Abstract
Small heat shock proteins (sHSPs) perform a fundamental role in protecting cells against a wide array of stresses but their biological function during viral infection remains unknown. Rice stripe virus (RSV) causes a severe disease of rice in Eastern Asia. OsHSP20 and its homologue (NbHSP20) were used as baits in yeast two-hybrid (YTH) assays to screen an RSV cDNA library and were found to interact with the viral RNA-dependent RNA polymerase (RdRp) of RSV. Interactions were confirmed by pull-down and BiFC assays. Further analysis showed that the N-terminus (residues 1–296) of the RdRp was crucial for the interaction between the HSP20s and viral RdRp and responsible for the alteration of the sub-cellular localization and distribution pattern of HSP20s in protoplasts of rice and epidermal cells of Nicotiana benthamiana. This is the first report that a plant virus or a viral protein alters the expression pattern or sub-cellular distribution of sHSPs.
Plant heat shock proteins (HSPs) are stimulated in response to a wide array of stress conditions and perform a fundamental role in protecting plants against abiotic stresses1,2,3. Generally, HSPs function as molecular chaperones, facilitating the native folding of proteins and preventing irreversible aggregation of denatured proteins during stress4,5. HSPs can be classified into five major categories based on molecular mass and sequence homology: HSPp100/ClpB, HSP90, 70 kDa heat shock protein (HSP70/DnaK), chaperonin (HSP60/GroEL), and small heat shock protein (sHSP). The sHSP family is one of the most abundant and complex groups and is characterized by a conserved α-crystallin domain (ACD) of 80–100 amino acids in the C-terminal region3,6. Most sHSPs are highly expressed under heat stress and often confer increased thermal tolerance by protecting proteins from irreversible denaturation7,8. The alpha-crystallin domain contains several beta-strands organized into two beta-sheets responsible for dimer formation, the basic building block of most sHSPs while other parts of the protein control oligomerisation, which is essential for sHSP function8. Heat shock granules that appear in the cell cytoplasm under stress conditions are largely composed of sHSPs together with the partially unfolded RNA-binding proteins and associated mRNAs that they are protecting9. Some studies have demonstrated that sHSPs act as ATP-independent molecular chaperones by binding proteins that are unfolding or denaturing and thereby preventing their aggregation and facilitating subsequent substrate refolding by ATP-dependent chaperone systems6,8,10,11. Although found in all domains of life, sHSPs are much more diverse in plants than in other organisms. Thus Arabidopsis thaliana has 19 and rice (Oryza sativa) has 23 sHSPs compared with 10 in humans, 4 in Drosophila melanogaster, and 2 in bacteria6. Plant sHSPs also protect cells against other environmental stresses, such as heavy metals, drought, cold, and oxidative stress7. There have been few reports of plant sHSP involvement in response to biotic stresses but it has been reported that a sHSP was induced in tobacco as a defense response to bacterial infection12. Stress granules are induced and then sometimes dispersed in some animal cells in response to virus infection13 but there have been no reports that SHSPs are involved in response to virus infection. In contrast, there are several reports that plant HSP70 proteins play roles during infection by various viruses including geminiviruses14,15 and potyviruses16.
Rice stripe disease is one of most devastating viral diseases of rice in East Asia17,18. Infected plants often have chlorotic stripes or mottling and necrotic streaks in the newly expanded leaves and growth is stunted17,19,20. The causal agent, Rice stripe virus (RSV), is one of best-studied rice viruses and is the type member of the genus Tenuivirus21. RSV is transovarially transmitted by small brown planthopper (SBPH) (Laodelphax striatellus) in a persistent and circulative-propagative manner22,23. Although RSV only infects rice and other poaceous plants in nature22, it can be transmitted experimentally to the model dicot Nicotiana benthamiana24,25.
