Abstract
Background
The rabies virus causes a fatal encephalitis and can be transmitted through organ transplantation. In 2013, a man developed rabies 18 months after receiving a kidney from a donor with rabies, who was not known to have been infected when the organs were procured. Three additional persons who received organs from the same donor (liver, kidney, heart), all of whom were not vaccinated for rabies before transplantation, received rabies post-exposure prophylaxis (PEP) with rabies immune globulin and 5 doses of rabies vaccine as soon as the diagnosis of rabies was made in the donor (18 months after their transplant surgeries). We describe their clinical management.
Methods
As the 3 recipients were all on immunosuppressive medications, post-vaccination serologic testing was performed using the rapid fluorescent focus inhibition test to measure rabies virus neutralizing antibodies (RVNAs). An acceptable antibody response to administration of rabies vaccine was defined as detection of RVNAs at a concentration ≥0.1 IU/mL from a serum specimen collected ≥7 days after the fifth vaccine dose.
Results
All 3 recipients demonstrated an acceptable antibody response despite their immunosuppressed states. More than 36 months have passed since their transplant surgeries, and all 3 recipients have no evidence of rabies.
Conclusions
The survival of 3 previously unvaccinated recipients of solid organs from a donor with rabies is unexpected. Although the precise factors that led to their survival remain unclear, our data suggest that PEP can possibly enhance transplant safety in settings in which donors are retrospectively diagnosed with rabies.
Keywords: rabies, solid organ transplantation, post-exposure prophylaxis
Rabies is a fatal encephalitis caused by the rabies virus. An estimated 55,000 humans die each year from rabies, and the majority of cases are related to bites from rabid dogs (1). Rabies can be prevented after conventional exposures, such as animal bites, through use of post-exposure prophylaxis (PEP), which for previously unvaccinated immunocompetent persons consists of rabies immune globulin (RIG) and 4 doses of rabies vaccine (2, 3).
The humoral response stimulated after administration of rabies vaccine involves production of rabies virus neutralizing antibodies (RVNAs) that can be measured with the rapid fluorescent focus inhibition test (RFFIT); administration of RIG provides an immediate supply of RVNAs while the host mounts this humoral response. According to the Advisory Committee on Immunization Practices (ACIP), an acceptable antibody response to administration of rabies vaccine can be documented if the host’s serum completely neutralizes challenge rabies virus at a serum dilution of 1:5; this corresponds to an approximate RVNA concentration of 0.1 IU/mL (2, 3). RVNA titers do not correlate with protection against rabies, and post-vaccination serologic testing is not routinely performed for immunocompetent persons because the majority will have an acceptable antibody response (2, 3). In contrast, immunosuppressed persons often have diminished antibody responses to rabies vaccines, although the precise factors associated with this suboptimal response are not well defined (2–7). ACIP therefore recommends that previously unvaccinated immunosuppressed persons receive RIG and 5 doses of vaccine, as well as postvaccination serologic testing to confirm that an acceptable antibody response has occurred (2).
While most rabies cases are associated with a history of direct exposure to rabid animals, rabies virus transmission can also occur through transplantation of tissues or organs that have been procured from infected human donors (8). For example, in 2004 and 2005, 2 separate outbreaks of rabies occurred among persons who had received solid organs procured from donors not known to have rabies at the time of transplantation (9, 10). During both events, all recipients who had not received pre-transplant rabies vaccination developed rabies symptoms within 6 weeks of transplantation and subsequently died; this included recipients who were given PEP after the retrospective diagnosis of rabies was made in the donor. Observations from these outbreaks suggest a high infectivity rate among recipients of organs that have been procured from donors with rabies and raise questions about the effectiveness of PEP in this setting (8, 9).
