Abstract
Objectives
To meta-analyze lipid outcomes from supervised exercise interventions among healthy adults.
Methods
Comprehensive search strategies identified trials testing supervised exercise interventions in samples of healthy adults. Data were coded and analyzed using random effects meta-analysis methods. Moderator analyses explored whether design, sample, or intervention characteristics were linked with lipid outcomes.
Results
Data were analyzed from 344 comparisons. The overall mean effect size for 2-group comparisons was 0.28, corresponding to reduced total cholesterol of 8.65 mg/dl. Study effect sizes were greater where participants were obese at baseline, and for interventions utilizing low-intensity exercise.
Conclusions
Supervised exercise interventions can improve lipid outcomes for healthy adults, with possible greater improvement for obese individuals, and through low-intensity exercise.
Keywords: exercise, physical activity, lipids, cholesterol, meta-analysis, systematic review
Cardiovascular disease remains the leading cause of death in the United States today.1 Hyperlipidemia is a well-established risk factor for cardiovascular disease.1-3 Control of lipid levels is one of the key strategies for primary and secondary prevention of cardiovascular disease.2 While pharmacologic approaches to lipid management are available, non-pharmacological treatments are the preferred and first-line approach to managing hyperlipidemia, particularly for primary prevention.4
Exercise is any structured, planned, bodily movement to increase or maintain physical fitness.5 Exercise is a key health behavior intervention to improve lipid levels and lower cardiovascular disease risk. Many trials have examined exercise interventions to improve lipid outcomes, with varying results.6,7 While a link has been established between weight loss and improved lipid levels,8 more recent work indicates that exercise may improve lipid levels independent of weight loss.9,10
Even among healthy adults, too few individuals engage in regular physical exercise. Only 48.4% of persons age 18 years and older obtain the recommended amount of aerobic exercise.11 Even individuals considered as healthy are frequently not active enough to meet the recommended levels of exercise to reduce the risk of developing chronic disease. Health care providers should play a key role in assisting individuals, particularly those at greater risk for cardiovascular disease, to increase the quantity and quality of their exercise behavior. Supervised exercise interventions, where participants exercise at a specified location under the supervision of an interventionist (eg, exercise researcher, trainer), have been effective at improving exercise behavior among healthy adults.12 The lipid-lowering effect of supervised exercise programs for healthy adults, however, has not been conclusively established.
Several recent literature syntheses have focused on the impact of exercise interventions on lipid outcomes.9,10,13-21 These studies have established the benefit of exercise overall as a component of lipid control for participants with cardiovascular and metabolic risk factors10,18,19,22 but the strength of the evidence for the effects of exercise on lipid outcomes for healthy adults is less clear. Published meta-analyses reporting lipid outcomes of exercise for healthy adults have mostly focused on specific sub-populations or limited intervention types. These meta-analyses have each synthesized small numbers of studies (ranging from 6 to 35 trials), and have had conflicting findings regarding the exercise characteristics associated with significant improvement in lipid levels. Finally, none of the meta-analyses identified in the past ten years examined the effect of supervised exercise on lipid outcomes of healthy adults.
This systematic review and meta-analysis aims to synthesize the results of supervised exercise interventions in populations of healthy adults, and to conduct moderator analyses to determine whether particular sample, study, or intervention characteristics are associated with greater improvement in lipid outcomes.
METHODS
We used accepted methods for conducting systematic reviews and meta-analyses to search, retrieve and screen potential studies, determine eligibility, extract and code data, and meta-analyze outcomes across all eligible studies.23,24
Inclusion Criteria
Studies were included if they reported lipid outcomes of a supervised exercise intervention. Lipid outcomes included total cholesterol level, high-density lipoprotein (HDL) level, low-density lipoprotein (LDL) level, triglyceride level, or the total cholesterol:HDL ratio. Primary studies were required to have samples of healthy adults (age 18 and older). Studies were restricted to supervised exercise interventions, where the exercise sessions were supervised by research project personnel. This type of intervention ensures that a consistent exercise dose was delivered to all participants within a study group. The sample included both published and unpublished studies, as publication status is not a reliable indicator of study quality, but rather is more likely to be correlated with the statistical significance of study findings.25 Studies were included regardless of sample size, as small, underpowered studies contribute to effect size (ES) estimates. The weights applied to effect size estimates ensured that studies with greater precision (typically also those with larger sample sizes) were weighted more heavily in pooled effect size estimates. Including unpublished and small-sample studies reduces risk for publication bias in meta-analysis findings. We included studies regardless of the location where the study was conducted, however studies where results were published in a language other than English were excluded.
