Abstract
Asian Americans and Pacific Islanders are twice as likely to be diagnosed with type 2 diabetes compared to Caucasians. The objective was to determine the effect of cognitive behavioral therapy on quality of life, general health perceptions, depressive symptoms, and glycemia in Asians and Pacific Islanders with type 2 diabetes. The design was a randomized controlled clinical trial comparing cognitive behavioral therapy to diabetes education and support for six weekly sessions. Participants were recruited from two endocrinology practices; 207 were enrolled. The cognitive behavioral therapy group was provided self-management tools which included biofeedback, breathing exercises, and stress relievers, while the diabetes education and support group included diabetes education and group discussions. Assessments of psychosocial and clinical outcomes were obtained before and after sessions and 12 months PostSession. Differences between the two groups were examined using linear mixed-effects models with linear contrasts. The cognitive behavioral therapy group had improved depressive symptom scores from PreSession to EndSession compared to the diabetes education and support group (P < .03), but the improvement did not extend to 12 months PostSession. Similar results were observed with misguided support scores in the Multidimensional Diabetes Questionnaire (P < .03) and susceptibility in health beliefs (P < .01), but no significant differences in HbA1c improvement were found between the two groups. Both interventions improved outcomes from baseline but were not sustained for 1 year.
Keywords: Cognitive behavioral therapy, self-management training, Asians, Pacific Islanders, type 2 diabetes
Introduction
Type 2 diabetes is a difficult disease with challenging behavioral requirements. In the United States, 8.3% of the population is afflicted with diabetes, with Asians and Pacific Islanders (API) having a greater prevalence of diabetes mellitus than the general population.1 Even after adjustment for body mass index, Asians are more likely to develop diabetes than non-Hispanic whites.2
Many barriers to effective diabetes self-management exist, particularly for API. For example, ethnic and cultural differences impact diabetes non-adherence for standards of care rates, which were found to be at 65% for Asian Americans and 87% for Pacific Islanders compared to 63% for Caucasians.3 Results of a focus group with Native Hawaiians and Pacific Islanders found that participants often felt anxious, angry, and depressed as a major barrier to proper diet and exercise.4 Some of the themes that emerged indicated a need for family involvement in diet, exercise, and educational programs. Another study on health beliefs reported that Asian patients view insulin as a poison rather than a lifesaver and think that insulin therapy means “the end of the road” and that they will die soon.5 Improved strategies, therefore, that focus not just on metabolic control but on modifying health beliefs, lifestyle behaviors, and psychological outcomes and processes such as decision making and moods, are needed for API with diabetes to enhance their self-management.6
Cognitive behavioral therapy (CBT) represents one strategy that may prove effective in modifying beliefs, attitudes, and behaviors to better manage diabetes among API.7–10 CBT includes the use of five strategies: (1) self-monitoring and goal setting; (2) stimulus control for the modification of behaviors and habits; (3) cognitive restructuring techniques that focus on challenging and modifying unrealistic or maladaptive thoughts or expectations; (4) stress management; and (5) social support. Studies of cognitive behavioral interventions in these groups, while few, have shown reductions in HbA1c levels, diastolic blood pressure (BP), and body mass index compared to regular education alone with a greater reduction in diastolic BP over 12 months.11 No large, long-term controlled studies of CBT in API groups,12 however, are available and consequently there is a need to examine CBT impact on API with diabetes.
The specific aim of the present study was to determine the effect of CBT in improving quality of life, general health perceptions, depressive symptoms, and glycemic control along with associated metabolic parameters. The comparison group included a Diabetes Education and Support (DES) intervention that focused on sharing experiences and reviewed diet, medications, and group suggested topics. The predictions for this study were that CBT improves psychosocial, behavioral, and clinical outcomes as compared to DES in API with diabetes.
Methods
Study Design
The study was a randomized controlled clinical trial with double blinding to condition. Patients and providers were not aware of the goals and outcomes of the study except for the interventions provided. Interventionists were unaware of what was being provided in the comparison group. The study was approved by the Committee on Human Studies (CHS #12473) and all participants signed informed consent prior to entry into this study (NCT01182701).
Participants
API patients with type 2 diabetes between the ages of 18–76 years were recruited through two practitioners in Hawai‘i. Individuals were eligible only if they had received individualized or group diabetes education and dietary counseling, performed self-monitoring of blood glucose, and maintained routine physician visits. Patients who had diabetes-related disabilities such as renal failure and blindness or were unable to ambulate and participate in an exercise program were excluded [New York Heart Assoc. Class 3 (symptomatic with daily activities) and 4 (symptomatic at rest)].13 Participants received sealed envelopes assigning them to either the CBT or DES group using random number sequencing generated by a psychologist. Baseline anthropometric and clinical data, medical knowledge test, and quality of life, psychosocial, and behavioral questionnaires (described below) were obtained prior to randomization and after consents were obtained. The CBT and DES group met for six successive weekly sessions that averaged 1–2 hours per session with a group size ranging between 2 and 6 individuals. Family members or a supportive friend were encouraged to attend and participate.
Cognitive Behavioral Therapy (CBT)
A six-session format was implemented to focus on behavioral practice that included modules on stress management, biofeedback assisted relaxation, mood management, cognitive restructuring, empowerment, values clarification, problem solving, and decision making.14–17 The components included contracting, behavioral rehearsal, reinforcements, and a stress prevention session on anticipating and coping with the problems of stress recurrence. Learned cognitive behavioral practices were reinforced at each follow-up session as these intervention programs have been reported to be an effective way of reducing self-care behavior deficit.17 After each session, participants were asked to practice the learned behavioral interventions on their own, and reviewed and discussed their experience during the following sessions. These sessions were facilitated by research assistants who were trained on the various aspects of CBT by the investigator (JI) with unannounced fidelity checks completed at various intervals. Participants consented to these unannounced visits. Similar checks were also conducted for the DES group based on a checklist for content coverage.