In 2013, we reported a third rabies virus transmission event through solid organ transplantation (11). In contrast to the 2 prior solid organ-associated outbreaks, out of a total of 4 previously unvaccinated solid organ recipients, only 1 (recipient of left kidney) developed rabies after an unusually long incubation period of 17 months. The 3 remaining recipients (liver, right kidney, heart) received PEP (with RIG and 5 doses of vaccine administered on PEP Days 0, 3, 7, 14, and 28) as soon as the diagnosis of rabies was made in the donor. Despite starting PEP 18 months after their transplant procedures, these 3 recipients have, to date, not developed rabies (2, 3). This article describes the clinical management of these 3 recipients after the organ donor was retrospectively diagnosed with rabies, with a focus on the challenges of managing PEP for immunosuppressed persons.
Methods
The details of the public health investigation have been previously published (11, 12). As the 3 surviving recipients were all on immunosuppressive medications, post-vaccination serologic testing was performed using RFFIT on serial serum specimens (11, 13–15). The lower limit of detection for RVNAs by RFFIT was 0.04 IU/mL. A recipient was considered to have had an acceptable antibody response to administration of rabies vaccine if RVNAs were detected from serum at a concentration ≥0.1 IU/mL at least 7 days after the fifth vaccine dose had been administered. We also evaluated a baseline serum specimen collected prior to the administration of PEP, to verify if there had been any previous exposure to rabies virus antigens. The baseline serum specimen was tested for RVNAs (using RFFIT) and for rabies virus-specific binding antibodies (using the indirect fluorescent antibody test) (14, 15).
Results
Liver recipient
The liver recipient was a 42-year-old man when he received his liver transplant for end-stage liver disease from Budd–Chiari syndrome; he had not been vaccinated for rabies before the time of transplantation. Immediately prior to receiving the organ, the patient was critically ill with a model for end-stage liver disease score of 40. The patient’s transplant surgery was uneventful, as was his immediate post-transplant course, aside from renal failure; he was discharged home on post-transplant day 11.
