Abstract
Linoleic acid (LA) is a bioactive fatty acid with diverse effects on human physiology and pathophysiology. LA is a major dietary fatty acid, and also one of the most abundant fatty acids in adipose tissue, where its concentration reflects dietary intake. Over the last half century in the United States, dietary LA intake has greatly increased as dietary fat sources have shifted toward polyunsaturated seed oils such as soybean oil. We have conducted a systematic literature review of studies reporting the concentration of LA in subcutaneous adipose tissue of US cohorts. Our results indicate that adipose tissue LA has increased by 136% over the last half century and that this increase is highly correlated with an increase in dietary LA intake over the same period of time.
Keywords: subcutaneous adipose tissue, US, linoleic acid, dietary linoleic acid, change over time
Introduction
Linoleic acid (LA) (18:2n–6) is an 18-carbon n–6 PUFA with diverse effects on human physiology. LA is linked to skin barrier (1), immune (2), cardiovascular (3, 4), and neurobiological (5) functions, and, as a precursor of arachidonic acid and its metabolites, to reproductive (6), thermoregulatory, and digestive functions (7). In addition, LA is a natural ligand for PPARs (8). PPARs are intimately involved in the regulation of metabolic functions, including lipid and glucose metabolism, and they have been implicated in obesity and cardiometabolic disease risk (9). PPARα agonism may contribute to the ability of dietary LA to reduce circulating concentrations of total and LDL cholesterol (10). Finally, LA can influence biological processes via its nonenzymatic oxidation products. Oxidation of lipids in LDL is a risk marker for coronary heart disease (11). Because of its abundance in LDL and susceptibility to oxidation, LA is the most commonly oxidized species in LDL (12). The LA content of LDL reflects dietary intake (13).
Many seed oils are rich in LA, and consumption in the United States has increased substantially over the last half century (14). Much of this increase has come from soybean oil, which contains between 50% and 60% of total FAs as LA (14). Adipose tissue concentration of LA is particularly responsive to dietary LA, as demonstrated by diet modification trials (10). As such, it is used as a biomarker of dietary intake (15). We hypothesized that increases in dietary LA in the US food system have led to increased adipose tissue concentrations of LA. To test this hypothesis, we conducted a systematic literature review of studies that have reported the LA concentration of subcutaneous adipose tissue. Our findings suggest that adipose tissue LA has more than doubled in the United States over the last half century and correlates strongly with LA in the US food supply, potentially influencing numerous aspects of human physiology and pathophysiology.
Methods
To identify studies, a systematic search was conducted in May 2015 with the use of the PubMed and Google Scholar databases. Search terms included “adipose tissue fatty acids,” “adipose tissue” and “fatty acids,” and “adipose linoleic acid” and “adipose 18:2.” Manuscripts were manually searched for additional references. All studies that reported adipose tissue LA concentration in US subjects before May 2015 were considered. Studies were excluded if subjects were infants, followed an atypical diet, or were diagnosed with a disease or had morbid obesity that could substantially influence FA concentrations in adipose tissue. We excluded nonsubcutaneous adipose depots because of potential differences in FA composition. In cases in which data from a study were reported in more than one publication, only results from the first report were included.
When provided in the manuscript, the date of adipose tissue sampling was used for analyses. When the date of sampling was not provided, the date of publication was used. Nineteen publications provided a sampling date, whereas 18 did not. More recent publications were more likely to provide a sampling date. In cases in which the date provided was a range, the mean value was used.
US LA intake data between 1909 and 1999 were estimated from USDA Economic Research Service food disappearance data, as previously described (14). A tabular version of the data was generously provided by Joseph R Hibbeln, MD (National Institute of Alcohol Abuse and Alcoholism). These data do not extend beyond 1999, and therefore the correlation analysis between LA intake and adipose tissue LA also does not extend beyond that date.
Statistics were performed in publicly available R software. Statistical significance was determined with the use of linear regression analysis.