We assumed study samples to be healthy unless the study report specifically stated otherwise. As a result, we included studies where participants may have been at risk for health concerns, such as being overweight or obese, but were not reported to actually have chronic health conditions.
Search Strategies
We used multiple approaches to maximize the number of identified eligible studies. Narrow searches may result in studies being overlooked, leading to biased meta-analysis results.26 An expert health sciences reference librarian conducted searches in 13 electronic databases (eg, MEDLINE, CINAHL, Scopus, PsycINFO). We also searched 36 research registries (eg, NIH RePORTER, National Health Service Clinical Trials Register) to identify potential studies that had not yet been published in indexed journals. The search taxonomy was developed to ensure no potentially eligible studies were overlooked (exercise: exercise, exercise therapy, exertion, physical activity, physical fitness, physical education and training, walking; intervention: adherence, behavior therapy, clinical trial, compliance, counseling, evaluation, evaluation study, evidence-based medicine, health care evaluation, health behavior, health education, health promotion, intervention, outcome and process assessment, patient education, program, program development, program evaluation, self care, treatment outcome, validation study). Eighty-two journals were hand searched to identify additional studies. Further computerized searches were done on the names of senior authors and principal investigators of eligible studies. Finally, we conducted ancestry searches on eligible study reference lists and on related review articles. Primary study authors were contacted when necessary to attempt to obtain missing data for calculating effect sizes.
Data Extraction and Measures
A codebook was developed using information from our previous meta-analyses, other published reviews, and examination of primary study reports. This led to a coding scheme permitting us to assess and code data about the reports, methods, samples, interventions and outcomes of each eligible study. Data were coded to the smallest possible level of detail to enhance validity and reliability.27 Studies were duplicate-coded by 2 independent trained coders directly from the primary study reports using a codebook and paper coding sheet. Coded data was then entered into a spreadsheet for comparison. Effect size data was further verified by a doctorally-prepared researcher. The duplicated electronic data sets were compared for each variable. Any discrepancies in coding were checked against the study reports to correct errors. Disagreements in coding were discussed to achieve consensus on coding decisions and to ensure accurate data entry.
Studies were categorized based on the data reported in the study reports. For example, studies were considered to consist of overweight participants if the report clearly indicated that greater than 50% of the study’s participants were overweight or had a body mass index (BMI) ≥ 25. Similarly, studies were coded as containing obese participants if the report stated that over half of the sample was obese or had a BMI ≥ 30.
Exercise intensity was coded as low, moderate, or high, based on the intensity reported by the primary study authors. If the primary authors did not categorize the exercise intensity, the intensity was determined from the intervention description in the report. For instance, exercise at 50-64% of maximum heart rate (MHR) would be categorized as low intensity, 65-80% of MHR would be moderate intensity, and >80% of MHR would be high intensity. If insufficient detail was provided to determine an intervention’s exercise intensity, it was coded as ‘not described’.