The RSV genome consists of four single-stranded RNA segments, designated RNA1 to RNA4 in the decreasing order of their molecular weight, which encode seven ORFs using a negative or ambisense coding strategy26. RNA1 (~9 kb) is the largest RNA segment and has a single ORF in the viral-complementary sense, encoding a 337-kDa protein that is an RNA-dependent RNA polymerase (RdRp)27,28. The other three segments (RNA2, 3.5 kb; RNA3, 2.5 kb; RNA4, 2.2 kb) each have two open reading frames (ORFs) one on the viral RNA (vRNA) and the other on the viral complementary strand (vcRNA)29,30,31. RSV vRNA2 encodes a membrane-associated protein p2 that reportedly acts as an RNA silencing suppressor32. The vcRNA2 encodes a glycoprotein pc2 of unknown function but which moves from the ER to the Golgi bodies in a manner strictly dependent on COP I and COP II early secretion pathways33,34. RNA3 encodes an RNA silencing suppressor p3 from the vRNA and a nucleocapsid protein pc3 from the vcRNA29,35. RSV vRNA4 encodes a disease-specific protein p4 that accumulates in both infected plant and insect cells36. The protein encoded by vcRNA4 (pc4) has been identified as a cell-to-cell movement protein24. Infection by RSV selectively modifies the expression of host genes and establishes a complex interaction with host cell components to block cellular defense mechanisms and hijack host cell machinery20,32,37,38. RSV p2 can bind to OsSGS3, a rice host protein32, PsbP, a 23 kDa oxygen-evolving complex protein of plants, has been shown to interact with p4, and its silencing resulted in more severe symptoms and the accumulation of viral RNAs37. Recently, host HSP70 has been shown to interact with RSV RdRp, and RSV RNAs were reduced in HSP70-silenced Nicotiana benthamiana38.
In this study we investigated the interactions between sHSPs and RSV-encoded proteins and found that the expression and sub-cellular localization of a host small heat shock protein 20 (HSP20) was significantly affected by RSV infection and that this was caused by its interaction with the viral RdRp.
Results
Expression of a conserved small heat shock protein (sHSP) was regulated by viral infection
Searches in both databases of rice expression profiles (http://cdna01.dna.affrc.go.jp and http://rice.plantbiology.msu.edu) showed that the expression of a rice small heat shock protein 20 (OsHSP20), encoded by Os03G026700, was significantly changed by infection with at least seven rice viruses, including RSV and also rice black-streaked dwarf virus (RBSDV), rice dwarf phytoreovirus (RDV), rice galled dwarf phytoreovirus (RGDV), rice grassy stunt tenuivirus (RGSV), rice tungro bacilliform virus (RTBV), and rice transitory yellowing virus (RTYV) (See Supplementary Materials Figure S1). Two published data sets with both Agilent and Affymetrix rice genome microarrays consistently showed that OsHSP20 accumulated in rice plants after RSV infection20,39, suggesting that OsHSP20 might be involved in the host response to RSV infection. We then performed quantitative RT-PCR (qPCR) and confirmed that OsHSP20 was significantly up-regulated by RSV infection in our rice plants (Fig. 1). N. benthamiana is an excellent experimental host of RSV that provides a simple viral inoculation system for further study of RSV-plant interactions24,25 and we confirmed that its HSP20 homologue was also up-regulated by RSV infection in N. benthamiana plants (data not shown). We then cloned both full-length coding regions of the OsHSP20 gene from rice and its homolog (NbHSP20) from N. benthamiana by RT-PCR with the respective primer pairs Os20F/Os20R and Nb20F/Nb20R (Table 1). Members of the HSP20 subfamily have 65–86% identity to one another and contain a conserved motif in the N-terminal part in addition to the characteristic sHSP alpha-crystallin domain (ACD) at the C-terminal region (See Supplementary Materials Figure S2).
Figure 1. Quantitative RT-PCR analysis showing that transcripts of OsHSP20 were more abundant in RSV-infected rice plants.