The patient’s induction immunosuppressive regimen consisted of methylprednisolone (1 intravenous [IV] dose of 1 g) and alemtuzumab (1 IV dose of 30 mg). His initial maintenance immunosuppressive regimen consisted of methylprednisolone tapered over the first 3 postoperative months and tacrolimus with initial serum trough level goals of 4–8 ng/mL. During post-transplant month 8, the patient developed herpes zoster of the lower extremity, which was treated successfully with acyclovir; no adjustments were made to his immunosuppressive regimen. Other post-transplant complications included chronic renal insufficiency, diabetes controlled with oral hypoglycemic agents, and hypertension. He has no episodes of rejection of his transplanted liver.
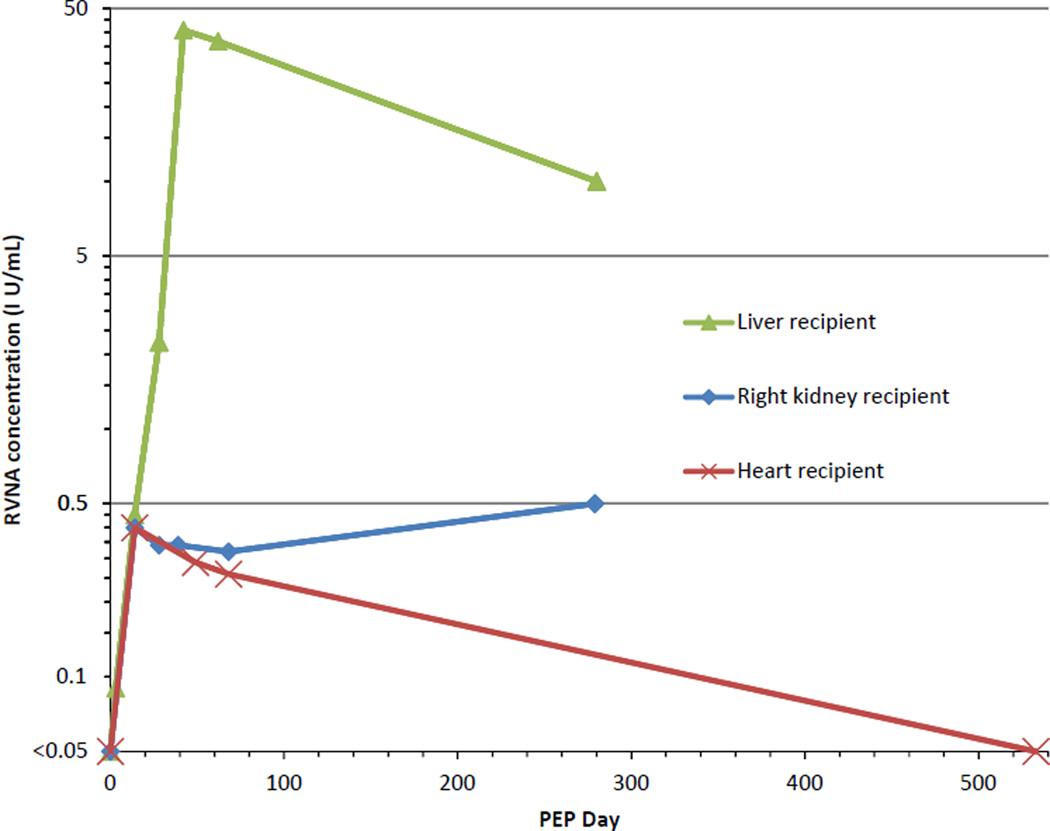
PEP consisted of RIG and 5 doses of purified chick embryo cell rabies vaccine (11). Serum collected 14 days after the fifth vaccine dose (PEP Day 42) demonstrated an acceptable antibody response to rabies vaccine; as of post-transplant month 28/PEP Day 280, RVNAs were still detectable at a concentration ≥0.1 IU/mL (Table 1, Fig. 1). The patient remains on maintenance immunosuppression with tacrolimus and has no clinical evidence of rabies.
Table 1.
Antibody responses to rabies post-exposure prophylaxis (PEP) and immunosuppressive medications used by 3 patients who received solid organs from a donor with rabies
| Organ recipient |
Months post- transplant |
PEP Day |
RVNA (IU/ mL) |
Immunosuppressive medications (serum trough level goals) |
|---|---|---|---|---|
| Liver | 18 | 0 | <0.04 | TAC (4–8 ng/mL) |
| 18 | 3 | 0.1 | TAC (4–8 ng/mL) | |
| 19 | 14 | 0.5 | TAC (4–8 ng/mL) | |
| 19 | 28 | 2.2 | TAC (4–8 ng/mL) | |
| 20 | 42 | 40.8 | TAC (4–8 ng/mL) | |
| 20 | 62 | 36.8 | TAC (4–8 ng/mL) | |
| 28 | 280 | 10.0 | TAC (4–8 ng/mL) | |
| Right kidney | 18 | 0 | <0.04 | TAC (6–8 ng/mL) MPA (1.5–2.5 µg/mL) |
| 19 | 14 | 0.4 | TAC (6–8 ng/mL) MPA (1.5–2.5 µg/mL) | |
| 19 | 28 | 0.3 | TAC (6–8 ng/mL) MPA (1.5–2.5 µg/mL) | |
| 20 | 39 | 0.3 | TAC (6–8 ng/mL) MPA (1.5–2.5 µg/mL) | |
| 21 | 68 | 0.3 | TAC (6–8 ng/mL) MPA (1.5–2.5 µg/mL) | |
| 28 | 279 | 0.5 | TAC (6–8 ng/mL) MPA (1.5–2.5 µg/mL) | |
| Heart | 18 | 0 | <0.04 | TAC (10–12 ng/mL) Prednisone 2.5 mg/day |
| 19 | 14 | 0.4 | TAC (10–12 ng/mL) Prednisone 2.5 mg/day | |
| 20 | 49 | 0.3 | TAC (10–12 ng/mL) Prednisone 2.5 mg/day | |
| 21 | 68 | 0.3 | TAC (10–12 ng/mL) Prednisone 2.5 mg/day | |
| 36 | 533 | <0.04 | TAC (6–8 ng/mL) Sirolimus (4–8 ng/mL) |
RVNA, rabies virus neutralizing antibody; TAC, tacrolimus; MPA, mycophenolic acid.