Diabetes Education and Support (DES)
Participants randomized to receive DES spent an equal number of meetings and amount of time in sessions as the CBT group, but primarily focused on sharing personal experiences and receiving a review of diabetes education.18 Sessions were facilitated by different research assistants who were trained by the investigator to provide education and manage group support. Participants were asked to share their own experiences with diabetes, their problems, and what needs they had. Diabetes education was provided by the assistants based on suggestions and needs of the group. Topics covered were manualized for consistency and included “taking care of your feet, dental care, sick day management, taking your diabetes on vacation, and insurance coverage.”
Health and Clinical Outcomes
Health outcomes were assessed at PreSession and EndSession, and 12 months after the end of the sessions. Anthropometric measurements of BP, height, weight, and calculated body mass index were obtained by standard methodology.19
Diabetes Quality of Life (DQOL) Survey
Psychosocial measures included the Diabetes Quality of Life (DQOL) measure, a 46-item multiple-choice assessment for adolescents and adults with insulin-dependent diabetes mellitus. This survey rates satisfaction with quality of life, impact of diabetes, diabetes worry, and social/vocational worry on a scale from 1 (Very Satisfied or No Impact/No Worry) to 5 (Very Dissatisfied or Very Impacted/Worried). There are four subscales in the DQOL measure focusing on satisfaction, impact, social worries, and diabetes worries. The range of scores for each subscale is from 0 to 100 with higher scores indicating better quality of life.20
Medical Outcome Study 36-Item Short Form Health Survey (SF-36)
The General Health subscale of the Medical Outcome Study 36-item Short-Form health survey (SF-36) which assesses self-appraised general health, was given and consists of five items (alpha = 0.78) rated on a 5-point scale, designed to measure functioning and well-being in people 14 years and older. The SF-36 scores have 8 subscales including physical function, health limitations, emotional limitations, fatigue, emotional well-being, social function, pain, and general health. There is a range from 0 to 100 for each subscale with higher scores defining a more favorable health state.21
Center for Epidemiologic Studies-Depression (CES-D) Scale
To measure depressive symptoms, the Center for Epidemiologic Studies-Depression (CES-D) scale, a 20-item, self-report scale was given. This scale measures current depressive symptomatology including depressed mood, feelings of guilt and worthlessness, helplessness and hopelessness, psychomotor retardation, loss of appetite, and sleep disturbance, and has been validated in the Native Hawaiian population. All items were summarized to form the CES-D scores, which have a range between 0 and 60. Higher scores on the CES-D indicate higher levels of distress. A CES-D score of ≥16 suggests a clinically significant level of psychological distress.22
Summary of Diabetes Self-Care Activities Questionnaire
Adherence was measured with various tools. The Summary of Diabetes Self-Care Activities questionnaire, a 12-instrument self-report measure of the frequency of completing different self-care activities over the preceding seven days, included subscales of general diet, specific diet, blood glucose, foot care, diet days, exercise, and medication taking. All subscales have scores ranging from 0 to 7, with higher values indicating better self-care activities.23
Multidimensional Diabetes Questionnaire (MDQ)
The Multidimensional Diabetes Questionnaire (MDQ, a measure of self-efficacy), designed to provide a comprehensive assessment of diabetes-related cognitive and social factors was used. There are 7 subscales in the MDQ measurement including interference, severity, social support, positive reinforcement, misguided support, self-efficacy, and outcome expectations. All scales have a range of 0 to 6 except self-efficacy and outcome expectations, which are ranged from 0 to 100. All scales have higher values indicating better results.24
Health Belief Scale
The Health Belief Scale is a 38-item Likert scale that assesses the severity or vulnerability and cost-benefit dimensions of the health belief model, and items are rated on a 4-point agree/disagree scale and summed to provide seven sub-scores including cues to action, health motivation, severity, susceptibility, psychology barriers, treatment benefits, and structural elements with higher indication more noncompliant.25
Clinical results such as the HbA1c and lipids were considered acceptable if the dates of measurement coincided with the dates of the initial session (PreSession), EndSession, and 12 months PostSession. However, a two-month allowance was made to accommodate various timing of tests ordered by the participant's physician(s). Most of the clinical measures were performed in local laboratories as requested by physicians with HbA1c levels determined by the Biorad Variance II Turbo Hemoglobin A1c Program, which is certified by the National Glycohemoglobin Standardization Program. Lipid profiles were measured on an Olympus analyzer according to National Cholesterol Education Program guidelines.
Statistical Analyses
Data analyses were performed using SAS software version 9.1 (SAS Institute Inc., Cary, NC). Descriptive statistics such as mean and standard deviations were used to describe the distributions of quantitative variables in our study. Two sample t-tests were used to compare the demographic and baseline quantitative variables between the CBT and DES groups and between subjects who completed the study and subjects who dropped out. Frequency distributions were used for nominal variables to show the frequencies of each category. Chi-square and Fisher's exact tests were used to examine the difference in nominal demographic variables between the CBT and DES groups, and between subjects who completed the study and subjects who dropped out to determine the missing patterns.26
Linear mixed-effects models were used to examine the effects of treatment and the interaction between treatment and significant demographic covariates on each of the outcomes from PreSession to 12 months PostSession using the Proc Mixed procedure in SAS 9.1. The residuals of the linear mixed-effects models were checked and no significant deviation from normal distribution assumption was found. The covariates were selected using the stepwise selection procedure with .05 as the cutoff for both entering and dropping from the model. The linear mixed-effects models account for correlations among repeated measurements within the same subject through the repeated statement in the Proc Mixed procedure. Compound symmetry variance-covariance structure, which was selected based on the Akaike's Information Criterion, was used to estimate the variance and covariance and account for the correlations within subjects over time. Linear contrasts for testing treatment effects at each follow-up time and for testing time trends within each intervention group were used to estimate the change of each outcome measurement over time. All significance tests were two-sided with significance level set at 5%.
