Abstract
Although there are many stimulus-responsive polymers, poly(N-isopropyl acrylamide) (pNIPAM) is of special interest due to the phase change it undergoes in a physiologically relevant temperature range that leads to the release of cells and proteins. The nondestructive release of cells opens up a wide range of applications, including the use of pNIPAM for cell sheet and tissue engineering. In this work, pNIPAM surfaces were generated that can be distinguished from the extracellular matrix. A polymerization technique was adapted that was previously used by Mendez, and the existing protocol was optimized for the culture of mammalian cells. The resulting surfaces were characterized with X-ray photoelectron spectroscopy and goniometry. The developed pNIPAM surfaces were further adapted by incorporation of 5-acrylamidofluorescein to generate fluorescent pNIPAM-coated surfaces. Both types of surfaces (fluorescent and nonfluorescent) sustained cellular attachment and produced cellular detachment of ∼90%, and are therefore suitable for the generation of cell sheets for engineered tissues and other purposes. These surfaces will be useful tools for experiments investigating cellular detachment from pNIPAM and the pNIPAM/cell interface.
I. INTRODUCTION
Poly(N-isopropyl acrylamide) (pNIPAM) is a thermoresponsive polymer widely used in bioengineering applications. Although there are many polymers that respond to a stimulus such as temperature, pH, light, or magnetic field,1 pNIPAM is of special interest due to the phase change it undergoes in a physiologically relevant temperature range, which leads to cell/protein release. PNIPAM has a lower critical solution temperature (LCST) of ∼32 °C. Above its LCST, pNIPAM is relatively hydrophobic. When grafted to a surface, it takes a globular, packed conformation. Below the LCST, the polymer is hydrated, and its chains become more extended.2 Mammalian cells can be easily cultured on pNIPAM at 37 °C (body temperature, and therefore the temperature at which cells are cultured in an incubator). When the temperature is lowered to below pNIPAM's LCST, the polymer's chains extend and cells detach as intact sheets. Since cell sheet detachment using pNIPAM preserves the cell sheet and its extracellular matrix (ECM),3,4 this detachment method may be preferred to enzymatic digestion or mechanical scraping. A detached cell sheet can be transferred to another surface and cultured for further use.5–10 The nondestructive release of cells opens up a wide range of applications, including the use of pNIPAM for cell sheet and tissue engineering.9
It has previously been demonstrated that the NIPAM monomer is toxic,11 and there have also been conflicting reports as to whether the polymerized form of NIPAM (pNIPAM) is toxic.12–18 We recently performed a comprehensive study of the cytotoxicity of pNIPAM and pNIPAM-coated surfaces, where robust pNIPAM surfaces were found to be noncytotoxic.19 In our previous work with pNIPAM, we also investigated the ECM before and after cell detachment from pNIPAM.3,4,20 From that work, it was apparent that although some ECM proteins remain on the surface (“rECM”),4 the majority of the ECM proteins detach with the cells during low-temperature cell release from pNIPAM-grafted surfaces.3,20–23 However, the identity and quantity of those proteins is not completely clear, as traditional surface characterization techniques such as X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (ToF-SIMS) are incapable of distinguishing between ECM proteins and pNIPAM.4,24
It is essential to know if any fragments of the polymer are removed with the cells, as small polymer fragments could have cytotoxic effects. This is especially important if these cells are to be used for the generation of tissue from cell sheets. Since previous work on pNIPAM did not yield surfaces that allow a clear distinction between pNIPAM and the ECM protein, we needed to generate pNIPAM surfaces that can be distinguished from the ECM.
For this work, the polymerization technique previously used by Mendez et al.25 was adapted. This technique has not been previously used for mammalian cell culture. Therefore, to obtain cellular attachment and detachment from the synthesized pNIPAM substrates, the existing protocol was adapted by varying parameters (e.g., initiator concentration, polymerization time). The surfaces were characterized with XPS and goniometry. The developed pNIPAM surfaces were further adapted by incorporation of 5-acrylamidofluorescein to generate fluorescent pNIPAM-coated surfaces. Both types of surfaces (fluorescent and nonfluorescent) sustained cellular attachment and produced cellular detachment of ∼90%, and are therefore suitable for the generation of cell sheets for engineered tissues and other purposes.
II. MATERIALS
N-isopropyl acrylamide (99%) was purchased from Acros Organics (Geel, Belgium). The HPLC-grade methanol, HPLC-grade dichloromethane, HPLC-grade acetone, hydrochloric acid (1 normal), and toluene were purchased from Honeywell Burdick & Jackson (Deer Park, TX). Concentrated sulfuric acid was obtained from EMD Chemicals (Gibbstown, NJ). 3-(trimethoxysilylpropyl)–2-bromo-2-methylpropionate was purchased from Gelest, Inc. (Morrisville, PA). The cooper (I) bromide, N,N,N′,N″,N″-pentamethyldiethylenetriamine, acryloyl chloride, and fluoresceinamine were purchased from Sigma Aldrich (St. Louis, MO). Round glass cover slips (15 mm) were purchased from Ted Pella, Inc. (Redding, CA). The silicon chips were obtained from Silitec (Salem, OR).
Dulbecco's modified eagle's medium (DMEM), minimum essential medium with alpha modification (αMEM), and Dulbecco's phosphate buffered saline without calcium or magnesium were purchased from HyCLone (Logan, UT). Bovine aortic endothelial cells (BAECs) were from Genlantis (San Diego, CA). Vero cells (CCL-81) were obtained from ATCC (Manassas, VA). Fetal bovine serum (FBS) and penicillin/streptomycin were from HyCLone (Logan, UT). Minimum essential medium non-essential amino acids solution (MEM NEAA) and 0.25% trypsin/EDTA were purchased from Gibco (Grand Island, NY).
III. METHODS
A. Surface preparation
Cell culture was performed on round glass cover slips, while surface analysis was performed on silicon chips. Silicon wafers were cut into 1 × 1 cm squares for XPS, and 0.8× 3 cm rectangles for goniometry. The Si chips were cleaned in an ultrasonic cleaner from VWR International (West Chester, PA) twice in each of the following solutions for 5 min: dichloromethane, acetone, and methanol. Glass cover slips were cleaned for 30 min with an acid wash, a 1:1 solution by volume of methanol and hydrochloric acid, rinsed with deionized water, and dried with nitrogen. Both types of surfaces were placed under nitrogen in a Petri dish sealed with Parafilm and stored in a desiccator for future experiments.
