr 2010;140:1267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zamora-Ros R, Shivappa N, Steck SE, Canzian F, Landi S, Alonso MH, Hebert JR, Moreno V. Dietary inflammatory index and inflammatory gene interactions in relation to colorectal cancer risk in the Bellvitge colorectal cancer case-control study. Genes Nutr 2015;10:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bamia C, Lagiou P, Buckland G, Grioni S, Agnoli C, Taylor AJ, Dahm CC, Overvad K, Olsen A, Tjonneland A, et al. . Mediterranean diet and colorectal cancer risk: Results from a European cohort. Eur J Epidemiol 2013;28:317–28. [DOI] [PubMed] [Google Scholar]
- 23.Fung TT, Hu FB, Wu K, Chiuve SE, Fuchs CS, Giovannucci E. The Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets and colorectal cancer. Am J Clin Nutr 2010;92:1429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarvandi S, Davidson NO, Schootman M. Increased risk of colorectal cancer in type 2 diabetes is independent of diet quality. PLoS One 2013;8:e74616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reedy J, Wirfalt E, Flood A, Mitrou PN, Krebs-Smith SM, Kipnis V, Midthune D, Leitzmann M, Hollenbeck A, Schatzkin A, et al. . Comparing 3 dietary pattern methods–cluster analysis, factor analysis, and index analysis–With colorectal cancer risk: The NIH-AARP Diet and Health Study. Am J Epidemiol 2010;171:479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shivappa N, Prizment AE, Blair CK, Jacobs DR Jr, Steck SE, Hebert JR. Dietary inflammatory index and risk of colorectal cancer in the Iowa Women’s Health Study. Cancer Epidemiol Biomarkers Prev 2014;23:2383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabung FK, Steck SE, Ma Y, Liese AD, Zhang J, Caan B, Hou L, Johnson KC, Mossavar-Rahmani Y, Shivappa N, et al. . The association between dietary inflammatory index and risk of colorectal cancer among postmenopausal women: results from the Women’s Health Initiative. Cancer Causes Control 2015;26:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wirth MD, Shivappa N, Steck SE, Hurley TG, Hebert JR. The dietary inflammatory index is associated with colorectal cancer in the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Br J Nutr 2015;113:1819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterkin BB. Dietary guidelines for Americans. J Am Diet Assoc 1990;90:1725–7. [PubMed] [Google Scholar]
- 30.Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: design and applications. J Am Diet Assoc 1995;95:1103–8. [DOI] [PubMed] [Google Scholar]
- 31.Dietary guidelines for Americans, 2005.6th Ed.: Department of Health and Human Services and Department of Agriculture, 2005. [Google Scholar]
- 32.McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr 2002;76:1261–71. [DOI] [PubMed] [Google Scholar]
- 33.Tabung FK, Steck SE, Zhang J, Ma Y, Liese AD, Agalliu I, Hingle M, Hou L, Hurley TG, Jiao L, et al. . Construct validation of the dietary inflammatory index among postmenopausal women. Ann Epidemiol 2015;25:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Tabung F, Hebert JR. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr 2014;17:1825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reedy J, Mitrou PN, Krebs-Smith SM, Wirfalt E, Flood A, Kipnis V, Leitzmann M, Mouw T, Hollenbeck A, Schatzkin A, et al. . Index-based dietary patterns and risk of colorectal cancer: the NIH-AARP Diet and Health Study. Am J Epidemiol 2008;168:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agnoli C, Grioni S, Sieri S, Palli D, Masala G, Sacerdote C, Vineis P, Tumino R, Giurdanella MC, Pala V, et al. . Italian Mediterranean Index and risk of colorectal cancer in the Italian section of the EPIC cohort. Int J Cancer 2013;132:1404–11. [DOI] [PubMed] [Google Scholar]
- 37.Pauwels EK. The protective effect of the Mediterranean diet: Focus on cancer and cardiovascular risk. Med Princ Pract 2011;20:103–11. [DOI] [PubMed] [Google Scholar]
- 38.Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, Willett WC, Hu FB. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 2005;82:163–73. [DOI] [PubMed] [Google Scholar]
- 39.Maisonneuve P, Shivappa N, Hebert JR, Bellomi M, Rampinelli C, Bertolotti R, Spaggiari L, Palli D, Veronesi G, Gnagnarella P. Dietary inflammatory index and risk of lung cancer and other respiratory conditions among heavy smokers in the COSMOS screening study. Eur J Nutr 2015;epub ahead of print:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shivappa N, Bosetti C, Zucchetto A, Montella M, Serraino D, La Vecchia C, Hebert JR. Association between dietary inflammatory index and prostate cancer among Italian men. Br J Nutr 2015;113:278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shivappa N, Bosetti C, Zucchetto A, Serraino D, La Vecchia C, Hebert JR. Dietary inflammatory index and risk of pancreatic cancer in an Italian case-control study. Br J Nutr 2015;113:292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rostom A, Dube C, Lewin G, Tsertsvadze A, Barrowman N, Code C, Sampson M, Moher D. Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: A systematic review prepared for the US Preventive Services Task Force. Ann Intern Med 2007;146:376–89. [DOI] [PubMed] [Google Scholar]
- 43.Dubé C, Rostom A, Lewin G, Tsertsvadze A, Barrowman N, Code C, Sampson M, Moher D. The use of aspirin for primary prevention of colorectal cancer: A systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med 2007;146:365–75. [DOI] [PubMed] [Google Scholar]
- 44.Bruce WR, Wolever TM, Giacca A. Mechanisms linking diet and colorectal cancer: The possible role of insulin resistance. Nutr Cancer 2000;37:19–26. [DOI] [PubMed] [Google Scholar]
- 45.Giovannucci E. Insulin and colon cancer. Cancer Causes Control 1995;6:164–79. [DOI] [PubMed] [Google Scholar]
- 46.Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr 2003; 78(3, Suppl)559S–69S. [DOI] [PubMed] [Google Scholar]
- 47.Schwingshackl L, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension Score, and Health Outcomes: A systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 2015;115:780–800.e5. [DOI] [PubMed] [Google Scholar]
- 48.George SM, Ballard-Barbash R, Manson JE, Reedy J, Shikany JM, Subar AF, Tinker LF, Vitolins M, Neuhouser ML. Comparing indices of diet quality with chronic disease mortality risk in postmenopausal women in the Women’s Health Initiative Observational Study: Evidence to inform national dietary guidance. Am J Epidemiol 2014;180:616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panagiotakos DB, Pitsavos C, Arvaniti F, Stefanadis C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev Med 2007;44:335–40. [DOI] [PubMed] [Google Scholar]
- 50.Kyrø C, Skeie G, Loft S, Overvad K, Christensen J, Tjonneland A, Olsen A. Adherence to a healthy Nordic food index is associated with a lower incidence of colorectal cancer in women: The Diet, Cancer and Health cohort study. Br J Nutr 2013;109:920–7. [DOI] [PubMed] [Google Scholar]
- 51.Roswall N, Li Y, Kyro C, Sandin S, Lof M, Adami HO, Weiderpass E. No association between adherence to a healthy Nordic food index and colorectal cancer: Results from a Swedish cohort study. Cancer Epidemiol Biomarkers Prev 2015;24:755–7. [DOI] [PubMed] [Google Scholar]
- 52.Liese AD, Krebs-Smith SM, Subar AF, George SM, Harmon BE, Neuhouser ML, Boushey CJ, Schap TE, Reedy J. The Dietary Patterns Methods Project: Synthesis of findings across cohorts and relevance to dietary guidance. J Nutr 2015;145:393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]