Abstract
Background
The present study is designed as a proof-of-concept study to evaluate light/chemical hardening technology and a newly formulated polymethylmethacrylate, polyhydroxyethylmethacrylate, and calcium hydroxide (PPCH) plus polyanhydride (PA) (PPCH-PA) composite graft material as a bone substitute compared to positive and negative controls in a minipig model.
Methods
PPCH-PA (composite graft); PPCH alone (positive control), PA alone (positive control), and no graft (negative control) were compared. Four mandibular premolar teeth per quadrant were extracted; a total of 48 implants were placed into sockets in three minipigs. Abutments were placed protruding into the oral cavity 4 mm in height for immediate loading. Crestal areas and intrabony spaces were filled with PPCH-PA, PPCH, or PA using a three-phase delivery system in which all graft materials were hardened by a light cure. In the negative control group, implant sites were left untreated. At 12 weeks, block sections containing implants were obtained. Evaluations included periodontal probing, pullout-force load, and stability measurements to determine implant stability, radiographs to examine bone levels, and scanning electron microscopy (SEM)–energy-dispersed spectroscopy to determine bone-to-implant contact.
Results
Probing measurements did not reveal any pathologic pocket formation or bone loss. Radiographs revealed that immediate implant placement and loading resulted in bone at or slightly apical to the first thread of the implant in all groups at 12 weeks. Stability test values showed a relative clinical stability for all implants (range: −7 to +1); however, implants augmented with PPCH-PA exhibited a statistically significantly greater stability compared to all other groups (P <0.05). The newly formed bone in PPCH-PA–treated sites was well organized with less marrow spaces and well-distributed osteocytes. SEM revealed a tighter implant–socket interface in the PPCH-PA group compared to other groups with reduced microfissures and implant–bone interface fractures during pullout testing, whereas implants treated with PA or no graft showed ≈10-μm microfissures between the implant and bone with fractures of the intrathread bone.
Conclusions
The newly formulated chemically hardened graft material PPCH-PA was useful in immediate implant placement after tooth extraction and resulted in greater stability and a well-organized implant–bone interface with immediate loading, especially in those areas where cancellous bone was present. The results of this proof-of-concept study warranted further research investigating different healing times and longer durations.
Keywords: Alveolar ridge augmentation, bone replacement material, dental implants, models, animal, tooth extraction
Primary implant stability and a lack of micromovement are considered to be two of the main factors that are necessary for predictably achieving a high success of osseointegrated oral implants.1 A successful osseointegrated oral implant is anchored directly to bone; however, in the presence of movement, a soft tissue interface may encapsulate the implant and cause its failure.2 To minimize the risk of a soft tissue encapsulation, it was recommended that implants be kept load free during the healing period (3 to 4 months in mandibles and 6 to 8 months in maxillae).3 However, placements of immediately and early loaded implants are commonly used techniques, particularly in mandibles with good bone quality.4–6
Although immediate implant placement was demonstrated to be as reliable as traditional surgical techniques, several problems relating to the primary stability, consistent management of soft tissue, and bone healing on implant surfaces at the coronal level were also reported.7 The main objective of immediate loading is to achieve a high mechanical stability to avoid micromovement during the course of osseointegration.8 For example, the standard implantplacement procedure in areas of weak bone quality with lower success rates9 was modified via undersized preparation to achieve a high primary stability.10 The osteotome technique was developed to increase the stability of implants11 and to improve implant success.12 A histologic study in rabbits showed that microfractures, which occurred because of the bone-condensing technique, improved peri-implant bone formation in the first weeks after implant placement through the release of cytokines.13 However, intensive use of the osteotomy technique can also harmthe bone and cause increased crestal bone resorption.14
Although it was claimed that the process of osseointegration requires, on average, 3 months in the mandible,3 there has been a demand for immediately loaded implants to achieve fast rehabilitation of partially and completely edentulous patients for both functional and esthetic reasons.15,16 As a prerequisite for osseointegration, mechanical loading of dental implants during healing was discouraged. It was suggested that the magnitude of the loading forces between the implant and the bone determined the implant success.17 Therefore, one key to the success of the titanium implant seems to be whether bone at the periphery of the implant adequately remodels. From the current literature,18–20 there are only clinical case reports with a minimal number of samples and a limited number of experimental animal trials available that provide data on bone-to-implant contact with immediately loaded implants. Previously, the peri-implant bone density and bone-mineral apposition rate were not quantified for immediately loaded implants.
Bone-to-implant contact is the basic histologic condition for osseointegration. Although the total amount of bone-to-implant contact necessary for successful osseointegration was not clearly defined,21,22 obtaining a bone-to-implant contact at the apical or coronal level at placement was recommended for the stability and prognosis of the implant.23,24
Many factors affect bone-to-implant contact, including the surgical technique, implant-surface morphology, presence or absence of bacterial contamination, loading, implant stability, and initial gap between the bone and implant. The bone-to-implant contact rate was predictable when the anatomy allowed a close fit insertion of the implant.25 In contrast, the osseointegration success was not predictable when the implant was placed without a close fit to the bone, such as in the vertical augmentation of the crest,26 split-crest technique,27,28 or into fresh extraction sockets.29,30
Recently, immediate implant placement into fresh extraction sockets was proposed to reduce healing periods and maintain the width of the bone crest.31–33 Although immediate implant placement was demonstrated to be as reliable as traditional surgical techniques, 31–34 several problems relating to the primary stability, consistent management of soft tissue, and bone healing on implant surfaces at the coronal level where a wide gap between bone and implant was present at the implantation time were reported to be associated with this technique.7,23,24 To eliminate these disadvantages, barrier membranes alone or in combination with bone substitutes were used;35–39 however, the results of these studies were conflicting. Although some studies35,36 reported improved osseointegration and stability when immediate implant placement was accompanied by guided bone regeneration with barrier membranes and/or bone grafts, several studies37–39 showed that the healing and osseointegration rate did not differ.
The synthetic bone-graft material composed of polymethylmethacrylate (PMMA), polyhydroxyethylmethacrylate (PHEMA), and calcium hydroxide (CH) (PPCH)¶ is a Food and Drug Administration (FDA)–approved bioabsorbable bone substitute used as a bone-void filler.40 Studies41–46 of the PPCH polymer composite graft material showed superior clinical results with regard to the horizontal defect fill in human Class II furcation defects compared to open flap debridement or guided tissue regeneration barriers alone. Polyanhydride (PA) (anhydride-co-imides) matrices are novel bioabsorbable polymeric materials with good mechanical properties designed specifically for orthopedic applications.47 This new family of PAs is capable of yielding high-strength, degradable networks after exposure to light.48 Resorption is by surface erosion into the water phase.48 Recently, a new technology where PPCH and PA formulations (PPCH-PA) were combined with a light/chemical hardening technology# placed with an easy-to-use three-phase delivery system was proposed to improve implant stability and promote longer and stronger host bone formation by the slow resorption of synthetic material when an immediate implant is placed. The present pilot study was designed as a proof-of-concept study to test the newly formulated composite graft material chemically hardened PPCH-PA as a bone substitute compared to individual chemically hardened PA and PPCH synthetic bone substitutes in a minipig model. This report encompassed an evaluation of implant stability and function during immediate implant placement and loading. Micro–computerized tomography (microCT), histologic, and histomorphometric analyses of the bone–implant contact and characteristics of newly formed bone around implants and in extraction sockets will be reported separately.
MATERIALS AND METHODS
Technology and Products
An FDA-cleared biocompatible microporous composite of PPCH-PA bone-replacement graft material** was obtained from the sponsor.†† PA is a rapidly bioabsorbable, synthetic bone-substitute material for applications in soft and hard tissue repair that can be light and/or chemically hardened, allowing for immediate function. The technology of light/chemical hardening‡‡ is a proprietary combination of processes that are light and chemical based that harden bone-substitute materials in situ. The three-phase system is a device to deliver individual doses of the synthetic bone graft materials PPCH, PA, or PPCH-PA to the surgical site. In this study, the PPCH-PA composite graft material and individual formulations of PPCH and PA were applied manually using the three-phase delivery system and hardened in situ with standard dental curing light to support immediate implants placed in fresh extraction sockets in minipigs. The material was radio-opaque due to a very small amount of barium sulfate that was intentionally added to the graft material to observe the degradation period and bone regeneration when it took place inside and outside of the porous material.