B. Surface-initiated atom transfer radical polymerization
Covalently bonded, reproducible pNIPAM surfaces (atrpNIPAM), were generated using surface-initiated atom transfer radical polymerization (ATRP). The protocol from Mendez et al. was used,26 with the exception that the concentration of the initiator, initiator attachment duration, as well as the polymerization time were varied to generate surfaces sustaining mammalian cell attachment and detachment. Round glass coverslips (for cell culture) and Si chips (for XPS and goniometry) were cleaned with sulfuric acid for 30 min. The hydroxylated surfaces were then exposed to the initiator, 3–(trimethoxysilylpropyl)–2-bromo-2-methylpropionate, dissolved in toluene, at varying concentrations. NIPAM monomer (10 g) was dissolved in water/methanol mixture (50 ml, 1:1 by weight). The metal catalyst, copper (I) bromide, 14 mg, and the ligand, N,N,N′,N″,N″-pentamethyldiethylenetriamine, 60 μl, were added to the NIPAM solution. The solution was then purged with nitrogen for at least 30 min. In a separate flask, glass cover slips and Si chips were purged with nitrogen. The solution with the reactants was then added to the flask with slides and the reaction was allowed to proceed for a desired amount of time. After the desired amount of time, the pNIPAM-coated surfaces were removed from the flask, rinsed with copious amount of water, dried with nitrogen, and placed under nitrogen in a Petri dish sealed with Parafilm.
C. Free radical polymerization of 5-acrylamidofluorescein
Free radical polymerization of 5-acrylamidofluorescein was adapted from Bruno et al.27 One gram of fluoresceinamine was suspended in 20 ml of acetone until fully dissolved. The solution was then purged with nitrogen. A second solution of 1.25 ml of acryloyl chloride, dissolved in 2 ml of acetone was added dropwise to the suspension over 10 min and stirred for 3 h at room temperature. The reaction occurred under nitrogen. A rotary evaporator was used to evaporate the remaining solvent in the flask containing 5-acrylamidofluorescein. The final product of 5-acrylamidofluorescein was confirmed using H1-NMR and stored under nitrogen and protected from light at ambient temperature.
D. Surface-initiated atom transfer polymerization with 5-acrylamidofluorescein
Fluorescent pNIPAM-coated surfaces (atrpNIPAM-5AF) were generated using surface initiated atom transfer polymerization. To generate fluorescent surfaces, 5-acrylamidofluorescein was added to the polymerization solution at three different concentrations (0.5, 0.1, and 0.05 mol. %). The polymerization solution was composed of NIPAM monomer (10 g), copper (I) bromide (14 mg), and N,N,N′,N″,N″-pentamethyldiethylenetriamine (60 μl), dissolved in water/methanol mixture (50 ml, 1:1 by weight). The polymerization was allowed to proceed for 15 min under nitrogen atmosphere. After 15 min, the surfaces were removed from the solution, rinsed with water, dried with nitrogen, and placed under nitrogen in a Petri dish sealed with Parafilm.
E. Goniometry
For goniometry measurements, pNIPAM was deposited onto silicon chips. Silicon chips were cut to the dimensions of 0.8 × 3 cm to fit into the sample holder of the goniometer used for the measurements. Contact angles were taken on these pNIPAM-coated Si surfaces. Uncoated Si-surfaces were used as controls. The measurements were performed using an Advanced Goniometer model 300-UPG from ramé-Hart Instrument Co. (Mountain Lakes, NJ) with an environmental chamber. The inverted (captive) bubble method was used for the measurements. The surface was placed facing down in a quartz cell filled with Millipore water (18 MΩ). Syringe with an inverted needle was used to place an air bubble on the surface. The angle between the surface and air bubble was measured using the DROPimage Standard program. Angles were obtained below the LCST, at room temperature (20 °C), and above the LCST, at body temperature (37 °C). The quartz cell was heated up to the body temperature using the Temp Controller model 100–500 connected to the environmental chamber. The results were compared to contact angles obtained on control surfaces.
F. X-ray photoelectron spectroscopy
XPS measurements were performed with a Kratos Axis Ultra DLD X-ray photoelectron spectrometer at the Center for Energy Emerging Technologies at UNM. A monochromatic Al Kα source operating at 225 W was used. High resolution spectra were acquired at 20 eV pass energy, survey spectra at 80 eV pass energy. Charge compensation was accomplished using low energy electrons. Standard operating conditions for good charge compensation were −3.1 V bias voltage, −1.0 V filament voltage, and filament current of 2.1 A. The data obtained are average of 3 different areas per sample. pNIPAM deposited onto silicon chips cut to the size of 1 × 1 cm were used for the analysis. Data analysis was carried out using Casa XPS. The binding energy scales of the high resolution spectra were calibrated by assigning the most intense C1s high resolution peak a binding energy of 285.0 eV. A linear function was used to model the background.
G. Cell culture
1. Bovine aortic endothelial cells
Bovine aortic endothelial cells (BAECs) were cultured according to previously established protocols.10 Dulbecco's modified eagle's medium was supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 1% minimum essential medium non-essential amino acids solution. Cells were incubated at 37 °C in a humid atmosphere with 5% CO2. When confluent, the cells were washed with Dulbecco's phosphate buffered saline without calcium or magnesium. Trypsin/EDTA (0.25%) was used to lift cells from cell culture flasks.
2. Vero epithelial cells
Monkey kidney epithelial cells (Vero, CCL-81) were obtained from ATCC (Manassas, VA). Epithelial cells were cultured in Dulbecco's modified eagle's medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Cells were incubated at 37 °C in a humid atmosphere with 5% CO2. When confluent, the cells were washed with 0.25% trypsin/EDTA and lifted from cell culture flasks using 0.25% trypsin/EDTA.