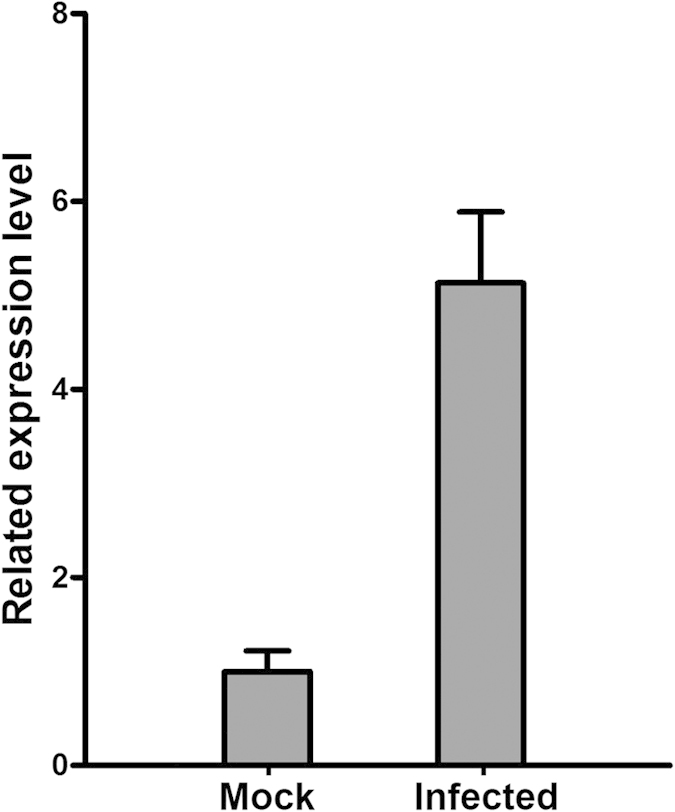
Table 1. Primers used in plasmid construction.
| Primers | Sequences (5′-3′) | Restriction sites (underlined) |
|---|---|---|
| R1-1F | GGAATTCCATATGACGACACCACCTCTCG | NdeI |
| R1-1R | CGCGGATCCTTACAGGACCCAGGTAGATCTG | BamHI |
| R1-2F | GGAATTCCATATGAAACCAGGAGGAAGCAGA | NdeI |
| R1-2R | CCGGAATTCCTCGGTGGCAAGATCAGAT | EcoRI |
| R1-3F | CGCGGATCCCTAGCCATCTCATTGTCC | BamHI |
| R1-3R | CGCGGATCCTGTCGTACAGGCAATCCA | BamHI |
| R1-4F | GGAATTCCATATGACACCTTTGGGAGAGAAG | NdeI |
| R1-4R | CGCGGATCCGTTCTCCTTCATTAGCTG | BamHI |
| R1-5F | CCGGAATTCACTCACCTAGGTGGTAGG | EcoRI |
| R1-5R | CGCGGATCCTCAGAAATCGAACTTATGGTC | BamHI |
| Os20F | GGAATTCCATATGTCGCTGATCCGCCGCAG | NdeI |
| Os20R | CGCGGATCCCTAGCCGGTAACCTGGATG | BamHI |
| Nb20F | GGAATTCCATATGTCTCTTATCCCAAGC | NdeI |
| Nb20R | CCGGAATTCTTAACCAGATATCTCAATGGC | EcoRI |
| B1-1F | CGCGGATCCATGACGACACCACCTCTCG | BamHI |
| B1-1R | GCGAGCTCTTACAGGACCCAGGTAGATCTG | SacI |
| BOs20F | CGCGGATCCATGTCGCTGATCCGCCGCAG | BamHI |
| BOs20R | GCGAGCTCCTAGCCGGTAACCTGGATG | SacI |
| BNb20F | CGGGGTACCATGTCTCTTATCCCAAGC | KpnI |
| BNb20R | GCGAGCTCTTAACCAGATATCTCAATGGC | SacI |
| G1-1R | ACGCGTCGACCAGGACCCAGGTAGATCTG | SalI |
| GOs20R | ACGCGTCGACGCCGGTAACCTGGATGGAC | SalI |
| GNb20R | CGGGGTACCACCAGATATCTCAATGGC | KpnI |