Power Analysis
Estimated treatment effects used the following sample size formula: N = [2*(Zα/2 + Zβ)2 * σ2] / delta2. N represents the sample size per group; Zα/2 and Zβ represents the standard normal deviates for type I and type II errors, σ2 represents the squared standard deviation, and delta2 represents the squared difference between the treatment and control groups. Effect sizes were calculated on the assumptions that there would be a 5% type I error and 20% type II error, providing a power of 80%. Published effects from research on behavioral interventions estimated that the treatment group would have ≥15% greater adherence to self-management behaviors than the control group,27 and a 10–30% improvement for other dependent variables.28,29 Treatment effects (delta), calculated based upon published means and standard deviations as well as upon the 164 participants (82 per group) predicted to complete the trial,30 estimated a ≥ 7.9% change from baseline for the diabetes self-management record. For dietary fat and exercise, treatment effects were detected at 15–21% and 34–40%, respectively.
Results
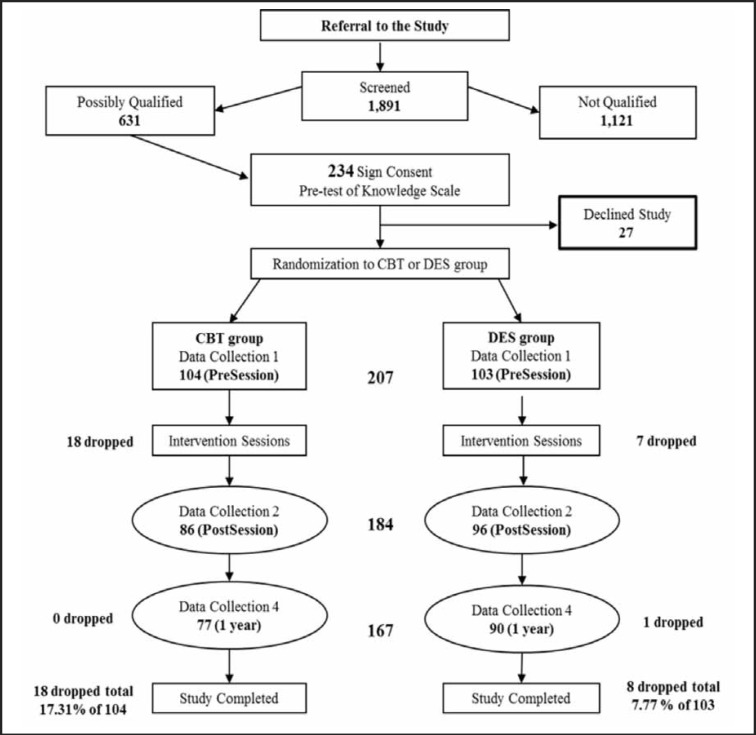
The two endocrinology practices identified 1,891 individuals with diabetes of whom 631 individuals met eligibility criteria for study participation. Two hundred and thirty-four patients signed informed consent but only 207 individuals were randomized (20 individuals withdrew, 3 could not be scheduled, and 4 relocated or passed away; Figure 1).
Figure 1.
Flow chart for subject recruitment and participation and data collection.
Demographic and PreSession Characteristics
The PreSession characteristics for the 207 participants are reported in Table 1. The mean age of participants was 57.3 years with higher proportion of female (55%) and married (70%) participants. A large proportion of the participants had education level of college or higher (45%) and had professional or managerial occupations (35%). Of the 207 participants, 150 participants were Asians and the remaining 57 participants were Hawaiians or other Pacific Islanders. No significant differences were observed by gender (P = .53), marital status (P = .69), occupational status (P = .69), and education (P = .34), or by clinical measures of glycemia, weight, BP, and lipid profiles between the CBT and DES groups (P > .05).
Table 1.
Baseline characteristics of participants in CBT and DES groups, completed interventions, and dropped out.
| Total (%) N = 207 | CBT (%) n = 104 | DES (%) n = 103 | P-value | Completed (%) n = 181 | Dropped out (%) n = 26 | P-value | |
| Mean age in years | 57.3±10.9 | 57.0±11.1 | 57.8±10.8 | .60 | 57.4 ±10.5 | 56.7±13.7 | .76 |
| Gender | |||||||
| Female | 113 (54.6%) | 59 (56.7%) | 54 (52.4%) | .53 | 86 (47.5) | 18 (69.2) | .06 |
| Male | 94 (45.4%) | 45 (43.7%) | 49 (47.6%) | 95 (52.5) | 8 (30.8) | ||
| Marital Status | |||||||
| Single | 26 (12.8%) | 15 (14.6%) | 11 (11.0%) | .69 | 22 (12.3%) | 4 (16.7%) | .70 |
| Married | 141 (69.5%) | 69 (67.0%) | 72 (72.0%) | 124 (67.0%) | 17 (72.0%) | ||
| Post Married | 36 (17.7%) | 19 (18.4%) | 17 (17.0%) | 33 (18.4%) | 3 (12.5%) | ||
| Occupational Status | |||||||
| Professional, Managerial | 68 (34.9%) | 32 (32.7%) | 36 (37.1%) | .69 | 61 (35.3%) | 7 (31.8%) | .93 |
| Technical, Clerical, Sales | 35 (17.9%) | 18 (18.4%) | 17 (17.5%) | 30 (17.3%) | 5 (22.7%) | ||
| Service | 21 (10.8%) | 13 (13.3%) | 8 (8.3%) | 19 (11.0%) | 2 (9.1%) | ||
| Other# | 71 (36.4%) | 35 (35.7%) | 36 (37.1%) | 63 (36.4%) | 8 (4.6%) | ||
| Education | |||||||
| Less than 12th grade | 19 (9.5%) | 11 (11.1%) | 8 (8.0%) | .34 | 17 (9.7%) | 2 (8.3%) | .92 |
| High School Graduate | 22 (11.0%) | 10 (10.1%) | 12 (12.0%) | 19 (10.8%) | 3 (12.5%) | ||
| Some College/Associate | 65 (32.7%) | 28 (28.3%) | 37 (37.0%) | 58 (33.0%) | 8 (33.3%) | ||
| Bachelor's Degree | 55 (27.6%) | 33 (33.3%) | 22 (22.0%) | 47 (26.7%) | 8 (33.3%) | ||
| Graduate School | 38 (19.1%) | 17 (17.2%) | 21 (21.0%) | 35 (19.9%) | 3 (12.5%) | ||
| Clinical Measures+ | |||||||
| Weight (lbs) | 199.7±65.2 | 208.1±71.5 | 190.2±56.4 | .15 | 199.6±64.5 | 202.2±80.4 | .92 |
| BMI (kg/M2) | 32.2±7.3 | 33.6±7.1 | 30.7±7.3 | .11 | 31.9±7.1 | 35.7±10.5 | .32 |
| Systolic BP (mmHg) | 138.7±20.0 | 137.9±18.6 | 139.6±21.6 | .65 | 139.1±20.5 | 132.7±7.6 | .42 |
| Diastolic BP (mmHg) | 84.3±13.0 | 85.4±1.5 | 83.1±2.0 | .35 | 84.3±13.2 | 84.3±9.3 | .99 |
| HbA1c level (%) | 8.0±1.6 | 8.1±0.2 | 7.8±0.2 | .18 | 7.9±1.6 | 8.6±2.0 | .08 |