3. Cell detachment
Cell detachment was performed in cold media without added supplements as previously described.10 Cells were first cultured in regular cell culture media. To initiate detachment, the medium was replaced with cold nonsupplemented medium. The well plates with cells in the cold medium were placed on a shaker table. The detachment was allowed to proceed for the desired amount of time (up to 2 h) at room temperature. Images of cells on pNIPAM surfaces and well as cells on uncoated control surfaces were taken before the beginning of detachment, and every 15 min after the detachment was started, up to 60 min. Cells were counted and averaged for each well. On average, seven images were taken per well. The amount of cells detached was determined by subtracting the amount of cells remaining on the surface from the number of cells present on the surface before the detachment. The percentage of detached cells was determined by dividing the number of detached cells by the number of cells initially presents on the surface.
IV. RESULTS AND DISCUSSION
A. Surface-initiated atom transfer radical polymerization of NIPAM (atrpNIPAM) and surface optimization
There are a variety of techniques for generating pNIPAM-coated surfaces, including plasma polymerization, spin-coating, electro-spinning, and electron beam irradiation.28 These techniques vary in cost, potential applications, and ease of use. For many of them, control of deposited polymer thickness—and therefore their applicability for cellular attachment and detachment—is limited. For investigations of the mechanism of cell detachment from pNIPAM-coated surfaces and of the pNIPAM/cell interface performed in this work, a polymerization technique was needed that would not only produce surfaces that are reproducible, with predictable surface chemistry and consistent detachment, but also one that would allow incorporation of fluorescent compounds for generation of fluorescent surfaces. To generate such a surface, surface-initiated ATRP was chosen.
ATRP has the advantage over other techniques (such as plasma polymerization or spin coating) in that it allows control over the degree of polymerization, and it generates surfaces with fairly uniform chemistry and controllable surface coverage. The polymer thickness is controlled by polymerization time, with longer polymerization times resulting in a thicker polymer layer.25 There are various approaches to synthesis of pNIPAM surfaces using ATRP. See supplementary material for a summary of some of the most recent studies using ATRP of NIPAM for cellular attachment and detachment.29 Although various polymerization techniques and conditions were used in these studies,30–42 there are a few common conclusions that can be made. In general, there is a limit to the length and density of pNIPAM brushes before cells will not adhere to the surface. Conversely, if the brushes are too short or insufficiently dense, adherent cells will not detach. There appears to be optimal film thickness and density that allow for reversible cell adhesion. Furthermore, the optimal parameters are dependent on the technique and the reagents used for the ATRP of NIPAM.
For this study, a polymerization method previously described by Mendez et al.26 was adapted. Since surfaces generated using this method were previously used only with bacterial cells, the first challenge was to adapt the protocol to obtain surfaces with surface thickness and density optimal for mammalian cell attachment and detachment. Preliminary studies were performed to determine the optimal polymerization conditions. Table I lists all parameters that were varied to optimize the surfaces for cell attachment and detachment. To control the thickness of atrpNIPAM surfaces (the pNIPAM chain length), polymerization time was varied from 5 to 30 min. Initiator concentration, initiation time, as well as the size of the dish in which the initiation took place, were varied to obtain different density of the initiator on the surfaces, and therefore, different densities of pNIPAM tethered to the surface (data not shown). To evaluate the coated surfaces, endothelial and epithelial cells were seeded and cultured on them, and detachment experiments were performed. In previous work on cytotoxicity of pNIPAM-coated surfaces, we found that endothelial cells showed increased sensitivity to pNIPAM-coated surfaces than epithelial cells.19
Table I.
Parameters varied for optimization of atrpNIPAM surfaces.
| Parameter varied | Parameter values |
|---|---|
| Initiation time (h) | 6, 18 |
| Dish size for initiation | Small (8 cm in diameter), large (18 cm in diameter) |
| Initiator concentration | 50 μl/50 ml; 100 μl/50 ml |
| Polymerization time (min) | 5, 10, 15, 30 |
Initiation time did not seem to affect cellular attachment or detachment. Both initiation time points resulted in similar amount of cells attaching to the surfaces, spreading, and proliferating, as well as in similar percentage of cells detaching from the dishes (estimated 70%–80% cells detached for the two different conditions). Dish size proved to be important, with cells not attaching to surfaces initiated in the smaller dish (higher pNIPAM density surfaces). Cells attached to atrpNIPAM surfaces that resulted from surfaces initiated in the larger dish. There was no significant difference between the two initiator concentrations tested, with the larger concentration resulting in a slightly better attachment of cells. Finally, 30 min polymerization time resulted in surfaces that were too thick for attachment of cells. Polymerization times of 5, 10, and 15 min produced surfaces that sustained cell attachment and detachment (data not shown). Based on several experiments and observations of cell attachment and detachment, atrpNIPAM surfaces that resulted from overnight initiation in the large dish, with 100 μl of initiator/50 ml of toluene, and 15 min polymerization time were chosen for further experiments. These parameters resulted in the best attachment and detachment of cells.
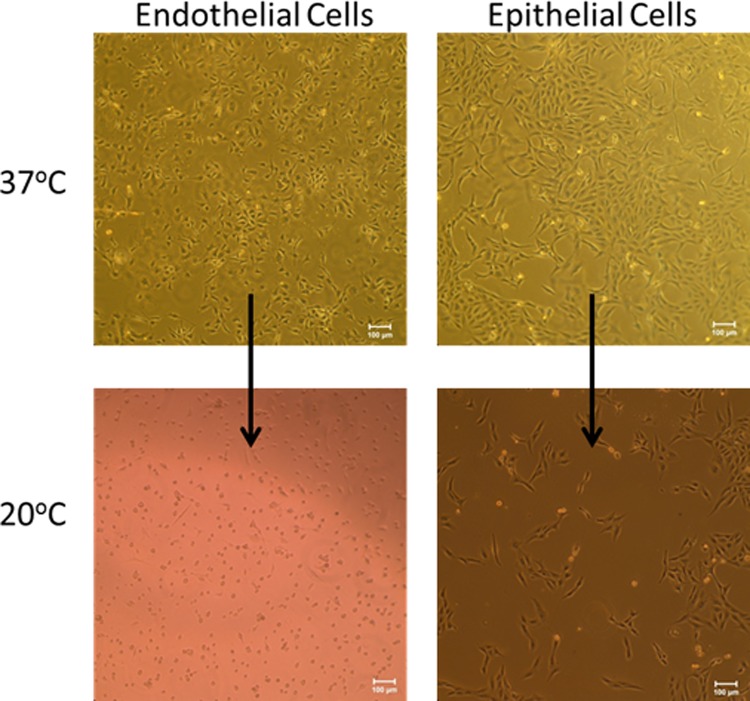
Figure 1 shows microscopy images of mammalian cells, endothelial cells on the left and epithelial cells on the right, growing on atrpNIPAM surfaces. The top row shows cells at 37 °C, the physiological temperature. The cells are spread and elongated, indicating that they are attached to the surface. After lowering the temperature below pNIPAM's LCST, the cells started to detach. The bottom row of Fig. 1 shows cells on atrpNIPAM surfaces after 2 h below the LCST. Endothelial cells have rounded morphology, and it appears that most cells have detached from the surface. Epithelial cells also attached to the surfaces, although they showed less cell detachment than endothelial cells. Since endothelial cells consistently showed ∼85%–90% detachment on the atrpNIPAM surfaces, this cell type was used for all remaining experiments.