| P1-1F | GGGGTACCATGGAGGAGCAGAAGCTGATC | KpnI |
| P1-1R | ATAAGAATGCGGCCGCTTACAGGACCCAGGTAGATCTG | NotI |
| POs20F | CGGAATTCATGTCGCTGATCCGCCGCAG | EcoRI |
| POs20R | CGGGGTACCTTACTTGTACAGCTCGTCCATG | KpnI |
| PNb20F | CGGAATTCATGTCTCTTATCCCAAGC | EcoRI |
| PNb20R | ATAAGAATGCGGCCGCTTACTTGTACAGCTCGTCCATG | NotI |
| GFPF | CGGAATTCATGGTGAGCAAGGGCGAGG | EcoRI |
| P53F | ACGCGTCGACATGGAGGAGCAGAAGCTGATC | SalI |
| P53R | ATAAGAATGCGGCCGCTCAGTCTGAGTCAGGCCC | NotI |
OsHSP20 and NbHSP20 self-interacted in yeast cells
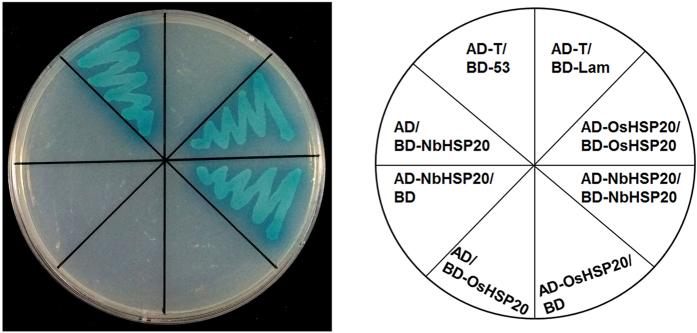
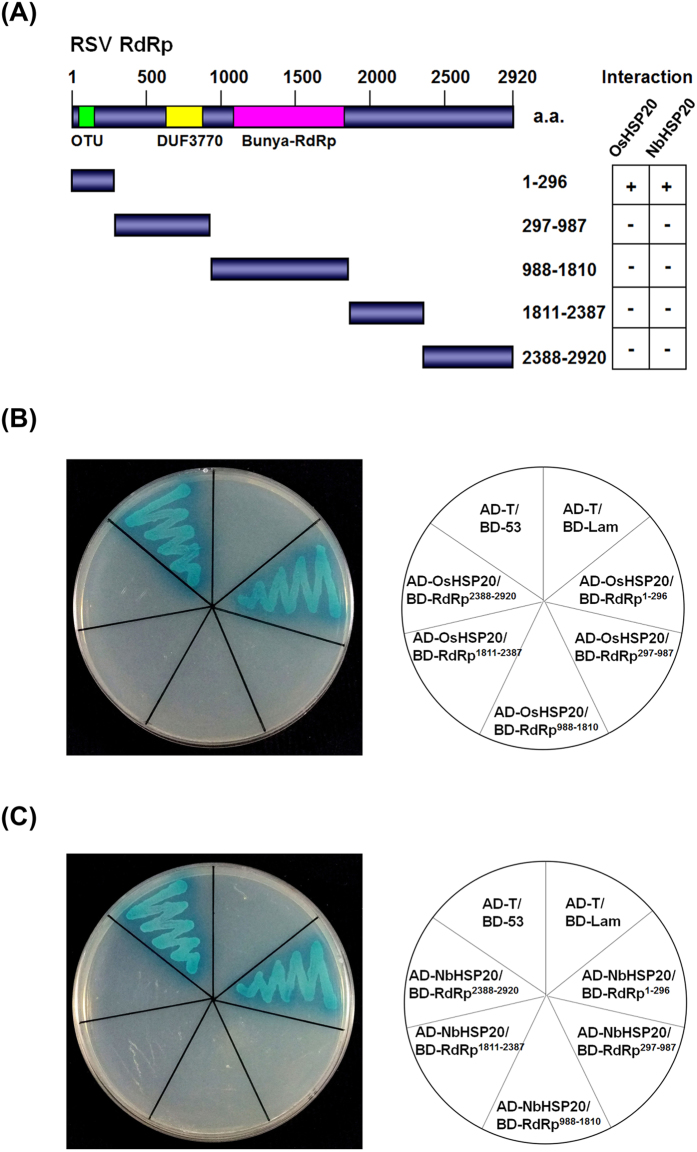
The full-length coding regions of OsHSP20 and NbHSP20 were separately cloned into the GAL4 binding domain vector pGBKT7 and activation domain vector pGADT7 and then combinations of plasmids expressing bait proteins BD-OsHSP20/AD-OsHSP20 or BD-NbHSP20/AD-NbHSP20 were co-transformed into S. cerevisiae Y2HGold cells after eliminating the autoactivation and toxicity. The resultant transformants were selected on SD/-Ade/-His/-Leu/-Trp/X-α-Gal/AbA medium. Both transformants of BD-OsHSP20/AD-OsHSP20 and BD-NbHSP20/AD-NbHSP20 as well as the positive control grew well and turned blue. In contrast, no growth was observed in the negative controls (Fig. 2). These results demonstrated that these HSP20 subfamily proteins self-interacted in yeast cells.