Animal Model
The study protocol and experimental design were reviewed and approved by Institutional Animal Care and Use Committee of the Boston University Medical Center (BUMC), Boston, Massachusetts. Three adult male minipigs (18 to 24 months old; average weight: 35 kg) were purchased from a breeder§§ for this particular experiment and acclimatized for 7 days at the BUMC Laboratory Animal Science Center (LASC) before any study procedures. The minipigs were housed in metal cages separately throughout the study at a temperature of 24°C ± 2°C, a relative humidity of 55%, and fed a regular minipig diet according to the manufacturer’s guidelines and tap water ad libitum. After implant surgeries, the animals were placed on a semiliquid diet, which was prepared by softening the regular diet with applesauce, for 2 weeks.
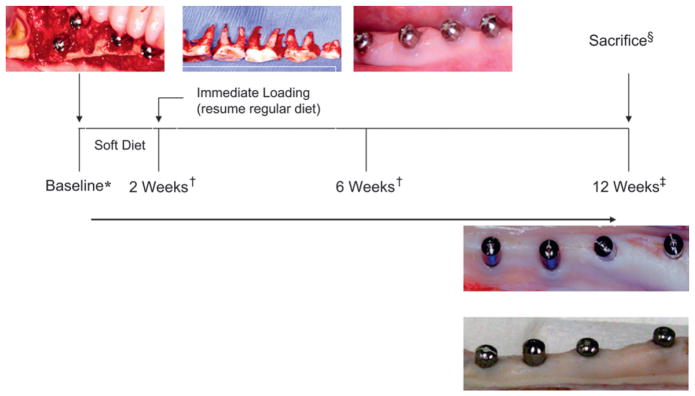
All surgical and postoperative oral-hygiene care procedures were performed in aseptic conditions and under general anesthesia performed by experienced veterinary personnel (Mimi Crowley, Sharron Kirchain, Michelle Callihan, and Rosa Yip) at Boston University Laboratory Animal Science Center, Boston, MA. Two surgeries were performed one week apart for each animal. After completion of surgical operations, the animals were anesthetized at 2, 6, and 12 weeks for postoperative oral care and implant-stability tests (Fig. 1).
Figure 1.
Study timeline. *All procedures were performed under general anesthesia; †oral hygiene, implant check, and stability test measurements; ‡stability test measurements and tissue harvesting; §animals were euthanized by an overdose of pentobarbital injections (120 mg/kg).
Implant Type
Parallel-wall, screw-type titaniumimplants of 3.25 mm in diameter and 11.5 and 13 mm in length,|||| healing abutments (4 mm in height), and a surgical drill system¶¶ were used in this study.
Surgical Procedures
Animals were premedicated subcutaneously with atropine (0.05 mg/kg) and thiopentone intravenously (IV) (2.5% solution and 20 mg/kg). Further, an endotracheal tube was for intubation, and a mixture of isoflurane (0.5% to 2%) and oxygen/nitrous oxide (1:1) was administered. A local anesthesia with 2% lidocaine with 1:100,000 epinephrine was also administered to surgical sites (0.5 cc per quadrant) to obtain local vasoconstriction and to prevent discomfort during extractions and implant placement.
In each minipig, maxillary and mandibular premolars (four premolars per quadrant) and molars (one molar per quadrant) were extracted. Extractions were performed by the elevation of a mucoperiosteal flap after sulcular and vertical release incisions at the distal aspect of the first molar and mesial aspect of the first premolar teeth, the separation of the roots using multi-blade burs and separation disks, and an incision of periodontal ligament with an Orban scalpel.## After the separation of the roots, periotome-assisted extractions were performed to prevent any additional trauma to the surrounding bone. Immediately after extractions, the sockets were irrigated with saline solution to remove any remnant of the roots separated. The implant-receiving sockets (the mesial root of each premolar tooth) were prepared, and implants were placed according to the manufacturer’s instructions for human use. The insertion torque was 40 Ncm for each implant. The placement torque force was increased by steps of 5 Ncm starting with 10 Ncm when the rotation of the implant stopped because of the friction with the jawbone. Where necessary, the placement was completed with a hand ratchet to optimally position the implant platform to maximize the primary stability. Implants were inserted until the coronal aspect was completely under the bone. A total of 48 implants, 16 implants in each pig, were inserted. Implants with a length of 11.5 mm were placed in the mesial sockets of first premolar teeth, whereas the sockets of the second, third, and fourth premolars received 13-mm implants based on anatomic considerations. Healing abutments (4-mm collar height)*** were tightly screwed into the implants (internal connection) 2 to 3 mm away from the opposing occlusion for controlled immediate loading and left unsplinted.
The distribution of implant augmentation per treatment group is shown in Table 1. Crestal areas and all bony sites at the boundaries of implants were filled with either the PPCH-PA, PPCH, or PA graft material using the three-phase delivery system, and dental curing light was used to harden the graft material for implant augmentation according to the manufacturer’s guidelines. Implant sites assigned to receive no graft (control) were left untreated. The placement of the graft material around implants and on the crestal areas constituted a modified ridge-augmentation procedure insuring tight closure without any exposure of the surface, and flaps were primarily closed using 4-0 bioabsorbable sutures. The primary closure of flaps was obtained by splitting the flap, where possible, and using matrix-suture techniques.
Table 1.
Distribution of Treatments by Site
| Group | Implants Placed (n) | Maxilla (n, implants) | Mandible (n, implants) |
|---|---|---|---|
|
| |||
| PPCH-PA | 16 | 8 | 8 |
| PA | 16 | 8 | 8 |
| PPCH | 8 | 4 | 4 |
| No graft | 8 | 4 | 4 |
Each animal received 16 implants that replaced four premolar teeth in each quadrant. Sites were randomly assigned to treatment groups: PPCH-PA, PPCH, PA, and no graft. Of the 12 quadrants in three minipigs, four quadrants (two maxillary and two mandibular, which were randomly selected) were treated with PPCH-PA or PA graft materials, whereas two quadrants (one maxillary and one mandibular) received either PPCH alone or no graft (Table 1).
Postoperative Care
Antibiotic (ceftiofur sodium, once a day, 3 to 5 mg/kg, intramuscular [IM])††† and analgesic therapies (buprenorphine perioperatively at 0.02 to 0.05 mg/kg IV q4 to 6 hours and then IM BID for 2 to 3 days postoperatively; ketoprofen 3 mg/kg IM intraoperatively and meloxicam 0.4 mg/kg by mouth, once a day for 3 to 4 days postoperatively) were administered.
Animals were checked daily for the first week after surgery to confirm safety. All animals were anesthetized using general anesthesia for a clinical follow-up and oral hygiene including brushing and irrigation with chlorhexidine digluconate at 2 and 6 weeks postoperatively. At 12 weeks, animals were initially anesthetized with ketamine HCL/xylazine IM and euthanized by a pentobarbital overdose (≥120 mg/kg, intravenous). Maxillae and mandibles were removed en bloc using an oscillating bone saw and high-speed cutting disk under continuous saline irrigation, and two bone blocks (right and left) per jaw were obtained. Each implant block containing 4 dental implants was further cut with a diamond saw under continuous-cooling water irrigation, and each implant was separated with the surrounding bone. One-half of the block sections (n = 23; one implant was lost at 6 weeks postoperatively) containing implants were used for clinical and mechanical analysis and then for undecalcified sectioning for further analysis by using scanning electron microscopy (SEM)–energy dispersed spectroscopy (EDS) (SEM-EDS). The remaining 24 implant–bone blocks were decalcified with an EDTA decalcification solution (10% EDTA solution in distilled water, pH 7.4, at 4°C) for ≈6 weeks for histologic and histomorphometric analyses. Four mandibles representing each treatment group were used for microCT evaluations of bone quality. Data from histologic, histomorphometric, and microCT evaluations will be reported in another article.