Fig. 1.
Endothelial cells (left) and epithelial cells (right) cultured on atrpNIPAM surfaces at 37 °C (top row) and after detachment at 20 °C (bottom row). Scale bar is 100 μm.
B. Synthesis of fluorescent pNIPAM surfaces (atrpNIPAM-5AF)
There are several studies reporting the synthesis of fluorescent pNIPAM, employing a number of different fluorescent dyes.43–46 To our knowledge, none of these studies used the resulting fluorescent pNIPAM for cell adhesion. For this study, 5-acrylamidofluorescein was used. This compound has recently been used in our lab to label pNIPAM-based microgels using free radical polymerization reaction, resulting in successful generation of fluorescent microgels (data not shown). The resulting fluorescent pNIPAM surfaces (atrpNIPAM-5AF) were tested for cell attachment and detachment using endothelial cells.
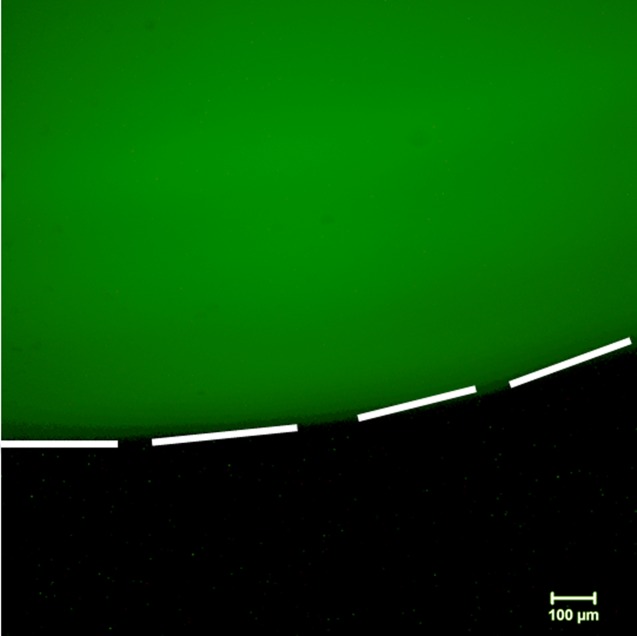
To synthesize fluorescent pNIPAM surfaces, the surface-initiated atom transfer polymerization technique described in Secs. III A–III G to generate pNIPAM films was modified to include 5-acrylamidofluorescein as one of the reagents. After optimizing the concentration of the fluorescent molecule to be used for the reaction (0.5, 0.1, and 0.05 mol. %), the final atrpNIPAM-5AF surfaces were synthesized with 0.05 mol. % of 5-acrylamidofluorescein. The formulation using the concentration of 0.05% of the fluorescent compound was chosen for the final polymerization recipe, as surfaces with this concentration still showed fluorescence and, at the same time, contained the smallest amount of the fluorescent compound. Figure 2 shows a glass cover slip that has been coated with fluorescent atrpNIPAM-5AF using this technique. The white dashed lines have been added to guide the eye to distinguish between the green fluorescence (from the 5-acrylamidofluorescein) on the glass slip against the Petri dish in which the surface was placed (which is not fluorescent, and therefore appears black).
Fig. 2.
Fluorescence microscopy image of an atrpNIPAM-5AF-coated glass cover slip resting in a Petri dish. The fluorescent surface appears in green; the Petri dish does not fluoresce, and appears in black. White dotted lines outline the edge of the coated cover slip. Scale bar is 100 μm.
