Abstract
A series of novel N-substituted carbazole imidazolium salt derivatives has been prepared and investigated for their cytotoxic activity against five human tumor cell lines by MTS assay. The results indicated that the existence of 5,6-dimethyl-benzimidazole ring, substitution of the imidazolyl-3-position with a 2-bromobenzyl or naphthylacyl group, as well as alkyl chain length between carbazole and imidazole ring were important for the antitumor activity. Compound 61, bearing a 2-bromobenzyl substituent at position-3 of the 5,6-dimethyl-benzimidazole, showed powerful inhibitory activities and was more selective to HL-60, SMMC-7721, MCF-7 and SW480 cell lines with IC50 values 0.51–2.48 μM. Mechanism of action studies revealed that this new compound could remarkably induce cell cycle arrest and apoptosis in SMMC-7721 cells. This work provides alternative novel way for future drug development based on carbazole and imidazolium salt scaffolds.
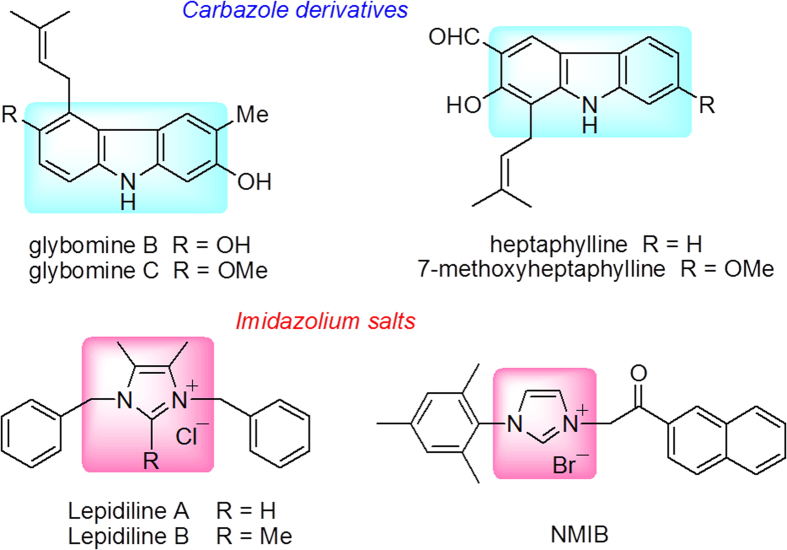
Carbazole and its derivatives are an important type of nitrogen-containing aromatic heterocyclic compounds with biological activity. Many natural products and drug molecules with the carbazole framework exhibit a broad range of biological and pharmacological activities1,2,3,4,5,6,7,8. In particular, carbazole derivatives show significant antitumor activity9,10,11. For example, glybomine B and C showed significant antitumor-promoting activity, which was confirmed by the inhibiting effect of these alkaloids in conjunction with the tumor promoter 12-O-tetradecanoylphorbol-13-acetate (TPA)12, while heptaphylline and 7-methoxyheptaphylline displayed strong cytotoxicity against NCI-H187 and KB cell lines (Fig. 1)13.
Figure 1. Representative structures of carbazole derivatives and imidazolium salts.
Recently, considerable attention has also been focused on imidazolium salts because of their remarkable array of biological activities, especially antitumor activity14,15,16,17. As exemplified in Fig. 1, natural compounds Lepidiline A and B, isolated from Lepidium meyenii, exhibited potent cytotoxic activity against a series of human cancer cell lines18. Meanwhile, the synthesis and potential cytotoxic activity of a series of new imidazolium salt derivatives, such as NMIB (Fig. 1), were reported in our previous literatures19,20,21,22,23. The antitumor mechanisms underlying arresting cell cycle progression and triggering tumour cell death by apoptosis have been validated for imidazolium salt derivatives22,23.
During the past 10 years, a pharmacophore hybrid approach for exploration of novel and highly bioactive compounds has been an effective and commonly used trend in the drug discovery field24,25,26,27,28. To validate synergistic integration of the anticancer activity of carbazole derivatives and the potent cytotoxic activity of imidazolium salts, we were interested in synthesizing a series of hybridizing compounds of carbazole with imidazole moieties. To the best of our knowledge, no reports concerning antitumor activity of carbazole imidazolium salt derivatives have been found in the literature.
In this paper, a series of novel N-substituted carbazole imidazolium salt derivatives were prepared. The purpose of this study was to investigate the antitumor activity of carbazole-based imidazole hybrids, with the final goal of developing potent antitumor agents.
Results and Discussion
Chemistry
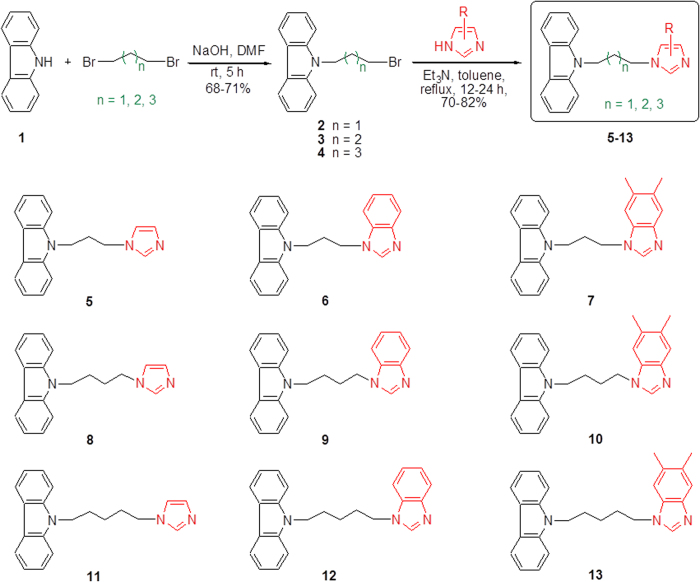
To prepare the N-substituted carbazole–imidazole hybrids (5–13), we used commercially available imidazole derivatives that were alkylated with N-alkyl bromide substituted carbazole, which was synthesized from readily available starting material carbazole 1 as depicted in Fig. 2. Straight chain alkyl groups (propyl, butyl and pentyl) were selected as linkers in the target compounds. Firstly, carbazole 1 reacted with dibromo alkane (1,3-dibromopropane, 1,4-dibromobutane or 1,5-dibromopentane) in the presence of sodium hydroxide to form the respective N-alkylbromide substituted carbazole 2–4 with 68–71% yield29. Next, bromide carbazole 2–4 was transformed to the corresponding nine N-substituted carbazole–imidazole hybrids 5–13 with imidazole or substituted benzimidazole (benzimidazole or 5,6-dimethyl-benzimidazole) by refluxing under acetone in 70–82% yields.
Figure 2. Synthesis of N-substituted carbazole–imidazole hybrids 5–13.
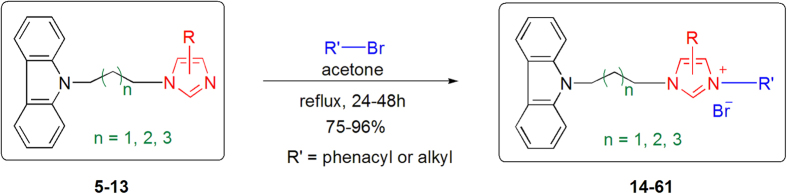
Finally, forty-eight N-substituted carbazole imidazolium salt derivatives 14–61 were synthesized with excellent yields by reaction of N-substituted carbazole–imidazole hybrids 5–13 with the corresponding alkyl and phenacyl bromides in refluxing acetone with 75–96% yields (Fig. 3). The structures and yields of imidazolium salt derivatives are listed in Table 1.
Figure 3. Synthesis of N-substituted carbazole imidazolium salt derivatives 14–61.
Table 1. Structures and yields of compounds 5–61.
| Entry | Compound | n | Imidazole ring | R’ | Molecular formula | m.p. (°C) | Yields (%) |
|---|---|---|---|---|---|---|---|
| 1 | 5 | 1 | imidazole | — | C18H17N3 | 101–103 | 68 |
| 2 | 6 | 1 | benzimidazole | — | C22H19N3 | 45–47 | 70 |
| 3 | 7 | 1 | 5,6-dimethyl-benzimidazole | — | C24H23N3 | 195–197 | 72 |
| 4 | 8 | 2 | imidazole | — | C19H19N3 | 267–269 | 70 |
| 5 | 9 | 2 | benzimidazole | — | C23H21N3 | 110–112 | 72 |
| 6 | 10 | 2 | 5,6-dimethyl-benzimidazole | — | C25H25N3 | 112–114 | 72 |
| 7 | 11 | 3 | imidazole | — | C20H21N3 | oil | 70 |
| 8 | 12 | 3 | benzimidazole | — | C24H23N3 | 149–151 | 70 |
| 9 | 13 | 3 | 5,6-dimethyl-benzimidazole | — | C26H27N3 | 103–105 | 72 |
| 10 | 14 | 1 | imidazole | phenacyl | C26H24BrN3O | 124–126 | 95 |
| 11 | 15 | 1 | imidazole | 4-methoxyphenacyl | C27H26BrN3O2 | 112–114 | 95 |
| 12 | 16 | 1 | imidazole | naphthylacyl | C30H26BrN3O | 136–138 | 94 |
| 13 | 17 | 1 | imidazole | 4-bromophenacyl | C26H23Br2N3O | 105–107 | 95 |
| 14 | 18 | 1 | imidazole | 4-bromobenzyl | C25H23Br2N3 | 64–66 | 80 |
| 15 | 19 | 1 | imidazole | 4-methylbenzyl | C26H26BrN3 | oil | 85 |
| 16 | 20 | 1 | benzimidazole | phenacyl | C30H26BrN3O | 120–122 | 95 |
| 17 | 21 | 1 | benzimidazole | 4-methoxyphenacyl | C31H28BrN3O2 | 144–146 | 95 |
| 18 | 22 | 1 | benzimidazole | naphthylacyl | C34H28BrN3O | 161–163 | 95 |
| 19 | 23 | 1 | benzimidazole | 4-bromobenzyl | C29H25Br2N3 | 222–224 | 85 |
| 20 | 24 | 1 | benzimidazole | 4-methylbenzyl | C30H28BrN3 | 201–203 | 85 |
| 21 | 25 | 1 | benzimidazole | 2-bromobenzyl | C29H25Br2N3 | 119–121 | 75 |
| 22 | 26 | 1 | 5,6-dimethyl-benzimidazole | naphthylacyl | C36H32BrN3O | 159–161 | 95 |
| 23 | 27 | 1 | 5,6-dimethyl-benzimidazole | 4-methoxyphenacyl | C33H32BrN3O2 | 176–178 | 94 |
| 24 | 28 | 1 | 5,6-dimethyl-benzimidazole | 4-methylbenzyl | C32H32BrN3 | 169–171 | 85 |
| 25 | 29 | 2 | imidazole | naphthylacyl | C32H29BrN3O | 107–109 | 95 |
| 26 | 30 | 2 | imidazole | 4-methoxyphenacyl | C28H28BrN3O2 | 90–92 | 96 |
| 27 | 31 | 2 | imidazole | 4-bromophenacyl | C27H25Br2N3O | 153–155 | 95 |
| 28 | 32 | 2 | imidazole | phenacyl | C24H26BrN3O | 96–98 | 94 |
| 29 | 33 | 2 | imidazole | 4-methylbenzyl | C27H28BrN3 | 174–176 | 85 |
| 30 | 34 | 2 | imidazole | 2-bromobenzyl | C26H25Br2N3 | 157–159 | 80 |
| 31 | 35 | 2 | benzimidazole | naphthylacyl | C35H30BrN3O | 239–241 | 95 |
| 32 | 36 | 2 | benzimidazole | 4-methoxyphenacyl | C32H30BrN3O2 | 182–184 | 96 |
| 33 | 37 | 2 | benzimidazole | 4-bromophenacyl | C31H27Br2N3O | 237–239 | 95 |
| 34 | 38 | 2 | benzimidazole | phenacyl | C31H28BrN3O | 179–181 | 95 |
| 35 | 39 | 2 | benzimidazole | 4-methylbenzyl | C31H30BrN3 | 196–198 | 95 |
| 36 | 40 | 2 | benzimidazole | 2-bromobenzyl | C30H27Br2N3 | 100–102 | 90 |
| 37 | 41 | 2 | 5,6-dimethyl-benzimidazole | naphthylacyl | C37H34BrN3O | 249–251 | 95 |
| 38 | 42 | 2 | 5,6-dimethyl-benzimidazole | 4-methoxyphenacyl | C34H34BrN3O2 | 156–158 | 96 |
| 39 | 43 | 2 | 5,6-dimethyl-benzimidazole | 4-bromophenacyl | C33H31Br2N3O | 230–232 | 94 |
| 40 | 44 | 2 | 5,6-dimethyl-benzimidazole | phenacyl | C33H32BrN3O | 152–154 | 90 |
| 41 | 45 | 2 | 5,6-dimethyl-benzimidazole | 2-bromobenzyl | C32H31Br2N3 | 129–131 | 85 |
| 42 | 46 | 2 | 5,6-dimethyl-benzimidazole | 4-methylbenzyl | C33H34BrN3 | 129–131 | 86 |
| 43 | 47 | 3 | imidazole | 4-methoxyphenacyl | C29H30BrN3O2 | oil | 90 |
| 44 | 48 | 3 | imidazole | naphthylacyl | C32H30BrN3O | 116–118 | 95 |
| 45 | 49 | 3 | imidazole | 4-methylbenzyl | C28H30BrN3 | oil | 80 |
| 46 | 50 | 3 | benzimidazole | phenacyl | C32H30BrN3O | 225–227 | 90 |
| 47 | 51 | 3 | benzimidazole | 4-methoxyphenacyl | C33H32BrN3O2 | 131–133 | 94 |
| 48 | 52 | 3 | benzimidazole | naphthylacyl | C36H32BrN3O | 120–122 | 90 |
| 49 | 53 | 3 | benzimidazole | 4-bromophenacyl | C32H29Br2N3 O | 187–189 | 94 |
| 50 | 54 | 3 | benzimidazole | 4-methylbenzyl | C32H32BrN3 | 193–195 | 90 |
| 51 | 55 | 3 | benzimidazole | 2-bromobenzyl | C31H29Br2N3 | 171–173 | 90 |
| 52 | 56 | 3 | 5,6-dimethyl-benzimidazole | phenacyl | C34H34BrN3O | 261–263 | 90 |
| 53 | 57 | 3 | 5,6-dimethyl-benzimidazole | 4-methoxyphenacyl | C35H36BrN3O2 | 228–230 | 95 |
| 54 | 58 | 3 | 5,6-dimethyl-benzimidazole | naphthylacyl | C38H36BrN3O | 205–207 | 96 |
| 55 | 59 | 3 | 5,6-dimethyl-benzimidazole | 4-bromophenacyl | C34H33Br2N3O | 196–198 | 90 |
| 56 | 60 | 3 | 5,6-dimethyl-benzimidazole | 4-methylbenzyl | C34H36BrN3 | 123–125 | 90 |
| 57 | 61 | 3 | 5,6-dimethyl-benzimidazole | 2-bromobenzyl | C33H33Br2N3 | 126–128 | 90 |
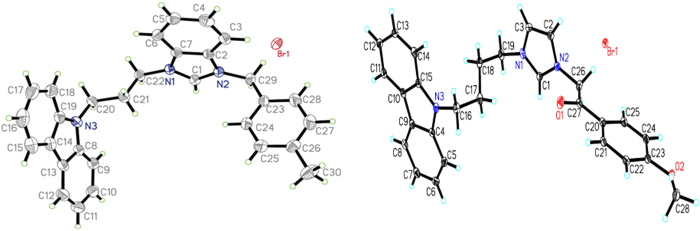
In order to confirm the chemical structures of the N-substituted carbazole imidazolium salt derivatives, compounds 24 and 30 were selected as the model compounds and determined by means of single-crystal X-ray diffraction analysis (the Cambridge Crystallographic Data Centre (CCDC) 1058661 and 1058662 contain the supplementary crystallographic data for compound 24 and 30. These data can be obtained free of charge from the Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/ data_request/cif). The molecular structures are shown in Fig. 4.
Figure 4. X-ray crystal structures of imidazolium salts 24 and 30.