| Total Cholesterol (mg/dl) | 167.5±41.8 | 165.9±4.7 | 168.9±4.6 | .64 | 167.8±41.6 | 164.4±45.0 | .77 |
| LDL Cholesterol (mg/dl) | 92.0±32.1 | 90.0±29.3 | 93.9±34.7 | .46 | 92.0±31.9 | 92.6±34.9 | .94 |
| Triglyceride (mg/dl) | 171.6±149.0 | 167.4±141.3 | 175.5±156.6 | .73 | 176.9±155.8 | 123.8±33.7 | <.01* |
| HDL Cholesterol (mg/dl) | 43.4±11.5 | 43.9±12.0 | 43.0±11.1 | .61 | 43.0±11.0 | 47.2±15.0 | .17 |
P-value < .05. # Other occupational status includes - agricultural, fishery, forestry, craft and repair; operators, miscellaneous.
HbA1c and Lipid profile results were available in fewer participants than the questionnaires and anthropometric measurements: CBT group N = 75; DES group N = 84; Completed group N = 167; and Dropped group N = 26.
Abbreviations: BMI- Body Mass Index, BP- Blood Pressure, HbA1c- hemoglobin A1c, LDL- Low Density Lipoprotein, HDL- High Density Lipoprotein.
Subjects who completed the study were compared with subjects who dropped out to determine missing patterns. No significant differences in gender (P = .06), marital status (P = .7), occupational status (P = .93), education (P = .92), and most clinical measures (P > .05) were found between subjects who completed the study and subjects who dropped out during the study period except for triglyceride levels (P < .01) (Table 1). The proportions of dropouts were not significantly different between the CBT group (17.31%) and the DES group (7.77%) (Figure 1; Fisher's exact test, P = .06).
Health Outcomes Changes with Intervention
A significant difference between the CBT and DES groups was found for depressive symptoms via the CES-D assessment from PreSession to EndSession (P = .03) (Table 2). The mean reduction in CES-D score for the CBT group was greater (8.03 − 10.48 = −2.45 in contrast to the DES group, which was almost unchanged (9.37 − 9.68 = −.31). At 12 months PostSession, no significant change in CES-D score from PreSession was found between the CBT and DES groups (P = .09). No significant differences in diabetes DQOL and SF-36 psychosocial measures were observed between the CBT group and the DES group during the study period (P > .05).
Table 2.
Expected means and standard errors in psychosocial and clinical measures comparing the CBT group with DES group from PreSession to EndSession and 12 months PostSession.
| Variables | CBT | DES | P-value from PreSession to End-Session (CBT vs DES) | P-value from PreSession to 12 months (CBT vs DES) | |||||
| PreSession | EndSession | Twelve months | PreSession | EndSession | Twelve months | ||||
| DQOL | Satisfaction | 61.10±1.66 | 65.20±1.76 | 62.77±1.85 | 62.46±1.65 | 66.63±1.68 | 65.20±1.72 | .98 | .65 |
| Impact | 76.49±1.26 | 77.56±1.32 | 77.23±1.38 | 72.96±1.25 | 73.72±1.27 | 76.24±1.30 | .84 | .11 | |
| Social Worries | 85.24±1.59 | 88.81±1.71 | 90.38±1.82 | 86.86±1.62 | 89.35±1.64 | 88.80±1.70 | .66 | .21 | |
| Diabetes worries | 75.62±2.06 | 77.80±2.19 | 78.05±2.30 | 72.68±2.04 | 75.29±2.09 | 78.24±2.14 | .89 | .29 | |
| SF-36 | Physical function | 71.23±2.63 | 73.78±2.78 | 73.73±2.84 | 71.83±2.65 | 72.32±2.70 | 72.89±2.73 | .49 | .63 |
| Health limitations | 67.30±3.95 | 74.11±4.24 | 67.56±4.39 | 67.27±3.92 | 69.41±4.03 | 92.97±4.08 | .37 | .31 | |
| Emotional limitations | 78.04±5.04 | 85.86±5.26 | 78.77±5.37 | 81.97±4.85 | 87.16±4.99 | 84.20±5.00 | .63 | .79 | |
| Fatigue | 58.15±1.88 | 65.01±2.02 | 61.94±2.09 | 57.71±1.87 | 61.31±1.92 | 62.16±1.94 | .18 | .80 | |
| Emotional well being | 79.11±1.44 | 83.52±1.55 | 81.22±1.61 | 78.68±1.44 | 80.26±1.47 | 79.82±1.49 | .15 | .63 | |
| Social function | 82.98±2.20 | 86.60±2.39 | 83.00±2.50 | 80.14±2.19 | 82.17±2.25 | 81.75±2.29 | .63 | .64 | |
| Pain | 73.82±2.31 | 75.02±2.46 | 69.07±2.54 | 67.86±2.29 | 73.83±2.34 | 68.37±2.38 | .10 | .08 | |
| General Health | 54.34±2.07 | 57.46±2.18 | 57.80±2.23 | 52.96±2.05 | 57.02±2.09 | 58.61±2.11 | .68 | .35 | |
| CESD | 10.48±0.83 | 8.03 ±0.88 | 9.33 ±0.90 | 9.68 ±0.83 | 9.37 ±0.85 | 10.21±0.86 | .03* | .09 | |
P-value <.05. Abbreviations: DQOL- Diabetes Quality of Life Measure, SF-36- General Health subscale of the Medical Outcome Study 36-Item Short-Form Health Survey, CES-D- Center for Epidemiologic Studies-Depression. The least squares means and standard errors of the means for each subscale of those measurements were estimated from the linear mixed effects models at each test point. P-values were obtained from linear contrasts of testing differences in means from PreSession to EndSession, and from PreSession to 12 months between CBT and DES group.