Fig. 1.
Antibody responses to rabies post-exposure prophylaxis (PEP) among 3 patients who received solid organs procured from a donor with rabies. The graph shows the concentration of rabies virus neutralizing antibodies (RVNAs) (Y-axis, logarithmic scale) at various time points after initiation of rabies PEP (X-axis) for 3 solid organ recipients who had received organs procured from a donor with rabies. The lower limit of detection for RVNAs by the laboratory assay we used is 0.04 IU/mL but, for simplicity, 0.05 IU/mL is indicated on this graph. The cutoff defined by the Advisory Committee on Immunization Practices for an acceptable antibody response to administration of rabies vaccine corresponds to an RVNA concentration of approximately 0.1 IU/mL; in contrast, the World Health Organization cutoff is 0.5 IU/mL.
Right kidney recipient
The right kidney recipient was a 41-year-old man with a history of hyperparathyroidism and Barrett’s esophagus when he received his renal transplant for hypertensive nephropathy; he had not been vaccinated for rabies before the time of transplantation. The patient’s transplant surgery was uneventful, as was his immediate post-transplant course aside from hypertension; he was discharged home on post-transplant day 3.
The patient’s induction immunosuppressive regimen consisted of methylprednisolone (rapid taper over 3 days starting with an IV dose of 500 mg) and alemtuzumab (1 IV dose of 30 mg). His initial maintenance immunosuppressive regimen consisted of tacrolimus and mycophenolic acid with serum trough level goals of 8–12 ng/mL and 2–3 µg/mL, respectively. During post-transplant month 3, he developed BK virus-associated nephropathy and BK viremia, prompting reductions in tacrolimus and mycophenolic acid doses to maintain serum trough level goals of 6–8 ng/mL and 1.5–2.5 µg/mL, respectively. A kidney biopsy obtained during post-transplant month 12 showed resolution of BK virus-associated nephropathy. He has not had any episodes of rejection of his transplanted kidney.
PEP consisted of RIG and a human diploid cell rabies vaccine for the first vaccine dose, and purified chick embryo cell rabies vaccine for the subsequent 4 vaccine doses (11). Serum collected 11 days after the fifth vaccine dose (PEP Day 39) demonstrated an acceptable antibody response to rabies vaccine; as of post-transplant month 28/PEP Day 279, RVNAs were still detectable at a concentration ≥0.1 IU/mL.
Several tests to measure immune function were performed during and after the course of PEP. T-cell analysis by flow cytometry at baseline (post-transplant month 18/PEP Day 0) revealed an absolute CD3 cell count of 189/mm3 (normal range 629–2465/mm3), absolute CD8 cell count of 46/mm3 (normal range 93–1025/mm3), and absolute CD4 cell count of 133/mm3 (normal range: 340–1568/mm3). Quantitative immunoglobulins (Igs) measured at the same time (post-transplant month 18/PEP Day 0) were as follows: IgG 748 mg/dL (normal range 700–1600 mg/dL), IgA 105 mg/dL (normal range 70–400 mg/dL), and IgM 52 mg/dL (normal range 40–230 mg/dL). Flow cytometry analysis on post-transplant month 21/PEP Day 68 revealed an absolute CD3 cell count of 205/mm3, absolute CD8 cell count of 68/mm3, and absolute CD4 cell count of 125/mm3. During post-transplant month 28/PEP Day 279, quantitative Ig measurement revealed the following: IgG 587 mg/dL, IgA 90 mg/dL, and IgM 36 mg/dL. The patient remains on maintenance immunosuppression with tacrolimus and mycophenolic acid and has no clinical evidence of rabies.