Figure 2. Self-interaction of OsHSP20 or NbHSP20 in yeast cells.
Yeast colonies co-expressing BD-OsHSP20 and AD-OsHSP20, or BD-NbHSP20 and AD-NbHSP20 grew well and turned blue on SD/-Ade/-His/-Leu/-Trp/X-α-Gal/AbA medium as did yeast colonies expressing BD-53 with AD-T, which was used as the positive control. Yeast co-transformed with BD-Lam and AD-T, BD-OsHSP20 and AD, BD and AD-OsHSP20, BD-NbHSP20 and AD, or BD and AD-NbHSP20 were used as negative controls.
OsHSP20 and NbHSP20 formed granules in vivo in the cytoplasm when expressed alone
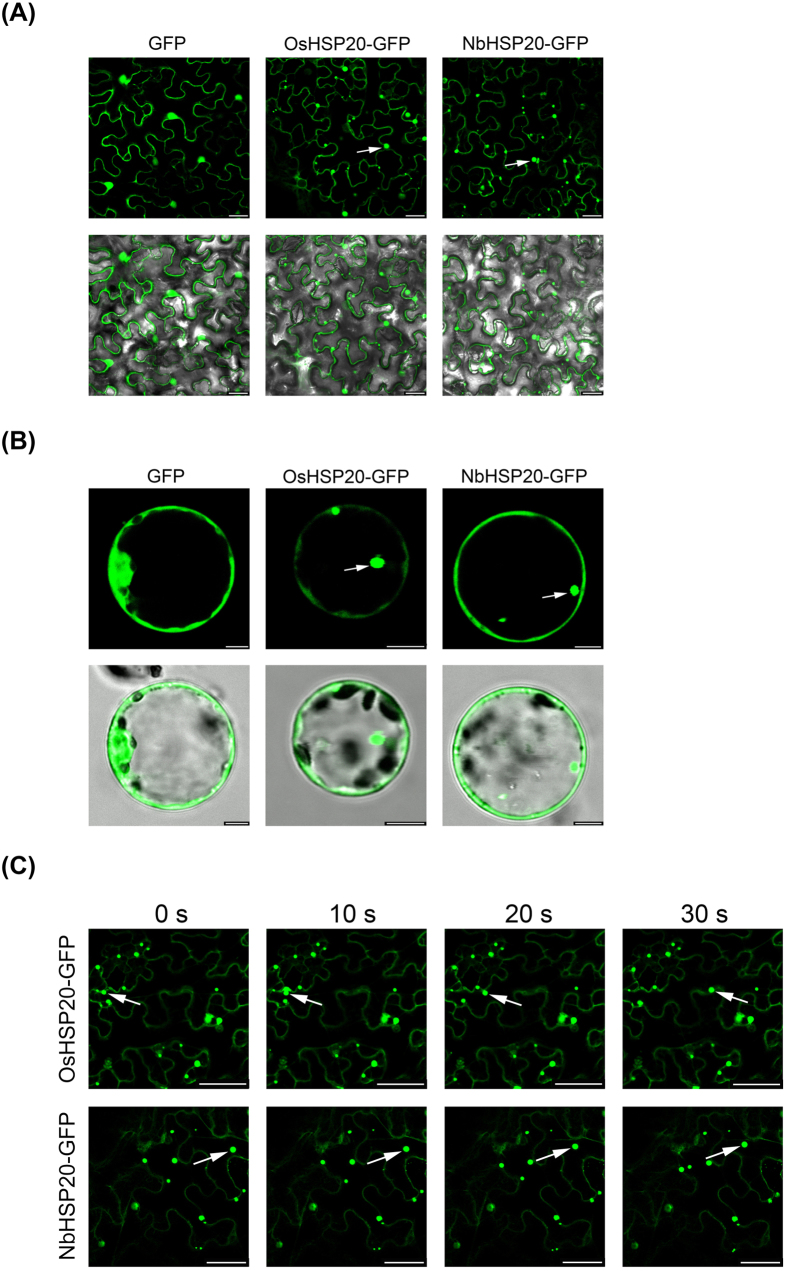
To examine the sub-cellular localization of both OsHSP20 and NbHSP20 proteins, constructs expressing OsHSP20 or NbHSP20 fused with eGFP at their C terminus (pCV-OsHSP20-GFP and pCV-NbHSP20-GFP) were constructed and introduced into N. benthamiana epidermal cells by Agrobacterium infiltration. In confocal microscopy 2 days post-infiltration (dpi) GFP fluorescence was localized to numerous granules of various sizes in the cytoplasm of cells expressing OsHSP20-GFP or NbHSP20-GFP. No fluorescence was seen in the nucleus. In the control, the non-fused GFP was distributed generally in the cytoplasm and nucleus, which indicated that the moiety GFP did not affect the localization of OsHSP20-GFP or NbHSP20-GFP (Fig. 3A). The same results were obtained when the plasmids were delivered into rice protoplasts via polyethylene glycol (PEG) transfection (Fig. 3B).