Data Analysis
Analyses were conducted using Comprehensive Meta-Analysis software. Coded study lipid outcome data were used to calculate a standardized mean difference effect size (ES) for each study. Study ESs were weighted by the inverse of the variance and synthesized using a random-effects model.24 A random-effects model assumes that the true intervention effect for each study is part of a random sample of true effects, allowing for expected between-study variation in effects, as well as the expected variation due to within study sampling error. Given the diversity of tested interventions and populations, between study variation is expected, making a random-effects model appropriate.24 Effect sizes could then be converted back to original metrics (eg, mg/dl) by multiplying by the pooled baseline standard deviation for each lipid measure.28
Heterogeneity of effect sizes was assessed using Q and I2 statistics. The Q statistic is the standard measure of the amount of variation observed across all studies in the meta-analysis. As the Q statistic is dependent on the number of studies in the analysis, meta-analysts commonly also use the I2 statistic. I2 represents the proportion of observed variance that is due to real differences in effect size across studies.24
As heterogeneity across studies is expected due to differences in intervention types, sample characteristics, and study designs, moderator analyses were conducted to further explore the heterogeneity inherent in the sample of studies.29,30 Both dichotomous and continuous moderator variables were examined using the Comprehensive Meta-Analysis software. Dichotomous moderators were analyzed using meta-analytic analogues of ANOVA, and continuous moderators using similar analogues of regression.24
RESULTS
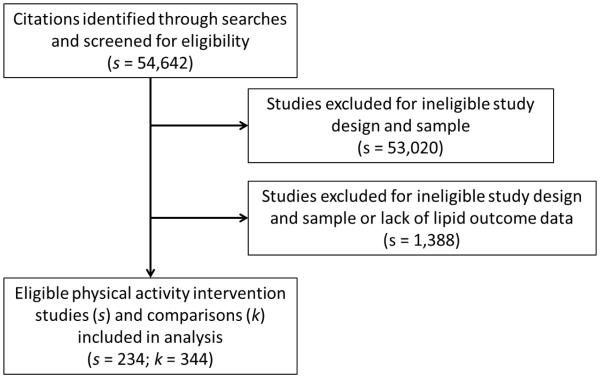
Comprehensive searching resulted in 54,642 reports screened for inclusion. Eligible lipid outcome data were coded from 87 2-group studies, reporting 133 treatment vs. control comparisons (see Figure 1). Supplementing these findings, we have also included 211 single-group, pre-post comparisons coded from 148 eligible studies (See Figure 1). The list of included studies is available from the primary author. The analyzed reports included 14,830 participants. The median sample size was 22 participants. The earliest study was published in 1961, with a median publication year of 1991. The median mean number of supervised exercise sessions per week was 3, and the median minutes per session was 48, suggesting that these interventions were close to but did not fully meet the current recommended guidelines for exercise behavior among adults. Characteristics of the included studies are reported in Table 1.
Figure 1.
Study Selection Flow Diagram
Note: s = number of studies; k = number of comparisons; A single study may have more than one treatment vs. control comparison, such as in the case of 2 distinct intervention groups.
Table 1.
Characteristics of Primary Studies Included in Lipid Outcomes Meta-Analyses
| Characteristic | k | Min | Q1 | Median | Q3 | Max |
|---|---|---|---|---|---|---|
| Mean age (years) | 264 | 18 | 32 | 41 | 53 | 80 |
| Total post-intervention sample size per study |
341 | 4 | 13 | 22 | 37 | 2044 |
| Treatment group post-intervention sample size per study |
341 | 2 | 11 | 15 | 27 | 2044 |
| Comparison group post-intervention sample size per study |
130 | 2 | 8 | 13 | 20 | 186 |
| Percentage female | 331 | 0 | 0 | 50 | 100 | 100 |
| Percentage racial or ethnic minority | 32 | 0 | 0 | 0 | 23 | 100 |
| Minutes of supervised exercise per session |
288 | 5 | 35 | 48 | 60 | 600 |
| Mean frequency per week of exercise sessions |
323 | 1 | 3 | 3 | 4 | 14 |
| Total number of supervised exercise sessions per study |
326 | 2 | 30 | 42 | 61 | 2028 |
Abbreviations: k = number of comparisons providing data on characteristic; Q1 = first quartile; Q3 = third quartile
Overall Effects of Interventions
Supervised exercise interventions significantly improved lipid outcome measures among healthy adults (Table 2). The mean lipid ES across all treatment versus control comparisons was 0.28 (S.E. = 0.04, 95% CI [0.20, 0.36], p < .001). For single-group, pre-post interventions, the mean ES was 0.19 (S.E. = 0.02, 95% CI [0.15, 0.23], p < .001). Analyzing intervention effects from baseline to outcome within treatment groups from studies designed as 2-group comparisons, the mean ES was similar to those from the single-group studies (ES = 0.19, S.E. = 0.03, 95% CI [0.13, 0.25], p < .001). In contrast, control groups did not show improvement in lipid outcomes from baseline to outcome (ES = −0.02, S.E. = 0.02, 95% CI [−0.07, 0.03], p = .456).