Biological evaluation and structure-activity relationship analysis
The cytotoxic potential of all newly synthesized imidazole and imidazolium salt derivatives toward five human tumor cell lines, HL-60 (myeloid leukaemia), SMMC-7721 (liver cancer), A549 (lung cancer), MCF-7 (breast cancer) and SW480 (colon cancer), were screened in vitro using MTS assay30. DDP (Cisplatin), as well as carbazole (1) and imidazole, were chosen as positive controls. The screening results are summarized in Table 2.
Table 2. Cytotoxic activities of compounds 5–61 in vitro against five tumor cell linesb (IC50, μMa).
| Entry | Compound | HL-60 | SMMC-7721 | A549 | MCF-7 | SW480 |
|---|---|---|---|---|---|---|
| 1 | 1 | >40 | >40 | >40 | >40 | >40 |
| 2 | imidazole | >40 | >40 | >40 | >40 | >40 |
| 3 | 5 | >40 | 27.31 | >40 | >40 | >40 |
| 4 | 6 | 7.91 | 21.59 | 25.96 | 13.99 | 25.84 |
| 5 | 7 | NDc | ND | ND | ND | ND |
| 6 | 8 | 21.89 | >40 | 37.38 | >40 | >40 |
| 7 | 9 | 3.11 | 3.21 | 12.36 | 5.06 | 18.25 |
| 8 | 10 | 20.58 | 20.31 | 19.36 | 17.80 | 20.01 |
| 9 | 11 | 14.43 | >40 | 31.86 | 20.74 | >40 |
| 10 | 12 | 14.11 | >40 | 37.97 | 27.65 | >40 |
| 11 | 13 | 14.71 | 14.10 | 17.64 | 18.79 | 17.47 |
| 12 | 14 | 6.23 | 24.62 | >40 | 12.39 | >40 |
| 13 | 15 | 2.44 | 13.83 | 25.11 | 8.78 | 19.61 |
| 14 | 16 | 2.79 | 6.99 | 15.44 | 4.60 | 9.53 |
| 15 | 17 | 3.38 | 11.89 | 19.62 | 8.74 | 12.49 |
| 16 | 18 | 3.09 | 13.48 | 24.78 | 8.25 | 12.20 |
| 17 | 19 | 2.15 | 13.65 | 19.82 | 6.90 | 14.98 |
| 18 | 20 | 3.22 | 15.79 | 25.87 | 13.99 | 15.00 |
| 19 | 21 | 2.28 | 11.58 | 15.57 | 5.92 | 12.26 |
| 20 | 22 | 2.80 | 3.27 | 5.65 | 2.69 | 3.28 |
| 21 | 23 | 2.95 | 15.67 | 18.19 | 3.88 | 9.57 |
| 22 | 24 | 1.17 | 10.24 | 12.66 | 3.85 | 5.22 |
| 23 | 25 | 1.94 | 8.54 | 12.24 | 3.78 | 7.41 |
| 24 | 26 | 1.74 | 3.19 | 3.89 | 2.66 | 3.32 |
| 25 | 27 | 1.99 | 6.59 | 11.11 | 2.46 | 3.38 |
| 26 | 28 | 9.93 | 4.89 | 9.14 | 10.10 | 13.67 |
| 27 | 29 | ND | ND | ND | ND | ND |
| 28 | 30 | 1.34 | 8.41 | 11.07 | 2.54 | 11.74 |
| 29 | 31 | 2.42 | 10.22 | 15.70 | 3.95 | 14.16 |
| 30 | 32 | 2.98 | 11.69 | 19.04 | 19.98 | 16.39 |
| 31 | 33 | 0.84 | 5.74 | 3.92 | 2.24 | 9.56 |
| 32 | 34 | 0.49 | 3.04 | 2.92 | 1.95 | 4.33 |
| 33 | 35 | 2.37 | 3.53 | 2.80 | 2.41 | 3.33 |
| 34 | 36 | 0.56 | 2.78 | 5.16 | 2.39 | 3.37 |
| 35 | 37 | 2.30 | 3.56 | 3.74 | 2.54 | 2.80 |
| 36 | 38 | 0.98 | 6.32 | 12.94 | 2.98 | 3.84 |
| 37 | 39 | 2.60 | 3.57 | 3.15 | 2.32 | 3.59 |
| 38 | 40 | 0.71 | 3.66 | 3.58 | 2.14 | 3.08 |
| 39 | 41 | 3.34 | 2.41 | 3.16 | 1.65 | 2.50 |
| 40 | 42 | 3.71 | 2.34 | 3.60 | 1.78 | 2.31 |
| 41 | 43 | 1.80 | 3.71 | 4.40 | 3.35 | 3.38 |
| 42 | 44 | 0.56 | 3.74 | 6.32 | 2.88 | 2.97 |
| 43 | 45 | 0.54 | 2.78 | 2.83 | 4.49 | 5.62 |
| 44 | 46 | 0.70 | 3.30 | 3.10 | 4.10 | 6.58 |
| 45 | 47 | 0.68 | 6.34 | 4.83 | 3.04 | 8.69 |
| 46 | 48 | 0.87 | 2.93 | 2.99 | 2.59 | 4.50 |
| 47 | 49 | 0.55 | 3.05 | 2.29 | 1.91 | 4.45 |
| 48 | 50 | 2.67 | 5.41 | 14.03 | 3.13 | 3.83 |
| 49 | 51 | 0.66 | 2.16 | 2.80 | 1.60 | 2.43 |
| 50 | 52 | 1.36 | 2.58 | 3.02 | 2.25 | 3.40 |
| 51 | 53 | 2.19 | 2.88 | 3.89 | 3.88 | 3.39 |
| 52 | 54 | 0.57 | 2.55 | 2.65 | 2.82 | 3.19 |
| 53 | 55 | 0.64 | 2.16 | 3.00 | 2.39 | 2.54 |
| 54 | 56 | 1.25 | 3.31 | 4.19 | 3.21 | 3.48 |
| 55 | 57 | 0.94 | 2.83 | 3.39 | 2.50 | 3.58 |
| 56 | 58 | 0.76 | 2.21 | 2.98 | 1.94 | 3.23 |
| 57 | 59 | 2.60 | 2.71 | 3.74 | 3.32 | 3.64 |
| 58 | 60 | 0.56 | 2.00 | 2.84 | 2.10 | 2.88 |
| 59 | 61 | 0.51 | 2.38 | 3.12 | 1.40 | 2.48 |
| 60 | DDP | 1.32 | 6.24 | 11.83 | 15.17 | 12.95 |
aCytotoxicity as IC50 for each cell line, is the concentration of compound which reduced by 50% the optical density of treated cells with respect to untreated cells using the MTT assay.
bData represent the mean values of three independent determinations.
cND: not determined.
As shown in Table 2, carbazole (1) and imidazole, as controls, lacked activity against all tumor cell lines investigated at the concentration of 40 μM. However, fifty-seven designed compounds (5–61) exhibited broad inhibitory effects against five tested cell lines. Obviously, the structures of carbazole-based imidazole derivatives and imidazolium salt derivatives have made a significant impact on their antitumor activity. N-substituted carbazole–imidazole hybrids 5–13 exhibited no inhibitory or very weak activities against five tested cell lines. In contrast, N-substituted carbazole–imidazolium salts 14–61 displayed moderate to good cytotoxic potential. This could be understandable because of the changes of molecular structure, charge distribution and water solubility31.
For the alkyl chain between carbazole and imidazole ring, the inhibitory activities of imidazolium salt derivatives against five tumor cell lines strengthened with the increase of alkyl chain length (n = 3 > 2 > 1, propyl in 14–28, butyl in 29–46 and pentyl in 47–61). Firstly, compounds 14–28 with propyl group showed relatively weak activities against five cell lines. Among them, compound 22, bearing naphthylacyl substituent at position-3 of the benzimidazole, displayed higher cytotoxic activities with IC50 values of 2.69–5.65 μM. Secondly, imidazolium salts 29–46 with butyl group displayed medium cytotoxic activities with IC50 values of 0.49–19.98 μM. Finally, compounds 47–61 with pentyl group exhibited strong cytotoxic activities with IC50 values below 4.50 μM and more active than DDP (except compounds 47 and 50).
For the imidazole ring (imidazole, benzimidazole or 5,6-dimethyl-benzimidazole), imidazolium salt derivatives 14–19, 29–34 and 47–49 with imidazole ring showed relatively low inhibitory activities against five cell lines. Most this kind compounds exhibited weak cytotoxic activities with IC50 values above 10.00 μM. Only compounds 47–49, with pentyl group between carbazole and imidazole ring, showed higher inhibitory activities with IC50 values of 0.55–8.69 μM. In comparison, imidazolium salt derivatives 20–25, 35–40 and 50–55 with benzimidazole ring exhibited higher inhibitory activities with IC50 values of 0.56–25.87 μM. Among them, there were one half of compounds (9/18) with IC50 values below 5.00 μM. Notably, imidazolium salt derivatives 26–28, 41–46 and 56–61 with 5,6-dimethyl-benzimidazole ring displayed strong inhibitory activities. Most this kind compounds showed powerful inhibitory activities with IC50 values below 5.00 μM and were significantly more active than DDP. Among them, compounds 61 and 62, bearing a 4-methylbenzyl or 2-bromobenzyl substituent at position-3 of the 5,6-dimethyl-benzimidazole, exhibited remarkable inhibitory activities with IC50 values of 0.51–3.12 μM against five test cell lines.
For the substituents of imidazolium salts, a phenacyl substituent at position-3 of imidazole ring, such as compounds 14, 20, 32, 38 and 50, decreased the inhibitory activities against five tumor cell lines, while a 4-bromophenacyl substituent, such as compounds 17, 23, 31, 37 and 53, could slightly improve the inhibitory activities. In contrast, a 4-methoxyphenacyl substituent in compounds 21, 27, 30, 42, 51 and 57, or a 4-methylbenzyl substituent in compounds 24, 28, 33, 45, 57 and 60 have positive effects on the inhibitory activities against tumor cell lines. Interestingly, compared with above substituents, a naphthylacyl substituent at position-3 of imidazole ring, such as compounds 22, 26, 35, 41, 48, 52 and 58, or a 2-bromobenzyl substituent in compounds 34, 40, 46, 55 and 61 could led to substantial improvement of the antitumor activity. It can be seen that most of these kinds of derivatives displayed strong cytotoxic activities and were much more active than DDP. Especially, compound 61, bearing a 2-bromobenzyl substituent at position-3 of the 5,6-dimethyl-benzimidazole, showed excellent inhibitory activities and was more selective to HL-60, SMMC-7721, MCF-7 and SW480 cell lines with IC50 values 0.51–2.48 μM.
The results indicated that the existence of 5,6-dimethyl-benzimidazole ring and substitution of the imidazolyl-3-position with a 2-bromobenzyl or naphthylacyl group were important for the antitumor activity. Moreover, the increase of alkyl chain length (n = 3 > 2 > 1) also led to enhance of the inhibitory activity. Overall, the structure-activity relationship (SAR) results of N-substituted carbazole imidazolium salt derivatives have been depicted in Fig. 5.
Figure 5. Structure-activity relationship of N-substituted carbazole imidazolium salt derivatives.
Apoptosis and arrest of the SMMC-7721 cells induced by selected derivative
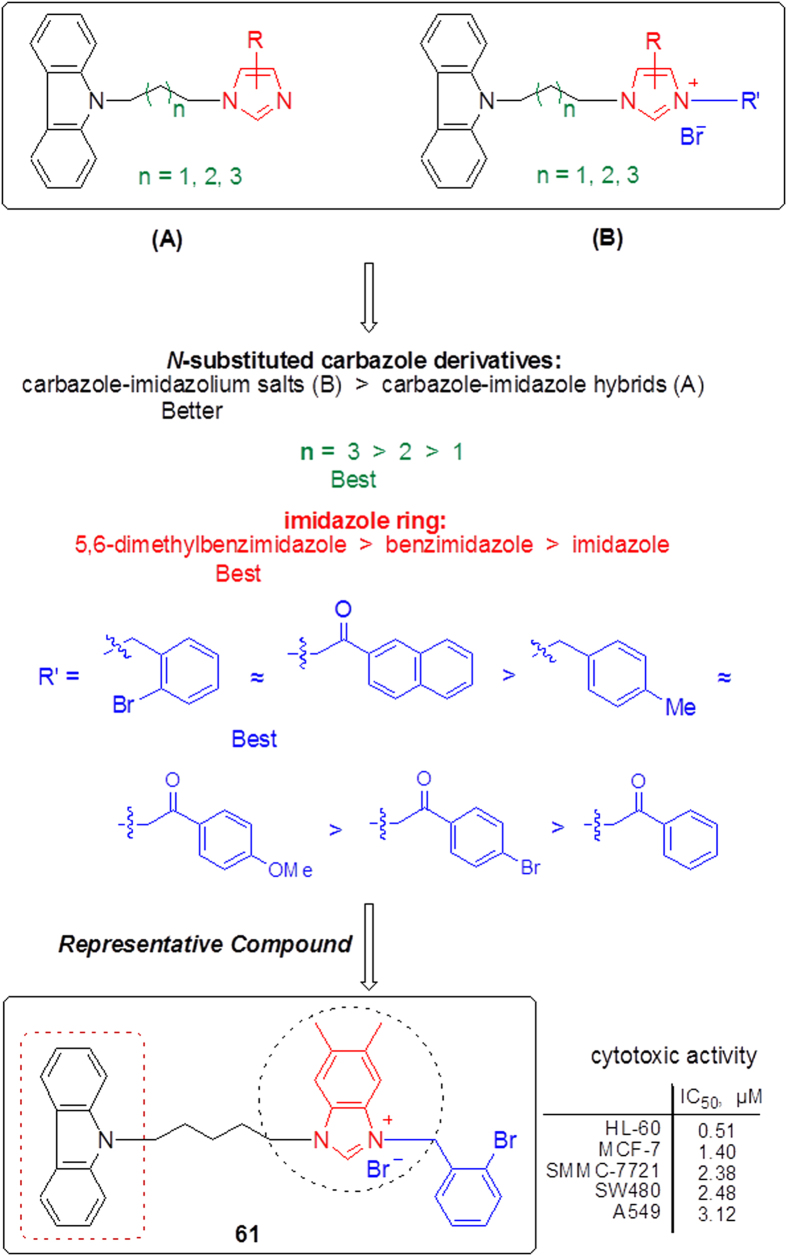
We then explored the mechanisms of action of these new N-substituted carbazole imidazolium salt derivatives. Initially, compound 61 was examined for apoptosis-induction ability. Apoptosis in SMMC-7721 cells was induced by treatment with compound 61 in a dose-dependent manner for 48 h. Apoptotic cell number increased to 14.83%, 22.26% and 84.5% when the cells were treated with compound 61 at 2, 4 and 6 μM, respectively, which were statistically different from the control (9.98%) (Fig. 6). These results showed that N-substituted carbazole imidazolium salt 61 can remarkably induce apoptosis of the SMMC-7721 cells.
Figure 6. Compound 61 induce significant apoptosis of SMMC-7721 cells.
(A) Cells were treated with 2, 4 and 6 μM compound 61 for 48 h. Treatment with 61 increased the early apoptotic (Annexin V+/PI−, lower right quadrant) and late apoptotic (Annexin V+/PI+, upper right quadrant) cells. (B) The quantification of cell apoptosis. Data represents the mean of three independent experiments.