For the MDQ, a statistically significant difference between the CBT and DES groups from PreSession to EndSession (P = .03) was observed for the misguided support score (Table 3), with the CBT group showing an increasing trend in mean misguided support scores compared with a decreasing trend for the DES group. A statistically significant difference between the CBT and DES groups was detected for susceptibility in the health beliefs scale from PreSession to EndSession (P < .01). The CBT group had a decreasing trend while the DES group had an increasing trend in their susceptibility during the intervention period. At 12 months, no significant change in susceptibility from PreSession was found between the two groups (P = .18). No other significant differences in scores by the SDSCA, MDQ, or health belief assessment were observed between the CBT group and the DES group during the study period (P > .05).
Table 3.
Expected means and standard errors in measures of adherence comparing the CBT group with DES group from PreSession to EndSession and 12 months PostSession.
| Variables | CBT | DES | P-value from PreSession to EndSession (CBT vs DES) | P-value from to PreSession to 12 months (CBT vs DES) | |||||
| PreSession | EndSession | Twelve months | PreSession | EndSession | Twelve months | ||||
| SDSCA | General diet | 3.61±0.12 | 4.15±0.13 | 3.83±0.14 | 3.79±0.12 | 3.98±0.13 | 4.00±0.13 | .07 | .96 |
| Specific diet | 3.52±0.12 | 3.58±0.13 | 3.61±0.14 | 3.47±0.12 | 3.42±0.12 | 3.64±0.13 | .57 | .67 | |
| Blood glucose | 4.24±0.23 | 4.69±0.25 | 4.65±0.26 | 4.68±0.24 | 5.21±0.24 | 5.05±0.25 | .80 | .89 | |
| Foot care | 3.57±0.15 | 4.23±0.16 | 4.34±0.17 | 3.60±0.15 | 4.46±0.16 | 4.51±0.16 | .30 | .49 | |
| Diet days | 3.52±0.21 | 4.14±0.23 | 3.60±0.24 | 3.78±0.21 | 4.13±0.22 | 3.90±0.22 | .43 | .91 | |
| Medications | 4.93±0.20 | 4.81±0.22 | 5.57±0.23 | 4.81±0.21 | 4.85±0.21 | 5.39±0.22 | .61 | 0.88 | |
| MDQ | Interference | 3.06±0.11 | 3.08±0.11 | 3.01±0.12 | 3.08±0.11 | 3.08±0.11 | 3.00±0.11 | .82 | .81 |
| Self-Efficacy# | 58.47±2.30 | 67.54±2.49 | 62.60±2.61 | 65.26±2.33 | 70.09±2.39 | 66.06±2.44 | .23 | .36 | |
| Severity | 1.22 ±0.15 | 1.14 ±0.16 | 1.16 ±0.17 | 1.29 ±0.15 | 1.32 ±0.16 | 1.16 ±0.16 | .57 | .73 | |
| Social support | 2.22 ±0.23 | 2.31 ±0.24 | 2.19 ±0.24 | 2.28 ±0.23 | 2.31 ±0.24 | 2.20 ±0.24 | .75 | .81 | |
| Positive reinforcement | 2.46 ±0.18 | 2.21 ±0.19 | 2.41 ±0.20 | 2.50 ±0.18 | 2.68 ±0.19 | 2.70 ±0.19 | .06 | .29 | |
| Misguided support | 2.07 ±0.17 | 1.79 ±0.18 | 1.92 ±0.19 | 2.09±0.18 | 2.27 ±0.18 | 2.35 ±0.18 | .03* | .07 | |
| Outcome expectancies | 90.00±1.36 | 94.69±1.49 | 92.72±1.58 | 93.70±1.38 | 95.71±1.43 | 93.17±1.46 | .25 | .18 | |
| Health Beliefs | Cues to action | 12.12±0.29 | 12.50±0.31 | 12.08±0.32 | 12.26±0.29 | 12.48±0.30 | 12.60±0.30 | .70 | .37 |
| Health motivation | 10.22±0.23 | 10.51±0.24 | 10.59±0.25 | 10.70±0.23 | 11.09±0.23 | 11.06±0.24 | .77 | .97 | |
| Severity | 12.96±0.40 | 13.38±0.43 | 13.36±0.45 | 13.24±0.40 | 13.16±0.41 | 13.56±0.42 | .40 | .89 | |
| Susceptibility | 19.51±1.01 | 18.23±1.03 | 19.21±1.04 | 18.67±1.02 | 19.38±1.03 | 19.28±1.03 | <.01* | .18 | |
| Psychology barriers | 8.74 ±0.23 | 8.61 ±0.25 | 8.50 ±0.26 | 9.03 ±0.23 | 8.83 ±0.24 | 8.37 ±0.24 | .85 | .24 | |
| Treatment benefits | 26.51±0.32 | 27.05±0.35 | 26.66±0.37 | 26.70±0.33 | 27.15±0.34 | 27.13±0.34 | .86 | .55 | |
| Structural elements | 12.64±0.25 | 13.35±0.27 | 12.93±0.28 | 12.95±0.25 | 13.46±0.26 | 13.51±0.27 | .58 | .45 | |
P-value <.05. Abbreviations: SDSCA- Summary of Diabetes Self-Care Activities, MDQ- Multidimensional Diabetes Questionnaire. The least squares means and standard errors of the means for each subscale of those measurements were estimated from the linear mixed effects models at each test point. P-values were obtained from linear contrasts of testing differences in means from PreSession to EndSession, and from PreSession to 12 months between CBT and DES group. # Significant difference at PreSession in Self-efficacy between CBT and DES groups; P = .05.