Results
We identified 37 studies that met inclusion criteria, reporting 38 measurements of subcutaneous adipose tissue LA (Table 1). Publication dates ranged from 1959 to 2008, with no studies identified after this period. The most common sampling site was the buttocks (n = 20), although data also represent abdominal (n = 7), breast adipose (n = 4), posterior iliac crest (n = 2), calf (n = 1), perineum (n = 1), and unspecified subcutaneous adipose tissue (n = 3).
TABLE 1.
Studies included in the analysis1
| Publication | Date | % LA | Sample date | Site | Notes |
| Hirsch et al. (16) | 1960 | 9.9 | NS | Buttocks | |
| Gellhorn et al. (17) | 1961 | 9.5 | NS | Unspecified SC | |
| Scott et al. (18) | 1962 | 9.5 | NS | Abdominal SC | |
| Hegsted et al. (19) | 1962 | 7.9 | NS | Unspecified SC | |
| Dayton et al. (20) | 1962 | 9 | 1959 | Buttocks | Baseline |
| Lee et al. (21) | 1962 | 12.1, 11.2 | NS | Buttocks | US whites and blacks |
| Lee et al. (22) | 1962 | 11.55 | NS | Buttocks | Nondiabetics |
| Sweeney et al. (23) | 1963 | 10.6 | NS | Perineum SC | Mothers |
| Remenchik et al. (24) | 1963 | 7.56 | NS | Abdominal SC | |
| Christakis et al. (25) | 1965 | 9.7 | NS | Buttocks | Baseline |
| Rabinowitz et al. (26) | 1965 | 8.7 | NS | Abdominal SC | Baseline |
| Dayton et al. (10) | 1966 | 11 | 1961 (estimated) | Buttocks | |
| Insull et al. (27) | 1967 | 9.2 | 1962 | Abdominal SC | |
| Fleischman et al. (28) | 1968 | 11 | NS | Buttocks | Baseline |
| Baker et al. (29) | 1969 | 8.2 | NS | Abdominal SC | Adults |
| Insull et al. (30) | 1969 | 10.21 | 1966 | Abdominal SC | |
| Insull et al. (31) | 1970 | 10.2 | 1962, 1966 | Abdominal SC | US whites and blacks |
| Witting et al. (32) | 1975 | 13 | 1973 | Buttocks | |
| Kokatnur et al. (33) | 1979 | 11.5 | 1969–1971 | Buttocks | |
| Fordyce et al. (34) | 1983 | 13.68 | NS | Posterior iliac crest | US-born Americans |
| Berry et al. (35) | 1986 | 16.3 | 1981–1982 | Buttocks | |
| Hill et al. (36) | 1987 | 15.8 | NS | Breast adipose | Controls |
| Handelman et al. (37) | 1988 | 18.2 | 1986 | Buttocks | |
| Malcom et al. (38) | 1989 | 15.37 | 1986–1987 | Buttocks | |
| Hudgins et al. (39) | 1991 | 16.91 | 1981 | Buttocks | |
| London et al. (40) | 1991 | 17.23 | 1986–1987 | Buttocks | |
| Hunter et al. (41) | 1992 | 19 | 1986–1987 | Buttocks | |
| London et al. (42) | 1993 | 17.72 | 1986–1988 | Buttocks | |
| Phinney et al. (43) | 1994 | 18.72 | NS | Unspecified SC | |
| Godley et al. (44) | 1996 | 17.57 | 1989–1991 | Buttocks | |
| Petrek et al. (45) | 1997 | 16.59 | 1987–1989 | Breast adipose | |
| Garland et al. (46) | 1998 | 18.5 | 1986–1987 | Buttocks | |
| Ullrich et al. (47) | 2001 | 18 | NS | Posterior iliac crest | Controls |
| Bagga et al. (48) | 2002 | 13.9 | 1995–1996 | Breast adipose | Controls |
| Knutsen et al. (49) | 2003 | 20.1 | NS | Buttocks | |
| Ren et al. (50) | 2008 | 23.4 | NS | Calf SC | |
| Yee et al. (51) | 2010 | 18 | 2006–2008 | Breast adipose | Baseline |
LA, linoleic acid; NS, not specified; SC, subcutaneous adipose tissue.