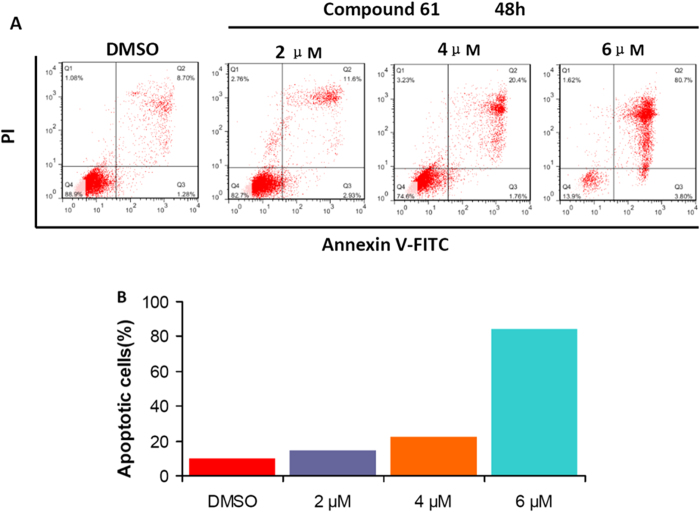
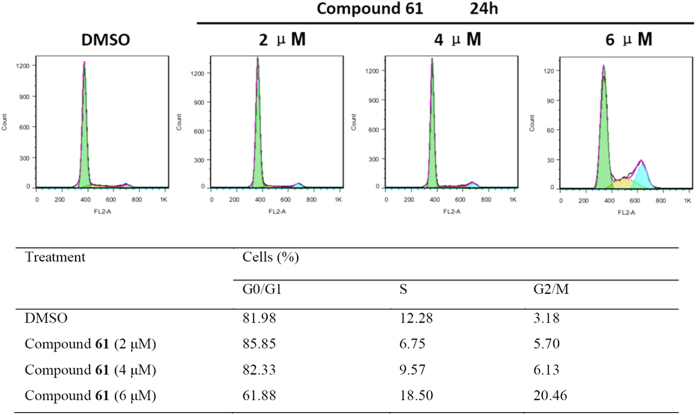
To further examine how new imidazolium salts suppressed the growth of SMMC-7721 cells, the effect of compound 61 on cell cycle distribution was investigated and the results of a typical experiment are shown in Fig. 7. SMMC-7721 cells were treated with compound 61 for 24 h, resulting in an obvious increase of the percentage of cells in G2/M phase when compared with the control. Compound 61 treatment caused 20.46% cells in G2/M phase as compared to control showing 3.18%. Inversely, G1 phase cell population was decreased to 61.88% as compared to control having 81.98%, while the proportion of S phase cells showed no significant change. These results suggested the role of cell cycle arrest in compound 61-induced growth inhibition of SMMC-7721 cells. This result is significant because disruption or malfunction of cell cycle control within the G2/M phase has been recognized as one of the most important biochemical phenomenon for tumor progression and tumorigenesis32.
Figure 7. Compound 61 induces G2/M phase arrest in SMMC-7721 cells.
(A) Cells were treated with 2, 4 and 6 μM of compound 61 for 24 h. Cell cycle was determined by PI staining and cell cytometry. (B) The percentages of cells in different phases were quantified. At least three independent experiments were performed and data of one representative experiment is shown.
In summary, a series of novel N-substituted carbazole imidazolium salt derivatives has been prepared in the present study and characterized by 1H-NMR, 13C-NMR, HRMS, IR, and single-crystal X-ray diffraction. All derivatives were evaluated in vitro against five human tumor cell lines for their cytotoxicity profile. The results indicated that the existence of 5,6-dimethyl-benzimidazole ring, substitution of the imidazolyl-3-position with a 2-bromobenzyl or naphthylacyl group, as well as alkyl chain length between carbazole and imidazole ring were important for the antitumor activity. Imidazolium salts 51, 52, 54, 55, 58, 60 and 61 were found to be the most potent compounds. Notably, compound 61, bearing a 2-bromobenzyl substituent at position-3 of the 5,6-dimethyl-benzimidazole, showed powerful inhibitory activities and was more selective to HL-60, SMMC-7721, MCF-7 and SW480 cell lines with IC50 values 0.51–2.48 μM. Mechanism of action studies revealed that this new compound could remarkably induce cell cycle arrest and apoptosis in SMMC-7721 cells. This work provides alternative novel way for future drug development based on carbazole and imidazolium salt scaffolds. Further studies on the mechanism and structural modifications of these N-substituted carbazole imidazolium salt derivatives are underway in our laboratories.
Methods
General procedures
Melting points were obtained on a XT-4 melting-point apparatus and were uncorrected. Proton nuclear magnetic resonance (1H-NMR) spectra were recorded on a Bruker Avance 300/400 spectrometer at 300/400 MHz. Carbon-13 nuclear magnetic resonance (13C-NMR) was recorded on Bruker Avance 300/400 spectrometer at 75/100 MHz. Chemical shifts are reported as δ values in parts per million (ppm) relative to tetramethylsilane (TMS) for all recorded NMR spectra. Low-resolution Mass spectra were recorded on a VG Auto Spec-3000 magnetic sector MS spectrometer. High Resolution Mass spectra were taken on AB QSTAR Pulsar mass spectrometer. X-Ray data was determined using a Bruker APEX JASCO P-1020 polarimeter. Silica gel (200–300 mesh) for column chromatography and silica GF254 for TLC were produced by Qingdao Marine Chemical Company (China). All air- or moisture-sensitive reactions were conducted under an argon atmosphere. Starting materials and reagents used in reactions were obtained commercially from Acros, Aldrich, Fluka and were used without purification, unless otherwise indicated.
Synthesis of compounds 2–4
To a mixture of carbazole 1 (1.5 g, 9 mmol) and NaOH (520 mg, 13 mmol) in DMF (30 mL) at 0 °C was added alkyl dibromide (27 mmol). The reaction mixture was stirred at room temperature for 5 h. Reaction progress was monitored by TLC, then diluted with water (50 mL), and extracted with ether (20 mL×3). The combined organic layers were washed with brine (20 mL), dried over anhydrous Na2SO4 and concentrated. The residue was purified by column chromatography (silica gel, petroleum ether 60–90 °C: EtOAc = 5:1) to afford 2–4 in 68–72% yield as white powder.
9-(3-Bromopropyl)-9H-carbazole (2)
Yield 68%. White powder, m.p. 148–150 °C. 1H NMR (300 MHz, CDCl3) δ: 8.06 (2H, d, J = 9.0 Hz), 7.47–7.44 (4H, m), 7.43–7.41 (2H, m), 4.42 (2H, t, J = 6.0 Hz), 3.31 (2H, t, J = 6.0 Hz), 2.40–2.32 (2H, m). 13C NMR (75 MHz, CDCl3): δ 140.04, 125.90, 123.02, 120.51, 119.20, 108.72, 41.03, 32.04, 30.91.
9-(4-Bromobutyl)-9H-carbazole (3)
Yield 68%. White powder, m.p. 104–106 °C. 1H NMR (300 MHz, CDCl3): δ 8.08 (2H, d, 004A = 6.0 Hz), 7.46 (2H, t, J = 6.0 Hz), 7.38–7.36 (2H, m), 7.19 (2H, t, J = 3.0 Hz), 4.26 (2H, t, J = 5.4 Hz), 3.30 (2H, t, J = 5.4 Hz), 1.90–1.77 (4H, m), 1.53–1.43 (2H, m). 13C NMR (75 MHz, CDCl3): δ140.39, 125.71, 122.91, 120.44, 118.90, 108.61, 42.82, 33.36, 32.50, 28.21, 25.93.
9-(5-Bromopentyl)-9H-carbazole (4)
Yield 72%. White powder, m.p. 51–53 °C. 1H NMR (300 MHz, CDCl3) δ: 8.08 (2H, d, J = 8.7 Hz), 7.47–7.41 (2H, m), 7.35 (2H, d, J = 8.1 Hz), 7.24–7.19 (2H, m), 4.26 (2H, t, J = 6.9 Hz), 3.30 (2H, t, J = 6.9 Hz), 1.90–1.77 (4H, m), 1.53–1.43 (2H, m). 13C NMR (75 MHz, CDCl3): δ 140.38, 125.71, 122.91, 120.44, 118.90, 108.61, 42.83, 33.37, 32.50, 28.21, 25.93.
Synthesis of compounds 5–13
A mixture of compound 2–4 (2 mmol) and imidazole or substituted imidazole (6 mmol) and Et3N (3 mmol) was stirred in tuloene (20 ml) at reflux for 12–24 h (monitored by TLC). After cooling to room temperature, the solvent was concentrated, and the residue was diluted with EtOAc (20 mL). The organic layer was washed with water (20 mL) and brine (20 mL), dried over anhydrous Na2SO4 and concentrated. The residue was purified by column chromatography (silica gel, petroleum ether 60–90 °C: EtOAc = 3:1) to afford 5–13 in 68–72% yield as powder or oil.
9-(3-(1H-Imidazol-1-yl)propyl)-9H-carbazole (5)
Yield 68%. Yellow powder, m.p. 101–103 °C. IR νmax (cm−1): 3425, 3106, 3050, 2947, 2872, 1595, 1487, 1453, 1338, 1227, 1163, 1074, 907, 822, 752, 663. 1H NMR (300 MHz, CDCl3) δ: 8.05 (2H, d, J = 7.8 Hz), 7.41 (2H, t, J = 7.8 Hz), 7.34 (1H, s), 7.23–7.15 (4H, m), 7.06 (1H, s), 6.79 (1H, s), 4.16 (2H, d, J = 6.9 Hz), 3.79 (2H, d, J = 6.9 Hz), 2.29–2.22 (2H, m). 13C NMR (75 MHz, CDCl3) δ: 140.07, 137.10, 129.77, 125.96, 123.04, 120.54, 119.36, 118.60, 108.36, 44.33, 39.66, 29.83. HRMS (ESI-TOF) m/z Calcd for C18H18N3 [M+1]+ 276.1501, found 276.1497.
9-(3-(1H-Benzo[d]imidazol-1-yl)propyl)-9H-carbazole (6)
Yield 70%. White powder, m.p. 45–47 °C. IR νmax (cm−1): 3401, 3051, 2932, 2876, 1599, 1490, 1453, 1376, 1332, 1254, 1215, 1163, 1063, 1008, 930, 889, 747, 624.1H NMR (300 MHz, CDCl3) δ: 8.08 (2H, d, J = 6.0 Hz), 7.81 (1H, d, J = 9.0 Hz), 7.75 (1H, s), 7.41 (2H, t, J = 7.5 Hz), 7.35–7.17 (6H, m), 7.12 (1H, d, J = 6.0 Hz), 4.26 (2H, t, J = 7.5 Hz), 4.04 (2H, t, J = 7.5 Hz), 2.47–2.40 (2H, m). 13C NMR (75 MHz, CDCl3) δ: 189.98, 155.93, 155.31, 136.70, 134.15, 131.33, 131.20, 130.42, 128.07, 126.60, 124.47, 124.09, 123.35, 123.16, 121.28, 120.84, 119.90, 116.38, 116.08, 112.19, 111.81, 58.72, 55.46, 20.85. HRMS (ESI-TOF) m/z Calcd for C22H20N3 [M+1]+ 326.1657, found 326.1649.
9-(3-(5,6-Dimethyl-1H-benzo[d]imidazol-1-yl)propyl)-9H-carbazole (7)
Yield 72%. White powder, m.p. 195–197 °C. IR νmax (cm−1): 3425, 3052, 3096, 1595, 1485, 1456, 1333, 1257, 1215, 1164, 1058, 1006, 845, 755, 618. 1H NMR (300 MHz, CDCl3) δ: 8.12–8.07 (2H, m), 7.69 (1H, s), 7.60 (1H, s), 7.46–7.40 (2H, m), 7.27–7.22 (4H, m), 6.84 (1H, s), 4.32 (2H, t, J = 6.6 Hz), 4.04 (2H, t, J = 7.2 Hz), 2.54–2.34 (2H, m), 2.35 (3H, s), 2.29 (3H, s). 13C NMR (75 MHz, CDCl3) δ: 142.64, 141.98, 140.11, 132.24, 132.06, 131.18, 125.95, 123.10, 120.59, 120.52, 119.35, 109.73, 108.45, 42.39, 39.94, 28.76, 20.52, 20.25. HRMS (ESI-TOF) m/z Calcd for C24H24N3 [M+1]+ 353.1970, found 354.1961.
9-(4-(1H-imidazol-1-yl)butyl)-9H-carbazole (8)
Yield 70%. White powder, m.p. 267–270 °C. IR νmax (cm−1): 3111, 3054, 2932, 2868, 1594, 1499, 1452, 1327, 1228, 1156, 1069, 1027, 911, 849, 747, 622. 1H NMR (300 MHz, DMSO) δ: 8.05 (2H, d, J = 7.8 Hz), 7.42 (2H, t, J = 8.1 Hz), 7.39 (1H, s), 7.24–7.15 (4H, m), 7.06 (1H, s), 6.79 (1H, s), 4.16 (2H, t, J = 6.9 Hz), 3.80 (2H, t, J = 6.9 Hz), 2.29–2.22 (2H, m). 13C NMR (75 MHz, DMSO) δ: 140.07, 137.10, 129.77, 125.97, 123.04, 120.54, 119.36, 118.59, 108.36, 44.33, 39.67, 29.83. HRMS (ESI-TOF) m/z Calcd for C19H20N3 [M+1]+ 290.1657, found 290.1652.
9-(4-(1H-benzo[d]imidazol-1-yl)butyl)-9H-carbazole(9)
Yield 72%. White powder, m.p. 110–112 °C. IR νmax (cm−1): 3414, 3050, 2934, 2867, 1598, 1489, 1453, 1372, 1334, 1239, 1159, 1067, 1007, 930, 860, 740, 626. 1H NMR (300 MHz, CDCl3) δ: 8.09 (2H, d, J = 7.8 Hz), 7.79 (1H, t, J = 5.7 Hz), 7.65 (1H, s), 7.44 (2H, t, J = 7.5 Hz), 7.32–7.29 (2H, m), 7.26–7.21 (5H, m), 4.26–4.24 (2H, m), 3.96–3.94 (2H, m), 1.88–1.86 (4H, m). 13C NMR (75 MHz, DMSO) δ: 143.87, 142.79, 140.22, 133.63, 125.85, 122.93, 122.13, 120.54, 119.15, 109.49, 108.51, 44.64, 42.35, 27.74, 26.28. HRMS (ESI-TOF) m/z Calcd for C23H22N3 [M+1]+ 340.1814, found 340.1810.
9-(4-(5,6-dimethyl-1H-benzo[d]imidazol-1-yl)butyl)-9H-carbazole (10)
Yield 72%. White powder, m.p. 112–114 °C. IR νmax (cm−1): 3425, 3051, 2944, 2864, 1594, 1489, 1454, 1335, 1216, 1161, 1061, 1008, 933, 844, 751, 618. 1H NMR (300 MHz, CDCl3) δ: 8.08 (2H, d, J = 7.5 Hz), 7.54 (2H, d, J = 6.3 Hz), 7.43 (2H, t, J = 8.1 Hz), 7.29 (2H, d, J = 8.1 Hz), 7.22 (2H, t, J = 7.5 Hz), 6.97 (1H, s), 4.25–4.22 (2H, m), 3.90–3.88 (2H, m), 2.34 (6H, s), 1.86–1.84 (4H, m). 13C NMR (75 MHz, CDCl3) δ: 142.53, 142.08, 140.24, 132.16, 132.07, 131.00, 125.83, 122.92, 120.50, 120.44, 119.11, 109.67, 108.53, 44.58, 42.35, 27.68, 26.27, 20.61, 20.26. HRMS (ESI-TOF) m/z Calcd for C25H26N3 [M+1]+ 368.2127, found 368.2118.
9-(5-(1H-imidazol-1-yl)pentyl)-9H-carbazole (11)
Yield 70%. Yellow oil. IR νmax (cm−1): 3419, 3049, 2933, 2859, 1595, 1486, 1456, 1328, 1229, 1154, 1077, 909, 818, 726, 664. 1H NMR (300 MHz, DMSO) δ: 8.07 (2H, d, J = 7.8 Hz), 7.46–7.41 (2H, m), 7.33–7.30 (3H, m), 7.24–7.19 (2H, m), 7.01 (1H, s), 6.74 (1H, s), 4.22 (2H, t, J = 6.9 Hz), 3.73 (2H, t, J = 6.9 Hz), 1.87–1.77 (2H, m), 1.71–1.61 (2H, m), 1.33–1.25 (2H, m). 13C NMR (75 MHz, DMSO) δ: 140.31, 136.97, 129.47, 125.73, 122.87, 120.45, 118.96, 118.73, 108.55, 46.69, 42.67, 30.92, 28.46, 24.36. HRMS (ESI-TOF) m/z Calcd for C20H22N3 [M+1]+ 304.1814, found 304.1810.