Table 2.
Lipid Outcome, Main Effects
| Comparisons | k | Mean ES | 95% CI | SE | Q | I2 |
|---|---|---|---|---|---|---|
| 2-group postintervention comparison | 133 | 0.28*** | 0.20, 0.36 | 0.04 | 224.04*** | 41.08 |
| 2-group pre-post comparison | 131 | 0.19*** | 0.13, 0.25 | 0.03 | 477.81*** | 72.79 |
| Treatment pre-post comparison | 211 | 0.19*** | 0.15, 0.23 | 0.02 | 1097.87*** | 80.87 |
| Control pre-post comparison | 131 | −0.02 | −0.07, 0.03 | 0.02 | 258.27*** | 49.67 |
k = number of comparisons
ES = estimated mean of true effect sizes (d index)
SE = standard error
Q = heterogeneity statistic (weighted squared deviations from summary effect)
I2 = index of heterogeneity beyond within-study sampling error
p < .05
p < .01
p < .001
This overall 2-group effect size equates to a decrease in the total cholesterol (TC) of 8.65 mg/dl (S.E. = 1.23), an increase in high-density lipoprotein (HDL) of 1.62 mg/dl (S.E. = 0.23), and a decrease in low-density lipoprotein (LDL) of 7.81 mg/dl (S.E. = 1.11). The same ES would equate to an improvement in the TC:HDL ratio of 0.34 (S.E. = 0.05).
Moderator Analyses
Moderator analyses were conducted on the 2-group, treatment versus control comparisons. The effect of supervised exercise interventions on lipid outcomes was not significantly different due to year of publication, publication source, presence of study funding, sample attrition, mean participant age, race, occupation, or mean baseline body mass index (BMI; see Table 3). Some sample characteristics, such as occupation, were infrequently reported, which limited the number and types of studies available to be included in moderator analyses. For example, only 2 eligible studies were conducted with samples of retired persons and one study with homemakers. The lack of detail in the data permitted us to only analyze between those samples reported as being employed versus studies of samples consisting of college students.
Table 3.
Continuous Moderators of Supervised Exercise Interventions
| Moderator | β | SE | p |
|---|---|---|---|
| Year of publication | −0.004 | 0.003 | .188 |
| Mean participant age | −0.004 | 0.002 | .116 |
| Mean baseline BMI | 0.001 | 0.013 | .937 |
| Mean baseline weight | 0.004 | 0.003 | .198 |
| Percent sample, female | −0.001 | 0.001 | .102 |
| Percent sample, black/African-American | 0.001 | 0.008 | .937 |
| Percent sample, Hispanic | −0.001 | 0.002 | .404 |
| Sample attrition, baseline to outcome | 0.001 | 0.001 | .285 |
| Frequency of supervised exercise sessions | −0.009 | 0.026 | .720 |
| Minutes of supervised exercise per intervention session |
0.005 | 0.002 | .010** |
β = meta-regression coefficient (unstandardized) for slope
SE = standard error
p = statistical significance of β
BMI = body mass index
p < .05
p < .01
p < .001
Minutes of supervised exercise per exercise session did modify the effect of supervised exercise on lipid outcomes. Every additional minute of exercise corresponds to an increase in ES of 0.005. Thus, an additional 10 minutes of exercise per session in the 2-group postintervention comparison would effectively increase the mean ES from 0.28 to 0.33.
Studies were equally effective among participants who were sedentary (0.29) and participants who were physically active (0.22) at baseline (p =.607). Studies where participants were obese at baseline, however, showed greater improvement in lipid outcomes than did studies where participants were not obese (0.84 vs. 0.10, p =.009; see Table 4). Too few studies were available that reported non-overweight samples to permit analysis of whether being overweight at baseline versus not overweight made a difference in the effect of supervised exercise interventions on lipid outcomes. The 29 studies reporting that most participants were overweight at baseline had a mean effect size of 0.40.