Heart recipient
The heart recipient was a 54-year-old man with a history of diabetes mellitus type II and hypertension when he received his heart transplant for idiopathic dilated cardiomyopathy; he had not been vaccinated for rabies before the time of transplantation. Immediately prior to receiving the organ, the patient was critically ill with methicillin-sensitive Staphylococcus aureus sepsis complicated by acute renal failure and recurrent ventricular tachycardia. The patient’s transplant surgery was uneventful, as was his immediate post-transplant course apart from renal failure; he was discharged home on post-transplant day 11.
The patient’s induction immunosuppressive regimen consisted of methylprednisolone (1 IV dose of 500 mg) and basilixmab (1 IV dose of 20 mg). He received an additional 1 g of methylprednisolone over the first 2 days postoperatively. His initial maintenance immunosuppressive regimen consisted of tacrolimus with initial serum trough level goals of 10–14 ng/mL, prednisone (50 mg daily) tapered over the first 45 postoperative days to 15 mg daily, and mycophenolate mofetil (1 g twice daily). During post-transplant month 2, he underwent a heart biopsy that showed grade 1A rejection; no adjustments were made to his immunosuppressive regimen. A heart biopsy during posttransplant month 3 showed grade 1B rejection; no adjustments were made to his immunosuppressive regimen. A heart biopsy during post-transplant month 4 showed resolution of rejection and his tacrolimus dose was titrated to target serum trough level goals of 10–12 ng/mL and his prednisone dose was subsequently weaned to 12.5 mg daily. The patient continued to have episodes of grade 1A rejection during post-transplant months 12 and 15, but no adjustments were made to his immunosuppressive regimen.
PEP consisted of RIG and a purified chick embryo cell rabies vaccine for the first 4 vaccine doses, and a human diploid cell rabies vaccine for the fifth vaccine dose (11). Serum collected 21 days after the fifth vaccine dose (PEP Day 49) demonstrated an acceptable antibody response to rabies vaccine; as of post-transplant month 36/PEP Day 533, RVNAs were not detectable. The patient remains on maintenance immunosuppression with tacrolimus and sirolimus, and has no clinical evidence of rabies.
Discussion
Three clusters of human rabies associated with solid organ transplantation have been reported globally, all of which occurred in countries with very low burdens of human rabies (9–11). In other parts of the world, including areas where canine rabies is endemic and human rabies is more common, organ transplantation is an increasingly utilized medical intervention (16). These circumstances underscore the need to investigate means of preventing transplant-associated rabies through primary prevention strategies, such as improving recognition of rabies among potential donors, and through secondary prevention strategies, such as PEP.
The survival of the 3 recipients described here, none of whom had been vaccinated for rabies at the time of their transplant surgeries, is unprecedented. Unfortunately, the precise factors that led to their survival remain unclear. Eighteen months had already passed when it was discovered that these 3 recipients had received organs from a donor with rabies and all 3 were asymptomatic at the time. One explanation for this is that these 3 recipients had received organs without any burden of rabies virus and were therefore not at risk for rabies, given that the typical incubation period of the rabies virus is <1 year. However, the long incubation period of 17 months experienced by the 1 recipient who did develop rabies is evidence against this hypothesis (11). Another possibility is that these 3 recipients were exposed to a subinfectious dose of rabies virus that had been cleared prior to PEP administration. Similar incidents have been reported among persons who have been bitten by rabid animals, but who do not develop rabies despite not receiving PEP (17, 18). A third possibility, and most concerning, is that these 3 recipients were within the incubation period of the rabies virus and would have experienced clinical disease had they not received PEP; therefore, we administered PEP according to a schedule recommended by ACIP. It is notable that the donor was infected with the raccoon rabies virus variant, and an additional question that remains unresolved is whether this might explain the different outcome of these 3 recipients, compared with the unvaccinated recipients in the 2 prior transplant-associated rabies outbreaks in 2004 and 2005 (9, 10). Raccoons are the most frequently reported rabid animal in the United States, yet only 3 cases of human infection with the raccoon rabies virus variant have ever been reported (11). While we know that infection with the raccoon rabies virus variant in humans is fatal, it is possible that this rabies virus variant has a different natural history in the human host (such as a longer incubation period) compared with other rabies virus variants (such as those from bats).