9-(5-(1H-benzo[d]imidazol-1-yl)pentyl)-9H-carbazole (12)
Yield 72%. Yellow powder, m.p. 149–151 °C. IR νmax (cm−1): 3436, 3051, 2933, 2862, 1923, 1807, 1738, 1598, 1487, 1451, 1368, 1331, 1243, 1203, 1155, 1119, 1074, 1009, 929, 880, 839, 744, 619. 1H NMR (300 MHz, CDCl3) δ: 8.08 (2H, d, J = 7.8 Hz), 7.81–7.78 (1H, m), 7.72 (1H, s), 7.46–7.41 (2H, m), 7.32–7.19 (7H, m), 4.21(2H, t, J = 6.9 Hz), 3.98 (2H, t, J = 6.9 Hz), 1.87–1.75 (4H, m), 1.35–1.30 (2H, m). 13C NMR (75 MHz, CDCl3): δ143.90, 142.82, 140.31, 133.75, 125.74, 122.89, 122.09, 120.46, 118.97, 109.57, 108.54, 44.77, 42.66, 29.78, 28.53, 24.71. HRMS (ESI-TOF) m/z Calcd for C24H24N3 [M+1]+ 354.1970, found 354.1957.
9-(5-(5,6-dimethyl-1H-benzo[d]imidazol-1-yl)pentyl)-9H-carbazole (13)
Yield 72%. White powder, m.p. 103–105 °C. IR νmax (cm−1): 3415, 3053, 2928, 2859, 1594, 1490, 1455, 1490, 1455, 1334, 1273, 1216, 1156, 1067, 1008, 841, 752, 618. 1H NMR (300 MHz, CDCl3) δ: 8.07 (2H, d, J = 7.8 Hz), 7.62 (1H, s), 7.56 (1H, s), 7.46–7.41 (2H, m), 7.31–7.29 (2H, m), 7.21 (2H, t, J = 7.5 Hz), 7.04 (1H, s), 4.19 (2H, d, J = 6.9 Hz), 3.93 (2H, d, J = 6.9 Hz), 2.36 (6H, s), 1.85–1.73 (4H, m), 1.36–1.31 (2H, m). 13C NMR (75 MHz, CDCl3) δ: 142.45, 142.09, 140.31, 132.26, 132.08, 131.01, 125.74, 122.86, 120.45, 120.36, 118.95, 109.77, 108.55, 44.74, 42.68, 29.74, 28.57, 24.66, 20.65, 20.29. HRMS (ESI-TOF) m/z Calcd for C26H28N3 [M+1]+ 382.2283, found 382.2777.
Synthesis of compounds 14–61
A mixture of substituted imidazole 5–13 (0.25 mmol) and phenacyl or alkyl (0.75 mmol) was stirred in acetone (10 ml) at reflux 24–48 h (10 ml). An insoluble substance was formed. After completion of the reaction as indicated by TLC, the precipitate was filtered and washed with acetone (3 × 10 ml), then dried to afford imidazolium salts 14–61 in 75–96% yields.
1-(3-(9H-carbazol-9-yl)propyl)-3-(2-oxo-2-phenylethyl)-1H-imidazol-3-iumbromide (14)
Yield 94%. Yellow powder, m.p. 124–126 °C. IR νmax (cm−1): 3395, 3134, 3065, 2963, 1697, 1595, 1454, 1338, 1229, 1166, 991, 754, 684. 1H NMR (300 MHz, MeOH) δ: 8.79 (1H, s), 8.05 (2H, d, J = 9.0 Hz), 7.98 (2H, d, J = 9.0 Hz), 7.65 (1H, d, J = 9.0 Hz), 7.55–7.50 (4H, m), 7.48–7.41 (4H, m), 7.19 (2H, d, J = 9.0 Hz), 5.76 (2H, s), 4.43 (2H, t, J = 6.0 Hz), 4.25 (2H, t, J = 6.0 Hz), 2.44–2.35 (2H, m). 13C NMR (75 MHz, MeOH) δ: 191.90, 141.41, 138.43, 135.81, 130.22, 129.41, 127.23, 125.33, 124.91, 122.93, 121.42, 120.48, 110.12, 56.71, 56.52, 40.76, 30.03. HRMS (ESI-TOF) m/z Calcd for C26H24N3O [M-Br]+ 394.1914, found 394.1910.
1-(3-(9H-carbazol-9-yl)propyl)-3-(2-(4-methoxyphenyl)-2-oxoethyl)-1H-imidazol-3-ium bromide (15)
Yield 95%. White powder, m.p. 112–114 °C. IR νmax (cm−1): 3415, 3141, 3054, 2965, 2839, 1684, 1600, 1454, 1335, 1240, 1167, 1025, 835, 755. 1H NMR (300 MHz, MeOH) δ: 8.74 (1H, s), 8.00 (2H, d, J = 6.0 Hz), 7.86 (2H, d, J = 9.0 Hz), 7.46–7.40 (4H, m), 7.36 (2H, s), 7.16 (2H, t, J = 7.5 Hz), 6.93 (2H, t, J = 9.0 Hz), 5.62 (2H, s), 4.36 (2H, t, J = 6.0 Hz), 4.19 (2H, t, J = 9.0 Hz), 3.36 (3H, s), 2.32 (2H, m). 13C NMR (75 MHz, MeOH) δ: 190.21, 166.20, 141.30, 138.44, 131.92, 127.62, 127.21, 125.33, 124.11, 122.81, 121.52, 120.51, 115.42, 110.22, 56.41, 56.10, 40.72, 30.02. HRMS (ESI-TOF) m/z Calcd for C27H26N3O2 [M-Br]+ 424.2020, found 424.2018.
1-(3-(9H-carbazol-9-yl)propyl)-3-(2-(naphthalen-2-yl)-2-oxoethyl)-1H-imidazol-3-ium bromide (16)
Yield 94%. White powder, m.p. 136–137 °C. IR νmax (cm−1): 3392, 3145, 3047, 2966, 1687, 1630, 1560, 1456, 1336, 1224, 1166, 1034, 935, 818, 753, 620. 1H NMR (300 MHz, MeOH) δ: 8.87 (1H, s), 8.67 (1H, s), 8.07 (3H, d, J = 6.0 Hz ), 8.00–7.89 (3H, m), 7.67–7.62 (2H, m), 7.59–7.23 (6H, m), 7.20 (2H, t, J = 6.0 Hz), 5.95 (2H, s), 4.49 (2H, t, J = 6.0 Hz), 4.31 (2H, t, J = 6.0 Hz), 2.47 (2H, m). 13C NMR (75 MHz, MeOH) δ: 191.85, 141.44, 138.65, 137.62, 133.91, 132.35, 131.82, 130.93, 130.49, 130.04, 128.97, 128.40, 127.12, 125.41, 124.25, 122.91, 120.39, 109.99, 56.50, 40.75, 29.91. HRMS (ESI-TOF) m/z Calcd for C30H26N3O [M-Br]+ 444.2070, found 444.2072.
1-(3-(9H-carbazol-9-yl)propyl)-3-(2-(4-bromophenyl)-2-oxoethyl)-1H-imidazol-3-ium bromide (17)
Yield 95%. White powder, m.p. 105–107 °C. IR νmax (cm−1): 3407, 3129, 3053, 2959, 1697, 1582, 1454, 1335, 1228, 1164, 1068, 994, 820, 755, 621. 1H NMR (300 MHz, CDCl3) δ: 8.78 (1H, s), 8.03 (2H, d, J = 6.0 Hz), 7.85 (2H, d, J = 6.0 Hz), 7.65 (2H, d, J = 6.0 Hz), 7.49–7.40 (6H, m), 7.18 (2H, t, J = 6.0 Hz), 5.72 (2H, s), 4.43 (2H, t, J = 6.0 Hz), 4,26 (2H, t, J = 6.0 Hz), 2.42–2.40 (2H, m). 13C NMR (75 MHz, CDCl3) δ: 191.11, 141.32, 138.41, 133.82, 133.52, 131.11, 130.72, 127.21, 125.33, 124.22, 122.93, 121.40, 120.51, 110.09, 56.40, 49.11, 40.72, 30.03. HRMS (ESI-TOF) m/z Calcd for C26H23BrN3O [M-Br]+ 472.1024, found 472.1022.
1-(3-(9H-carbazol-9-yl)propyl)-3-(4-bromobenzyl)-1H-imidazol-3-ium bromide (18)
Yield 80%. White powder, m.p. 64–66 °C. IR νmax (cm−1): 3411, 3137, 3049, 2949, 1595, 1486, 1453, 1333, 1154, 1067, 1011, 809, 753, 613. 1H NMR (300 MHz, CDCl3) δ: 9.95 (1H, s), 7.96 (2H, d, J = 6.0 Hz), 7.38–7.29 (6H, m), 7.23 (2H, d, J = 6.0 Hz), 7.15 (2H, m), 7.05 (1H, s), 6.98 (1H, s), 5.27 (2H, s), 4.42 (2H, t, J = 6.0 Hz), 4.28 (2H, t, J = 6.0 Hz), 2.43–2.45 (2H, m). 13C NMR (75 MHz, CDCl3) δ: 139.80, 136.11, 132.42, 131.81, 130.74, 126.23, 123.72, 122.73, 121.93, 121.63, 120.31, 119.42, 108.92, 52.31, 47.92, 40.03, 29.03. HRMS (ESI-TOF) m/z Calcd for C25H23BrN3 [M-Br]+ 444.1070, found 444.1065.
1-(3-(9H-carbazol-9-yl)propyl)-3-(4-methylbenzyl)-1H-imidazol-3-ium bromide (19)
Yield 85%. Yellow oil. IR νmax (cm−1): 3403, 3129, 3051, 2964, 1599, 1562, 1454, 1334, 1230, 1155, 1054, 832, 755, 626. 1H NMR (300 MHz, CDCl3) δ: 9.96 (1H, s), 7.98 (2H, d, J = 9.0 Hz), 7.44–7.36 (4H, m), 7.19–7.14 (4H, m), 7.08–7.04 (3H, m), 6.92 (1H, s), 5.19 (2H, s), 4.48–4.46 (2H, m), 4.36–4.34 (2H, m), 2.50–2.48 (2H, m), 2.25 (3H, s). 13C NMR (75 MHz, CDCl3) δ: 139.81, 139.51, 136.12, 130.03, 129.52, 128.92, 126.11, 122.73, 121.81, 121.30, 119.32, 109.00, 53.02, 47.92, 40.02, 29.10, 21.11. HRMS (ESI-TOF) m/z Calcd for C26H26N3 [M-Br]+ 380.2127, found 380.2121.
1-(3-(9H-carbazol-9-yl)propyl)-3-(2-oxo-2-phenylethyl)-1H-benzo[d]imidazol-3-ium bromide (20)
Yield 95%. White powder, m.p. 120–122 °C. IR νmax (cm−1): 3425, 3049, 2965, 1695, 1600, 1565, 1482, 1453, 1339, 1229, 1049, 987, 754, 684. 1H NMR (300 MHz, DMSO) δ: 9.74 (1H, s), 8.16–8.11 (5H, m), 8.07–8.04 (1H, m), 7.19 (1H, t, J = 7.2 Hz), 7.69–7.64 (6H, m), 7.44 (2H, t, J = 7.5 Hz), 7.20 (2H, t, J = 7.5 Hz), 6.36 (2H, s), 4.76 (2H, t, J = 7.2 Hz), 4.61 (2H, t, J = 6.9 Hz), 2.47–2.44 (2H, m). 13C NMR (75 MHz, DMSO) δ: 191.68, 143.79, 140.21, 135.08, 134.15, 132.38, 131.11, 129.54, 128.90, 127.26, 127.03, 126.25, 122.65, 120.80, 119.45, 114.43, 114.04, 109.70, 53.68, 54.20, 28.58. HRMS (ESI-TOF) m/z Calcd for C30H26N3O [M-Br]+ 444.2076, found 444.2072.
1-(3-(9H-carbazol-9-yl)propyl)-3-(2-(4-methoxyphenyl)-2-oxoethyl)-1H-benzo[d]imidazol-3-ium bromide (21)
Yield 95%. Yellow powder, m.p. 144–146 °C. IR νmax (cm−1): 3431, 3378, 3035, 2933, 2847, 1683, 1600, 1565, 1454, 1331, 1239, 1175, 1024, 983, 837, 755. 1H NMR (300 MHz, DMSO) δ: 9.81 (1H, s), 8.16–8.05 (6H, m), 7.69–7.67 (4H, m), 7.44 (2H, t, J = 7.2 Hz), 7.23–7.16 (4H, m), 6.34 (2H, s), 4.80–4.78 (2H, m), 4.63–4.62 (2H, m), 3.89 (3H, s), 2.49–2.47 (2H, m). 13C NMR (75 MHz, DMSO) δ: 197.37, 164.18, 143.40, 139.73, 131.91, 130.85, 130.64, 126.70, 126.50, 125.72, 122.16, 120.39, 118.93, 114.30, 113.90, 113.56, 109.20, 55.78, 52.76, 44.67, 39.47, 28.12. HRMS (ESI-TOF) m/z Calcd for C31H28N3O2 [M-Br]+ 474.2182, found 474.2174.
1-(3-(9H-carbazol-9-yl)propyl)-3-(2-(naphthalen-2-yl)-2-oxoethyl)-1H-benzo[d]imidazol-3-ium bromide (22)
Yield 95%. Yellow powder, m.p. 161–163 °C. IR νmax (cm−1): 3429, 3129, 3048, 2952, 1688, 1625, 1564, 1454, 1339, 1221, 1187, 1008, 938, 862, 821, 753. 1H NMR (300 MHz, DMSO) δ: 9.87 (1H, s), 8.96 (1H, s), 8.24 (1H, d, J = 7.5 Hz), 8.17–8.12 (5H, m), 8.08–8.06 (2H, m), 7.77–7.68 (6H, m), 7.45 (2H, d, J = 7.5 Hz), 7.21 (2H, d, J = 7.5 Hz), 6.55 (2H, s), 4.81 (2H, t, J = 6.9 Hz), 4.64 (2H, t, J = 6.9 Hz), 2.49–2.48 (2H, m). 13C NMR (75 MHz, DMSO) δ: 191.11, 143.42, 139.74, 135.55, 131.95, 130.97, 130.68, 129.70, 129.36, 128.68, 127.85, 127.37, 126.77, 126.54, 125.75, 123.29, 122.17, 120.32, 118.95, 113.97, 113.60, 109.21, 53.23, 44.71, 39.47, 28.14. HRMS (ESI-TOF) m/z Calcd for C34H28N3O [M-Br]+ 494.2232, found 494.2227.
1-(3-(9H-carbazol-9-yl)propyl)-3-(4-bromobenzyl)-1H-benzo[d]imidazol-3-ium bromide (23)
Yield 95%. Yellow powder, m.p. 222–224 °C. IR νmax (cm−1): 3425, 3031, 2953, 1600, 1563, 1485, 1453, 1335, 1225, 1069, 806, 750. 1H NMR (300 MHz, DMSO) δ: 9.96 (1H, s), 8.15 (2H, d, J = 7.8 Hz), 8.11–8.08 (1H, m), 7.92–7.89 (1H, m), 7.70 (2H, d, J = 8.1 Hz), 7.65–7.57 (4H, m), 7.49–7.23 (4H, m), 7.21 (2H, t, J = 7.5 Hz), 5.71 (2H, s), 4.66–4.64 (4H, m), 2.49–2.47 (2H, m). 13C NMR (75 MHz, DMSO) δ: 142.25, 139.77, 133.28, 131.76, 131.24, 130.57, 126.60, 126.50, 125.70, 122.14, 121.99, 120.29, 118.91, 113.72, 109.29, 49.13, 44.78, 39.51, 28.02. HRMS (ESI-TOF) m/z Calcd for C29H25 BrN3 [M-Br]+ 494.1232, found 494.1226.