Table 4.
Dichotomous Moderators of Supervised Exercise Interventions
| Moderator | k1 | ES1 | k0 | ES0 | Q btwn | p |
|---|---|---|---|---|---|---|
| Publication source | 118 | 0.29 | 14 | 0.18 | 0.98 | .321 |
| Journal article (1) dissertation/thesis (0) | ||||||
| Presence of funding | 87 | 0.27 | 46 | 0.32 | 0.28 | .598 |
| Funded (1) vs. no/not reported (0) | ||||||
| Randomization | 92 | 0.29 | 41 | 0.27 | 0.03 | .874 |
| Individual (1) vs. group/site (0) | ||||||
| Occupation | 27 | 0.36 | 17 | 0.21 | 1.82 | .177 |
| College students (1) vs. employed (0) | ||||||
| Fitness testing | 17 | 0.37 | 116 | 0.26 | 1.70 | .192 |
| Yes (1) vs. no (0) | ||||||
| Exercise prescription | 79 | 0.30 | 54 | 0.26 | 0.23 | .628 |
| Yes (1) vs. no (0) | ||||||
| Active prior to intervention | 14 | 0.22 | 119 | 0.29 | 0.26 | .607 |
| Active (1) vs. sedentary (0) | ||||||
| Overweight at baseline | 29 | 0.40 | 103 | 0.25 | 2.04 | .154 |
| Overweight (1) vs. not reported (0) | ||||||
| Obese at baseline | 10 | 0.84 | 10 | 0.10 | 6.91 | .009** |
| Obese (1) vs. not obese (0) | ||||||
| Exercise Intensity | 6 | 0.40 | 29 | 0.13 | 4.69 | .030* |
| Low (1) vs. high (0) | ||||||
| Flexibility exercises | 37 | 0.21 | 96 | 0.28 | 0.63 | .429 |
| Yes (1) vs. no (0) | ||||||
| Resistance exercises | 13 | 0.10 | 120 | 0.28 | 0.37 | .542 |
| Yes (1) vs. no (0) |
k = number of comparisons
ES = effect size (d index)
Qbtwn = heterogeneity statistic (weighted squared deviations from summary effect)
p = test of statistical significance
p < .05
p < .01
p < .001
Exercise intensity was the only significant intervention moderator. Studies with low-intensity supervised exercise led to greater improvements in lipid outcomes than did studies using high-intensity exercise. The mean effect size for low-intensity exercise studies was 0.40, while for the high-intensity studies the mean ES was 0.13 (p = .030).
All of the supervised exercise interventions included aerobic exercise. No effect size differences were observed based on the other types of exercise (ie, flexibility, resistance) that were included in some of the interventions.
Interestingly, there was no greater impact on lipid outcomes when the intervention sought to change both exercise and diet versus targeting exercise behavior alone (0.28 vs. 0.26, p = .947). This remained the case even when analyzing studies containing overweight participants (0.28 vs. 0.49, p = .146), but the difference was significant among the subset of studies targeting obese participants (0.42 vs. 1.49, p = .033).
DISCUSSION
This meta-analysis found that supervised exercise interventions of limited duration and frequency have modest but significant effects on lipid outcomes in healthy adults. Previous meta-analyses of exercise interventions found lipid outcome ESs ranging from −0.05 to 0.21.9,10,14,16 The overall ESs in this meta-analysis were larger than those prior studies. This may be due to more rigorous search methods, which led to including more eligible studies, or possibly this project’s focus on supervised exercise interventions, as opposed to other types of exercise interventions.
The median frequency and duration of exercise sessions of studies included in this meta-analysis did not meet national recommendations for exercise levels for healthy adults.31 The magnitude of the effect of supervised exercise on lipid outcomes in the present study may be an underestimate of the true benefit of exercise performed at the recommended levels. As future studies test the effects on lipid outcomes of supervised exercise performed at the recommended dose, synthesized ESs may be larger.