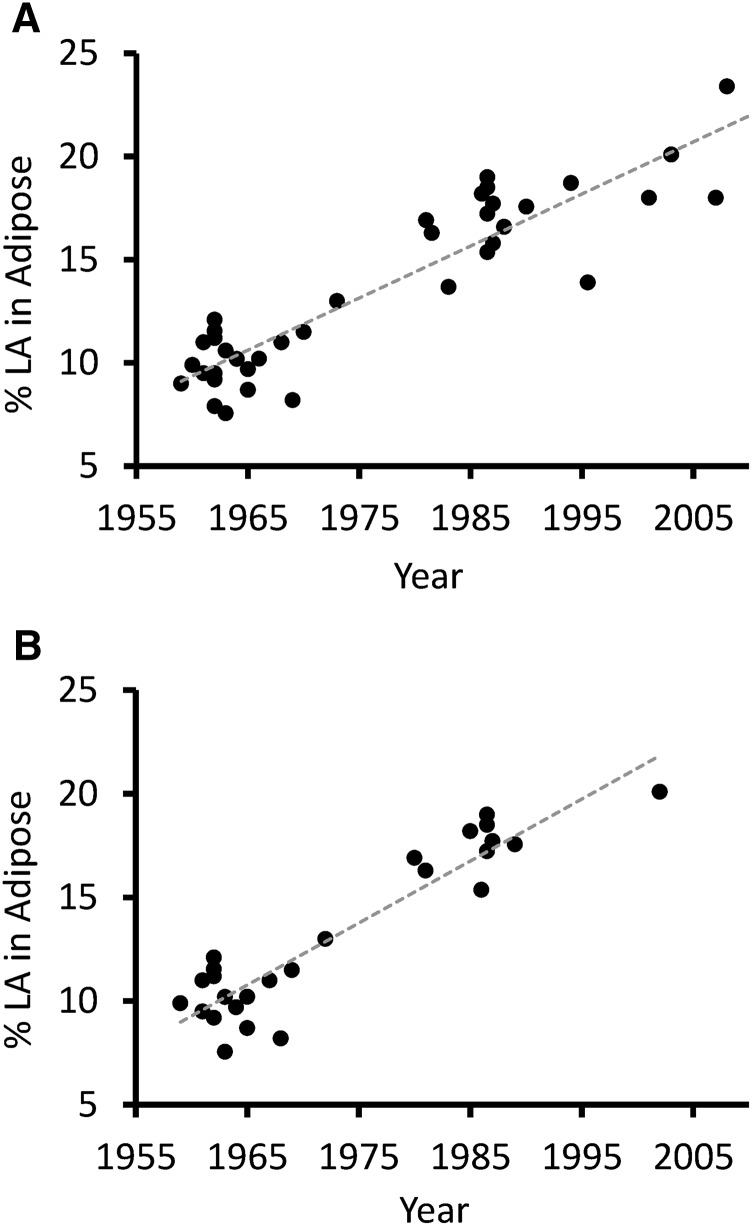
When considering all subcutaneous adipose sites, LA concentration increased in a linear manner over time (R2 = 0.83; P < 0.001) (Figure 1A). The best-fit line for adipose tissue LA increased from 9.1% in 1959 to 21.5% in 2008, representing a 136% increase.
FIGURE 1.
US adipose tissue LA concentration, 1959–2008. Increase in adipose tissue LA concentration over time across all subcutaneous sites (R2 = 0.83; P < 0.001) (A). Increase in adipose tissue LA concentration over time, buttocks and abdominal subcutaneous only (R2 = 0.86; P < 0.001) (B). LA, linoleic acid.
To eliminate possible confounding because of differences in adipose tissue composition across subcutaneous sites, we repeated the analysis including only data from the buttocks and abdominal tissue, which have a comparable FA composition (38). This had little impact on the strength of the relation between time and adipose tissue LA (R2 = 0.86; P < 0.001) (Figure 1B).