C. Surface characterization: Goniometry and XPS
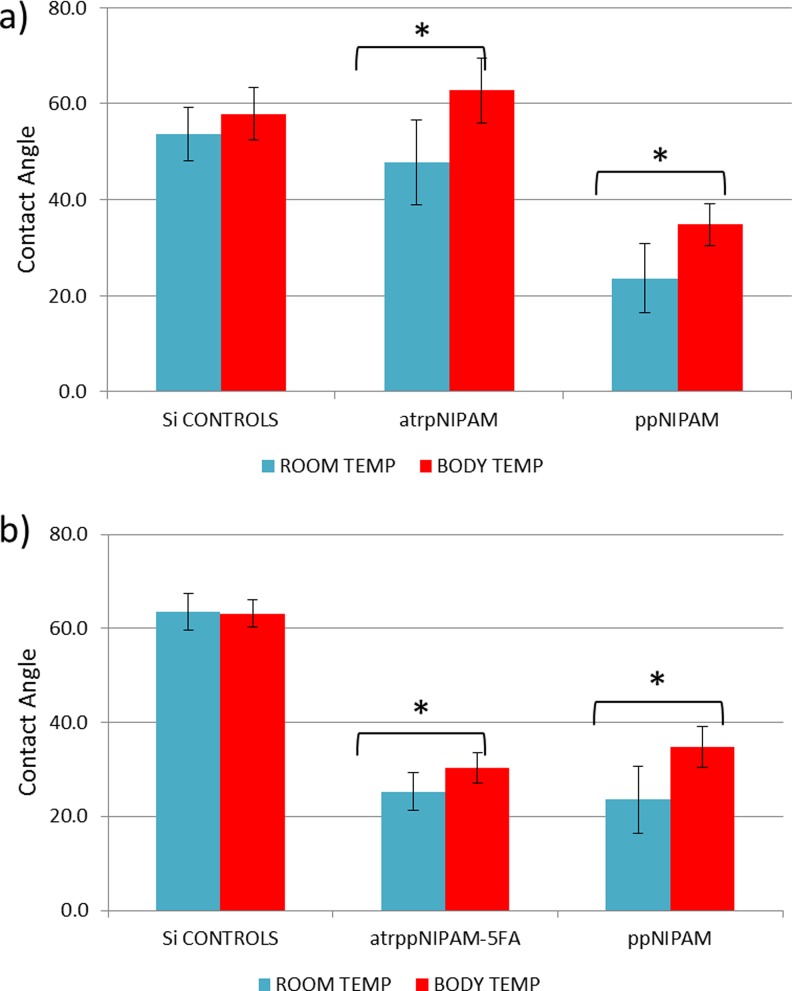
The atrpNIPAM surfaces were analyzed using goniometry and XPS data analysis. Figure 3(a) shows the results of inverted bubble contact angle measurements for controls (uncoated Si chips), atrpNIPAM surfaces, and plasma polymerized surfaces (ppNIPAM, surfaces that we developed in our previous work47). The measurements were taken at room temperature (20 °C) and at body temperature (37 °C). Control samples (uncoated Si chips) did not show any thermoresponse, with average values of ∼54 and 58° at room and body temperature, respectively. The atrpNIPAM surfaces, however, showed a large difference between average values at room temperature (48°), and at body temperature (62°). By comparison, ppNIPAM surfaces had average values of 24° at room temperature and 35° at body temperature. The difference between the contact angle values for atrpNIPAM and ppNIPAM results from different surface chemistry and topography. It has been demonstrated, that pNIPAM surfaces of different surface chemistry, surface thickness, and concentrations of pNIPAM will result in different contact angles (see supplementary material29 for a list of articles exemplifying how different experimental conditions results in different properties of atrpNIPAM surfaces). Overall, contact angle measurements proved that the atrpNIPAM surfaces synthesized in this work are thermoresponsive.
Fig. 3.
Inverted bubble contact angles of atrpNIPAM and ppNIPAM (plasma polymerized NIPAM) surfaces (a) and atrpNIPAM-5AF-coated surfaces and ppNIPAM surfaces (b) obtained at room and body temperature in ultrapure water.
The thermoresponse of atrpNIPAM-5AF surfaces was also tested using contact angle goniometry. Figure 3(b) shows the results of these measurements. Controls (uncoated Si chips) showed no thermoresponse. As expected, the atrpNIPAM surfaces had lower contact angles at room temperature (with an average value of 25°) than at body temperature (30°). While these values are similar to the ones for ppNIPAM surfaces, they are lower than the contact angles of nonfluorescent atrpNIPAM surfaces (∼48° at room temperature, and 62° at body temperature). This change in the contact angle is not unexpected, as a new compound was added to the films, altering their resulting chemistry. Most importantly, the fluorescently tagged pNIPAM surfaces retained their thermoresponse, indicating that the films will still be suitable for use to reversibly adhere cells.
To confirm deposition of pNIPAM onto the surfaces, XPS analysis was performed. The survey spectra (to determine elemental composition of the outer ∼100 Å of the sample), and high resolution C1s spectra (to determine molecular bonding environment), were obtained, and compared to the theoretical composition of pNIPAM-coated surfaces, as calculated by the stoichiometry of the monomer. Table II shows the results of the XPS analysis for the atrpNIPAM surfaces. Analysis of both the elemental composition (top) and the carbon bonding environment (bottom) shows close adherence to the theoretical composition. In addition, no silicon was detected from the underlying substrate, indicating that the films were pinhole-free. Therefore, XPS analysis indicates that pNIPAM was successfully deposited onto the surface, and the coverage was uniform.
Table II.
Elemental composition (survey spectra) and molecular bonding environment (high resolution C1s spectra) of atrpNIPAM surfaces from XPS data analysis. N = 3 with a standard deviation of ±1%.
| Relative atomic % | |||||||
|---|---|---|---|---|---|---|---|
| Surface type | Survey spectra | High resolution C1s spectra | |||||
| C | N | O | Si | C-H | C-OH/C-N | N-C=O | |
| Theoretical | 75 | 12.5 | 12.5 | 0 | 66.7 | 16.7 | 16.7 |
| atrpNIPAM | 77.9 | 9.7 | 12.4 | 0 | 66.8 | 18.6 | 14.7 |
D. Cell attachment and detachment from atrpNIPAM
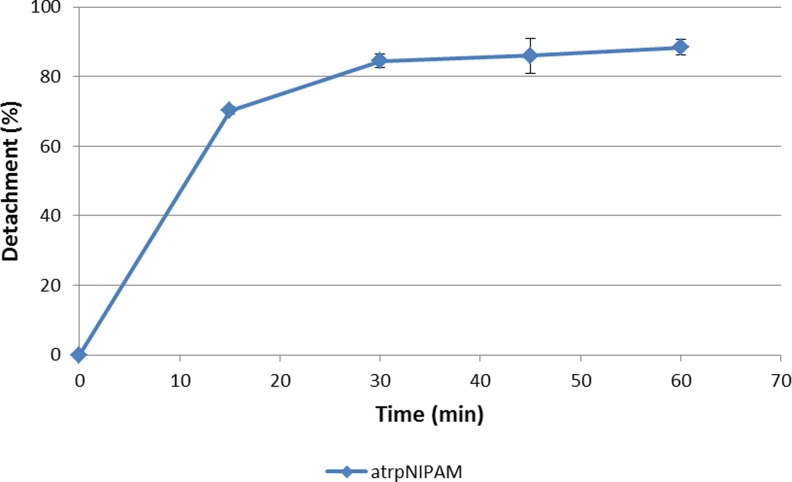
The optimized atrpNIPAM surfaces were used for cell culture with bovine aortic endothelial cells. As future experiments with these surfaces will require not only cell attachment but also consistent cell detachment, detachment experiments were performed to determine the efficiency of these surfaces at releasing cells when the temperature is lowered to below pNIPAM's LCST. Cell detachment experiments were performed in cold nonsupplemented medium at room temperature. It was found that these specific conditions result in the quickest detachment of endothelial cells from pNIPAM surfaces.10 Figure 4 shows a plot of percent detachment versus time. The surfaces produced cell detachment of ∼70% after a 15-min treatment at lower temperature. After 60 min, close to 90% of cells detached from the surface. This maximum percent detachment is similar to the maximum detachment obtained from commercially available pNIPAM surfaces (which is 90%).48 Therefore, these surfaces proved to be capable of producing high percent of cell detachment in a relatively short amount of time and of producing large cell sheets useful for cell sheet engineering.