Clinical and Macroscopic Evaluations
Clinical and macroscopic evaluations included a visual inspection, periodontal probing of the peri-implant sulcus, application of force (pullout-force load), and measurements using an electronic mobility testing device‡‡‡ to determine implant stability (mobility) and radiographic evaluations to examine radiographic bone levels.
Stability test readings
Each of the implants placed in three animals was tested 2 weeks after immediate loading and repeated at 6 and 12 weeks using the electronic mobility testing device according to the manufacturer’s instructions. The measurement recorded by the device was converted by a mathematical formula to a stability test value (STV), which is a unit of mobility. The STV scale ranged from −8 (clinically firm) to +50 (very loose). It was reported that the range of −8 to +9 STV units corresponded to a clinical assessment of 0 on the Miller tooth mobility index49 used in the clinic. STVs in the negative range usually indicated ankylotic healing of an osseointegrated dental implant. For the dental implant, an STV of +10 or greater generally meant that osseointegration was not achieved. The calibration of the electronic mobility testing device and positioning of the handpiece parallel to the floor and perpendicular to the surface of the implant abutment were carefully done prior to each procedure. Measurements were carried out at the same location and in the same tapping direction. To avoid an intra-examiner variability, the same examiner (HH) performed all measurements with the electronic mobility testing device throughout the study. Mean STVs were determined for all implants at 2, 6, and 12 weeks for all groups. Mean STVs were determined separately for implants in maxillary and mandibular sites. The values recorded were between −7 and +1.
Soft tissue probing (peri-implant probing depth)
A periodontal probe§§§ was used to measure the probing attachment level at six sites (mesio-buccal, mid-buccal, disto-buccal, mesio-lingual, mid-lingual, and disto-lingual), of each implant at 12 weeks, immediately after removal of the jaws. The same examiner (HH) performed all probing measurement to avoid an intraexaminer variability. The periodontal probe was placed into the soft tissue sulcus around the implant and the mean probing depth was calculated for all groups.
Radiographs
Immediately after sacrifice, maxillae and mandibles were harvested en bloc, split in half, and fixed in 10% formalin. Before block sectioning, radiographs were taken with a digital x-ray|||||| from all implant sites. Digital radiographs were evaluated using software¶¶¶ and the radiologic bone loss around implants, which was evaluated by counting the implant threads outside of bone at both sides (mesial and distal), was measured to calculate the crestal bone loss.
Mechanical (pullout-force) test
After taking radiographic images, the soft tissue surrounding the neck of the implants was carefully removed with a Gracey curet. Block specimens were produced by cutting transverse sections (4 to 9 mm thick) from the central region of the bone specimens containing implants using a diamond saw.### Abutments were tightened and secured to prevent the possible collapse of the implant neck during the pullout test. Biomechanical testing was performed with a universal testing machine**** on fresh sections to determine the pullout-force load strength of the bone–implant interface. The abutment was clasped in a vise grip, and the bone was also secured with a vise grip. The vise grip was positioned to apply a pure axial load such that stress would be applied to the bone–implant interface. The implant abutment was pulled apart from the integrated bone block, and peak loads were recorded by the load transducer. At the point of implant failure, the test was stopped immediately to prevent complete damage of the interface. The maximum force load, displacement at maximum load, load at autobreak, and displacement at autobreak were calculated and transferred to a computer file by using a software program.†††† The full-scale load range was 10,000 kN, and the crosshead speed was 0.5 mm per minute. In total, 23 implant blocks (seven PPCH-PA, eight PA alone, four PPCH alone, and four no graft) were tested.
SEM and Electron Microprobe Analyses
For SEM, implant–bone blocks (n = 23) were sagittally cut using a parallel diamond-band saw‡‡‡‡ and gradually dehydrated. For the optimum preservation of details, surfaces were left uncoated and analyzed using a scanning electron microscope.§§§§ An electron microscope analysis was conducted using the same scanning electron microscope with an energy dispersive x-ray (EDX) system and a light-element x-ray detector|||||||| (15 mm working distance). The elemental composition was determined as the weight percentage and calcium/phosphate (Ca/P) ratios were calculated from five measurements per selected area.
Energy Dispersive X-Ray Spectroscopy (EDS) Assessment
Implants were evaluated to determine the chemical composition of the implant–bone interface by an EDX system connected to a scanning electron microscope. The system was able to detect atoms with an atomic weight equal to or greater to that of boron and allowed the semiquantitative determination of the composition of a surface within a thickness range of ≈1 μm with a high resolution. EDS was used to identify and evaluate the relative concentrations of all chemical elements present in tissues and was carried out using point-analysis, line-scan, and mapping facilities with an EDX system. More than 200-point analyses were carried out on the sections. These included ≥3 sites within bone in areas with no visible adjacent biomaterial in each section to determine the elemental composition of the natural bone as a baseline for comparison. Additional information was obtained from line scans and elemental maps.
Statistical Analyses
Data obtained by direct measurements during morphologic assessment were used in multiple statistical analyses. The unit of measurements was the treated site (n = 16 for PPCH-PA and PA alone; n =8 for PPCH and no graft). Mean values for probing depth measurements, readings of the electronic mobility testing device, and values of pullout load were used to determine changes in implant stability among test groups. Mean values were compared using one-way analysis of variance (ANOVA) with post hoc least significant difference (LSD) correction. In addition, the Ca/P ratios were analyzed using ANOVA with LSD correction. All statistical evaluations were performed using a statistical package.¶¶¶¶
RESULTS
Clinical and Macroscopic Analyses
All sites treated with implants with or without augmentation materials healed without major complications. Some sites exhibited some minor gingival inflammation because of heavy calculus deposition around implants at each time point (2, 6, and 12 weeks). Loose abutments were tightened, while lost abutments were replaced during 2- and 6-week postoperative care. At 6 weeks, one implant was lost most probably because of trauma after 2-week evaluations. The animal had an anesthesia complication during the evaluation and had to be intubated by mouth. No further health problem was observed; none of the implants became loose or lost during this period. Occlusal surfaces of abutments exhibited contact points (tattered and shining), which indicated function. At sacrifice, all implants showed good healing results and clinical stability except the one lost at 6 weeks (Table 2).
Table 2.
Treatment Sites and Implant Survival
| Animals (n) | Implants Placed (n) | Implants Lost or Failed (n) | Observation Period |
|---|---|---|---|
|
| |||
| 3 | 48 | 1 (at 6 weeks) | 12 weeks |
Probing depth and radiologic assessments
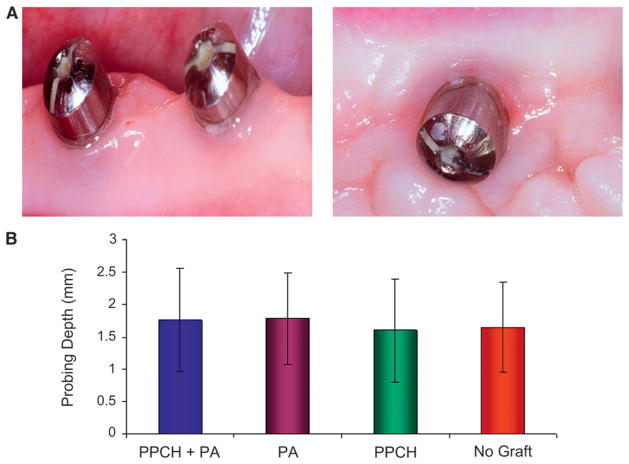
Figures 2 and 3 show the clinical soft tissue and radiographic bone examinations. Probing depths around implants did not indicate any pathologic pocket formation and associated bone loss. PPCH-PA was slightly better than the other groups, but there was no statistically significant difference among groups with respect to probing depths (P >0.05) (Fig. 2).
Figure 2.
Soft tissue around implants. A) Soft tissue surrounding the neck of the implants showed normal characteristics. B) Soft tissue probing was made at six points around each implant. Means ± SDs were compared among groups. No statistically significant difference was found among groups (P >0.05).