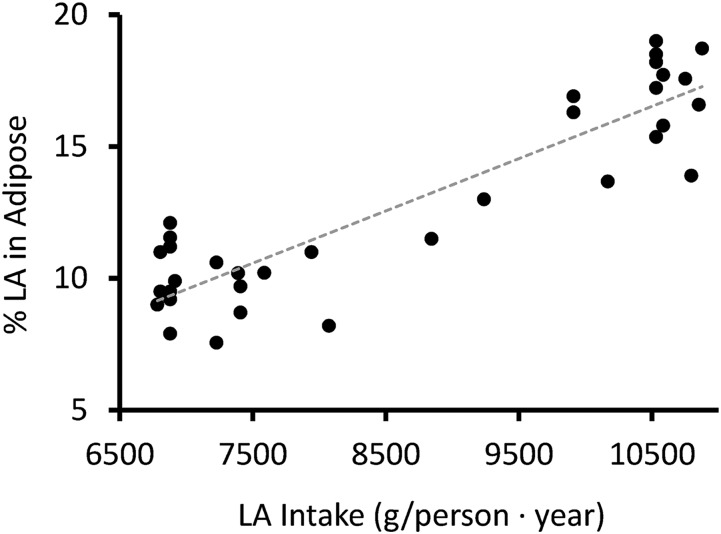
Dietary LA has been shown to influence adipose tissue LA concentration in diet modification trials; however, to our knowledge, this hypothesis has never been evaluated by interrogating the correlation between LA intake and adipose tissue LA in a free-living population over a long period of time. Considering all subcutaneous sites, dietary intake of LA was highly correlated with adipose tissue LA (R2 = 0.81; P < 0.001) (Figure 2). For every kg increase of yearly per capita LA intake, adipose tissue concentration of LA increased by 2%.
FIGURE 2.
Correlation between adipose tissue LA concentration (all sites) and dietary LA intake, 1959–1999. Adipose tissue LA concentration is strongly correlated with dietary LA intake (R2 = 0.81; P < 0.001). LA, linoleic acid.
Conclusions
These findings support the hypothesis that adipose tissue LA concentration has greatly increased in the United States over the last half century. Between 1959 and 2008, adipose tissue LA increased by 136%, representing a major shift in the adipose tissue concentration of a bioactive FA.
Because of its influence on multiple physiologic and pathophysiologic processes, LA has the potential to play an important role in human health. At the same time that the LA content of adipose tissue has been increasing, the United States has experienced substantial changes in disease prevalence. Cardiovascular disease risk has declined (52), whereas the prevalence of obesity (53), diabetes (54), and asthma (55) have increased. Our aim was not to evaluate the potential causal role of LA in these pathophysiologic processes, but simply to highlight that a role for LA in these trends is mechanistically possible and worthy of further investigation.
Our findings suggest that adipose tissue LA concentration is strongly correlated with dietary LA intake. This provides additional independent support for the hypothesis that long-term adipose tissue LA concentration is tied to dietary LA intake, and for the first time demonstrates that this relation applies to long-term population trends. Our analysis may also provide a basis for modeling the relation between population LA intake and adipose tissue LA. For each kg increase of estimated yearly per capita LA intake, adipose tissue LA increased by 2%. Because the half-life of LA incorporation into adipose tissue is ∼680 d (10), changes in LA intake would take several years to result in a stable increase of adipose tissue LA.