Clinical Outcomes
There were no reportable adverse events. There were no significant differences in health outcomes between the CBT and DES groups at baseline for all measures except significantly lower self-efficacy scores were found in the CBT group compared to the DES group (MDQ, self-efficacy) (P = .05, Table 3). Results of glycemia, lipid profile, and weight were minimally changed with both interventions from PreSession to EndSession and 12 months PostSession (Table 4). Accordingly, there were no significant differences observed between the CBT and DES groups for weight, body mass index, and BP changes (P > .05). There was a small improvement of less than 0.3% in A1c levels at EndSession in both CBT and DES groups, but there was no change (less than 0.1%) from PreSession to 12 months (P > .05). Changes in lipid profile from PreSession were similar in the two groups, but not significant between groups post-intervention.
Table 4.
Comparison of clinical outcomes among participants in the CBT and DES groups from PreSession to EndSession and 12 months.
| CBT (N) | DES (N) | P-value from PreSession to EndSession (CBT vs DES) | P-value from to PreSession to 12 months (CBT vs DES) | |||||
| Variables | PreSession | EndSession | Twelve months | PreSession | EndSession | Twelve months | ||
| Weight (lbs) | 185.6 (91) | 190.1 (74) | 191.8 (75) | 188.8 (91) | 190.1 (83) | 191.2 (81) | .36 | .17 |
| BMI (kg/M2) | 31.3 (87) | 31.7 (73) | 32.0 (72) | 31.4 (90) | 31.2 (81) | 31.7 (81) | .59 | .13 |
| Systolic BP (mmHg) | 136.0 (91) | 141.3 (74) | 135.4 (75) | 136.5 (92) | 135.2 (84) | 136.2 (81) | .07 | .82 |
| Diastolic BP (mmHg) | 84.1 (91) | 84.2 (74) | 81.3 (75) | 80.1 (92) | 80.6 (84) | 81.4 (81) | .66 | .08 |
| A1c level (%) | 7.93 (75) | 7.64 (58) | 7.84 (71) | 7.79 (84) | 7.61 (61) | 7.79 (57) | .91 | .66 |
| Total Cholesterol (mg/dl) | 165.5 (70) | 160.0 (53) | 174.1 (63) | 167.6 (78) | 165.6 (55) | 172.8 (55) | .38 | .91 |
| LDL Cholesterol (mg/dl) | 89.3 (68) | 80.6 (51) | 86.9 (60) | 93.3 (74) | 90.1 (51) | 87.8 (51) | .97 | .60 |
| Triglyceride (mg/dl) | 162.7 (70) | 146.3 (52) | 163.7 (62) | 175.2 (78) | 166.0 (54) | 177.2 (55) | .50 | .65 |
| HDL Cholesterol (mg/dl) | 44.4 (70) | 42.4 (53) | 43.5 (62) | 42.5 (78) | 42.1 (53) | 45.8 (55) | .42 | .24 |
Discussion
This study was initiated to compare CBT to DES with the hypothesis that CBT would decrease depressive symptoms, increase self-efficacy, and improve quality of life and general health perceptions. The missing pattern analysis did not show any significant differences between subjects who completed the study and subjects who dropped out during the study period except for triglyceride levels. The proportion of drop outs was not significantly different between the CBT and DES groups. Thus, the missing at random assumption of the linear mixed-effects models is satisfied and the estimated coefficients from the Proc Mixed procedure in SAS 9.1 are unbiased and valid.
Implications
Both CBT and DES improved (at least in the short-term) PostSession quality of life and adherence measures and HbA1c levels from baseline. There were also improvements in CES-D scores at the end of the sessions in the CBT group as compared to the DES group, but this difference did not persist to 12 months PostSession. Another study found that CES-D scores were lower among specific Asian groups such as mixed Asians as compared to Japanese,31 and differences in depressive symptoms measures were noted between API.22,32 Differences in CES-D scores among API at baseline could have impacted the effect of CBT on depressive symptoms score changes in this study. However, a separate analysis of the intervention effect could not be performed between API with diabetes due to small sample size. Changes in misguided support scores and susceptibility in the Health Beliefs Scale, components of adherence measures also improved with CBT as compared to DES at EndSession, but again failed to be maintained long-term at 12 months. The CBT interventions in this study attempted to focus on patient-centered models and included social support, behavioral skills training, attitudinal change, and cognitive interventions for change which could have impacted depressive symptoms scores and measures of adherence. However, other multilevel factors of CBT such as social and environmental resources were not addressed33 and should be included in future studies. In general, all changes measured showed initial improvement at EndSession with recidivism occurring further away from the intervention.
Collectively, the results of this study demonstrate that among API participants with type 2 diabetes, CBT reduces depressive symptoms scores and improves multiple components of adherence measures, but the improvements are short-lived. Unfortunately, the changes were not associated with long-term improvements in quality of life, depressive symptoms, and adherence scores beyond comparator intervention of DES. Moreover, changes in clinical parameters of glycemia and weight were similar between the two groups, despite perceived improvements in psychosocial and behavioral aspects with CBT at EndSession.