Fig. 4.
Bovine aortic endothelial cell detachment from atrpNIPAM surfaces. Time points were 15, 30, 45, and 60 min.
E. Cell attachment and detachment from fluorescent pNIPAM surfaces
The atrpNIPAM-5AF surfaces were tested for cellular attachment and detachment using endothelial cells. The cells were seeded on the surfaces, and after they reached desired confluency, they were detached from the surfaces using cold serum-free media, with the detachment occurring at room temperature.
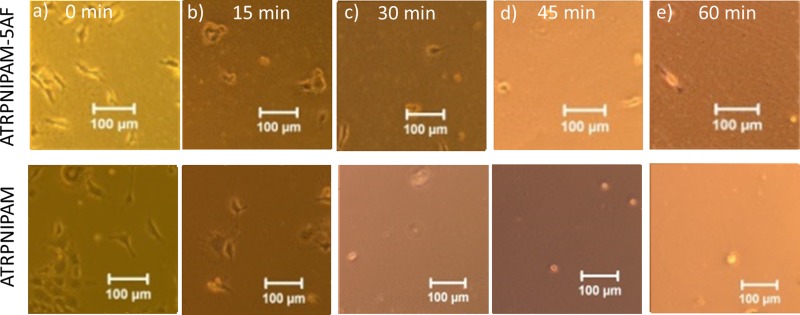
To ensure that the surfaces still behave the same as the nonfluorescent atrpNIPAM surfaces and that the addition of the fluorescent molecule did not affect the performance of pNIPAM, the detachment was observed over time, at time points of 15, 30, 45, and 60 min. Figure 5 shows images of cells growing on the atrpNIPAM-5AF surfaces prior to detachment, at 37 °C, as well as after 15 min (b), 30 min (c), 45 min (d), and 60 min (e) of detachment. At first, the cells appear spread and attached to the surface, as has previously been observed with this cell type [see Fig. 6(a)]. After the introduction of the media at low temperature was introduced, the cells became more rounded, and start detaching from the surface [see Fig. 6(b)]. Almost complete detachment was achieved after 60 min [see Fig. 6(e)].
Fig. 5.
Bright phase microscopy of endothelial cells cultured on atrpNIPAM-5AF surfaces (top row) and atrpNIPAM surfaces (bottom row) during detachment at room temperature after 0 min (a), after 15 min (b), after 30 min (c), after 45 min (d), and after 60 min (e). Scale bar is 100 μm.
Fig. 6.
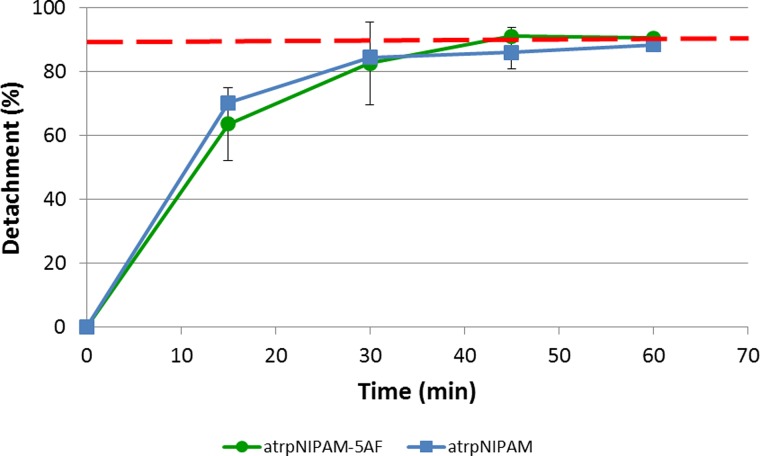
Comparison of the detachment of endothelial cells from atrpNIPAM-5AF surfaces (green circles) and atrpNIPAM surfaces (blue squares). Time points were 15, 30, 45, and 60 min. The red dashed line indicates 90% detachment.
Next, the number of detached cells was calculated by counting the cells that remained attached to the surface, and subtracting that number from the number of cells attached to the surface before the detachment was started. The percentage detachment was plotted against time. Figure 6 compares the detachment from atrpNIPAM-5AF to the detachment from atrpNIPAM (nonfluorescent surfaces). Figure 6 indicates that detachment of endothelial cells from the fluorescent atrpNIPAM films is almost identical to those cells cultured on nonfluorescent counterparts. For example, the initial rate of detachment of cells (indicated by the slope of the linear region of the graph) is 4.2%/min, which is very similar to the detachment at room temperature from atrpNIPAM (which was 4.7%/min). In addition, the detachment reaches its maximum after 45 min, at 90% of detached cells, which is similar to those detached from nonfluorescent atrpNIPAM. Together, these results indicate that the presence of the fluorescent tag did not alter the dynamics of how detachment occurs from pNIPAM.