Limitations of this study relate primarily to the nature of the underlying data. The data were not the result of longitudinal random sampling of the US population, but rather were primarily small samples representing varied regions of the United States. The underlying assumption is that local data, to some extent, represent broader population trends. However, local differences in dietary habits could reduce the applicability of individual data points to the United States as a whole. Furthermore, the results shown here are from laboratories that used different methods (e.g., packed compared with liquid-phase capillary columns or total adipose FAs compared with adipose tissue TG FAs) and reporting (e.g., weight percentage or area percentage). Some of the earlier studies may not have accounted for the longer chain (20- and 22-carbon) PUFAs, effectively increasing the proportion of LA in studies reported in the earlier years. Despite differences in methods that exist in the studies included in this cross-sectional study, we are unaware of any that would produce a large and systematic increase of LA. Our results are also limited by the fact that there are gaps in years of reported studies. In addition, 18 of 37 studies did not provide a sampling date, and therefore we used the publication date in analyses. In these cases, the sampling date occurred before the publication date, but the time difference is unknown. These limitations would all be expected to increase the variability of the data and decrease the likelihood of identifying statistically significant correlations. The fact that we were nevertheless able to identify strong positive relations between adipose tissue LA concentration and time (R2 = 0.81–0.90; P < 0.001) argues that the data were of sufficient quality to address the hypotheses in question. Finally, estimates of LA intake used in Figure 2 are based on food disappearance data (14), which are not a direct measure of food intake. However, food disappearance estimates are likely more accurate than self-reported estimates, such as those from the CDC NHANES surveys, which are the primary alternative (56).
Our findings suggest that adipose tissue LA has increased substantially in the United States over the last half century, and, to our knowledge, for the first time demonstrate that this increase is highly correlated with increased dietary LA intake over the same period. Interestingly, 3 recent investigations (2013–2015) of adipose tissue LA in European countries found concentrations of 11% (57), 12.5% (58), and 10.6% (59). These values are similar to earlier reports from the United States. Because LA is in involved in numerous physiologic and pathophysiologic processes, these changes have potentially significant implications for public health. These findings call for further research into the consequences, positive or negative, of such a major shift in the adipose tissue concentration of a single bioactive FA occurring over a relatively short period of time in the United States.
Acknowledgments
We thank Joseph R Hibbeln for generously providing us with tabular data on US dietary LA intake, and Elizabeth Sosik for statistical consultation. Both authors read and approved the final manuscript.
References
- 1.Ziboh VA. Prostaglandins, leukotrienes, and hydroxy fatty acids in epidermis. Semin Dermatol 1992;11:114–20. [PubMed] [Google Scholar]
- 2.Harbige LS. Dietary n-6 and n-3 fatty acids in immunity and autoimmune disease. Proc Nutr Soc 1998;57:555–62. [DOI] [PubMed] [Google Scholar]
- 3.Farvid MS, Ding M, Pan A, Sun Q, Chiuve SE, Steffen LM, Willett WC, Hu FB. Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Circulation 2014;130:1568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramsden CE, Zamora D, Leelarthaepin B, Majchrzak-Hong SF, Faurot KR, Suchindran CM, Ringel A, Davis JM, Hibbeln JR. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ 2013;346:e8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibson RA, Muhlhausler B, Makrides M. Conversion of linoleic acid and alpha-linolenic acid to long-chain polyunsaturated fatty acids (LCPUFAs), with a focus on pregnancy, lactation and the first 2 years of life. Matern Child Nutr 2011;7:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thorburn GD. The placenta, PGE2 and parturition. Early Hum Dev 1992;29:63–73. [DOI] [PubMed] [Google Scholar]