Figure 3. Sub-cellular localization of OsHSP20 and NbHSP20 proteins.
(A) GFP fluorescence in N. benthamiana leaf epidermal cells agroinfiltrated with pCV-GFP-N1, pCV-OsHSP20-GFP and pCV-NbHSP20-GFP, respectively. The results were observed 48 h after infiltration. Scale bar, 25 μm. (B) GFP fluorescence in rice protoplasts transfected with pCV-GFP-N1, pCV-OsHSP20-GFP and pCV-NbHSP20-GFP, respectively. The results were observed 18 h after transfection. Scale bar, 5 μm. The white arrow points to a granule. The fluorescence and merged images are depicted in the upper and lower panels, respectively. (C) Images recording the movement of OsHSP20-GFP or NbHSP20-GFP in N. benthamiana epidermal cells. In each local field (upper and lower), four sequential pictures detecting green fluorescence were taken at 0, 10, 20 and 30 s. The mobile GFP granules are marked with white arrows. Scale bar, 50 μm.
In confocal microscopy, the OsHSP20-GFP and NbHSP20-GFP granules moved in the cytoplasm. To record the movement of OsHSP20-GFP or NbHSP20-GFP granules, four sequential photographs were taken over a period of 30 s (Fig. 3C). In addition, a video recording the movement of OsHSP20-GFP and NbHSP20-GFP under the GFP channel was taken. The granules moved at different speeds and often moved into another focal plane (See Supplementary Materials Videos S1 and S2).
Distribution patterns of both OsHSP20 and NbHSP20 were affected by RSV infection
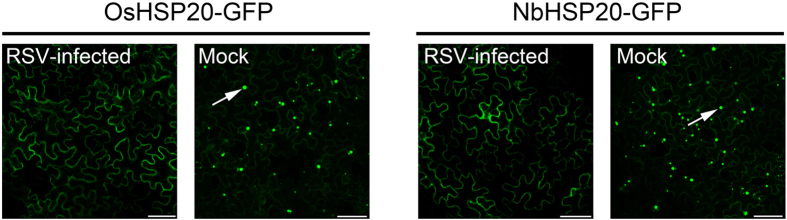
To further determine the relationship between the HSP20s and RSV infection, plasmids expressing OsHSP20-GFP or NbHSP20-GFP were introduced into healthy or RSV-infected N. benthamiana epidermal cells by Agrobacterium infiltration. Both proteins were localized in the cytoplasm of RSV-infected cells (Fig. 4) and no fluorescence was seen in the nucleus, as in healthy cells (Fig. 3). However, while numerous granules with a variety of sizes were observed in the cytoplasm of non-infected cells almost no GFP granules were detectable in the cytoplasm of RSV-infected cells (Fig. 4).
Figure 4. Localization of OsHSP20 or NbHSP20 was affected by RSV infection.
GFP fluorescence in healthy (Mock) or RSV-infected N. benthamiana leaf epidermal cells agroinfiltrated with pCV-OsHSP20-GFP (left) and pCV-NbHSP20-GFP (right), respectively. The white arrow points to a granule. The results were observed 48 h after infiltration. Scale bar, 50 μm.