V. CONCLUSIONS
For this investigation, a new technique that allows generation of fluorescently labeled pNIPAM-coated surfaces via atom transfer radical polymerization to study cell/surface integration was developed. This novel technique was not previously used for cell culture. The adapted polymerization technique allowed to synthesize fluorescent and nonfluorescent atrpNIPAM surfaces which were optimized for cell attachment and detachment using bovine aortic endothelial cells. Both types of surfaces were tested for cell attachment and detachment, to ensure that they have the same characteristics (i.e., thermoresponse and similar detachment profile). It was found that the inclusion of the fluorescent tag in the atrpNIPAM-5AF surfaces does not affect the thermoresponsive and reversibly cell adherent nature of the films, as similar cell attachment and detachment profiles of cells cultured on their nonfluorescent atrpNIPAM counterparts was observed. Both types of surfaces sustained cell attachment and proliferation and produced cell detachment that reached 90% after a 60-min treatment at a temperature below pNIPAM's LCST. These surfaces will be useful tools for further experiments investigating cellular detachment from pNIPAM and the pNIPAM/cell interface, as well as for yielding cell sheets for cell sheet engineering. Most importantly, these surfaces will make it possible to visualize via fluorescence microscopy, if any pNIPAM fragments remained attached to the detached cells and cell sheets.
ACKNOWLEDGMENTS
The authors would like to thank Matt Rush, Kent Coombs, and Lyndsay Stapleton from the University of New Mexico, and Phanindhar Shivapooja from Duke University for helpful discussions. The authors thank Kateryna Artyushkova at the Center for Energy Emerging Technologies at UNM for acquisition of XPS data. Financial support for this study was provided by the National Science Foundation Graduate Fellowship Program, NSF PREM award #DMR-0611616, and the UNM Center for Biomedical Engineering.
References
- 1. Gil E. S. and Hudson S. A., Prog. Polym. Sci. 29, 1173 (2004). 10.1016/j.progpolymsci.2004.08.003 [DOI] [Google Scholar]
- 2. Schild H. G., Prog. Polym. Sci. 17, 163 (1992). 10.1016/0079-6700(92)90023-R [DOI] [Google Scholar]
- 3. Canavan H. E., Cheng X., Graham D. J., Ratner B. D., and Castner D. G., J. Biomed. Mater. Res. A 75, 1 (2005). 10.1002/jbm.a.30297 [DOI] [PubMed] [Google Scholar]
- 4. Canavan H. E., Cheng X., Ratner B. D., and Castner D. G., Langmuir 21, 1949 (2005). 10.1021/la048546c [DOI] [PubMed] [Google Scholar]
- 5. Shimizu T., Yamato M., Kikuchi A., and Okano T., Biomaterials 24, 2309 (2003). 10.1016/S0142-9612(03)00110-8 [DOI] [PubMed] [Google Scholar]
- 6. Hirose M., Kwon O. H., Yamato M., Kikuchi A., and Okano T., Biomacromolecules 1, 377 (2000). 10.1021/bm0002961 [DOI] [PubMed] [Google Scholar]
- 7. Nishida K. et al. , Transplantation 77, 379 (2004). 10.1097/01.TP.0000110320.45678.30 [DOI] [PubMed] [Google Scholar]
- 8. Yang J., Yamato M., Shimizu T., Sekine H., Ohashi K., Kanzaki M., Ohki T., Nishida K., and Okano T., Biomaterials 28, 5033 (2007). 10.1016/j.biomaterials.2007.07.052 [DOI] [PubMed] [Google Scholar]
- 9. Cooperstein M. A. and Canavan H. E., Langmuir 26, 7695 (2010). 10.1021/la902587p [DOI] [PubMed] [Google Scholar]
- 10. Reed J. A., Lucero A. E., Cooperstein M. A., and Canavan H. E., J. Appl. Biomater. Biomech. 6, 81 (2008). [PMC free article] [PubMed] [Google Scholar]
- 11.MSDS No. 91139 [Online]; Acros Organics: Geel, Belgium; June, 20, 2009, see http://www.acros.com/DesktopModules/Acros_Search_Results/Acros_Search_Results.aspx?search_type=CatalogSearch&SearchString=N-isopropylacrylamide (accessed 20 January 2013).
- 12. Vihola H., Laukkanen A., Valtola L., Tenhu H., and Hirvonen J., Biomaterials 26, 3055 (2005). 10.1016/j.biomaterials.2004.09.008 [DOI] [PubMed] [Google Scholar]
- 13. Panayiotou M. and Freitag R., Polymer 46, 6777 (2005). 10.1016/j.polymer.2005.06.060 [DOI] [Google Scholar]
- 14. Wadajkar A. S., Koppolu B., Rahimi M., and Nguyen K. T., J. Nanopart. Res. 11, 1375 (2009). 10.1007/s11051-008-9526-5 [DOI] [Google Scholar]
- 15. Naha P. C., Bhattacharya K., Tenuta T., Dawson K. A., Lynch I., Gracia A., Lyng F. M., and Byrne H. J., Toxicol. Lett. 198, 134 (2010). 10.1016/j.toxlet.2010.06.011 [DOI] [PubMed] [Google Scholar]
- 16. Xu F. J., Zhu Y., Liu F. S., Nie J., Ma J., and Yang W. T., Bioconjugate Chem. 21, 456 (2010). 10.1021/bc900337p [DOI] [PubMed] [Google Scholar]
- 17. Mortisen D., Peroglio M., Alini M., and Eglin D., Biomacromolecules 11, 1261 (2010). 10.1021/bm100046n [DOI] [PubMed] [Google Scholar]
- 18. Li Y. Y., Zhang X. Z., Cheng H., Zhu J. L., Li U. N., Cheng S. X., and Zhuo R. X., Nanotechnology 18, 505101 (2007). 10.1088/0957-4484/18/50/505101 [DOI] [Google Scholar]
- 19. Cooperstein M. A. and Canavan H. E., Biointerphases 8, 19 (2013). 10.1186/1559-4106-8-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Canavan H. E., Graham D. J., Cheng X. H., Ratner B. D., and Castner D. G., Langmuir 23, 50 (2007). 10.1021/la062330o [DOI] [PubMed] [Google Scholar]
- 21. Kushida A., Yamato M., Konno C., Kikuchi A., Sakurai Y., and Okano T., J. Biomed. Mater. Res. 45, 355 (1999). [DOI] [PubMed] [Google Scholar]
- 22. Kushida A., Yamato M., Kikuchi A., and Okano T., J. Biomed. Mater. Res. 54, 37 (2001). [DOI] [PubMed] [Google Scholar]
- 23. Ide T., Nishida K., Yamato M., Sumide T., Utsumi M., Nozaki T., Kikuchi A., Okano T., and Tano Y., Biomaterials 27, 607 (2006). 10.1016/j.biomaterials.2005.06.005 [DOI] [PubMed] [Google Scholar]
- 24. Cole M. A., Voelcker N. H., Thissen H., and Griesser H. J., Biomaterials 30, 1827 (2009). 10.1016/j.biomaterials.2008.12.026 [DOI] [PubMed] [Google Scholar]
- 25. Andrzejewski B. P. Ph.D. thesis, University of New Mexico, 2009. [Google Scholar]
- 26. Mendez S., Curro J. G., McCoy J. D., and Lopez G. P., Macromolecules 38, 174 (2005). 10.1021/ma048156x [DOI] [Google Scholar]
- 27. Bruno A. E., Barnard S., Rouilly M., Waldner A., Berger J., and Ehrat M., Anal. Chem. 69, 507 (1997). 10.1021/ac960855n [DOI] [PubMed] [Google Scholar]
- 28. Silva R. M. P. Da, Mano J. F., and Reis R. L., Trends Biotechnol. 25, 577 (2007). 10.1016/j.tibtech.2007.08.014 [DOI] [PubMed] [Google Scholar]
- 29.See supplementary material at http://dx.doi.org/10.1116/1.4894530E-BJIOBN-10-301501 for a summary of several recent studies using ATRP of NIPAM for cellular attachment and detachment is found in Table I in the supplementary material; and for a list of articles exemplifying how different experimental conditions results in different properties of atrpNIPAM surfaces.