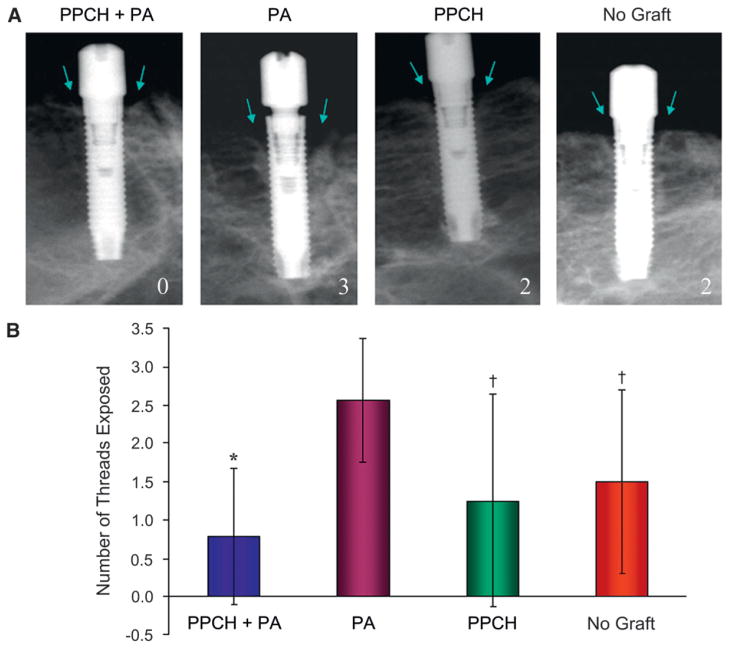
Figure 3.
A) Exposed-thread numbers at the crestal level were calculated as previously described to calculate the radiological bone loss at both mesial and distal sites of each implant. As shown in the respective radiographs for each group, zero indicates no exposure, while 2 and 3 refer to “two and three” threads exposed, respectively. Arrows depict the area evaluated for exposed implant threads. B) Based on counting of the threads exposed mesially and distally for each implant, the PA-alone group showed the highest number of threads exposed (mean ± SD: 2.6 ± 0.8; P < 0.05),† while PPCH-PA group exhibited the least number of threads exposed (0.8 ± 0.9; P < 0.001).*
Digital radiographs were evaluated using software#### in which the radiologic bone density and amount were evaluated. The bone loss around implants was calculated by counting the exposed implant threads. Radiographs were evaluated for the assessment of implant threads in the bone and any radiolucency between implant–bone interfaces.50 Overall, radiographs revealed that immediate implant placement and immediate loading resulted in bone at or slightly apical to the first thread of the implant in all groups at 12 weeks. Radiographs did not reveal any significant radiolucency at the crestal or apical bone or at the implant–bone interface along the implant (Fig. 3A). Exposed-thread numbers at the crestal level varied between zero and three, and the PA-alone group demonstrated the highest number of threads exposed among the groups (mean ± SD: 2.6 ± 0.8; P <0.05), whereas implants augmented with PPCH-PA had thread exposure at a very minimal level (0.8 ± 0.9) (Fig. 3B).
Measurements of electronic mobility testing device
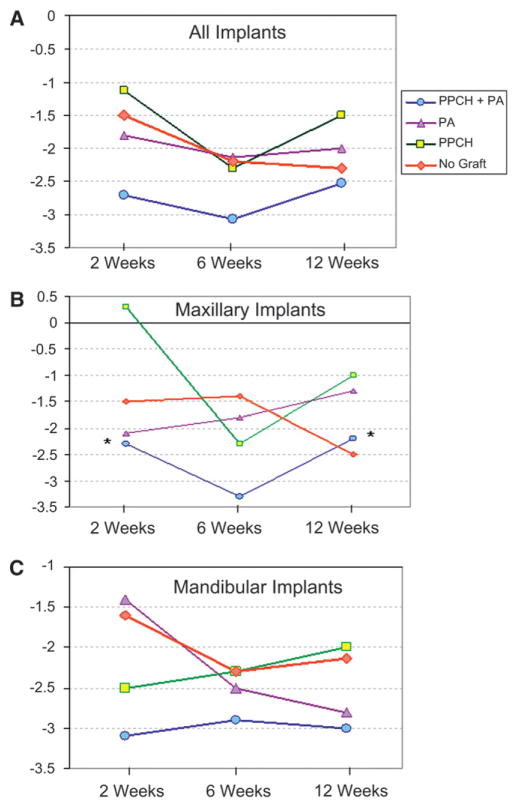
STVs recorded in the study ranged between −7 and +1, which all showed the clinical stability for all implants. STVs were recorded at 2 weeks after immediate loading and repeated at 6 and 12 weeks. The mean STV was calculated among groups for all implants (Table 3; Fig. 4A). Based on the analysis with the electronic mobility testing device, chemically-hardened PPCH-PA displayed a higher stability, on average, compared to all other groups throughout the study (P = 0.004, P = 0.03, and P = 0.04 compared PA, PPCH, and no graft, respectively). Considering all implants placed either in the maxilla or mandible, the PPCH-PA group showed better STVs at each time point starting at 2 weeks, but the difference was not statistically significant compared to the other groups (P >0.05) (Fig. 4A). The mean STV was also calculated separately for implants placed in the maxilla and mandible (Figs. 4B and 4C). At 2 and 12 weeks, STVs of implants augmented with PPCH-PA were statistically significantly better compared to the PPCH-alone group when only maxillary implants were evaluated (Fig. 4B). In implants placed into the mandible, PPCH-PA displayed a better stability at 2 weeks compared to the no-graft group (P = 0.05). There was no statistically significant difference between the no-graft group and any of the other graft materials tested (Fig. 4C).
Table 3.
Changes (mean [SD]) in STVs for All Implants at Each Recording Period
| Treatment | n | 2 Weeks | 6 Weeks | 12 Weeks | Average |
|---|---|---|---|---|---|
|
| |||||
| No graft | 8 | −1.5 (2.0) | −2.8 (1.7) | −2.3 (2.0) | −2.2 |
| PPCH | 8 | −1.1 (2.0) | −2.3 (0.5) | −1.5 (1.3) | −1.6 |
| PA | 16 | −1.8 (1.4) | −2.1 (1.3) | −2.0 (1.4) | −2.0 |
| PPCH-PA | 16 | −2.7 (1.9) | −3.1 (1.4) | −2.5 (1.4) | −2.8 |
Figure 4.
Implant stability. Stability test measurements were recorded at 2, 6, and 12 weeks. A) All implants B) Maxillary implants only C) Mandibular only.
Biomechanical testing (pullout test)
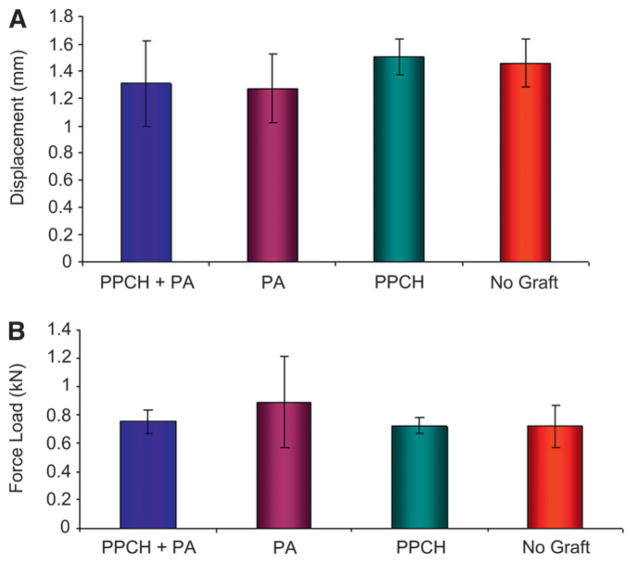
Mechanical pullout tests of all the implants showed similar bond strength. Figure 5 shows a typical force load and pullout testing for bond strength for PPCH-PA and no graft, respectively, at 12 weeks. Mean and SD peak values were calculated by the mechanical analysis software,***** and statistical analysis were performed. According to this evaluation, the mean maximum load at breakage for PPCH-PA, PA, PPCH, and no graft was 0.78 ± 0.05, 0.69 ± 0.15, 0.71 ± 0.06, and 0.69 ± 0.14, respectively. Although, PPCH-PA–treated bone showed slightly more strength during pullout test (Fig. 5A), there was no statistical difference among groups (Fig. 5B). The displacement distance at the maximum load was also similar for all groups (Fig. 5A).