- 7.Funk CD. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001;294:1871–5. [DOI] [PubMed] [Google Scholar]
- 8.Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lehhard JM, et al. . Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ. Proc Natl Acad Sci USA 1997;94:4318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev 1999;20:649–88. [DOI] [PubMed] [Google Scholar]
- 10.Dayton S, Hashimoto S, Dixon W, Pearce ML. Composition of lipids in human serum and adipose tissue during prolonged feeding of a diet high in unsaturated fat. J Lipid Res 1966;7:103–11. [PubMed] [Google Scholar]
- 11.Meisinger C, Baumert J, Khuseyinova N, Loewel H, Koenig W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation 2005;112:651–7. [DOI] [PubMed] [Google Scholar]
- 12.Jira W, Spiteller G, Carson W, Schramm A. Strong increase in hydroxy fatty acids derived from linoleic acid in human low density lipoproteins of atherosclerotic patients. Chem Phys Lipids 1998;91:1–11. [DOI] [PubMed] [Google Scholar]
- 13.Kratz M, Cullen P, Kannenberg F, Kassner A, Fobker M, Abuja PM, Assmann G, Wahrburg U. Effects of dietary fatty acids on the composition and oxidizability of low-density lipoprotein. Eur J Clin Nutr 2002;56:72–81. [DOI] [PubMed] [Google Scholar]
- 14.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century123. Am J Clin Nutr 2011;93:950–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008;47:348–80. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch J, Farquhar JW, Ahrens EH, Peterson ML, Stoffel W. Studies of adipose tissue in man. A microtechnic for sampling and analysis. Am J Clin Nutr 1960;8:499–511. [DOI] [PubMed] [Google Scholar]
- 17.Gellhorn A, Marks PA. The composition and biosynthesis of lipids in human adipose tissues. J Clin Invest 1961;40:925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott RF, Daoud AS, Gittelsohn A, Opalka E, Florentin R, Goodale F. Lack of correlation between fatty acid patterns in adipose tissue and amount of coronary arteriosclerosis. Am J Clin Nutr 1962;10:250–6. [DOI] [PubMed] [Google Scholar]
- 19.Hegsted DM, Jack CW, Stare FJ. The composition of human adipose tissue from several parts of the world. Am J Clin Nutr 1962;10:11–8. [DOI] [PubMed] [Google Scholar]
- 20.Dayton S, Pearce ML, Hashimoto S, Fakler LJ, Hiscock E, Dixon WJ. A controlled clinical trial of a diet high in unsaturated fat. Preliminary observations. N Engl J Med 1962;266:1017–23. [DOI] [PubMed] [Google Scholar]
- 21.Lee K, Shaper A, Scott R, Goodale F, Thomas W. Geographic studies pertaining to arteriosclerosis: Comparison of fatty acid patterns of adipose tissue and plasma lipids in East Africans with those of North American white and negro groups. Arch Pathol 1962;74:481–8.
- 22.Lee KT, Scott RF, Morrison ES, Thomas WA. Chemi-co-anatomic studies of arteriosclerosis and thrombosis in diabetics. II. Fatty acids of adipose tissue and plasma lipids in several groups of North American diabetics and nondiabetics. Exp Mol Pathol 1962;1:364–76. [DOI] [PubMed] [Google Scholar]
- 23.Sweeney MJ, Etteldorf JN, Throop LJ, Timma DL, Wrenn EL. Diet and fatty acid distribution in subcutaneous fat and the cholesterol-triglyceride fraction of serum of young infants. J Clin Invest 1963;42:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Remenchik AP, Bernsohn J. Relationships between the composition of adipose tissue and the total fat content of the body. Ann N Y Acad Sci 1963;110:754–9. [DOI] [PubMed] [Google Scholar]
- 25.Christakis GJ, Rinzler SH, Archer M, Hashim SA, Vanitallie TB. Effect of serum cholesterol-lowering diet on composition of depot fat in man. Am J Clin Nutr 1965;16:243–51. [DOI] [PubMed] [Google Scholar]