We performed post-vaccination serologic testing for the 3 recipients, given their immunosuppressed status, as recommended by ACIP. ACIP does not specify whether a single serum sample or serial samples should be evaluated, though some authors have argued that a single sample is sufficient, if target antibody levels are demonstrated (19). This approach for management of PEP for immunosuppressed persons is often reasonable for conventional rabies virus exposures, such as animal bites, in which there is a discrete, finite exposure. However, we considered the 3 recipients as potentially being at continuous risk for rabies virus exposures, given the possibility that their transplanted organs still harbored rabies virus. Furthermore, the sites of their exposure (their transplanted organs) could not be washed, as is typically done for bite wounds (an action that reduces the risk of rabies even in the absence of PEP), and because their transplanted organs could not be directly infiltrated with RIG (2, 20, 21). We therefore collected serial serum samples from all 3 recipients as part of their post-vaccination serologic testing.
The criteria we used to establish whether an acceptable antibody response had occurred was consistent with that recommended by ACIP: complete neutralization of challenge rabies virus at a serum dilution of 1:5 using RFFIT, which roughly corresponds to an RVNA concentration of 0.1 IU/mL. In contrast, the World Health Organization (WHO) recommends that a concentration of 0.5 IU/mL be used as a cutoff (22). Ultimately, these are arbitrary standards and having an RVNA concentration above these levels does not confer “protection” against rabies (5, 19). It is estimated that as many as 36,000 persons receive PEP in the United States each year, and although PEP is not always properly administered and despite using a lower RVNA cutoff for an acceptable antibody response than WHO, there have been no reports in the United States of failures of PEP with the currently licensed cell-culture rabies vaccines (2, 23). The difference between the ACIP and WHO criteria are important to consider, however, because the heart recipient never developed a concentration of RVNAs ≥0.5 IU/mL, and the right kidney recipient did not develop a concentration of RVNAs ≥0.5 IU/mL until the last serum draw on PEP Day 279. Few data are available to guide management of PEP for immunosuppressed persons when neither of these RVNA cutoffs is reached, and greatly divergent strategies have been proposed, including repeat administration of the entire PEP series and doubling of the vaccine dose (4, 24). Until more data are available to help standardize management of PEP for immunosuppressed persons, each case will have to be managed individually, often in consultation with appropriate public health officials. Adverse events can occur as a result of receiving rabies vaccine, and therefore the benefits and risks of administering extra doses of vaccine beyond the 5 doses recommended by ACIP should be carefully considered (3).
An ongoing challenge in managing PEP in immunosuppressed persons remains our inability to predict which of these persons is likely to have a poor antibody response. While it was not surprising to see that the liver recipient had the most robust antibody response among the 3 recipients, given that he was taking fewer immunosuppressive medications than the other 2 recipients, the overwhelming superiority of his response seems out of proportion to differences in immunosuppressive medications. Indeed, the antibody response mounted by the liver recipient exceeds that reported for other liver transplant recipients at a similar point following completion of vaccination (36.8 IU/mL vs. 11.69 IU/mL in a liver transplant recipient aged 17 years) (7). These observations suggest that other intrinsic host factors might also play a role in how a person responds to PEP.
Another uncertainty in managing PEP for immunosuppressed persons is the duration over which they should demonstrate a target RVNA level. For example, the liver and heart recipients both had declining antibody titers as of the final serum sample that was evaluated; in contrast, the right kidney recipient had an increased antibody titer, despite not having recently received rabies vaccine or having had any changes in his immunosuppressive medication regimen. Whether this situation, particularly for the liver and heart recipients, necessitates ongoing serologic evaluation remains unclear.
While some data are available regarding PEP in persons with human immunodeficiency virus, additional investigations of PEP responses are urgently needed among other classes of immunosuppressed persons, such as transplant recipients (25, 26). From a laboratory perspective, evaluation of T- and B-cell responses and other markers of immune function after administration of rabies vaccine should be a priority area for further investigation (27, 28). An international registry to systematically report experiences with PEP for immunosuppressed individuals could help clinicians and public health officials make evidence-based treatment recommendations (5). Finally, prospective studies that aim to enroll transplant recipients and measure immune responses to PEP should be conducted; the advantage of such an approach is that it will allow recruitment of a more homogenous study population focused on the transplant setting (6).