Figure 5.
Biomechanical (pullout) test. Peak loads during the pullout test were recorded by the load transducer and automatically transferred to a computer software program attached to the machine. A) Comparison of the displacement distance at the maximum load (10,000 kN) among groups. B) Force load at the time of the displacement. No statistically significant difference was found among groups (P >0.05).
SEM Analyses
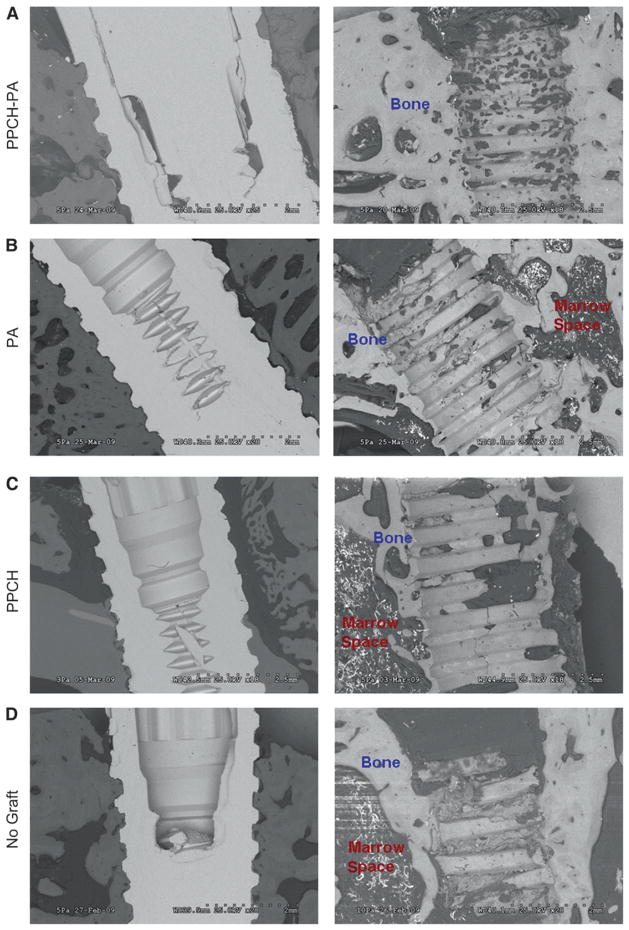
Implants were evaluated using field-emission SEM. SEM microphotographs revealed well-osseointegrated implant surfaces in PPCH-PA–treated sites. A healthy ultrastructure and appearance of the interface were found at places of direct contact between the implant and bone. The newly formed bone in PPCH-PA treated sites appeared to be well organized with fewer marrow spaces and well-distributed osteocytes (Fig. 6A), whereas the peri-implant bone of the PA- and PPCH-treated sites appeared rich in marrow spaces and less organized with poorly distributed osteocytes (Figs. 6B and 6C). Sites without augmentation showed large bone-marrow spaces and poorly distributed osteocytes around the bone-to-implant contact area (Fig. 6D).
Figure 6.
The implant–bone interface and composition of newly formed bone around implants were evaluated using field-emission SEM in each group. A) The micrographs represent the implant–bone interface in PPCH-PA in lower and higher magnifications. There were no obvious marrow spaces for the PPCH-PA group. Peri-implant bone of the PA- (B) and PPCH(C)-treated sites are shown. D) Micrographs of bone-to-implant contact area in sites without augmentation. Original magnifications A through D×20.
Energy Dispersive X-Ray (EDX) System
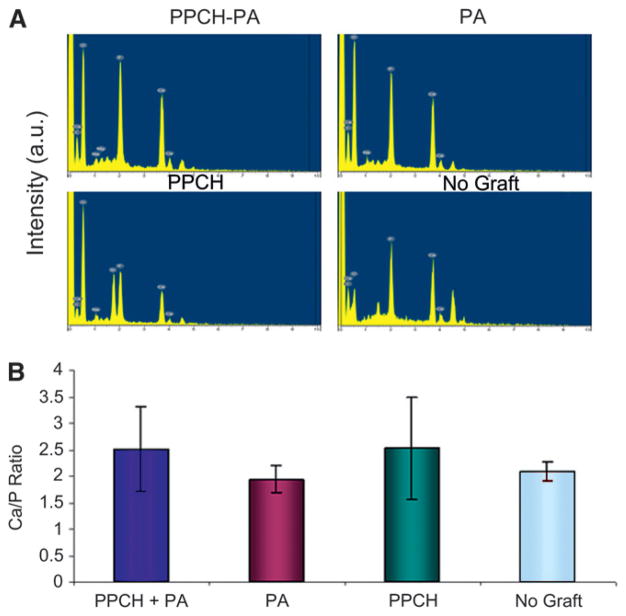
EDX analysis demonstrated that the surface was mainly composed of oxygen, calcium, phosphorus, nitrogen, and carbon elements. EDX results showed that the surface of implants without augmentation was mainly composed of calcium and phosphorus and little oxygen, whereas the PPCH-PA–, and PA-treated sites were mainly composed of calcium (Ca) and phosphorus (P) and were also rich in oxygen levels (Fig. 7A). PPCH sites showed high oxygen levels; however, calcium and phosphorus levels were less (Fig. 7A). Using EDS, the Ca/P ratio was also calculated (Fig. 7B). Scanning electron micrographs revealed a tighter implant–socket interface in the PPCH-PA group compared to PA-alone, PPCH-alone, and no-graft groups, with reduced microfissures and dentate-bone interface fractures. In detail, the SEM analysis revealed that sites treated with PPCH-PA showed the fewest microfissures between the implant and bone and the fewest number of fractures in the implant–bone interface after the pullout test. Conversely, implants treated with PA showed ≈10 μm microfissures between the implant and bone and a brittle-bone interface with fractures due to the pullout test. In parallel, the PPCH group also resulted in ≈10 μm microfissures between the implant and bone with a fractured implant–bone interface; however, this group showed better integration compared to the no-graft group, which showed ≈20 μm microfissures between the implant and bone and a fractured dentate bone interface due to the pullout test.
Figure 7.
EDX analysis. A) Composition of implant–bone surfaces in all groups shown in intensity. B) Ca/P ratios were calculated at five areas for each sample, and the mean ± SD was used for statistical comparisons. a.u. = arbitrary units.
DISCUSSION
After tooth extraction, immediate implant placement was suggested for the prevention of alveolar bone loss and reduction of the overall treatment period.31 In a recent review, Esposito et al.51 evaluated the success, function, complications, and patient satisfaction among immediate, immediate-delayed, and delayed implants and concluded that immediate and immediate-delayed implantsmay offer some advantages over delayed implants in terms of patient satisfaction and esthetics, possibly by preserving the alveolar bone.
To minimize the risk of implant failures, it was suggested that immediate postextraction implants should be kept load-free during the healing period.3 However, it is well known that immediate loading is a predictable procedure, which does not impair but rather enhances bone formation.52 In a review53 in which different times for the loading of dental implants were examined, the authors concluded that it was possible to successfully load dental implants immediately or early after their placement in selected patients, but a high degree of primary implant stability seemed to be one of the prerequisites for a successful immediate/early loading procedure.