- 26.Rabinowitz JL, Reimenschneider RW, Myerson RM. The effect of L-triiodothyronine on the fatty acid composition of human adipose tissue. Metabolism 1965;14:612–8. [DOI] [PubMed] [Google Scholar]
- 27.Insull W, Bartsch GE. Fatty acid composition of human adipose tissue related to age, sex, and race. Am J Clin Nutr 1967;20:13–23. [DOI] [PubMed] [Google Scholar]
- 28.Fleischman AI, Hayton T, Bierenbaum ML, Watson P. The effect of a polyunsaturated diet upon adipose-tissue fatty acids in young coronary males. A five-year cohort study. Lipids 1968;3:147–50. [DOI] [PubMed] [Google Scholar]
- 29.Baker GL. Human adipose tissue composition and age. Am J Clin Nutr 1969;22:829–35. [DOI] [PubMed] [Google Scholar]
- 30.Insull W, Lang PD, Hsi BP, Yoshimura S. Studies of arteriosclerosis in Japanese and American men. J Clin Invest 1969;48:1313–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Insull W, Lang PD, Hsi BP. Adipose tissue fatty acid differences in American men between 1962 and 1966. Am J Clin Nutr 1970;23:17–26. [DOI] [PubMed] [Google Scholar]
- 32.Witting LA, Lee L. Recommended dietary allowance for vitamin E: relation to dietary, erythrocyte and adipose tissue linoleate. Am J Clin Nutr 1975;28:577–83. [DOI] [PubMed] [Google Scholar]
- 33.Kokatnur MG, Oalmann MC, Johnson WD, Malcom GT, Strong JP. Fatty acid composition of human adipose tissue from two anatomical sites in a biracial community. Am J Clin Nutr 1979;32:2198–205. [DOI] [PubMed] [Google Scholar]
- 34.Fordyce MK, Christakis G, Kafatos A, Duncan R, Cassady J. Adipose tissue fatty acid composition of adolescents in a U.S.–Greece cross-cultural study of coronary heart disease risk factors. J Chronic Dis 1983;36:481–6. [DOI] [PubMed] [Google Scholar]
- 35.Berry EM, Hirsch J, Most J, McNamara DJ, Thornton J. The relationship of dietary fat to plasma lipid levels as studied by factor analysis of adipose tissue fatty acid composition in a free-living population of middle-aged American men. Am J Clin Nutr 1986;44:220–31. [DOI] [PubMed] [Google Scholar]
- 36.Hill P, Wynder EL. Comparison of mammary adipose fatty acid composition in Japanese and American breast cancer patients. Eur J Cancer Clin Oncol 1987;23:407–10. [DOI] [PubMed] [Google Scholar]
- 37.Handelman GJ, Epstein WL, Machlin LJ, van Kuijk FJ, Dratz EA. Biopsy method for human adipose with vitamin E and lipid measurements. Lipids 1988;23:598–604. [DOI] [PubMed] [Google Scholar]
- 38.Malcom GT, Bhattacharyya AK, Velez-Duran M, Guzman MA, Oalmann MC, Strong JP. Fatty acid composition of adipose tissue in humans: differences between subcutaneous sites. Am J Clin Nutr 1989;50:288–91. [DOI] [PubMed] [Google Scholar]
- 39.Hudgins LC, Hirsch J, Emken EA. Correlation of isomeric fatty acids in human adipose tissue with clinical risk factors for cardiovascular disease. Am J Clin Nutr 1991;53:474–82. [DOI] [PubMed] [Google Scholar]
- 40.London SJ, Sacks FM, Caesar J, Stampfer MJ, Siguel E, Willett WC. Fatty acid composition of subcutaneous adipose tissue and diet in postmenopausal US women. Am J Clin Nutr 1991;54:340–5. [DOI] [PubMed] [Google Scholar]
- 41.Hunter DJ, Rimm EB, Sacks FM, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Comparison of measures of fatty acid intake by subcutaneous fat aspirate, food frequency questionnaire, and diet records in a free-living population of US men. Am J Epidemiol 1992;135:418–27. [DOI] [PubMed] [Google Scholar]
- 42.London SJ, Sacks FM, Stampfer MJ, Henderson IC, Maclure M, Tomita A, Wood WC, Remine S, Robert NJ, Dmochowski JR. Fatty acid composition of the subcutaneous adipose tissue and risk of proliferative benign breast disease and breast cancer. J Natl Cancer Inst 1993;85:785–93. [DOI] [PubMed] [Google Scholar]
- 43.Phinney SD, Stern JS, Burke KE, Tang AB, Miller G, Holman RT. Human subcutaneous adipose tissue shows site-specific differences in fatty acid composition. Am J Clin Nutr 1994;60:725–9. [DOI] [PubMed] [Google Scholar]