1-(3-(9H-carbazol-9-yl)propyl)-3-(4-methylbenzyl)-1H-benzo[d]imidazol-3-ium bromide (24)
Yield 85%. White powder, m.p. 201–203 °C. IR νmax (cm−1): 3411, 3117, 3025, 2956, 1606, 1564, 1453, 1336, 1224, 1143, 1028, 931, 752. 1H NMR (300 MHz, DMSO) δ: 9.87 (1H, s), 8.15 (2H, d, J = 7.8 Hz), 8.05 (1H, d, J = 4.8 Hz), 7.89 (1H, t, J = 5.0 Hz), 7.68–7.61 (4H, m), 7.47–7.37 (4H, m), 7.23–7.17 (4H, m), 5.63 (2H, s), 4.64–4.62 (4H, m), 2.49–2.48 (2H, m), 2.64 (3H, s). 13C NMR (75 MHz, DMSO) δ: 142.30, 139.76, 138.09, 131.24, 130.82, 129.41, 128.26, 126.54, 125.70, 122.15, 120.31, 118.93, 113.63, 109.21, 49.68, 44.73, 39.49, 27.99, 20.65. HRMS (ESI-TOF) m/z Calcd for C30H28N3 [M-Br]+ 430.2283, found 430.2278.
1-(3-(9H-carbazol-9-yl)propyl)-3-(2-bromobenzyl)-1H-benzo[d]imidazol-3-ium bromide (25)
Yield 75%. Yellow powder, m.p. 119–121 °C. IR νmax (cm−1): 3129, 3045, 2937, 1707, 1600, 1562, 1452, 1336, 1223, 1147, 1027, 753, 609. 1H NMR (300 MHz, CDCl3) δ: 9.91 (1H, s), 8.24 (1H, s), 8.15–8.12 (3H, m), 7.87–7.85 (1H, m), 7.72–7.69 (3H, m), 7.64–7.62 (2H, m), 7.46–7.33 (5H, m), 7.20 (2H, t, J = 7.5 Hz), 5.78 (2H, s), 4.75–4.65 (4H, m), 2.48–2.47 (2H, m). 13C NMR (75 MHz, CDCl3) δ: 142.96, 139.74, 133.17, 132.54, 131.09, 130.94, 130.64, 128.36, 126.81, 126.60, 125.70, 122.96, 122.13, 120.28, 118.91, 113.90, 113.62, 109.30, 50.33, 44.86, 39.77, 28.14. HRMS (ESI-TOF) m/z Calcd for C29H25BrN3 [M-Br]+ 494.1232, found 494.1228.
1-(3-(9H-carbazol-9-yl)propyl)-5,6-dimethyl-3-(2-(naphthalen-2-yl)-2-oxoethyl)-1H-benzo[d] imidazol-3-ium bromide (26)
Yield 95%. White powder, m.p. 159–161 °C. IR νmax (cm−1): 3129, 3045, 2960, 1688, 1625, 1564, 1454, 1335, 1220, 1128, 1013, 935, 829, 754, 683. 1H NMR (300 MHz, DMSO) δ: 9.70 (1H, s), 8.96 (1H, s), 8.24 (1H, d, J = 7.92 Hz), 8.17–8.13 (3H, m), 8.08–8.06 (2H, m), 7.90 (1H, s), 7.78–7.69 (5H, m), 7.47 (2H, t, J = 7.4 Hz), 7.22 (2H, t, J = 7.4 Hz), 6.47 (2H, s), 4.71 (2H, t, J = 7.1 Hz), 4.64 (2H, t, J = 6.8 Hz), 2.48–2.46 (2H, m), 2.36–2.35 (6H, m). 13C NMR (75 MHz, DMSO) δ: 191.63, 142.64, 140.24, 136.93, 136.03, 132.50, 131.49, 131.42, 130.91, 130.19, 129.84, 129.56, 129.17, 128.35, 127.86, 126.24, 123.79, 122.67, 120.82, 119.44, 113.88, 113.45, 109.74, 53.60, 45.04, 28.67, 20.43. HRMS (ESI-TOF) m/z Calcd for C36H32N3O [M-Br]+ 522.2540, found 522.2541.
1-(3-(9H-carbazol-9-yl)propyl)-3-(2-(4-methoxyphenyl)-2-oxoethyl)-5,6-dimethyl-1H-benzo[d] imidazol-3-ium bromide (27)
Yield 94%. White powder, m.p. 176–179 °C. IR νmax (cm−1): 3128, 3015, 2966, 1682, 1601, 1566, 1454, 1337, 1240, 1181, 1020, 956, 840, 752, 690, 603. 1H NMR (400 MHz, DMSO) δ: 9.67 (1H, s), 8.16–8.09 (4H, m), 7.82 (1H, s), 7.77 (1H, s), 7.68 (2H, d, J = 8.2 Hz), 7.45 (2H, t, J = 7.4 Hz), 7.23–7.17 (4H, m), 6.26 (2H, s), 4.69 (2H, t, J = 7.2 Hz), 4.62 (2H, t, J = 6.8 Hz), 3.90 (3H, s), 2.48–2.46 (2H, m), 2.36–2.35 (6H, m). 13C NMR (100 MHz, DMSO) δ: 189.91, 164.66, 142.60, 140.22, 136.86, 136.65, 131.35, 130.88, 129.52, 127.02, 126.21, 122.64, 120.80, 119.42, 114.78, 113.80, 113.42, 109.74, 56.28, 53.15, 44.99, 39.99, 28.65, 20.41. HRMS (ESI-TOF) m/z Calcd for C33H32N3O2 [M-Br]+ 502.2489, found 502.2492.
1-(3-(9H-carbazol-9-yl)propyl)-5,6-dimethyl-3-(4-methylbenzyl)-1H-benzo[d]imidazol-3-ium bromide (28)
Yield 85%. White powder, m.p. 169–171 °C. IR νmax (cm−1): 3124, 3023, 2961, 1600, 1563, 1453, 1336, 1221, 1126, 1014, 845, 755, 673. 1H NMR (400 MHz, DMSO) δ: 9.73 (1H, s), 8.15–8.13 (2H, m), 7.69–7.67 (4H, m), 7.46–7.44 (2H, m), 7.35–7.33 (2H, m), 7.20–7.17 (4H, m), 5.55 (2H, s), 4.62–4.55 (4H, m), 2.35–2.30 (6H, m), 2.26–2.25 (2H, m), 2.08 (3H, s). 13C NMR (100 MHz, DMSO) δ: 141.51, 140.24, 138.50, 136.69, 131.54, 130.07, 129.90, 129.68, 128.61, 126.19, 122.62, 120.80, 119.42, 113.55, 109.75, 49.92, 45.09, 39.99, 31.16, 28.48, 21.15, 20.43. HRMS (ESI-TOF) m/z Calcd for C32H32N3 [M-Br]+ 458.2596, found 458.2591.
1-(4-(9H-fluoren-9-yl)butyl)-3-(2-(naphthalen-2-yl)-2-oxoethyl)-1H-imidazol-3-ium bromide (29)
Yield 95%. White powder, m.p. 107–109 °C. IR νmax (cm−1): 3051, 2943, 2859, 1692, 1625, 1593, 1564, 1455, 1335, 1226, 1166, 1029, 939, 861, 753, 626. 1H NMR (400 MHz, DMSO) δ: 9.29 (1H, s), 8.86 (1H, s), 8.22–8.11 (4H, m), 8.7–8.02 (2H, m), 7.92 (1H, s), 7.83 (1H, s), 7.75–7.65 (4H, m), 7.47 (2H, t, J = 7.4 Hz), 7.21 (2H, t, J = 7.4 Hz), 6.27 (2H, s), 4.48 (2H, t, J = 6.7 Hz), 4.37 (2H, t, J = 6.7 Hz), 1.95–1.92 (2H, m), 1.82–1.79 (2H, m). 13C NMR (100 MHz, DMSO) δ: 191.74, 140.38, 137.82, 135.99, 132.51, 131.43, 131.06, 130.17, 129.81, 129.25, 128.35, 127.86, 126.23, 124.71, 123.65, 122.56, 122.51, 120.80, 119.26, 109.78, 56.02, 49.13, 42.10, 27.68, 25.69. HRMS (ESI-TOF) m/z Calcd for C31H28N3O [M-Br]+ 457.2227, found 457.2226.
1-(4-(9H-carbazol-9-yl)butyl)-3-(2-(4-methoxyphenyl)-2-oxoethyl)-1H-imidazol-3-ium bromide (30)
Yield 96%. White powder, m.p. 90–92 °C. IR νmax (cm−1): 3054, 2937, 2835, 1688, 1599, 1564, 1499, 1455, 1340, 1240, 1169, 1026, 935, 835, 757, 627. 1H NMR (400 MHz, DMSO) δ: 9.22 (1H, s), 8.16 (2H, d, J = 7.7 Hz), 8.03 (2H, d, J = 8.6 Hz), 7.88 (1H, s), 7.76 (1H, s), 7.65 (2H, d, J = 8.2 Hz), 7.47 (2H, t, J = 7.4 Hz), 7.21(2H, t, J = 7.4 Hz), 7.15 (2H, d, J = 8.6 Hz), 6.05 (2H, s), 4.48 (2H, t, J = 6.7 Hz), 4.33 (2H, t, J = 6.7 Hz), 3.41 (3H, s), 1.93–1.90 (2H, m), 1.80–1.77 (2H, m). 13C NMR (100 MHz, DMSO) δ: 190.03, 164.56, 140.37, 137.77, 131.07, 126.94, 126.21, 124.67, 122.54, 122.39, 120.79, 119.24, 114.82, 109.77, 56.26, 55.56, 49.08, 42.08, 27.67, 25.67. HRMS (ESI-TOF) m/z Calcd for C28H28N3O2 [M-Br]+ 438.2176, found 438.2177.
1-(4-(9H-carbazol-9-yl)butyl)-3-(2-(4-bromophenyl)-2-oxoethyl)-1H-imidazol-3-ium bromide (31)
Yield 95%. White powder, m.p. 153–155 °C. IR νmax (cm−1): 3141, 3049, 2933, 2851, 1698, 1582, 1455, 1389, 1230, 1166, 1069, 993, 823, 755, 622. 1H NMR (400 MHz, DMSO) δ: 9.25–9.22 (1H, m), 8.16 (2H, d, J = 7.7 Hz), 7.98 (2H, d, J = 7.9 Hz), 7.89–7.84 (3H, m), 7.77 (1H, s), 7.65 (2H, d, J = 8.2 Hz), 7.46 (2H, t, J = 7.5 Hz), 7.20 (2H, t, J = 7.4 Hz), 6.10 (2H, s), 4.47 (2H, t, J = 6.7 Hz), 4.34 (2H, t, J = 6.7 Hz), 1.92–1.90 (2H, m), 1.80–1.77 (2H, m). 13C NMR (100 MHz, DMSO) δ: 191.21, 140.36, 137.73, 133.18, 132.66, 130.60, 129.11, 126.20, 124.62, 122.54, 122.48, 120.79, 119.24, 109.76, 55.93, 49.11, 42.09, 27.67, 25.67. HRMS (ESI-TOF) m/z Calcd for C27H25BrN3O [M-Br]+ 486.1181, found 486.1176.
1-(4-(9H-carbazol-9-yl)butyl)-3-(2-oxo-2-phenylethyl)-1H-imidazol-3-ium bromide (32)
Yield 94%. White powder, m.p.96–97 °C. IR νmax (cm−1): 3133, 3054, 2942, 2859, 1903, 1696, 1593, 1565, 1453, 1337, 1231, 1165, 1119, 990, 818, 756, 686. 1H NMR (400 MHz, DMSO) δ: 9.21 (1H, s), 8.16 (2H, d, J = 7.7 Hz), 8.05 (2H, d, J = 7.6 Hz), 7.88 (1H, s), 7.78–7.74 (2H, m), 7.66–7.61 (4H, m), 7.46 (2H, t, J = 7.5 Hz), 7.21 (2H, t, J = 7.4 Hz), 6.11 (2H, s), 4.48 (2H, t, J = 6.7 Hz), 4.34 (2H, t, J = 6.7 Hz), 1.93–1.90 (2H, m), 1.80–1.77 (2H, m). 13C NMR (100 MHz, DMSO) δ: 191.81, 140.37, 137.75, 134.99, 134.12, 129.57, 128.63, 126.21, 124.66, 122.54, 122.46, 120.80, 119.24, 109.76, 55.94, 49.10, 42.08, 27.67, 25.67. HRMS (ESI-TOF) m/z Calcd for C27H26N3O [M-Br]+ 408.2076, found 408.2072.
1-(4-(9H-carbazol-9-yl)butyl)-3-(4-methylbenzyl)-1H-imidazol-3-ium bromide (33)
Yield 85%. Yellow powder, m.p. 174–176 °C. IR νmax (cm−1): 3133, 2948, 2864, 1598, 1558, 1453, 1334, 1231, 1153, 1027, 826, 754, 622. 1H NMR (400 MHz, DMSO) δ: 9.42 (1H, s), 8.15 (2H, d, J = 7.7 Hz), 7.81 (2H, d, J = 7.9 Hz), 7.62 (2H, d, J = 8.2 Hz), 7.44 (2H, t, J = 7.4 Hz), 7.29 (2H, d, J = 7.8 Hz), 7.22–7.14 (4H, m), 5.39 (2H, s), 4.44 (2H, t, J = 6.7 Hz), 4.23 (2H, t, J = 6.7 Hz), 2.27 (3H, s), 1.90–1.86 (2H, m), 1.74–1.72 (2H, m). 13C NMR (100 MHz, DMSO) δ: 140.33, 138.60, 136.47, 132.44, 129.95, 128.76, 126.18, 123.14, 123.00, 122.53, 120.79, 119.22, 109.71, 52.10, 49.09, 42.06, 27.43, 25.66, 21.18. HRMS (ESI-TOF) m/z Calcd for C27H28N3 [M-Br]+ 394.2278, found 394.2274.
1-(4-(9H-carbazol-9-yl)butyl)-3-(2-bromobenzyl)-1H-imidazol-3-ium bromide (34)
Yield 80%. Yellow powder, m.p.157–159 °C. IR νmax (cm−1): 3099, 2955, 2851, 1594, 1559, 1453, 1336, 1226, 1160, 1057, 880, 739, 648. 1H NMR (400 MHz, DMSO) δ: 9.36 (1H, s), 8.15 (2H, d, J = 7.7 Hz), 7.87 (1H, s), 7.77 (1H, s), 7.68 (1H, d, J = 7.8 Hz), 7.62 (2H, d, J = 8.2 Hz), 7.46–7.36 (5H, m), 7.20 (2H, t, J = 7.4 Hz), 5.51 (2H, s), 4.46 (2H, t, J = 6.7 Hz), 4.27 (2H, t, J = 6.7 Hz), 1.90–1.87 (2H, m), 1.74–1.70 (2H, m). 13C NMR (100 MHz, DMSO) δ: 140.33, 137.17, 133.96, 133.61, 131.52, 131.39, 128.96, 126.17, 123.62, 123.31, 123.25, 122.53, 120.79, 119.22, 109.72, 52.70, 49.14, 42.06, 27.56, 25.65. HRMS (ESI-TOF) m/z Calcd for C26H26BrN3 [M-Br]+ 458.1232, found 458.1226.
1-(4-(9H-carbazol-9-yl)butyl)-3-(2-(naphthalen-2-yl)-2-oxoethyl)-1H-benzo[d]imidazol-3-ium bromide (35)
Yield 95%. White powder, m.p. 239–241 °C. IR νmax (cm−1): 3109, 3020, 2947, 2884, 1795, 1682, 1624, 1557, 1455, 1338, 1255, 1124, 1078, 933, 818, 753, 678. 1H NMR (400 MHz, DMSO) δ: 9.81 (1H, s), 8.94 (1H, s), 8.24 (1H, d, J = 7.5 Hz), 8.16–8.04 (7H, m), 7.76–7.70 (4H, m), 7.65 (2H, t, J = 7.6 Hz), 7.45 (2H, t, J = 6.9 Hz), 7.20 (2H, t, J = 6.9 Hz), 6.55 (2H, s), 4.68–4.66 (2H, m), 4.51–4.49 (2H, m), 2.05–2.03 (2H, m), 1.92–1.90 (2H, m). 13C NMR (100 MHz, DMSO) δ: 191.62, 143.84, 140.37, 136.05, 132.49, 131.44, 131.13, 130.20, 129.88, 129.19, 128.38, 127.90, 127.29, 127.08, 126.18, 123.81, 122.53, 120.78, 119.23, 114.50, 114.22, 109.79, 53.73, 47.11, 42.13, 26.76, 25.93. HRMS (ESI-TOF) m/z Calcd for C35H30N3O [M-Br]+ 508.2383, found 508.2385.