In most extraction cases, bony defects or crestal bone deficiency are a major problem if an immediate implant placement is the goal. This problem can even be more expected when immediate loading is planned. Although the primary stability is the prerequisite for a successful implant in these conditions, the augmentation of crestal areas is also recommended due to the gap that occurs between the implant neck and socket walls after a tooth extraction. The aim of the present study is to test the efficacy of a newly formulated chemically hardened PPCH-PA graft material in the stability and function of immediately loaded implants placed in fresh extraction sockets. The results demonstrated that the augmentation with the light/chemically hardened PPCH-PA graft material after immediate implant placement was useful in promoting bone formation at crestal areas and supporting the stability of the implants. The findings of the study are consistent with data reported in current literature51 with respect to STVs when immediate loading was tested. However, to our knowledge, there are only some case reports and case series that tested STVs of immediate implants for which augmentation materials were used to support immediate loading.20,44
The goal of bone-augmentation procedures is to stimulate or at least facilitate the growth of new bone into the augmented site. For many years, the gold standard for bone grafting was autogenous bone from intra- or extraoral donor sites.54 The search for suitable bone-substitute materials intensified due to the shortcomings of autografts, mainly donor-site morbidity and limited available bone volume. A bone-substitute material can be used instead of or in combination with an autograft to increase the graft volume. Irrespective of the use, the bone substitute must be biocompatible and should promote the proliferation of new vessels and, ultimately, the growth of new bone into the augmented area. The ideal bone substitute maintains this biologic and mechanical support during healing and is gradually replaced by the newly formed bone.
To assess the individual treatment success as well as the evaluation of treatment quality, the evaluation of radiographs is essential. Threaded implants exhibit obvious measuring points with a known distance between two adjacent threads to which marginal bone loss could be related. Therefore, in clinical practice, the most natural quality assessment of an implant treatment with threaded implants would be to relate marginal bone loss to implant threads. Thus, to possibly avoid inherent errors connected with radiographic assessments of marginal bone loss in millimeters, it was decided to use the implant thread as a measuring gauge. This method was in accordance with other recent clinical evaluations.55–57 The material used in this study could be observed if the graft material was still at the site, as its appearance was distinct from the bone surface. It appeared as opaque hollow circles within the bone. The material was radio-opaque due to a very small amount of barium sulfate that was intentionally added to the graft material to observe the degradation period and bone regeneration when it took place inside and outside of the porous material.
PPCH and PA are completely degradable. The degradation time can be changed based on the indication. Depending on the specific application, the time required can be manipulated based on a number of factors, e.g., the ratio of the bone substitute and the cross-linkable prepolymer. When the cross-linkable prepolymer contains more than one type of monomer, the ratio of the monomers also plays a crucial role in the degradation/resorption time. In addition, because the bone substitute is PMMA/PHEMA based, which is known to be very slowly degradable, increasing the proportion of the bone substitute increases the degradation time. The other factors that affect degradation time are the function of the pH, hydrophobicity/hydrophilicity of the components, and geometrical shape and thickness. It was estimated that, at the time of sacrifice, 40% to 50% of this particular material used in this study was degraded, whereas ≈90% to 96% was expected to resorb in 2 to 4 years. Another study,58 on PMMA/PHEMA, in which biopsy specimens were taken from five patients, showed that 0.001% to 0.002% of the original material was still present after 11 years with no inflammation or adverse reactions, and it was concluded that this material served as a scaffold for bone and contained the ideal conditions of biocompatibility, biodegradability, and osteoconduction. In a previous study,59 PMMA was also shown to be biocompatible, and no tissue reaction was observed histologically. The presence of PMMA/PHEMA particles in the histologic samples from patients with >11 years of follow-up indicated that it maintained the scaffold role over long time periods. The PA used in the present study was formulated to resorb in 4 to 6 months and both PA and PPCH materials had no negative effect on osseointegration and acted as a scaffold for bone growth. Because both were good osseoconductive materials and also allowed new bone formation around and inside porous structures, the importance of using these materials was to eliminate the micromovement during the initial healing and immediate function period and eventually to regenerate new bone.
The three-phase delivery system enabled the entire mixture to be cured completely and at one time instead of having to layer the material and curing each layer separately (e.g., composite filling materials). Until it was ready to be used, the light and chemical curing aspects were not prematurely mixed. Hence, during its use, the system allowed for the preparation and placement of the material at the site. As opposed to other known light or chemically cured materials (composite fillings), the PPCH-PA was hydrophilic and did not interfere with the blood or moistness at the environment. Bleeding/moisture control can be done by a gauze application, but it is not essential because it is important to get the marrow cells (potentially osteogenic precursor cells) from the blood into the pores and mixture for bone regeneration. In fact, the hydrophilic structure and the −10-mV charge of the materials attracted these cell to the surface of the materials.
CONCLUSIONS
The results of the present study show that the newly formulated light/chemically hardened graft material PPCH-PA was beneficial in immediate implant placement after tooth extraction and resulted in greater stability during the immediate loading over a 3-month period. Within the limits of the small sample size, these findings showed that the light/chemically hardened PPCH-PA composite bone-replacement graft material could be safely and successfully used to perform crestal ridge augmentations around implants and in extraction sockets. In addition, the PPCH-PA graft material has the potential to provide an implant stability and more bone formation during immediate loading. Additional studies in animals evaluating different healing times and longer durations and well-controlled human clinical studies need to be carried out for further evaluation of this newly formulated bone-substitute material.
Acknowledgments
This study was supported, in part, by Bioplant R&D, Westport, Connecticut and the United States Public Health Service, NIH, Bethesda, Maryland (grant DE019938; to Dr. Van Dyke). The authors thank Dr. Devon A. Shipp, Department of Chemistry and Biomolecular Science, Clarkson University, Potsdam, New York; the veterinary and animal care personnel, Mimi Crowley, Sharron Kirchain, Michelle Callihan, Rosa Yip, and Sergio Maura at the BUMC LASC; and Ronald L’Herault, laboratory supervisor, Boston University School of Dental Medicine Biomaterials Laboratory, Boston, Massachusetts, for their technical assistance during the study. Implants and the implant equipment were kindly donated by Biomet 3i, Palm Beach Gardens, Florida. Specialty surgical instruments were a gift from Hu-Friedy, Chicago, Illinois. Dr. Ashman, chief executive officer of Bioplant R&D, is the inventor of and has issued patents on the light and chemically cured materials (PPCH-PA [composite graft of hard tissue replacement and polyanhydride with light/chemical hardening technology]) tested in this study. Drs. Hasturk, Kantarci, Ghattas, Schmidt, Giordano, Diekwisch, and Van Dyke declare no financial interests related to this study.
Footnotes
Hard-Tissue Replacement, Bioplant, Westport, CT.
LCH, Bioplant R&D.
Bioplant, Kerr Corporation; a division of Sybron Dental Specialties, Orange, CA.
Bioplant R&D.
LCH, Bioplant R&D.
Göttingen Minipigs, Marshall BioResources, North Rose, NY.
Osseotite Certain MicroMiniplant, Biomet 3i, Palm Beach Gardens, FL.
OSSEOCISION, Biomet 3i.
Hu-Friedy, Chicago, IL
Biomet 3i, Implant Innovations, Palm Beach Gardens, FL.
Naxcel, Pfizer, New York, NY.
Periotest, Medizintechnik Gulden, Modautal, Germany.
UNC-15 probe, Hu-Friedy.
Schick Technologies, Long Island City, NY.
CDR DICOM, Schick Technologies, Long Island City, NY.
Buehler Ecomet, Buehler, Lake Bluff, IL.
Instron Model 4202, Instron, Canton, MA.
Instron Software, Instron.
Exakt 300 Diamond Band Saw, EXAKT, Norderstedt, Germany.
Hitachi S-3000N, Hitachi Instruments Engineering, Ibarki, Japan.
Oxford Inca, Oxford Instruments Analytical, High Wycombe, U.K.
SPSS version. 11.0 for Windows, IBM, Chicago, IL.
CDR DICOM, Schick Technologies.
Instron Software, Instron.