The effect of supervised exercise interventions on lipid levels is likely to be greater for those persons with higher BMIs at the start of an exercise program, as effect sizes were significantly greater for studies where participants were obese at baseline. Effect sizes also trended toward greater improvement in lipids in studies where participants were overweight (but not obese) at baseline. These findings are consistent with prior evidence that exercise improves lipid levels in overweight and obese persons.7 Across the studies included in this meta-analysis, the ESs for patients who are overweight or obese would be equivalent to a mean improvement in TC:HDL ratio of 0.5 to 1.0 , a mean improvement in total cholesterol of 12.3 to 25.9 mg/dl, in HDL of 2.3 to 4.9 mg/dl, or in LDL of 11.1 to 23.4 mg/dl. Cholesterol goals vary depending on individual patient risk factors, however this evidence suggests that healthy adults with higher BMIs prior to starting a supervised exercise program may see significantly greater improvement in lipid levels than those with lower BMIs. Clinicians should recommend increased exercise for obese patients regardless of whether the exercise leads to changes in weight.
While Kelly4 hypothesized that the combination of exercise and diet interventions would work synergistically to achieve greater improvements in lipid levels, this meta-analysis did not find a significant effect size difference between studies focusing solely on exercise versus those focusing on changes in both exercise and diet. For studies enrolling overweight and/or obese participants, however, the results trended toward interventions focusing on exercise alone as having greater effects on lipid outcomes. This finding may be due to higher baseline lipid levels among overweight and obese participants who may have been less healthy than non-overweight and non-obese samples. With higher baseline lipid levels, these comparisons would then be more likely to show improvement in lipid outcomes from increased exercise activity. The link with overweight and obese participants should be considered strictly exploratory, however, as the number of comparisons in these particular moderator analyses was small.
The larger effect size for low-intensity supervised exercise as compared to high-intensity exercise is an interesting finding that may reflect a number of potential factors. While all of the studies in this meta-analysis characterized their samples as being healthy adults, it is possible that low-intensity exercise studies may have been more likely to enroll participants with lower levels of overall fitness at baseline, and who therefore had greater room for improvement. Low-intensity programs may also have included greater numbers of obese participants, who had greater improvement in lipid levels. Finally, attendance rates may have been greater in low-intensity versus high-intensity programs, particularly for sedentary and/or overweight/obese participants. Previous research on lipid outcomes at different exercise intensities has yielded conflicting results,14,15 although recent evidence supports low-intensity exercise as being superior to high intensity for controlling lipid levels.32,33 Further research is needed to conclusively determine the impact of exercise intensity and dose on lipid outcomes.
Limitations
Meta-analysis findings are considered observational research, and the findings of moderator comparisons, while useful for proposing further research in this area, cannot be considered indicative of causal relationships. Further, the findings of this meta-analysis are limited in that it includes studies of healthy persons. The impact of physical exercise on lipid levels may be different in persons with chronic conditions that change physiological responses to exercise or who have co-morbidities that may interact with exercise and hyperlipidemia.
Further, meta-analyses are limited by the data contained in the primary study reports and whatever additional data can be obtained from primary study authors. This leads otherwise eligible studies to be excluded due to inadequate data for calculation of effect size. Frequently, eligible studies have poorly described samples and interventions, providing scant data for coding moderator variables and limiting sample sizes for moderator analyses. Further, the potential exists for publication bias, where potentially eligible studies are not included because they were not identified or were never published in the first place.23,34 Although this meta-analysis project utilized extensive search strategies to locate all available eligible studies, some studies may have been missed. No differences were found in effect size related to publication status, however, and funnel plots for publication bias indicated a balanced distribution of studies.23
This project also pooled lipid measures from the primary studies as a single outcome. Exercise may influence different lipid measures in different ways, limiting the generalizability of these findings. We did not, however, observe significant differences in effect size based on the lipid measure reported.