Limitations and Implications for Future Studies and Practice
A significant limitation was the decrease in contact for both groups after the initial intervention. While all participants were seen in group sessions at the 12-month time period, no other interactions took place. “Booster” sessions that assist with long-term benefits and better outcomes have been recommended by others, and perhaps may have provided more positive results at 12 months in this study.34
Other limitations and a possible explanation for the lack of difference between interventions may be due to the intervention properties of the DES group. They were given time for group support and discussions as well as review of previous diabetes self-management classes not specifically related to self-management but more towards their care. Studies have found that social support has a positive impact on changing motivation to change health outcomes such as quality of life.35 Another explanation was the high satisfaction measures and a low dropout rate for both intervention groups indicating a selection bias with motivated participants in both groups.
One study found Asians had the least difficulty with physical activity and blood sugar monitoring, worried least about their future, and had good control of their disease compared to the other groups.36 These behavioral and psychological factors could play an important role in influencing treatment adherences and was not taken into account in this study. However, this could be addressed in the future with a larger sample of the different ethnic groups.
Finally, this program of six weeks may not have met the threshold necessary for more dramatic change to occur. Still, the results suggest CBT and mediators such as depressive symptoms and self-efficacy do influence health outcomes.37 Another paper in review reports on the effects depressive symptoms have on responses to interventions as well as the association with glycemic control (VU, JI, JD, & RFA, unpublished data, 2015). This has implications for clinical practice as addressing depressive symptoms may have a positive effect on management of their disease. For the investigation of CBT on diabetes self-management for long-term effects, future studies could examine disaggregated ethnic groups to address the differences between API, which will require a larger cohort of participants. Additionally, future studies could include a true control group, assess motivation to change, tailor a longer intervention period to specific ethnic groups, and include environmental influences on attempts to self-manage.
In conclusion, API patients with type 2 diabetes who were recruited from endocrinologist's practices and have had diabetes for many years, initially thought that they would receive very little benefit from participating in this study. Yet, improvements in psychosocial and clinical outcomes albeit small and short-term, suggest that motivated individuals produce outcomes (self-selected, a bias) regardless of the intervention. Clinicians could take heart in the results of this study and support their patients' participation in diabetes education and self-management training programs offered in the community. Understanding the difficulty in maintaining improvement for a year as shown in our study raises the need to provide ongoing support to improve overall psychosocial, adherence, and clinical outcomes in their patients.
Acknowledgements
This project was funded by NINR 5R01NR007883 to JI. RA discloses that he has received research funding from Eli Lilly and Company, AbbVie, and Merck. RA also discloses that he is a consultant and on the speaker's bureau for AstraZeneca. No other authors reported any financial disclosures.
Conflict of Interest
None of the authors identify a conflict of interest.
References
- 1.King GL, McNeely MJ, Thorpe LE, et al. Understanding and addressing unique needs of diabetes in Asian Americans, native Hawaiians, and Pacific Islanders. Diabetes Care. 2012;35(5):1181–1188. doi: 10.2337/dc12-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oza-Frank R, Ali MK, Vaccarino V, Narayan KM. Asian Americans: diabetes prevalence across U.S. and World Health Organization weight classifications. Diabetes Care. 2009;32(9):1644–1646. doi: 10.2337/dc09-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heisler M, Faul JD, Hayward RA, Langa KM, Blaum C, Weir D. Mechanisms for racial and ethnic disparities in glycemic control in middle-aged and older Americans in the health and retirement study. Arch Intern Med. 2007;167(17):1853–1860. doi: 10.1001/archinte.167.17.1853. [DOI] [PubMed] [Google Scholar]
- 4.Beckham S, Bradley S, Washburn A, Taumua T. Diabetes management: utilizing community health workers in a Hawaiian/Samoan population. J Health Care Poor Underserved. 2008;19(2):416–427. doi: 10.1353/hpu.0.0012. [DOI] [PubMed] [Google Scholar]
- 5.Burden M. Approaches to managing diabetes in Asian people. Community Nurse. 1998;4(4):31–34. [PubMed] [Google Scholar]
- 6.Nam S, Chesla C, Stotts NA, Kroon L, Janson SL. Barriers to diabetes management: patient and provider factors. Diabetes Res Clin Pract. 2011;93(1):1–9. doi: 10.1016/j.diabres.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet. 2004;363(9421):1589–1597. doi: 10.1016/S0140-6736(04)16202-8. [DOI] [PubMed] [Google Scholar]
- 8.Safren SA, Gonzalez JS, Wexler DJ, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in patients with uncontrolled type 2 diabetes. Diabetes Care. 2014;37(3):625–633. doi: 10.2337/dc13-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snoek FJ, van der Ven NC, Lubach CH, et al. Effects of cognitive behavioural group training (CBGT) in adult patients with poorly controlled insulin-dependent (type 1) diabetes: a pilot study. Patient Educ Couns. 2001;45(2):143–148. doi: 10.1016/s0738-3991(01)00113-6. [DOI] [PubMed] [Google Scholar]