References
- 1.Albrektsson T, Brånemark PI, Hansson HA, Lindström J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981;52:155–170. doi: 10.3109/17453678108991776. [DOI] [PubMed] [Google Scholar]
- 2.Brunski JB, Moccia AF, Jr, Pollack SR, Korostoff E, Trachtenberg DI. The influence of functional use of endosseous dental implants on the tissue-implant interface. I. Histological aspects. J Dent Res. 1979;58:1953–1969. doi: 10.1177/00220345790580100201. [DOI] [PubMed] [Google Scholar]
- 3.Brånemark PI, Hansson BO, Adell R, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl. 1977;16:1–132. [PubMed] [Google Scholar]
- 4.Brånemark PI, Engstrand P, Ohrnell LO, et al. Brånemark kovum: A new treatment concept for rehabilitation of the edentulous mandible. Preliminary results from a prospective clinical follow-up study. Clin Implant Dent Relat Res. 1999;1:2–16. doi: 10.1111/j.1708-8208.1999.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 5.Becker W, Becker BE, Huffstetlert S. Early functional loading at 5 days for Brånemark implants placed into edentulous mandibles: A prospective, open-ended, longitudinal study. J Periodontol. 2003;74:695–702. doi: 10.1902/jop.2003.74.5.695. [DOI] [PubMed] [Google Scholar]
- 6.Chiapasco M, Gatti C, Rossi E, Haefliger W, Markwalder TH. Implant-retained mandibular overdentures with immediate loading. A retrospective multicenter study on 226 consecutive cases. Clin Oral Implants Res. 1997;8:48–57. doi: 10.1111/j.1600-0501.1997.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 7.Rosenquist B. A comparison of various methods of soft tissue management following the immediate placement of implants into extraction sockets. Int J Oral Maxillofac Implants. 1997;12:43–51. [PubMed] [Google Scholar]
- 8.Brunski JB. Avoid pitfalls of overloading and micromotion of intraosseous implants. Dent Implantol Update. 1993;4:77–81. [PubMed] [Google Scholar]
- 9.Jaffin RA, Berman CL. The excessive loss of Brånemark fixtures in type IV bone: A 5-year analysis. J Periodontol. 1991;62:2–4. doi: 10.1902/jop.1991.62.1.2. [DOI] [PubMed] [Google Scholar]
- 10.Horiuchi M, Ichikawa T, Kanitani H, Wigianto R, Kawamoto N, Matsumoto N. Pilot-hole preparation for proper implant positioning and the enhancement of bone formation. J Oral Implantol. 1995;21:318–324. [PubMed] [Google Scholar]
- 11.Summers RB. A new concept in maxillary implant surgery: The osteotome technique. Compendium. 1994;15:152, 154–156, 158. passim; quiz 162. [PubMed] [Google Scholar]
- 12.Piattelli A, Ruggeri A, Franchi M, Romasco N, Trisi P. An histologic and histomorphometric study of bone reactions to unloaded and loaded non-submerged single implants in monkeys: A pilot study. J Oral Implantol. 1993;19:314–320. [PubMed] [Google Scholar]
- 13.Nkenke E, Kloss F, Wiltfang J, et al. Histomorphometric and fluorescence microscopic analysis of bone remodelling after installation of implants using an osteotome technique. Clin Oral Implants Res. 2002;13:595–602. doi: 10.1034/j.1600-0501.2002.130604.x. [DOI] [PubMed] [Google Scholar]
- 14.Strietzel FP, Nowak M, Küchler I, Friedmann A. Periimplant alveolar bone loss with respect to bone quality after use of the osteotome technique: Results of a retrospective study. Clin Oral Implants Res. 2002;13:508–513. doi: 10.1034/j.1600-0501.2002.130510.x. [DOI] [PubMed] [Google Scholar]
- 15.Brånemark PI. Osseointegration and its experimental background. J Prosthet Dent. 1983;50:399–410. doi: 10.1016/s0022-3913(83)80101-2. [DOI] [PubMed] [Google Scholar]
- 16.Ledermann PD, Schenk RK, Buser D. Long-lasting osseointegration of immediately loaded, bar-connected TPS screws after 12 years of function: A histologic case report of a 95-year-old patient. Int J Periodontics Restorative Dent. 1998;18:552–563. [PubMed] [Google Scholar]
- 17.Hoshaw SJ, Fyhrie DP, Takano Y, Burr DB, Milgrom C. A method suitable for in vivo measurement of bone strain in humans. J Biomech. 1997;30:521–524. doi: 10.1016/s0021-9290(96)00176-5. [DOI] [PubMed] [Google Scholar]
- 18.Piatelli A, Croce A, Tete S, Artese L. Primary non-Hodgkin’s lymphoma of the mandible: A case report. J Oral Maxillofac Surg. 1997;55:1162–1166. doi: 10.1016/s0278-2391(97)90300-1. [DOI] [PubMed] [Google Scholar]
- 19.Zubery Y, Bichacho N, Moses O, Tal H. Immediate loading of modular transitional implants: A histologic and histomorphometric study in dogs. Int J Periodontics Restorative Dent. 1999;19:343–353. [PubMed] [Google Scholar]
- 20.Testori T, Szmukler-Moncler S, Francetti L, et al. Immediate loading of Osseotite implants: A case report and histologic analysis after 4 months of occlusal loading. Int J Periodontics Restorative Dent. 2001;21:451–459. [PubMed] [Google Scholar]
- 21.Albrektsson T, Eriksson AR, Friberg B, et al. Histologic investigations on 33 retrieved Nobelpharma implants. Clin Mater. 1993;12:1–9. doi: 10.1016/0267-6605(93)90021-x. [DOI] [PubMed] [Google Scholar]
- 22.Nyström E, Kahnberg KE, Albrektsson T. Treatment of the severely resorbed maxillae with bone graft and titanium implants: Histologic review of autopsy specimens. Int J Oral Maxillofac Implants. 1993;8:167–172. [PubMed] [Google Scholar]
- 23.Ivanoff CJ, Sennerby L, Lekholm U. Influence of mono- and bicortical anchorage on the integration of titanium implants. A study in the rabbit tibia. Int J Oral Maxillofac Surg. 1996;25:229–235. doi: 10.1016/s0901-5027(96)80036-1. [DOI] [PubMed] [Google Scholar]
- 24.Sennerby L, Thomsen P, Ericson LE. A morphometric and biomechanic comparison of titanium implants inserted in rabbit cortical and cancellous bone. Int J Oral Maxillofac Implants. 1992;7:62–71. [PubMed] [Google Scholar]
- 25.Esposito M, Thomsen P, Ericson LE, Lekholm U. Histopathologic observations on early oral implant failures. Int J Oral Maxillofac Implants. 1999;14:798–810. [PubMed] [Google Scholar]
- 26.Malmquist JP. Successful implant restoration with the use of barrier membranes. J Oral Maxillofac Surg. 1999;57:1114–1116. doi: 10.1016/s0278-2391(99)90336-1. [DOI] [PubMed] [Google Scholar]
- 27.Scipioni A, Bruschi GB, Giargia M, Berglundh T, Lindhe J. Healing at implants with and without primary bone contact. An experimental study in dogs. Clin Oral Implants Res. 1997;8:39–47. doi: 10.1111/j.1600-0501.1997.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 28.Scipioni A, Bruschi GB, Calesini G, Bruschi E, De Martino C. Bone regeneration in the edentulous ridge expansion technique: Histologic and ultrastructural study of 20 clinical cases. Int J Periodontics Restorative Dent. 1999;19:269–277. [PubMed] [Google Scholar]
- 29.Rosenquist B, Ahmed M. The immediate replacement of teeth by dental implants using homologous bone membranes to seal the sockets: Clinical and radiographic findings. Clin Oral Implants Res. 2000;11:572–582. doi: 10.1034/j.1600-0501.2000.011006572.x. [DOI] [PubMed] [Google Scholar]
- 30.Nemcovsky CE, Artzi Z, Moses O. Rotated split palatal flap for soft tissue primary coverage over extraction sites with immediate implant placement. Description of the surgical procedure and clinical results. J Periodontol. 1999;70:926–934. doi: 10.1902/jop.1999.70.8.926. [DOI] [PubMed] [Google Scholar]