Conclusion
Supervised exercise interventions can positively impact lipid outcomes for healthy adults. While the mean effect sizes are small to moderate, they represent clinically significant improvement in lipid levels. Greater improvement may be seen by obese individuals, and through utilizing low-intensity exercise, although the dynamics of the relationship between exercise intensity and lipid outcomes requires further study.
Acknowledgements
This research was supported by the National Institutes of Health (Grant number R01NR009656 to Vicki Conn). The authors would also like to acknowledge the support of the John A. Hartford Foundation to Todd Ruppar (Claire M. Fagin Fellow) and Jo-Ana Chase (Patricia Archbold Scholar). Dr. Ruppar is also a Robert Wood Johnson Foundation Nurse Faculty Scholar. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the John A. Hartford Foundation, or the Robert Wood Johnson Foundation.
Footnotes
Human Subjects Statement
This project analyzed completed studies as the unit of analysis. No individual patient data was collected, thus this project is considered exempt from IRB review.
Conflict of Interest Statement
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Hoyert DL, Xu J. National Vital Statistics Reports. National Center for Health Statistics; Hyattsville, MD: 2012. Deaths: Preliminary Data for 2011. [PubMed] [Google Scholar]
- 2.Kraus WE, Slentz CA. Exercise training, lipid regulation, and insulin action: a tangled web of cause and effect. Obesity. 2009;17(Suppl 3):S21–26. doi: 10.1038/oby.2009.384. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Cleeman JI, Merz CN. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 4.Kelly RB. Diet and exercise in the management of hyperlipidemia. Am Fam Physician. 2010;81(9):1097–1102. [PubMed] [Google Scholar]
- 5.McArdle W, Katch FI, Katch VL. Exercise Physiology: Nutrition, Energy, and Human Performance. Lippincott Williams & Wilkins; Philadelphia: 2010. [Google Scholar]
- 6.Mauricio D, Orozco LJ, Buchleitner AM, et al. Exercise or exercise and diet for preventing type 2 diabetes mellitus. Cochrane Database Syst Rev. 2008;(3):CD003054. doi: 10.1002/14651858.CD003054.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Shaw K, Gennat H, O'Rourke P, et al. Exercise for overweight or obesity. The Cochrane database of systematic reviews. 2006;(4):CD003817. doi: 10.1002/14651858.CD003817.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992;56(2):320–328. doi: 10.1093/ajcn/56.2.320. [DOI] [PubMed] [Google Scholar]
- 9.Kelley GA, Kelley KS, Tran ZV. Walking, lipids, and lipoproteins: a meta-analysis of randomized controlled trials. Prev Med. 2004;38(5):651–661. doi: 10.1016/j.ypmed.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Carroll S, Dudfield M. What is the relationship between exercise and metabolic abnormalities? A review of the metabolic syndrome. Sports Med. 2004;34(6):371–418. doi: 10.2165/00007256-200434060-00004. [DOI] [PubMed] [Google Scholar]
- 11.National Center for Health Statistics . Health, United States, 2012. U.S. Department of Health and Human Services; Hyattsville, MD: 2013. [Google Scholar]
- 12.Conn VS, Hafdahl AR, Mehr DR. Interventions to increase physical activity among healthy adults: meta-analysis of outcomes. Am J Public Health. 2011;101(4):751–758. doi: 10.2105/AJPH.2010.194381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebrahim S, Taylor F, Ward K, et al. Multiple risk factor interventions for primary prevention of coronary heart disease. Cochrane Database Syst Rev. 2011;(1):CD001561. doi: 10.1002/14651858.CD001561.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halbert JA, Silagy CA, Finucane P, et al. Exercise training and blood lipids in hyperlipidemic and normolipidemic adults: a meta-analysis of randomized, controlled trials. Eur J Clin Nutr. 1999;53(7):514–522. doi: 10.1038/sj.ejcn.1600784. [DOI] [PubMed] [Google Scholar]
- 15.Hata Y, Nakajima K. Life-style and serum lipids and lipoproteins. J Atheroscler Thromb. 2000;7(4):177–197. doi: 10.5551/jat1994.7.177. [DOI] [PubMed] [Google Scholar]