The survival of 3 previously unvaccinated recipients of solid organs from a donor with rabies is an unexpected and unprecedented situation. Although we do not know the exact circumstances that led to their survival, our data suggest for the first time that PEP can possibly enhance transplant safety in settings in which donors are retrospectively diagnosed with rabies.
Acknowledgements
Thanks: We thank the 3 patients described in this report and the Transplant-Associated Rabies Virus Transmission Investigation Team for its assistance with this investigation.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflicts of interest: The authors declare no conflicts of interest.
References
- 1.Knobel DL, Cleaveland S, Coleman PG, et al. Re-evaluating the burden of rabies in Africa and Asia. Bull World Health Organ. 2005;83(5):360–368. [PMC free article] [PubMed] [Google Scholar]
- 2.Manning SE, Rupprecht CE, Fishbein D, et al. Human rabies prevention–United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2008;57(RR-3):1–28. [PubMed] [Google Scholar]
- 3.Rupprecht CE, Briggs D, Brown CM, et al. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the advisory committee on immunization practices. MMWR Recomm Rep. 2010;59(RR-2):1–9. [PubMed] [Google Scholar]
- 4.Rodriguez-Romo R, Morales-Buenrostro LE, Lecuona L, et al. Immune response after rabies vaccine in a kidney transplant recipient. Transpl Infect Dis. 2011;13(5):492–495. doi: 10.1111/j.1399-3062.2011.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopel E, Oren G, Sidi Y, David D. Inadequate antibody response to rabies vaccine in immunocompromised patient. Emerg Infect Dis. 2012;18(9):1493–1495. doi: 10.3201/eid1809.111833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckerle I, Rosenberger KD, Zwahlen M, Junghanss T. Serologic vaccination response after solid organ transplantation: a systematic review. PLoS One. 2013;8(2):e56974. doi: 10.1371/journal.pone.0056974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cramer CH, Shieck V, Thomas SE, Kershaw DB, Magee JC, Lopez MJ. Immune response to rabies vaccination in pediatric transplant patients. Pediatr Transplant. 2008;12(8):874–877. doi: 10.1111/j.1399-3046.2008.00936.x. [DOI] [PubMed] [Google Scholar]
- 8.Vetter JM, Frisch L, Drosten C, et al. Survival after transplantation of corneas from a rabies-infected donor. Cornea. 2011;30(2):241–244. doi: 10.1097/ICO.0b013e3181e4572a. [DOI] [PubMed] [Google Scholar]
- 9.Maier T, Schwarting A, Mauer D, et al. Management and outcomes after multiple corneal and solid organ transplantations from a donor infected with rabies virus. Clin Infect Dis. 2010;50(8):1112–1119. doi: 10.1086/651267. [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan A, Burton EC, Kuehnert MJ, et al. Transmission of rabies virus from an organ donor to four transplant recipients. N Engl J Med. 2005;352(11):1103–1111. doi: 10.1056/NEJMoa043018. [DOI] [PubMed] [Google Scholar]
- 11.Vora NM, Basavaraju SV, Feldman KA, et al. Raccoon rabies virus variant transmission through solid organ transplantation. JAMA. 2013;310(4):398–407. doi: 10.1001/jama.2013.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace RM, Stanek D, Griese S, et al. A large-scale, rapid public health response to rabies in an organ recipient and the previously undiagnosed organ donor. Zoonoses Public Health. 2014;61(8):560–570. doi: 10.1111/zph.12105. [DOI] [PubMed] [Google Scholar]
- 13.Rabies Serology. [accessed on January 24, 2015]; Available at: http://www.cdc.gov/rabies/specific_groups/doctors/serology.html.