1-(4-(9H-carbazol-9-yl)butyl)-3-(2-(4-methoxyphenyl)-2-oxoethyl)-1H-benzo[d]imidazol-3-ium bromide (36)
Yield 95%. White powder, m.p. 182–184 °C. IR νmax (cm−1): 3028, 2930, 2839, 1796, 1678, 1600, 1560, 1453, 1334, 1238, 1175, 1025, 983, 832, 754. 1H NMR (300 MHz, DMSO) δ: 9.93 (1H, s), 8.16–8.13 (3H, m), 8.11–8.09 (3H, m), 7.66–7.64 (4H, m), 7.44 (2H, t, J = 7.5 Hz), 7.21–7.16 (4H, m), 6.44 (2H, s), 4.66–4.63 (2H, m), 4.50–4.46 (2H, m), 3.89 (3H, s), 2.04–2.01 (2H, m), 1.92–1.90 (2H, m). 13C NMR (75 MHz, DMSO) δ: 189.94, 164.66, 143.76, 140.37, 132.43, 131.43, 131.07, 127.18, 127.01, 126.19, 122.53, 120.77, 119.23, 114.78, 114.48, 114.18, 109.83, 56.31, 53.46, 47.06, 42.15, 26.73, 25.89. HRMS (ESI-TOF) m/z Calcd for C32H30N3O2 [M-Br]+ 488.2338, found 488.2332.
1-(4-(9H-carbazol-9-yl)butyl)-3-(2-(4-bromophenyl)-2-oxoethyl)-1H-benzo[d]imidazol-3-ium bromide (37)
Yield 95%. White powder, m.p. 237–239 °C. IR νmax (cm−1): 3021, 2931, 2876, 1795, 1688, 1580, 1552, 1452, 1386, 1221, 1169, 1070, 984, 822, 753, 618. 1H NMR (300 MHz, DMSO) δ: 9.80 (1H, s), 8.16–8.05 (6H, m), 7.89 (2H, d, J = 8.1 Hz), 7.69–7.64 (4H, m), 7.44 (2H, t, J = 7.5 Hz), 7.19 (2H, t, J = 7.3 Hz), 6.43 (2H, s), 4.67–4.64 (2H, m), 4.50–4.47 (2H, m), 2.03–2.01 (2H, m), 1.90–1.88 (2H, m). 13C NMR (75 MHz, DMSO): δ 191.12, 143.71, 140.36, 133.24, 132.61, 132.41, 131.08, 130.89, 129.20, 127.24, 127.06, 126.17, 122.52, 120.77, 119.22, 114.54, 114.20, 109.80, 53.76, 47.09, 42.13, 26.75, 25.91. HRMS (ESI-TOF) m/z Calcd for C31H27BrN3O [M-Br]+ 536.1338, found 536.1330.
1-(4-(9H-carbazol-9-yl)butyl)-3-(2-oxo-2-phenylethyl)-1H-benzo[d]imidazol-3-ium bromide (38)
Yield 95%. White powder, m.p. 179–181 °C. IR νmax (cm−1): 3024, 2936, 1795, 1692, 1596, 1563, 1452, 1337, 1229, 1180, 1074, 930, 823, 754, 615. 1H NMR (300 MHz, DMSO) δ: 9.86 (1H, s), 8.16–8.11 (6H, m), 7.79 (1H, t, J = 7.2 Hz), 7.68–7.64 (6H, m), 7.45 (2H, t, J = 7.4 Hz), 7.20 (2H, t, J = 7.4 Hz), 6.47 (2H, s), 4.66 (2H, t, J = 6.7 Hz), 4.49 (2H, t, J = 6.7 Hz), 2.04–2.01 (2H, m), 1.91–1.90 (2H, m). 13C NMR (75 MHz, DMSO) δ: 191.74, 143.74, 140.37, 135.06, 134.19, 132.43, 131.09, 129.53, 128.95, 127.23, 127.04, 126.18, 122.52, 120.77, 119.22, 114.53, 114.20, 109.82, 53.81, 47.08, 42.14, 26.74, 25.90. HRMS (ESI-TOF) m/z Calcd for C31H28N3O [M-Br]+ 458.2232, found 458.2230.
1-(4-(9H-carbazol-9-yl)butyl)-3-(4-methylbenzyl)-1H-benzo[d]imidazol-3-ium bromide (39)
Yield 95%. White powder, m.p. 196–198 °C. IR νmax (cm−1): 3113, 3023, 1815, 1599, 1559, 1453, 1376, 1216, 1180, 1024, 754, 610. 1H NMR (400 MHz, DMSO) δ: 10.12 (1H, s), 8.14 (2H, d, J = 7.6 Hz), 8.05 (1H, t, J = 3.2 Hz), 7.96 (1H, t, J = 5.2 Hz), 7.66–7.60 (4H, m), 7.45–7.39 (4H, m), 7.19 (2H, t, J = 7.4 Hz), 7.13 (2H, t, J = 7.6 Hz), 5.73 (2H, s), 4.57–4.54 (2H, m), 4.49–4.46 (2H, m), 2.25 (3H, m), 2.02–2.01 (2H, m), 1.87–1.85 (2H, m). 13C NMR (100 MHz, DMSO) δ: 142.71, 140.34, 138.55, 131.69, 131.46, 131.20, 129.92, 128.96, 127.06, 126.15, 122.52, 120.77, 119.20, 114.41, 114.30, 109.79, 50.10, 47.03, 42.16, 26.57, 25.96, 21.17. HRMS (ESI-TOF) m/z Calcd for C31H30N3 [M-Br]+ 444.2440, found 444.2427.
1-(4-(9H-carbazol-9-yl)butyl)-3-(2-bromobenzyl)-1H-benzo[d]imidazol-3-ium bromide (40)
Yield 95%. Yellow powder, m.p.100–102 °C. IR νmax (cm−1): 3117, 3043, 2942, 1600, 1563, 1453, 1335, 1226, 1024, 753, 665, 615. 1H NMR (300 MHz, DMSO) δ: 9.93 (1H, s), 8.14 (2H, d, J = 7.7 Hz), 8.09 (1H, d, J = 8.1 Hz), 7.88 (1H, t, J = 7.9 Hz), 7.69 (1H, d, J = 7.7 Hz), 7.65–7.61 (4H, m), 7.44–7.35 (5H, m), 7.19 (2H, t, J = 7.4 Hz), 5.81 (2H, s), 4.59 (2H, t, J = 6.7 Hz), 4.47 (2H, t, J = 6.7 Hz), 2.02–1.98 (2H, m), 1.87–1.84 (2H, m). 13C NMR (100 MHz, DMSO) δ: 143.42, 140.34, 133.76, 133.00, 131.57, 131.49, 131.37, 128.89, 127.33, 127.18, 126.15, 123.59, 122.51, 120.77, 119.21, 114.45, 114.26, 109.77, 50.93, 47.08, 42.13, 26.76, 25.93. HRMS (ESI-TOF) m/z Calcd for C30H27BrN3 [M-Br]+ 508.1383, found 508.1382.
1-(4-(9H-carbazol-9-yl)butyl)-5,6-dimethyl-3-(2-(naphthalen-2-yl)-2-oxoethyl)-1H-benzo[d]imidazol-3-ium bromide (41)
Yield 95%. White powder, m.p.249–251 °C. IR νmax (cm−1): 3024, 2949, 1808, 1685, 1625, 1593, 1562, 1454, 1336, 1217, 1186, 1011, 933, 858, 753, 617. 1H NMR (300 MHz, DMSO) δ: 9.58 (1H, s), 8.86 (1H, s), 8.15 (1H, d, J = 7.9 Hz), 8.05 (3H, d, J = 8.0 Hz), 7.97 (2H, t, J = 10.0 Hz), 7.81 (1H, s), 7.76 (1H, s), 7.69–7.61 (2H, m), 7.55 (2H, d, J = 8.2 Hz), 7.36 (2H, t, J = 7.5 Hz), 7.11 (2H, t, J = 7.4 Hz), 6.40 (2H, s), 4.51 (2H, t, J = 6.3 Hz), 4.40 (2H, t, J = 6.6 Hz), 2.31 (3H, s), 2.27 (3H, s), 1.94–1.91 (2H, m), 1.82–1.80 (2H, m). 13C NMR (75 MHz, DMSO) δ: 191.63, 142.51, 140.35, 136.99, 136.78, 136.03, 132.50, 131.48, 131.42, 130.96, 130.19, 129.85, 129.52, 129.16, 128.37, 127.88, 126.16, 123.80, 122.51, 120.75, 119.21, 113.91, 113.56, 109.78, 53.62, 46.98, 42.09, 26.66, 25.83, 20.45. HRMS (ESI-TOF) m/z Calcd for C37H34N3O [M-Br]+ 536.2696, found 536.2697.
1-(4-(9H-carbazol-9-yl)butyl)-3-(2-(4-methoxyphenyl)-2-oxoethyl)-5,6-dimethyl-1H-benzo[d]imidazol-3-ium bromide (42)
Yield 96%. White powder, m.p. 156–158 °C. IR νmax (cm−1): 3051, 3015, 2936, 1683, 1599, 1565, 1454, 1336, 1239, 1176, 1016, 955, 838, 755, 601. 1H NMR (300 MHz, DMSO) δ: 9.62 (1H, s), 8.13 (2H, d, J = 7.7 Hz), 8.09 (2H, d, J = 8.5 Hz), 7.83–7,82 (2H, m), 7.63 (2H, d, J = 8.2 Hz), 7.43 (2H, t, J = 7.4 Hz), 7.21–7.17 (4H, m), 6.28 (2H, s), 4.57 (2H, t, J = 6.0 Hz), 4.47 (2H, t, J = 6.6 Hz), 3.90 (3H, s), 2.38 (3H, s), 2.34 (3H, s), 1.99–1.97 (2H, m), 1.88–1.87 (2H, m). 13C NMR (75 MHz, DMSO) δ: 189.91, 164.66, 142.49, 140.34, 136.92, 136.72, 131.35, 130.92, 129.48, 127.01, 126.14, 122.50, 120.73, 119.19, 114.77, 113.83, 113.52, 109.77, 56.29, 53.16, 46.92, 42.07, 26.65, 25.80, 20.42. HRMS (ESI-TOF) m/z Calcd for C34H34N3O2 [M-Br]+ 516.2646, found 516.2648.
1-(4-(9H-carbazol-9-yl)butyl)-3-(2-(4-bromophenyl)-2-oxoethyl)-5,6-dimethyl-1H-benzo[d]imidazol-3-ium bromide (43)
Yield 94%. White powder, m.p. 230–232 °C. IR νmax (cm−1): 3015, 2934, 1694, 1582, 1454, 1336, 1229, 1180, 1071, 957, 819, 753, 611. 1H NMR (300 MHz, DMSO) δ: 8.03–7.95 (4H, m), 7.78–7.64 (3H, m), 7.44–7.37 (8H, m), 7.32–7.24 (2H, m), 7.09–7.07 (2H, m), 6.01 (2H, s), 3.81 (3H, s), 2.52 (3H, s). 13C NMR (75 MHz, DMSO) δ: 191.18,142.48, 140.40, 137.03, 136.84, 133.31, 132.67, 130.92, 129.55, 129.23, 126.21, 122.57, 120.81, 119.27, 114.00, 113.63, 109.84, 53.65, 47.03, 46.13, 42.14, 26.72, 25.88, 20.53. HRMS (ESI-TOF) m/z Calcd for C33H31 BrN3 [M-Br]+ 564.1645, found 564.1638.
1-(4-(9H-carbazol-9-yl)butyl)-5,6-dimethyl-3-(2-oxo-2-phenylethyl)-1H-benzo[d]imidazol-3-ium bromide (44)
Yield 90%. White powder, m.p. 152–153 °C. IR νmax (cm−1): 3121, 3043, 2936, 1694, 1599, 1564, 1453, 1337, 1230, 1187, 1001, 955, 848, 755, 612. 1H NMR (300 MHz, DMSO) δ: 9.63 (1H, s), 8.13 (4H, t, J = 7.2 Hz), 7.85 (2H, d, J = 4.9 Hz), 7.80–7.78 (1H, m), 7.68–7.62 (4H, m), 7.44 (2H, t, J = 7.6 Hz), 7.19 (2H, t, J = 7.4 Hz), 6.36 (2H, s), 4.59 (2H, t, J = 6.6 Hz), 4.48 (2H, t, J = 6.8 Hz), 2.39 (3H, s), 2.35 (3H, s), 2.01–1.98 (2H, m), 1.89–1.87 (2H, m). 13C NMR (75 MHz, DMSO) δ: 191.71, 142.43, 140.34, 136.95, 136.75, 135.04, 134.18, 130.91, 129.51, 128.90, 126.14, 122.50, 120.74, 119.19, 113.91, 113.54, 109.77, 53.60, 46.95, 42.08, 26.66, 25.81, 20.45. HRMS (ESI-TOF) m/z Calcd for C33H32N3O [M-Br]+ 486.2545, found 486.2535.
1-(4-(9H-carbazol-9-yl)butyl)-3-(2-bromobenzyl)-5,6-dimethyl-1H-benzo[d]imidazol-3-ium bromide (45)
Yield 85%. Yellow powder, m.p. 129–131 °C. IR νmax (cm−1): 3137, 3047, 2939, 1600, 1562, 1453, 1337, 1228, 1184, 1021, 947, 844, 755, 608. 1H NMR (300 MHz, DMSO) δ: 9.69 (1H, s), 8.12 (2H, d, J = 7.7 Hz), 7.82 (1H, s), 7.70 (1H, d, J = 7.5 Hz), 7.66 (1H, s), 7.60 (2H, d, J = 8.2 Hz), 7.43–7.37 (4H, m), 7.34–7.32 (1H, m), 7.18 (2H, t, J = 7.5 Hz), 5.71 (2H, s), 4.50 (2H, t, J = 6.6 Hz), 4.45 (2H, t, J = 6.8 Hz), 2.36 (3H, s), 2.34 (3H, s), 1.98–1.95 (2H, m), 1.84–1.82 (2H, m). 13C NMR (75 MHz, DMSO) δ: 142.03, 140.31, 137.06, 136.99, 133.72, 133.20, 131.38, 130.97, 130.00, 129.91, 128.88, 126.11, 123.44, 122.49, 120.73, 119.17, 113.80, 113.62, 109.73, 50.71, 46.96, 42.06, 26.64, 25.82, 20.49, 20.45. HRMS (ESI-TOF) m/z Calcd for C32H32BrN3 [M-Br]+ 536.1701, found 536.1698.
1-(4-(9H-carbazol-9-yl)butyl)-5,6-dimethyl-3-(4-methylbenzyl)-1H-benzo[d]imidazol-3-ium bromide (46)
Yield 86%. White powder, m.p. 129–131 °C. IR νmax (cm−1): 3128, 3041, 2936, 1599, 1559, 1454, 1337, 1228, 1183, 1012, 944, 840, 755, 609. 1H NMR (300 MHz, CDCl3) δ: 9.87 (1H, s), 8.13 (2H, d, J = 7.7 Hz), 7.76 (1H, s), 7.73 (1H, s), 7.61 (2H, d, J = 8.2 Hz), 7.42 (2H, t, J = 7.5 Hz), 7.35–7.32 (2H, m), 7.18 (2H, t, J = 7.5 Hz), 7.12 (2H, d, J = 7.7 Hz), 5.62 (2H, s), 4.50–4.44 (4H, m), 2.34 (6H, s), 2.25 (3H, s), 2.00–1.97 (2H, m), 1.84–1.81 (2H, m). 13C NMR (75 MHz, CDCl3) δ: 141.34, 140.29, 138.47, 136.84, 132.90, 131.62, 130.01, 129.91, 129.70, 128.54, 126.12, 122.48, 120.73, 119.18, 113.72, 113.65, 109.72, 52.32, 49.86, 46.91, 40.17, 26.46, 25.84, 21.15, 20.47, 20.43. HRMS (ESI-TOF) m/z Calcd for C34H34N3 [M-Br]+ 472.2747, found 472.2742.