- 16.Kodama S, Tanaka S, Saito K, et al. Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: a meta-analysis. Arch Intern Med. 2007;167(10):999–1008. doi: 10.1001/archinte.167.10.999. [DOI] [PubMed] [Google Scholar]
- 17.Tambalis K, Panagiotakos DB, Kavouras SA, et al. Responses of blood lipids to aerobic, resistance, and combined aerobic with resistance exercise training: a systematic review of current evidence. Angiology. 2009;60(5):614–632. doi: 10.1177/0003319708324927. [DOI] [PubMed] [Google Scholar]
- 18.Pattyn N, Cornelissen VA, Eshghi SR, et al. The effect of exercise on the cardiovascular risk factors constituting the metabolic syndrome: a meta-analysis of controlled trials. Sports Med. 2013;43(2):121–133. doi: 10.1007/s40279-012-0003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashino Y, Jackson JL, Fukumori N, et al. Effects of supervised exercise on lipid profiles and blood pressure control in people with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2012;98(3):349–360. doi: 10.1016/j.diabres.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Kelley GA, Kelley KS, Roberts S, et al. Efficacy of aerobic exercise and a prudent diet for improving selected lipids and lipoproteins in adults: a meta-analysis of randomized controlled trials. BMC Med. 2011;9:74. doi: 10.1186/1741-7015-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelley GA, Kelley KS, Roberts S, et al. Comparison of aerobic exercise, diet or both on lipids and lipoproteins in adults: a meta-analysis of randomized controlled trials. Clin Nutr. 2012;31(2):156–167. doi: 10.1016/j.clnu.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark AM, Haykowsky M, Kryworuchko J, et al. A meta-analysis of randomized control trials of home-based secondary prevention programs for coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2010;17(3):261–270. doi: 10.1097/HJR.0b013e32833090ef. [DOI] [PubMed] [Google Scholar]
- 23.Cooper H, Hedges L, Valentine J. The Handbook of Research Synthesis and Meta-Analysis. Russell Sage Foundation; New York: 2009. [Google Scholar]
- 24.Borenstein M, Hedges L, Higgins J, et al. Introduction to Meta-Analysis. John Wiley & Sons; West Sussex: 2009. [Google Scholar]
- 25.Rothstein HR, Hopewell S. Grey literature. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. Russell Sage Foundation; New York: 2009. pp. 103–125. [Google Scholar]
- 26.White H, Cooper H, Hedges L, et al. Scientific communication and literature retrieval. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. Russell Sage Foundation; New York: 2009. pp. 51–71. [Google Scholar]
- 27.Orwin R, Vevea J, Cooper H, et al. Cooper H, Hedges L, Valentine J, editors. Evaluating coding decisions. The Handbook of Research Synthesis and Meta-Analysis [Google Scholar]
- 28.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. (Version 5.1.0). The Cochrane Collaboration 2011. Available at: http://www.cochrane-handbook.org. [Google Scholar]
- 29.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP. Commentary: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37(5):1158–1160. doi: 10.1093/ije/dyn204. [DOI] [PubMed] [Google Scholar]
- 31.U.S. Department of Health and Human Services . Physical Activity Guidelines for Americans. Available at: http://www.health.gov/paguidelines/guidelines/ [Google Scholar]
- 32.Botero JP, Prado WL, Guerra RL, et al. Does aerobic exercise intensity affect health-related parameters in overweight women? Clin Physiol Funct Imaging. 2014;34(2):138–142. doi: 10.1111/cpf.12076. [DOI] [PubMed] [Google Scholar]
- 33.Duvivier BM, Schaper NC, Bremers MA, et al. Minimal intensity physical activity (standing and walking) of longer duration improves insulin action and plasma lipids more than shorter periods of moderate to vigorous exercise (cycling) in sedentary subjects when energy expenditure is comparable. PLoS One. 2013;8(2):e55542. doi: 10.1371/journal.pone.0055542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conn VS, Valentine JC, Cooper HM, et al. Grey literature in meta-analyses. Nurs Res. 2003;52(4):256–261. doi: 10.1097/00006199-200307000-00008. [DOI] [PubMed] [Google Scholar]