- 44.Godley PA, Campbell MK, Gallagher P, Martinson FE, Mohler JL, Sandler RS. Biomarkers of essential fatty acid consumption and risk of prostatic carcinoma. Cancer Epidemiol Biomark Prev 1996;5:889–95. [PubMed] [Google Scholar]
- 45.Petrek JA, Hudgins LC, Ho M, Bajorunas DR, Hirsch J. Fatty acid composition of adipose tissue, an indication of dietary fatty acids, and breast cancer prognosis. J Clin Oncol 1997;15:1377–84. [DOI] [PubMed] [Google Scholar]
- 46.Garland M, Sacks FM, Colditz GA, Rimm EB, Sampson LA, Willett WC, Hunter DJ. The relation between dietary intake and adipose tissue composition of selected fatty acids in US women. Am J Clin Nutr 1998;67:25–30. [DOI] [PubMed] [Google Scholar]
- 47.Ullrich NF, Purnell JQ, Brunzell JD. Adipose tissue fatty acid composition in humans with lipoprotein lipase deficiency. J Investig Med 2001;49:273–5. [PubMed] [Google Scholar]
- 48.Bagga D, Anders KH, Wang H-J, Glaspy JA. Long-chain n-3-to-n-6 polyunsaturated fatty acid ratios in breast adipose tissue from women with and without breast cancer. Nutr Cancer 2002;42:180–5. [DOI] [PubMed] [Google Scholar]
- 49.Knutsen SF, Fraser GE, Beeson WL, Lindsted KD, Shavlik DJ. Comparison of adipose tissue fatty acids with dietary fatty acids as measured by 24-hour recall and food frequency questionnaire in Black and White Adventists: the Adventist Health Study. Ann Epidemiol 2003;13:119–27. [DOI] [PubMed] [Google Scholar]
- 50.Ren J, Dimitrov I, Sherry AD, Malloy CR. Composition of adipose tissue and marrow fat in humans by 1H NMR at 7 Tesla. J Lipid Res 2008;49:2055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yee LD, Lester JL, Cole RM, Richardson JR, Hsu JC, Li Y, Lehman A, Belury MA, Clinton SK. ω-3 Fatty acid supplements in women at high risk of breast cancer have dose-dependent effects on breast adipose tissue fatty acid composition. Am J Clin Nutr 2010;91:1185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Finegold JA, Asaria P, Francis DP. Mortality from ischaemic heart disease by country, region, and age: Statistics from World Health Organisation and United Nations. Int J Cardiol 2013;168:934–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fryar CD, Carroll MD, Ogden CL. Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1960–1962 through 2007. Natl Cent Health Stat 2008;2012:1–6. [Google Scholar]
- 54.Geiss LS, Wang J, Cheng YJ, Thompson TJ, Barker L, Li Y, Albright AL, Gregg EW. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA 2014;312:1218–26. [DOI] [PubMed] [Google Scholar]
- 55.Allan K, Devereux G. Diet and asthma: nutrition implications from prevention to treatment. J Am Diet Assoc 2011;111:258–68. [DOI] [PubMed] [Google Scholar]
- 56.Schoeller DA, Thomas D, Archer E, Heymsfield SB, Blair SN, Goran MI, Hill JO, Atkinson RL, Corkey BE, Foreyt J, et al. . Self-report-based estimates of energy intake offer an inadequate basis for scientific conclusions. Am J Clin Nutr 2013;97:1413–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cottet V, Vaysse C, Scherrer M-L, Ortega-Deballon P, Lakkis Z, Delhorme J-B, Deguelte-Lardière S, Combe N, Bonithon-Kopp C. Fatty acid composition of adipose tissue and colorectal cancer: a case-control study. Am J Clin Nutr 2015;101:192–201. [DOI] [PubMed] [Google Scholar]
- 58.Kunešová M, Hlavatý P, Tvrzická E, Staňková B, Kalousková P, Viguerie N, Larsen TM, van Baak MA, Jebb SA, Martinez JA, et al. . Fatty acid composition of adipose tissue triglycerides after weight loss and weight maintenance: the DIOGENES study. Physiol Res 2012;61:597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hellmuth C, Demmelmair H, Schmitt I, Peissner W, Blüher M, Koletzko B. Association between Plasma Nonesterified Fatty Acids Species and Adipose Tissue Fatty Acid Composition. PLoS ONE [Internet]. 2013;8 [cited 2015 Jul 29]. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3788793/. [DOI] [PMC free article] [PubMed]