1-(5-(9H-carbazol-9-yl)pentyl)-3-(2-(4-methoxyphenyl)-2-oxoethyl)-1H-imidazol-3-ium bromide (47)
Yield 95%. Yellow oil. IR νmax (cm−1): 3137, 3054, 2936, 1685, 1599, 1454, 1335, 1241, 1167, 1023, 983, 835, 755, 628. 1H NMR (300 MHz, MeOH) δ: 8.80 (1H, s), 8.02 (2H, d, J = 7.8 Hz), 7.95 (2H, d, J = 8.7 Hz), 7.45 (1H, s), 7.42–7.35 (5H, m), 7.17–7.12 (2H, 3), 6.98 (2H, d, J = 8.7 Hz), 5.75 (2H, s), 4.21 (2H, t, J = 13.5 Hz), 3.92 (2H, t, J = 7.2 Hz), 3.77 (3H, s), 1.78–1.70 (2H, m), 1.67–1.59 (2H, m), 1.19–1.18 (2H, m). 13C NMR (75 MHz, MeOH) δ: 190.06, 166.10, 141.57, 138.29, 131.75, 127.64, 126.85, 125.22, 123.85, 122.83, 121.22, 119.95, 115.30, 110.13, 56.27, 56.03, 50.46, 43.29, 30.53, 29.18, 24.58. HRMS (ESI-TOF) m/z Calcd for C29H30N3O2 [M-Br]+ 452.2333, found 452.2327.
1-(5-(9H-carbazol-9-yl)pentyl)-3-(2-(naphthalen-2-yl)-2-oxoethyl)-1H-imidazol-3-ium bromide (48)
Yield 95%. White powder, m.p. 116–118 °C. IR νmax (cm−1): 3137, 3051, 2939, 1693, 1625, 1564, 1455, 1335, 1223, 1166, 98, 822, 753, 628. 1H NMR (300 MHz, DMSO) δ: 9.26 (1H, s), 8.87 (1H, s), 8.21–8.06 (6H, m), 7.88–7.81 (2H, m), 7.72–7.62 (4H, m), 7.47–7.45 (2H, m), 7.21–7.20 (2H, m), 6.26 (2H, s), 4.42–4.26 (4H, m), 1.86–1.84 (4H, m), 1.36–1.34 (2H, m). 13C NMR (75 MHz, DMSO) δ: 191.76, 140.42, 137.76, 136.01, 132.52, 131.45, 131.06, 130.17, 129.82, 129.27, 128.36, 127.87, 126.17, 124.60, 123.66, 122.52, 120.76, 119.16, 109.75, 55.97, 49.22, 42.51, 29.75, 28.36, 23.58. HRMS (ESI-TOF) m/z Calcd for C32H30N3O [M-Br]+ 472.2383, found 472.2386.
1-(5-(9H-carbazol-9-yl)pentyl)-3-(4-methylbenzyl)-1H-imidazol-3-ium bromide (49)
Yield 80%. Yellow oil. IR νmax (cm−1): 3129, 3048, 2937, 1600, 1557, 1454, 1334, 1227, 1154, 1026, 831, 755, 627. 1H NMR (300 MHz, MeOH) δ: 8.89 (1H, s), 8.01 (2H, d, J = 7.8 Hz), 7.38–7.37 (5H, m), 7.27 (1H, s), 7.23–7.21 (2H, m), 7.17–7.11 (4H, m), 5.20 (2H, s), 4.20 (2H, t, J = 6.6 Hz), 3.87 (2H, t, J = 7.2 Hz), 2.27 (3H, s), 1.75–1.70 (2H, m), 1.64–1.59 (2H, m), 1.16–1.11 (2H, m). 13C NMR (75 MHz, MeOH) δ: 141.71, 140.50, 136.74, 132.15, 131.01, 129.79, 126.94, 123.97, 123.78, 123.51, 121.29, 120.06, 110.23, 53.87, 50.58, 43.33, 30.62, 29.29, 24.73, 21.35. HRMS (ESI-TOF) m/z Calcd for C28H30N3 [M-Br]+ 408.2434, found 408.2436.
1-(5-(9H-carbazol-9-yl)pentyl)-3-(2-oxo-2-phenylethyl)-1H-benzo[d]imidazol-3-ium bromide (50)
Yield 90%. White powder, m.p. 225–227 °C. IR νmax (cm−1): 3129, 3027, 2937, 1695, 1598, 1565, 1452, 1336, 1222, 1115, 987, 753, 690. 1H NMR (300 MHz, DMSO) δ: 9.89 (1H, s), 8.18–8.06 (6H, m), 7.80 (1H, t, J = 5.4 Hz), 7.70–7.66 (4H, m), 7.60 (2H, d, J = 6.2 Hz), 7.43 (2H, t, J = 5.6 Hz), 7.19 (2H, t, J = 5.6 Hz), 6.51 (2H, s), 4.56 (2H, t, J = 5.1 Hz), 4.40 (2H, t, J = 4.8 Hz), 1.95 (2H, t, J = 6.0 Hz), 1.86 (2H, d, J = 6.0 Hz), 1.45–1.43 (2H, m). 13C NMR (75 MHz, DMSO) δ: 191.78, 143.71, 140.41, 135.08, 134.22, 132.41, 131.13, 129.54, 128.96, 127.21, 127.02, 126.15, 122.51, 120.74, 119.14, 114.48, 114.21, 109.72, 53.80, 47.12, 42.56, 28.96, 28.46, 23.90. HRMS (ESI-TOF) m/z Calcd for C32H30N3O [M-Br]+ 472.2383, found 472.2383.
1-(5-(9H-carbazol-9-yl)pentyl)-3-(2-(4-methoxyphenyl)-2-oxoethyl)-1H-benzo[d]imidazol-3-ium bromide (51)
Yield 94%. White powder, m.p. 131–133 °C. IR νmax (cm−1): 3137, 3011, 2936, 2323, 1684, 1600, 1566, 1454, 1336, 1238, 1174, 1022, 984, 836, 755. 1H NMR (300 MHz, DMSO) δ: 9.86 (1H, s), 8.15–8.13 (4H, m), 8.08–8.04 (2H, m), 7.67–7.65 (2H, m), 7.60–7.58 (2H, m), 7.45–7.41 (2H, m), 7.20–7.21 (4H, m), 6.42 (2H, s), 4.45 (2H, t, J = 6.0 Hz), 4.39 (2H, d, J = 6.0 Hz), 3.90 (3H, s), 1.96–1.91 (2H, m), 1.86–1.81 (2H, m), 1.44–1.42 (2H, m). 13C NMR (75 MHz, DMSO) δ: 189.94, 164.70, 143.74, 140.40, 132.41, 131.11, 127.19, 127.02, 126.15, 122.50, 120.73, 119.14, 114.81, 114.40, 114.17, 109.70, 56.31, 53.34, 47.10, 42.55, 28.94, 28.44, 23.89. HRMS (ESI-TOF) m/z Calcd for C33H32N3O2 [M-Br]+ 502.2489, found 502.2489.
1-(5-(9H-carbazol-9-yl)pentyl)-3-(2-(4-methoxyphenyl)-2-oxoethyl)-1H-benzo[d]imidazol-3-ium bromide (52)
Yield 90%. White powder, m.p. 120–122 °C. IR νmax (cm−1): 3145, 3039, 2936, 2855, 1688, 1617, 1564, 1454, 1336, 1220, 1185, 997, 936, 821, 753. 1H NMR (300 MHz, DMSO) δ: 9.87 (1H, s), 8.99 (1H, s), 8.25 (1H, d, J = 7.8 Hz), 8.15 (4H, d, J = 6.9 Hz), 8.09 (3H, d, J = 7.7 Hz), 7.78–7.71 (2H, m), 7.70–7.67 (2H, m), 7.60 (2H, d, J = 8.2 Hz), 7.44 (2H, t, J = 7.4 Hz), 7.19 (2H, t, J = 7.4 Hz), 6.60 (2H, s), 4.58 (2H, t, J = 6.9 Hz), 4.42 (2H, t, J = 6.6 Hz), 1.98 (2H, t, J = 6.7 Hz), 1.87 (2H, t, J = 7.0 Hz), 1.46–1.45 (2H, m). 13C NMR (75 MHz, DMSO) δ: 191.67, 143.79, 140.42, 136.07, 132.52, 132.46, 131.51, 131.18, 130.21, 129.88, 129.20, 128.38, 127.90, 127.25, 127.06, 126.15, 123.82, 122.51, 120.75, 119.14, 114.46, 114.25, 109.71, 53.76, 47.16, 42.57, 28.98, 28.47, 23.93. HRMS (ESI-TOF) m/z Calcd for C36H32N3O [M-Br]+ 522.2540, found 522.2538.
1-(5-(9H-carbazol-9-yl)pentyl)-3-(2-(4-bromophenyl)-2-oxoethyl)-1H-benzo[d]imidazol-3-ium bromide (53)
Yield 94%. White powder, m.p. 187–189 °C. IR νmax (cm−1): 3011, 2962, 2925, 1694, 1583, 1452, 1387, 1335, 1225, 1200, 1070, 985, 820, 750, 624. 1H NMR (400 MHz, DMSO) δ: 9.83 (1H, s), 8.15 (2H, d, J = 7.7 Hz), 8.10 (4H, d, J = 6.5 Hz), 7.91 (2H, d, J = 8.3 Hz), 7.69–7.66 (2H, m), 7.60 (2H, d, J = 8.2 Hz), 7.44 (2H, t, J = 7.4 Hz), 7.19 (2H, t, J = 7.4 Hz), 6.47 (2H, s), 4.57 (2H, t, J = 6.9 Hz), 4.41 (2H, t, J = 6.6 Hz), 1.98 (2H, t, J = 6.7 Hz), 1.87 (2H, t, J = 7.0 Hz), 1.46–1.45 (2H, m). 13C NMR (100 MHz, DMSO) δ: 191.16, 143.67, 140.41, 133.27, 132.63, 132.38, 131.13, 130.91, 129.22, 127.22, 127.04, 126.14, 122.50, 120.74, 119.13, 114.48, 114.22, 109.71, 53.75, 47.14, 42.56, 28.97, 28.47, 23.91. HRMS (ESI-TOF) m/z Calcd for C32H29BrN3O [M-Br]+ 550.1489, found 550.1484.
1-(5-(9H-carbazol-9-yl)pentyl)-3-(4-methylbenzyl)-1H-benzo[d]imidazol-3-ium bromide (54)
Yield 90%. White powder, m.p. 193–195 °C. IR νmax (cm−1): 3117, 3020, 2933, 2864, 2323, 1600, 1557, 1453, 1376, 1335, 1218, 1180, 1023, 844, 756.1H NMR (400 MHz, DMSO) δ: 10.08 (1H, s), 8.15 (2H, d, J = 7.7 Hz), 8.02–8.00 (1H, m), 7.98–7.96 (1H, m), 7.64–7.62 (2H, m), 7.60–7.57 (2H, m), 7.44–7.41 (4H, m), 7.21–7.17 (4H, m), 5.74 (2H, s), 4.46 (2H, t, J = 7.2 Hz), 4.40 (2H, t, J = 6.8 Hz), 2.28 (3H, s), 1.99–1.92 (2H, m), 1.88–1.81 (2H, m), 1.45–1.37 (2H, m). 13C NMR (100 MHz, DMSO) δ: 142.68, 140.40, 138.60, 131.74, 131.46, 131.19, 129.95, 128.82, 127.04, 126.12, 122.49, 120.73, 119.13, 114.35, 114.31, 109.69, 50.13, 47.09, 42.53, 28.89, 28.51, 23.99, 21.18. HRMS (ESI-TOF) m/z Calcd for C32H32N3 [M-Br]+ 458.2591, found 458.2592.
1-(5-(9H-carbazol-9-yl)pentyl)-3-(2-bromobenzyl)-1H-benzo[d]imidazol-3-ium bromide (55)
Yield 90%. White powder, m.p. 171–173 °C. IR νmax (cm−1): 3121, 3043, 3015, 2936, 2864, 2323, 1600, 1562, 1453, 1376, 1335, 1222, 1024, 754, 614. 1H NMR (400 MHz, MeOH) δ: 9.97 (1H, s), 8.15 (2H, d, J = 8.0 Hz), 8.08–8.06 (1H, m), 7.93–7.91 (1H, m), 7.75 (1H, d, J = 8.0 Hz), 7.67–7.65 (2H, m), 7.58 (2H, d, J = 8.0 Hz), 7.46–7.37 (5H, m), 7.19 (2H, t, J = 8.0 Hz), 6.60 (2H, s), 4.51 (2H, t, J = 6.9 Hz), 4.40 (2H, t, J = 6.6 Hz), 1.95 (2H, t, J = 6.7 Hz), 1.85 (2H, t, J = 7.0 Hz), 1.42–1.41 (2H, m). 13C NMR (100 MHz, MeOH) δ: 143.37, 140.39, 133.77, 133.03, 131.61, 131.51, 131.36, 128.92, 127.31, 127.18, 126.12, 123.61, 122.49, 120.74, 119.12, 114.47, 114.22, 109.69, 50.90, 47.14, 42.54, 29.01, 28.51 23.92. HRMS (ESI-TOF) m/z Calcd for C31H29BrN3 [M-Br]+ 522.1545, found 522.1542.
1-(5-(9H-carbazol-9-yl)pentyl)-5,6-dimethyl-3-(2-oxo-2-phenylethyl)-1H-benzo[d]imidazol-3-ium bromide (56)
Yield 90%. White powder, m.p. 261–263 °C. IR νmax (cm−1): 3127, 3031, 2944, 1696, 1597, 1565, 1482, 1452, 1336, 1222, 1151, 999, 958, 848, 755, 613. 1H NMR (400 MHz, DMSO) δ: 9.66 (1H, s), 8.15 (4H, t, J = 5.2 Hz), 7.87 (2H, d, J = 4.0 Hz), 7.80 (1H, t, J = 7.4 Hz), 7.68 (2H, t, J = 7.6 Hz), 7.59 (2H, d, J = 8.2 Hz), 7.42 (2H, t, J = 7.2 Hz), 7.19 (2H, t, J = 7.6 Hz), 6.39 (2H, s), 4.50 (2H, t, J = 7.6 Hz), 4.40 (2H, t, J = 7.6 Hz), 2.40 (3H, s), 2.36 (3H, s), 1.96–1.92 (2H, m), 1.87–1.83 (2H, m), 1.42–1.40 (2H, m). 13C NMR (100 MHz, DMSO) δ: 191.76, 142.41, 140.40, 136.93, 136.77, 135.07, 134.22, 130.90, 129.61, 129.54, 128.92, 126.11, 122.49, 120.72, 119.11, 113.87, 113.63, 109.69, 53.59, 46.98, 42.56, 28.94, 28.46, 23.86, 20.44. HRMS (ESI-TOF) m/z Calcd for C34H34N3O [M-Br]+ 500.2696, found 500.2691.
1-(5-(9H-carbazol-9-yl)pentyl)-3-(2-(4-methoxyphenyl)-2-oxoethyl)-5,6-dimethyl-1H-benzo[d] imidazol-3-ium bromide (57)
Yield 95%. White powder, m.p. 228–230 °C. IR νmax (cm−1): 3125, 2938, 1684, 1600, 1566, 1454, 1334, 1240, 1177, 1017, 958, 840, 754, 600. 1H NMR (400 MHz, DMSO) δ: 9.70 (1H, s), 8.14 (4H, d, J = 7.9 Hz), 7.86 (2H, s), 7.59 (2H, d, J = 8.2 Hz), 7.42 (2H, t, J = 7.4 Hz), 7.21–7.17 (4H, m), 6.35 (2H, s), 4.49 (2H, d, J = 6.7 Hz), 4.39 (2H, d, J = 6.4 Hz), 3.91 (3H, s), 2.40 (3H, s), 2.36 (3H, s), 1.96–1.92 (2H, m), 1.87–1.83 (2H, m), 1.41–1.39 (2H, m). 13C NMR (100 MHz, DMSO) δ: 189.97, 164.68, 142.45, 140.39, 136.88, 136.72, 131.39, 130.91, 129.59, 127.05, 126.11, 122.49, 120.71, 119.10, 114.79, 113.82, 113.60, 109.68, 56.31, 53.21, 46.95, 42.56, 28.92, 28.46, 23.84, 20.42. HRMS (ESI-TOF) m/z Calcd for C35H36N3O2 [M-Br]+ 530.2802, found 530.2795.