- 10.Welschen LM, van Oppen P, Dekker JM, Bouter LM, Stalman WA, Nijpels G. The effectiveness of adding cognitive behavioural therapy aimed at changing lifestyle to managed diabetes care for patients with type 2 diabetes: design of a randomised controlled trial. BMC Public Health. 2007;7:74. doi: 10.1186/1471-2458-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridgeway NA, Harvill DR, Harvill LM, Falin TM, Forester GM, Gose OD. Improved control of type 2 diabetes mellitus: a practical education/behavior modification program in a primary care clinic. South Med J. 1999;92(7):667–672. doi: 10.1097/00007611-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Yomogida J, Inouye J, Li D, Davis J. The effect of a cognitive-behavioral intervention on diet and exercise among Asian Americans and Pacific Islanders with type 2 diabetes. Asian Pac Island Nurs J. 2015 doi: 10.1177/2373665815581368. [DOI] [Google Scholar]
- 13.The Criteria Committee of the New York Heart Association, author. Functional capacity and objective assessment. In: Dolgin M, editor. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. 9th ed. Boston, MA: Little, Brown and Company; 1994. pp. 253–255. [Google Scholar]
- 14.Aikens JE, Kiolbasa TA, Sobel R. Psychological predictors of glycemic change with relaxation training in non-insulin-dependent diabetes mellitus. Psychother Psychosom. 1997;66(6):302–306. doi: 10.1159/000289152. [DOI] [PubMed] [Google Scholar]
- 15.Bailey BK, McGrady AV, Good M. Management of a patient with insulin-dependent diabetes mellitus learning biofeedback-assisted relaxation. Diabetes Educ. 1990;16(3):201–204. doi: 10.1177/014572179001600310. [DOI] [PubMed] [Google Scholar]
- 16.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 17.Peyrot M, McMurry JF, Jr, Kruger DF. A biopsychosocial model of glycemic control in diabetes: stress, coping and regimen adherence. J Health Soc Behav. 1999;40(2):141–158. [PubMed] [Google Scholar]
- 18.American Association of Diabetes Educators, author. AADE position statement: AADE7 TM self-care behaviors. Diabetes Educat. 2008;34:445–448. [Google Scholar]
- 19.Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 20.DCCT Research Group, author. Reliability and validity of a diabetes quality-of-life measure for the diabetes control and complications trial (DCCT). The DCCT Research Group. Diabetes Care. 1988;11(9):725–732. doi: 10.2337/diacare.11.9.725. [DOI] [PubMed] [Google Scholar]
- 21.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 22.Kaholokula JK, Haynes SN, Grandinetti A, Chang HK. Ethnic differences in the relationship between depressive symptoms and health-related quality of life in people with type 2 diabetes. Ethn Health. 2006;11(1):59–80. doi: 10.1080/13557850500391287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toobert DJ, Glasgow RE. Assessing diabetes self-management: The summary of diabetes self-care activities questionnaire. In: Bradley C, editor. Handbook of psychology and diabetes: A guide to psychological measurement in diabetes research. Chur, Switzerland: Harwood Academic; 1994. pp. 351–375. [Google Scholar]
- 24.Talbot F, Nouwen A, Gingras J, Gosselin M, Audet J. The assessment of diabetes-related cognitive and social factors: the Multidimensional Diabetes Questionnaire. J Behav Med. 1997;20(3):291–312. doi: 10.1023/a:1025508928696. [DOI] [PubMed] [Google Scholar]
- 25.Harris R, Linn MW, Skyler JS, Sandifer R. Development of the diabetes health belief scale. Diabetes Educ. 1987;13(3):292–297. doi: 10.1177/014572178701300310. [DOI] [PubMed] [Google Scholar]
- 26.Stokes ME, Davis CS, Koch GG. Categorical data analysis using the SAS system. Cary, NC: SAS Institute Inc.; 1995. [Google Scholar]
- 27.Mendez FJ, Belendez M. Effects of a behavioral intervention on treatment adherence and stress management in adolescents with IDDM. Diabetes Care. 1997;20(9):1370–1375. doi: 10.2337/diacare.20.9.1370. [DOI] [PubMed] [Google Scholar]
- 28.Grey M, Boland EA, Davidson M, Yu C, Sullivan-Bolyai S, Tamborlane WV. Short-term effects of coping skills training as adjunct to intensive therapy in adolescents. Diabetes Care. 1998;21(6):902–908. doi: 10.2337/diacare.21.6.902. [DOI] [PubMed] [Google Scholar]
- 29.Lutgendorf SK, Antoni MH, Ironson G, et al. Cognitive-behavioral stress management decreases dysphoric mood and herpes simplex virus-type 2 antibody titers in symptomatic HIV-seropositive gay men. J Consult Clin Psychol. 1997;65(1):31–43. doi: 10.1037//0022-006x.65.1.31. [DOI] [PubMed] [Google Scholar]
- 30.Bielamowicz MK, Miller WC, Elkins E, Ladewig HW. Monitoring behavioral changes in diabetes care with the diabetes self-management record. Diabetes Educ. 1995;21(5):426–431. doi: 10.1177/014572179502100506. [DOI] [PubMed] [Google Scholar]
- 31.Inouye J, Li D, Davis J, Arakai R. Ethnic and gender differences in psychosocial factors in Native Hawaiian, other Pacific Islanders and Asian Americans with type 2 diabetes. J Health Dispar Res Prac. 2012;5(3):1–11. [Google Scholar]
- 32.Aczon-Armstrong M, Inouye J, Reyes-Salvail F. Depression and chronic illness: Asian/Pacific Islander adults in Hawaii. Issues Ment Health Nurs. 2013;34(3):169–179. doi: 10.3109/01612840.2012.738356. [DOI] [PubMed] [Google Scholar]
- 33.Whittemore R. Behavioral interventions for diabetes self-management. Nurs Clin North Am. 2006;41(4):641–654. viii. doi: 10.1016/j.cnur.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 34.Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care. 2001;24(3):561–587. doi: 10.2337/diacare.24.3.561. [DOI] [PubMed] [Google Scholar]
- 35.Chaveepojnkamjorn W, Pichainarong N, Schelp FP, Mahaweerawat U. A randomized controlled trial to improve the quality of life of type 2 diabetic patients using a self-help group program. Southeast Asian J Trop Med Public Health. 2009;40(1):169–176. [PubMed] [Google Scholar]
- 36.Misra R, Lager J. Ethnic and gender differences in psychosocial factors, glycemic control, and quality of life among adult type 2 diabetic patients. J Diabetes Complications. 2009;23(1):54–64. doi: 10.1016/j.jdiacomp.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Sarkar U, Fisher L, Schillinger D. Is self-efficacy associated with diabetes self-management across race/ethnicity and health literacy? Diabetes Care. 2006;29(4):823–829. doi: 10.2337/diacare.29.04.06.dc05-1615. [DOI] [PubMed] [Google Scholar]