- 30. Chen B. Y., Xu F. J., Fang N., Neoh K. G., Kang E. T., Chen W. N., and Chan V., Acta Biomater. 4, 218 (2008). 10.1016/j.actbio.2007.09.002 [DOI] [PubMed] [Google Scholar]
- 31. Gunnewiek M. K., Luca A. Di, Sui X., van Blitterswijk C. A., Moroni L., and Vancso G. J., Isr. J. Chem. 52, 339 (2012). 10.1002/ijch.201100118 [DOI] [Google Scholar]
- 32. Ke Z., Dai B., Li L., Yan G., and Zhou D., J. Appl. Polym. Sci. 115, 976 (2010). 10.1002/app.30996 [DOI] [Google Scholar]
- 33. Kim D. J., Kong B., Jung Y. H., Kim K. S., Kim W. J., Lee K. B., Kang S. M., Jeon S., and Choi I. S., Bull. Korean Chem. Soc. 25, 1629 (2004). 10.5012/bkcs.2004.25.11.1629 [DOI] [Google Scholar]
- 34. Li L., Zhu Y., Li B., and Gao C., Langmuir 24, 13632 (2008). 10.1021/la802556e [DOI] [PubMed] [Google Scholar]
- 35. Li L., Wu J., and Gao C., Colloids Surf., B 85, 12 (2011). 10.1016/j.colsurfb.2010.09.028 [DOI] [PubMed] [Google Scholar]
- 36. Mizutani A., Kikuchi A., Yamato M., Kanazawa H., and Okano T., Biomaterials 29, 2073 (2008). 10.1016/j.biomaterials.2008.01.004 [DOI] [PubMed] [Google Scholar]
- 37. Nagase K., Kimura A., Shimizu T., Matsuura K., Yamato M., Takeda N., and Okano T., J. Mater. Chem. 22, 19514 (2012). 10.1039/c2jm31797d [DOI] [Google Scholar]
- 38. Tamura A., Nishi M., Kobayashi J., Nagase K., Yajima H., Yamato M., and Okano T., Biomacromolecules 13, 1765 (2012). 10.1021/bm300256e [DOI] [PubMed] [Google Scholar]
- 39. Sui X., Luca A. Di, Gunnewiek M. K., Kooij E. S., van Blitterswijk C. A., Moroni L., Hempenius M. A., and Vancso G. J., Aust. J. Chem. 64, 1259 (2011). 10.1071/CH11168 [DOI] [Google Scholar]
- 40. Xu F. J., Zheng Y. Q., Zhen W. J., and Yang W. T., Colloids Surf., B 85, 40 (2011). 10.1016/j.colsurfb.2010.09.027 [DOI] [PubMed] [Google Scholar]
- 41. Zhang C., Vernier P. T., Wu Y.-H., and Yang W., J. Biomed. Mater. Res., Part B 100, 217 (2012). 10.1002/jbm.b.31941 [DOI] [PubMed] [Google Scholar]
- 42. Nagase K., Watanabe M., Kikuchi A., Yamato M., and Okano T., Macromol. Bio. 11, 400 (2011). 10.1002/mabi.201000312 [DOI] [PubMed] [Google Scholar]
- 43. Ottaviani M. F., Winnik F. M., Bossmann S. H., and Turro N. J., Helv. Chim. Acta 84, 2476 (2001). [DOI] [Google Scholar]
- 44. Matsumura Y. and Iwai K., Polymer 46, 10027 (2005). 10.1016/j.polymer.2005.08.050 [DOI] [Google Scholar]
- 45. Matsumura Y. and Katoh A., J. Lumin. 128, 625 (2008). 10.1016/j.jlumin.2007.10.012 [DOI] [Google Scholar]
- 46. Laurenti M., Lopez-Cabarcos E., Garcia-Blanco F., Frick B., and Rubio-Retama J., Langmuir 25, 9579 (2009). 10.1021/la900864a [DOI] [PubMed] [Google Scholar]
- 47. Lucero A. E. M.S. thesis, University of New Mexico, 2009. [Google Scholar]
- 48.Information Brochure [Online]; Thermo Scientific, Waltham, MA. See https://static.fishersci.com/cmsassets/downloads/segment/Scientific/pdf/Cell_Culture/ProductSpecific_ReferenceMaterial/TS_NalgeneNunc/upcell_brochure.pdf (accessed 22 April 2014).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- See supplementary material at http://dx.doi.org/10.1116/1.4894530E-BJIOBN-10-301501 for a summary of several recent studies using ATRP of NIPAM for cellular attachment and detachment is found in Table I in the supplementary material; and for a list of articles exemplifying how different experimental conditions results in different properties of atrpNIPAM surfaces.