- 14.Noah DL, Drenzek CL, Smith JS, et al. Epidemiology of human rabies in the United States, 1980 to 1996. Ann Intern Med. 1998;128(11):922–930. doi: 10.7326/0003-4819-128-11-199806010-00012. [DOI] [PubMed] [Google Scholar]
- 15.Warner CK, Zaki SR, Shieh WJ, et al. Laboratory investigation of human deaths from vampire bat rabies in Peru. Am J Trop Med Hyg. 1999;60(3):502–507. doi: 10.4269/ajtmh.1999.60.502. [DOI] [PubMed] [Google Scholar]
- 16.Sun Q, Gao X, Wang H, Ko DS, Li XC. A new era for organ transplantation in China. Lancet. 2014;383(9933):1971–1972. doi: 10.1016/S0140-6736(14)60953-3. [DOI] [PubMed] [Google Scholar]
- 17.Sitthi-Amorn C, Jiratanavattana V, Keoyoo J, Sonpunya N. The diagnostic properties of laboratory tests for rabies. Int J Epidemiol. 1987;16(4):602–605. doi: 10.1093/ije/16.4.602. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert AT, Petersen BW, Recuenco S, et al. Evidence of rabies virus exposure among humans in the Peruvian Amazon. Am J Trop Med Hyg. 2012;87(2):206–215. doi: 10.4269/ajtmh.2012.11-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibbons RV, Rupprecht CE. Postexposure rabies prophylaxis in immunosuppressed patients. JAMA. 2001;285(12):1574–1575. doi: 10.1001/jama.285.12.1574. [DOI] [PubMed] [Google Scholar]
- 20.Dean DJ, Baer GM, Thompson WR. Studies on the local treatment of rabies-infected wounds. Bull World Health Organ. 1963;28(4):477–486. [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan MM, Cohen D, Koprowski H, Dean D, Ferrigan L. Studies on the local treatment of wounds for the prevention of rabies. Bull World Health Organ. 1962;26:765–775. [PMC free article] [PubMed] [Google Scholar]
- 22.WHO Expert Consultation on Rabies. [accessed on January 24 2015];2013 Available at http://apps.who.int/iris/bitstream/10665/85346/1/9789240690943_eng.pdf.
- 23.Christian KA, Blanton JD, Auslander M, Rupprecht CE. Epidemiology of rabies post-exposure prophylaxis–United States of Americam, 2006–2008. Vaccine. 2009;27(51):7156–7161. doi: 10.1016/j.vaccine.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 24.Hay E, Derazon H, Bukish N, Scharf S, Rishpon S. Postexposure rabies prophylaxis in a patient with lymphoma. JAMA. 2001;285(2):166–167. doi: 10.1001/jama.285.2.166. [DOI] [PubMed] [Google Scholar]
- 25.Thisyakorn U, Pancharoen C, Ruxrungtham K, et al. Safety and immunogenicity of preexposure rabies vaccination in children infected with human immunodeficiency virus type 1. Clin Infect Dis. 2000;30(1):218. doi: 10.1086/313610. [DOI] [PubMed] [Google Scholar]
- 26.Jaijaroensup W, Tantawichien T, Khawplod P, Tepsumethanon S, Wilde H. Postexposure rabies vaccination in patients infected with human immunodeficiency virus. Clin Infect Dis. 1999;28(4):913–914. doi: 10.1086/517241. [DOI] [PubMed] [Google Scholar]
- 27.Gelinck LB, Jol-van der Zijde CM, Jansen-Hoogendijk AM, et al. Restoration of the antibody response upon rabies vaccination in HIV-infected patients treated with HAART. AIDS. 2009;23(18):2451–2458. doi: 10.1097/QAD.0b013e328331a43b. [DOI] [PubMed] [Google Scholar]
- 28.Moon HH, Kim TS, Roh YN, et al. Can immune function assay predict infection or recovery? Transplant Proc. 2012;44(4):1048–1051. doi: 10.1016/j.transproceed.2012.04.001. [DOI] [PubMed] [Google Scholar]