- 31.Rosenquist B, Grenthe B. Immediate placement of implants into extraction sockets: Implant survival. Int J Oral Maxillofac Implants. 1996;11:205–209. [PubMed] [Google Scholar]
- 32.Polizzi G, Grunder U, Goené R, et al. Immediate and delayed implant placement into extraction sockets: A 5-year report. Clin Implant Dent Relat Res. 2000;2:93–99. doi: 10.1111/j.1708-8208.2000.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 33.Grunder U, Polizzi G, Goené R, et al. A 3-year prospective multicenter follow-up report on the immediate and delayed-immediate placement of implants. Int J Oral Maxillofac Implants. 1999;14:210–216. [PubMed] [Google Scholar]
- 34.Schwartz-Arad D, Grossman Y, Chaushu G. The clinical effectiveness of implants placed immediately into fresh extraction sites of molar teeth. J Periodontol. 2000;71:839–844. doi: 10.1902/jop.2000.71.5.839. [DOI] [PubMed] [Google Scholar]
- 35.Schliephake H, Dard M, Planck H, Hierlemann H, Jakob A. Guided bone regeneration around endosseous implants using a resorbable membrane vs a PTFE membrane. Clin Oral Implants Res. 2000;11:230–241. doi: 10.1034/j.1600-0501.2000.011003230.x. [DOI] [PubMed] [Google Scholar]
- 36.Prosper L, Gherlone EF, Redaelli S, Quaranta M. Four-year follow-up of larger-diameter implants placed in fresh extraction sockets using a resorbable membrane or a resorbable alloplastic material. Int J Oral Maxillofac Implants. 2003;18:856–864. [PubMed] [Google Scholar]
- 37.Schultes G, Gaggl A. Histologic evaluation of immediate versus delayed placement of implants after tooth extraction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:17–22. doi: 10.1067/moe.2001.115464. [DOI] [PubMed] [Google Scholar]
- 38.Botticelli D, Berglundh T, Buser D, Lindhe J. The jumping distance revisited: An experimental study in the dog. Clin Oral Implants Res. 2003;14:35–42. doi: 10.1034/j.1600-0501.2003.140105.x. [DOI] [PubMed] [Google Scholar]
- 39.Becker W, Becker BE, Handelsman M, Ochsenbein C, Albrektsson T. Guided tissue regeneration for implants placed into extraction sockets: A study in dogs. J Periodontol. 1991;62:703–709. doi: 10.1902/jop.1991.62.11.703. [DOI] [PubMed] [Google Scholar]
- 40.Ashman A, Bruins P. Prevention of alveolar bone loss postextraction with HTR grafting material. Oral Surg Oral Med Oral Pathol. 1985;60:146–153. doi: 10.1016/0030-4220(85)90282-8. [DOI] [PubMed] [Google Scholar]
- 41.Yukna RA. HTR polymer grafts in human periodontal osseous defects. I. 6-month clinical results. J Periodontol. 1990;61:633–642. doi: 10.1902/jop.1990.61.10.633. [DOI] [PubMed] [Google Scholar]
- 42.Yukna RA. Clinical evaluation of HTR polymer bone replacement grafts in human mandibular Class II molar furcations. J Periodontol. 1994;65:342–349. doi: 10.1902/jop.1994.65.4.342. [DOI] [PubMed] [Google Scholar]
- 43.Yukna RA, Yukna CN. Six-year clinical evaluation of HTR synthetic bone grafts in human grade II molar furcations. J Periodontal Res. 1997;32:627–633. doi: 10.1111/j.1600-0765.1997.tb00572.x. [DOI] [PubMed] [Google Scholar]
- 44.Yukna RA, Saenz AM, Shannon M, Mayer ET, Castellon P. Use of HTR synthetic bone as an augmentation material in conjunction with immediate implant placement: A case report. J Oral Implantol. 2003;29:24–28. doi: 10.1563/1548-1336(2003)029<0024:UOHSBA>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 45.Yukna RA, Sayed-Suleyman A, Finley JM, Hochstedler J, Mayer ET. Use of HTR synthetic bone grafts in conjunction with immediate dental implants. Compend Contin Educ Dent. 2003;24:649–652. 654, 657. quiz 658. [PubMed] [Google Scholar]
- 46.Calongne KB, Aichelmann-Reidy ME, Yukna RA, Mayer ET. Clinical comparison of microporous biocompatible composite of PMMA, PHEMA and calcium hydroxide grafts and expanded polytetrafluoroethylene barrier membranes in human mandibular molar Class II furcations. A case series. J Periodontol. 2001;72:1451–1459. doi: 10.1902/jop.2001.72.10.1451. [DOI] [PubMed] [Google Scholar]
- 47.Uhrich KE, Ibim SE, Larrier DR, Langer R, Laurencin CT. Chemical changes during in vivo degradation of poly(anhydride-imide) matrices. Biomaterials. 1998;19:2045–2050. doi: 10.1016/s0142-9612(98)00110-0. [DOI] [PubMed] [Google Scholar]
- 48.Lu S, Anseth KS. Photopolymerization of multilaminated poly(HEMA) hydrogels for controlled release. J Control Release. 1999;57:291–300. doi: 10.1016/s0168-3659(98)00125-4. [DOI] [PubMed] [Google Scholar]
- 49.d’Hoedt B, Lukas D, Mühlbradt L, et al. Periotest methods – Development and clinical trial (in German) Dtsch Zahnarztl Z. 1985;40:113–125. [PubMed] [Google Scholar]
- 50.Kallus T, Bessing C, Homsi G, Eklund I. Five-year evaluation of Lifecore Restore implants: A retrospective comparison with Nobel Biocare MK II implants. Clin Implant Dent Relat Res. 2009;11:167–177. doi: 10.1111/j.1708-8208.2008.00115.x. [DOI] [PubMed] [Google Scholar]
- 51.Esposito M, Grusovin MG, Coulthard P, Worthington HV. The efficacy of various bone augmentation procedures for dental implants: A Cochrane systematic review of randomized controlled clinical trials. Int J Oral Maxillofac Implants. 2006;21:696–710. [PubMed] [Google Scholar]
- 52.Schenk RK, Buser D, Hardwick WR, Dahlin C. Healing pattern of bone regeneration in membrane-protected defects: A histologic study in the canine mandible. Int J Oral Maxillofac Implants. 1994;9:13–29. [PubMed] [Google Scholar]
- 53.Esposito M, Grusovin MG, Willings M, Coulthard P, Worthington HV. The effectiveness of immediate, early, and conventional loading of dental implants: A Cochrane systematic review of randomized controlled clinical trials. Int J Oral Maxillofac Implants. 2007;22:893–904. [PubMed] [Google Scholar]
- 54.Hjørting-Hansen E. Bone grafting to the jaws with special reference to reconstructive preprosthetic surgery. A historical review (in German) Mund Kiefer Gesichtschir. 2002;6:6–14. doi: 10.1007/s10006-001-0343-6. [DOI] [PubMed] [Google Scholar]
- 55.Moberg LE, Köndell PA, Sagulin GB, Bolin A, Heimdahl A, Gynther GW. Brånemark system and ITI dental implant system for treatment of mandibular edentulism. A comparative randomized study: 3-year follow-up. Clin Oral Implants Res. 2001;12:450–461. doi: 10.1034/j.1600-0501.2001.120504.x. [DOI] [PubMed] [Google Scholar]
- 56.Roos-Jansåker AM, Lindahl C, Renvert H, Renvert S. Nine-to fourteen-year follow-up of implant treatment. Part I: Implant loss and associations to various factors. J Clin Periodontol. 2006;33:283–289. doi: 10.1111/j.1600-051X.2006.00907.x. [DOI] [PubMed] [Google Scholar]
- 57.Fransson C, Lekholm U, Jemt T, Berglundh T. Prevalence of subjects with progressive bone loss at implants. Clin Oral Implants Res. 2005;16:440–446. doi: 10.1111/j.1600-0501.2005.01137.x. [DOI] [PubMed] [Google Scholar]
- 58.Froum S, Orlowski W. Ridge preservation utilizing an alloplast prior to implant placement–clinical and histological case reports. Pract Periodontics Aesthet Dent. 2000;12:393–402. quiz 404. [PubMed] [Google Scholar]
- 59.Stahl SS, Froum SJ, Tarnow D. Human clinical and histologic responses to the placement of HTR polymer particles in 11 intrabony lesions. J Periodontol. 1990;61:269–274. doi: 10.1902/jop.1990.61.5.269. [DOI] [PubMed] [Google Scholar]