1-(5-(9H-carbazol-9-yl)pentyl)-5,6-dimethyl-3-(2-(naphthalen-2-yl)-2-oxoethyl)-1H-benzo[d]imidazol-3-ium bromide (58)
Yield 96%. White powder, m.p. 205–207 °C. IR νmax (cm−1): 3035, 2933, 1686, 1625, 1564, 1454, 1335, 1221, 1188, 1010, 933, 827, 752, 602. 1H NMR (400 MHz, DMSO) δ: 9.55 (1H, s), 8.87 (1H, s), 8.16 (1H, d, J = 7.9 Hz), 8.09–8.06 (3H, m), 8.02–7.99 (2H, m), 7.81 (2H, d, J = 8.5 Hz), 7.71–7.62 (2H, m), 7.52 (2H, d, J = 8.0 Hz), 7.35 (2H, t, J = 8.0 Hz), 7.11 (2H, t, J = 8.0 Hz), 6.40 (2H, s), 4.44 (2H, d, J = 7.2 Hz), 4.34 (2H, d, J = 6.8 Hz), 2.33 (3H, s), 2.30 (3H, s), 1.91–1.83 (2H, m), 1.81–1.76 (2H, m), 1.38–1.31 (2H, m). 13C NMR (100 MHz, DMSO) δ: 191.65, 142.50, 140.41, 136.99, 136.81, 136.06, 132.51, 131.51, 131.39, 130.95, 130.19, 129.88, 129.66, 129.20, 128.39, 127.91, 126.12, 123.81, 122.50, 120.74, 119.13, 113.85, 113.67, 109.68, 53.55, 47.02, 42.57, 28.95, 28.47, 23.89, 20.45. HRMS (ESI-TOF) m/z Calcd for C38H36N3O [M-Br]+ 550.2853, found 550.2848.
1-(5-(9H-carbazol-9-yl)pentyl)-3-(2-(4-bromophenyl)-2-oxoethyl)-5,6-dimethyl-1H-benzo[d]imidazol-3-ium bromide (59)
Yield 96%. Yellow powder, m.p. 196–198 °C. IR νmax (cm−1): 3015, 2926, 1694, 1582, 1452, 1386, 1335, 1227, 1069, 1009, 958, 821, 748, 612. 1H NMR (400 MHz, DMSO) δ: 9.63 (1H, s), 8.14 (2H, d, J = 7.7 Hz), 8.07 (2H, d, J = 8.3 Hz), 7.92–7.86 (4H, m), 7.58 (2H, d, J = 8.2 Hz), 7.43 (2H, t, J = 7.4 Hz), 7.19 (2H, t, J = 7.5 Hz), 6.37 (2H, s), 4.49 (2H, t, J = 7.0 Hz), 4.40 (2H, t, J = 6.8 Hz), 2.40 (3H, s), 2.36 (3H, s), 1.96–1.92 (2H, m), 1.87–1.83 (2H, m), 1.42–1.40 (2H, m). 13C NMR (100 MHz, DMSO) δ: 191.16, 142.36, 140.40, 136.94, 136.97, 133.27, 132.62, 130.87, 129.60, 129.20, 126.11, 122.49, 120.72, 119.11, 113.89, 113.63, 109.68, 53.57, 47.00, 42.56, 28.94, 28.46, 23.86, 23.86, 20.44. HRMS (ESI-TOF) m/z Calcd for C34H33BrN3O [M-Br]+ 578.1802, found 578.1806.
1-(5-(9H-carbazol-9-yl)pentyl)-5,6-dimethyl-3-(4-methylbenzyl)-1H-benzo[d]imidazol-3-ium bromide (60)
Yield 90%. White powder, m.p. 123–125 °C. IR νmax (cm−1): 3117, 2937, 1598, 1558, 1474, 1453, 1337, 1224, 1125, 1013, 954, 846, 754, 718, 607. 1H NMR (400 MHz, DMSO) δ: 9.70 (1H, s), 8.13 (2H, d, J = 7.7 Hz), 7.89 (2H, d, J = 8.4 Hz), 7.57 (2H, d, J = 8.2 Hz), 7.43–7.40 (4H, m), 7.21–7.17 (4H, m), 5.69 (2H, s), 4.41–4.37 (4H, m), 2.35–2.33 (6H, m), 2.27 (3H, s), 1.97–1.90 (2H, m), 1.87–1.80 (2H, m), 1.42–1.35 (2H, m). 13C NMR (100 MHz, DMSO) δ: 141.31, 140.38, 138.51, 136.84, 136.80, 131.68, 130.14, 129.93, 129.69, 128.67, 126.07, 122.47, 120.69, 119.09, 113.71, 109.66, 49.90, 46.95, 42.52, 31.17, 28.84, 28.50. 23.92, 21.17, 20.48, 20.40. HRMS (ESI-TOF) m/z Calcd for C34H36BrN3 [M-Br]+ 486.2909, found 486.2902.
1-(5-(9H-carbazol-9-yl)pentyl)-3-(2-bromobenzyl)-5,6-dimethyl-1H-benzo[d]imidazol-3-ium bromide (61 )
Yield 90%. White powder, m.p. 126–127 °C. IR νmax (cm−1): 3125, 3051, 2938, 2876, 2353, 1599, 1561, 1453, 1335, 1218, 1155, 1029, 953, 845, 752, 611. 1H NMR (400 MHz, DMSO) δ: 9.75 (1H, s), 8.12 (2H, d, J = 7.5 Hz), 7.86 (1H, s), 7.74 (1H, d, J = 7.5 Hz), 7.70 (1H, s), 7.55 (2H, t, J = 8.0 Hz), 7.42–7.36 (5H, m), 7.18 (2H, t, J = 7.2 Hz), 5.76 (2H, s), 4.43–4.41 (2H, m), 4.38–4.37 (2H, m), 2.37 (3H, s), 2.35 (3H, s), 1.93–1.90 (2H, m), 1.83–1.80 (2H, m), 1.38–1.35 (2H, m). 13C NMR (100 MHz, MeOH) δ: 141.98, 140.37, 137.04, 133.73, 133.22, 131.41, 130.99, 130.04, 130.00, 128.92, 126.08, 123.47, 122.47, 120.71, 119.09, 113.89, 113.59, 109.66, 50.70, 47.01, 42.53, 28.96, 28.51, 23.86, 20.50, 20.42. HRMS (ESI-TOF) m/z Calcd for C33H33BrN3 [M-Br]+ 550.1852, found 550.1854.
MTS assay
Cytotoxicity was determined by performing MTS assay. Briefly, 100 ml of cells suspension were seeded in 96-well cell culture plates and allowed to adhere overnight. The cells were treated with drugs for 48 hours, and then 20 ml of CellTiter 96® AQueous One Solution Reagent (Promega, Madison, USA) was added and the cells were further incubated at 37 °C for 1–2 h. Cell viability was measured by reading the absorbance at a wavelength of 490 nm. Concentrations of 50% inhibition of growth (IC50) were calculated on the basis of the relative survival curve.
Cell apoptosis assay
To analyze the cells for apoptosis, cells were plated and allowed to adhere overnight. Cells were treated with drugs indicated for 48 hours and then analyzed for apoptosis using Annexin-V-FITC/Propidium iodide staining. Cells were trypsinized, pelleted, washed in PBS, and resuspended in 1×binding buffer containing Annexin-V-FITC and propidium iodide (BD Pharmingen) according to the manufacturer’s instructions. The samples were analyzed for the apoptosis using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ).
Cell cycle analysis
To analyze the DNA content by flow cytometry, cells were collected and washed twice with PBS. Cells were fixed with 70% ethanol overnight. Fixed cells were washed with PBS, and then stained with a 50 μg/ml propidium iodide (PI) solution containing 50 μg/ml RNase A for 30 min at room temperature. Fluorescence intensity was analyzed by FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). The percentages of the cells distributed in different phases of the cell cycle were determined using ModFIT LT 2.0.
Additional Information
How to cite this article: Liu, L.-X. et al. Synthesis and antitumor activity of novel N-substituted carbazole imidazolium salt derivatives. Sci. Rep. 5, 13101; doi: 10.1038/srep13101 (2015).
Supplementary Material
Acknowledgments
This work was supported by grants from the Program for Changjiang Scholars and Innovative Research Team in University (IRT13095), Natural Science Foundation of China (21462049, 21332007 and U1402227), Yunnan Province (2013FA028, 2012FB113 and 2010GA014), Education Department of Yunnan Province (ZD2014010) and Program for China Scholarship Council (201408535034) and excellent young talents of Yunnan University.
Footnotes
The authors declare no competing financial interests.
Author Contributions L.X.L., X.Q.W., B.Z. and L.J.Y. conducted the experiments of the chemistry. Y.L. conducted the experiments of biology. X.D.Y., H.B.Z. and Y.L. designed experiments, analyzed and interpreted the data, and wrote the manuscript. All authors have given approval to the final version of the manuscript.
References
- Schmidt A. W., Reddy K. R. & Knölker H. J. Occurrence, biogenesis, and synthesis of biologically active carbazole alkaloids. Chem. Rev. 112, 3193–3328 (2012). [DOI] [PubMed] [Google Scholar]
- Głuszyńska A. Biological potential of carbazole derivatives. Eur. J. Med. Chem. 94, 405–426 (2015). [DOI] [PubMed] [Google Scholar]
- Knolker H. J. & Reddy K. R. in The alkaloids. Chemistry and biology, vol. 65 (ed Cordell, ) Ch. 4, 181–193 (Academic Press, 2008). [DOI] [PubMed] [Google Scholar]
- Knölker H. J. & Reddy K. R. Isolation and synthesis of biologically active carbazole alkaloids. Chem. Rev. 102, 4303–4427 (2002). [DOI] [PubMed] [Google Scholar]
- Knölker H. J. Transition metal complexes in organic synthesis. Part 70: Synthesis of biologically active carbazole alkaloids using organometallic chemistry. Curr. Org. Synth. 1, 309–331 (2004). [Google Scholar]
- Choi T. A. et al. Synthesis and activity of carbazole derivatives against Mycobacterium tuberculosis. Chem Med Chem 1, 812–815 (2006). [DOI] [PubMed] [Google Scholar]
- Choi T. A. et al. Transition metal complexes in organic synthesis. Part 70: Synthesis of biologically active carbazole alkaloids using organometallic chemistry. Med. Chem. Res. 17, 374–385 (2008). [Google Scholar]
- Forke R., Jäger A. & Knolker H. J. First total synthesis of clausine L and pityriazole, a metabolite of the human pathogenic yeast Malassezia furfur. Org. Biomol. Chem. 6, 2481–2483 (2008). [DOI] [PubMed] [Google Scholar]
- Hou S. et al. Novel carbazole inhibits phospho-STAT3 through induction of protein-tyrosine phosphatase PTPN6. J. Med. Chem. 57, 6342–6353 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh M. S. et al. Current perspective of natural alkaloid carbazole and its derivatives as antitumor agents. Anti-Cancer Agents Med. Chem. 10.2174/1871520615666150113105405 (2015). [DOI] [PubMed] [Google Scholar]
- Christian A. & Martine D. Antitumor carbazoles. Anti-Cancer Agents Med. Chem. 7, 247–267 (2007). [DOI] [PubMed] [Google Scholar]
- Ito C. et al. Chemical constituents of Glycosmis arborea: Three new carbazole alkaloids and their biological activity. J. Nat. Prod. 67, 1488–1491 (2004). [DOI] [PubMed] [Google Scholar]
- Songsiang U., Thongthoom T., Boonyarat C. & Yenjai C. Claurailas A-D, cytotoxic carbazole alkaloids from the roots of Clausena harmandiana. J. Nat. Prod. 74, 208–212 (2011). [DOI] [PubMed] [Google Scholar]
- Zhang L., Peng X. M., Damu G. L. V., Geng R. X. & Zhou C. H. Comprehensive review in current developments of imidazole-based medicinal chemistry. Med. Res. Rev. 34, 340–437 (2014). [DOI] [PubMed] [Google Scholar]
- Riduan S. N. & Zhang Y. Imidazolium salts and their polymeric materials for biological applications. Chem. Soc. Rev. 42, 9055–9070 (2013). [DOI] [PubMed] [Google Scholar]
- Ramos L. M. et al. The biginelli reaction with an imidazolium-tagged recyclable iron catalyst: Kinetics, mechanism, and antitumoral activity. Chem. Eur. J. 19, 4156–4168 (2013). [DOI] [PubMed] [Google Scholar]
- Fortuna C. G., Barresi V., Berellini G. & Musumarra G. Design and synthesis of trans 2-(furan-2-yl)vinyl heteroaromatic iodides with antitumour activity. Bioorg. Med. Chem. 16, 4150–4159 (2008). [DOI] [PubMed] [Google Scholar]
- Cui B., Zheng B. L., He K. & Zheng Q. Y. Imidazole alkaloids from Lepidium meyenii. J. Nat. Prod. 66, 1101–1103 (2003). [DOI] [PubMed] [Google Scholar]
- Xu X. L. et al. Synthesis and antitumor activity of novel 2-substituted indoline imidazolium salt derivatives. Org. Biomol. Chem. 13, 1550–1557 (2015). [DOI] [PubMed] [Google Scholar]
- Sun C. J. et al. Design, synthesis and antitumor activity of novel 8-substituted 2,3,5,6-tetrahydrobenzo[1,2-b:4,5-b’] difuran imidazolium salt derivatives. RSC Adv. 4, 16312–16319 (2014). [Google Scholar]
- Wang X. Q. et al. Design, synthesis and biological evaluation of novel hybrid compounds of imidazole scaffold-based 2-benzylbenzofuran as potent anticancer agents. Eur. J. Med. Chem. 62, 111–121 (2013). [DOI] [PubMed] [Google Scholar]
- Liu L. X. et al. Synthesis and antitumor activities of novel dibenzo [b, d] furane imidazole hybrid compounds. Eur. J. Med. Chem. 66, 423–437 (2013). [DOI] [PubMed] [Google Scholar]
- Zeng X. H., Yang X. D., Zhang Y. L., Qing C. & Zhang H. B. Synthesis and antitumor activity of 1-mesityl-3-(2-naphthoylmethano)-1H-imidazolium bromide. Bioorg. Med. Chem. Lett. 2010, 20, 1844–1847 [DOI] [PubMed] [Google Scholar]
- Fortin S. & Berube G. Advances in the development of hybrid anticancer drugs. Expert Opin. Drug Dis. 8, 1029–1047 (2013). [DOI] [PubMed] [Google Scholar]
- Morphy B. Hybrid molecules with a dual mode of action: dream or reality? Acc. Chem. Res. 41, 69–77 (2007). [DOI] [PubMed] [Google Scholar]
- Viegas C. Jnr, Danuello A., Bolzani V. S., Barreiro E. J. & Fraga C. A. M. Molecular hybridization: A useful tool in the design of new drug prototypes. Curr. Med. Chem. 14, 1829–1852 (2007). [DOI] [PubMed] [Google Scholar]
- Vilanova C. et al. Design and synthesis of pironetin analogue/colchicine hybrids and study of their cytotoxic activity and mechanisms of interaction with tubulin. J. Med. Chem. 57, 10391–10403 (2014). [DOI] [PubMed] [Google Scholar]
- Li L. et al. Synthesis, in vitro and in vivo antitumor activity of scopoletin-cinnamic acid hybrids. Eur. J. Med. Chem. 93, 300–307 (2015). [DOI] [PubMed] [Google Scholar]
- Wei Z. L. et al. Isoxazolyl-serine-based agonists of peroxisome proliferator-activated receptor: Design, synthesis, and effects on cardiomyocyte differentiation. J. Am. Chem. Soc. 126, 16714–16715 (2004). [DOI] [PubMed] [Google Scholar]
- Liu W. et al. Two mixed-NH3/amine platinum (II) anticancer complexes featuring a dichloroacetate moiety in the leaving group. Sci. Rep. 3, 24641 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranke J. et al. Design of sustainable chemical products-the example of ionic liquids. Chem. Rev. 107, 2183–2206 (2007). [DOI] [PubMed] [Google Scholar]
- Löbrich M. & Jeggo P. A. The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nat. Rev. Cancer 7, 861–869 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.