Both HSP20s interacted with RSV RdRp
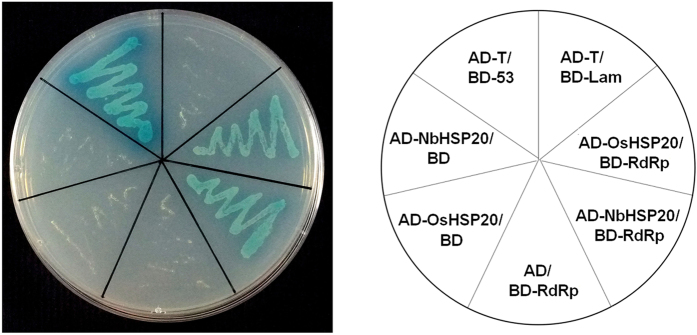
To investigate the interaction between the HSP20 and viral proteins, the full-length coding regions of OsHSP20 and NbHSP20 were cloned into the GAL4 binding domain vector pGBKT7 as bait for screening a prey library of RSV cDNA in yeast two-hybrid (YTH) assays. More than 10 independent clones were recovered following growth on selective media and sequences of all these clones were 100% identical to RSV genomic segment RNA1 encoding the viral RdRp. Combinations of plasmids expressing bait proteins BD-OsHSP20 or -NbHSP20 and prey protein AD-RdRp were then co-transformed into S. cerevisiae using the plasmid combinations BD-Lam/AD-T, BD/AD-RdRp, BD/AD -OsHSP20 or BD/AD -NbHSP20 as negative controls and BD-53/AD-T as a positive control. Only transformants of BD-OsHSP20/AD-RdRp, BD-NbHSP20/AD-RdRp and the positive control grew well on the selective medium and turned blue. In contrast, no growth was observed in the negative controls (Fig. 5). These results consistently indicated a strong interaction between the HSP20 proteins and RSV RdRp.
Figure 5. Interactions of RSV RdRp with the OsHSP20 or NbHSP20 in co-transformed yeast cells grown on SD/-Ade/-His/-Leu/-Trp/X-α-Gal/AbA.
Yeast colonies expressing BD-RdRp with AD-OsHSP20 or AD-NbHSP20 were able to grow well and turned blue on SD/-Ade/-His/-Leu/-Trp/X-α-Gal/AbA medium as did yeast colonies expressing BD-53 with AD-T, which was used as the positive control. Yeast co-transformed with BD-Lam and AD-T, BD-RdRp and AD, BD and AD-OsHSP20, or BD and AD-NbHSP20 were used as negative controls.
The N-terminal part of RSV RdRp was responsible for its interactions with OsHSP20 and NbHSP20 in vivo and in vitro
The large RdRp protein of RSV has multiple functional motifs and domains40. Five fragments of the RdRp, divided on the basis of the conserved domains (RdRp1–296, RdRp297–987, RdRp988–1810, RdRp1811–2387 and RdRp2388–2920), were then tested separately by cloning into the yeast vectors to determine which part was involved in the interaction with HSP20 (Fig. 6A). Only transformants expressing BD-RdRp1–296/AD-OsHSP20, BD-RdRp1–296/AD-NbHSP20 and the positive control grew well on the selective medium and turned blue, suggesting that the N-terminal part of RdRp should be crucial for the interaction between the HSP20s and the viral RdRp (Fig. 6B,C).
Figure 6. Interactions of OsHSP20 or NbHSP20 with fragments of RSV RdRp in co-transformed yeast cells grown on SD/-Ade/-His/-Leu/-Trp/X-α-Gal/AbA.
(A) Five peptides were selected to cover the whole RSV RdRp based around the conserved domains (OUT, DUF3770 and Bunya-RdRp domain) of the protein. The numbers denote RSV RdRp amino acid positions. The ability of RSV RdRp fragments to interact with both OsHSP20 and NbHSP20 in YTH assays is shown on the right (+, positive; −, negative). (B) Yeast colonies expressing BD-RdRp1–296 with AD-OsHSP20 grew well on the selective medium, but those expressing BD-RdRp297–987, BD-RdRp988–1810, BD-RdRp1811–2387 or BD-RdRp2388–2920 with AD-OsHSP20 did not. (C) Yeast colonies expressing BD-RdRp1–296 with AD-NbHSP20 grew well on the selective medium, but those expressing BD-RdRp297–987, BD-RdRp988–1810, BD-RdRp1811–2387 or BD-RdRp2388–2920 with AD-NbHSP20 did not. Yeast co-transformed with BD-53 and AD-T, and BD-Lam and AD-T were used as the positive and negatives control, respectively.
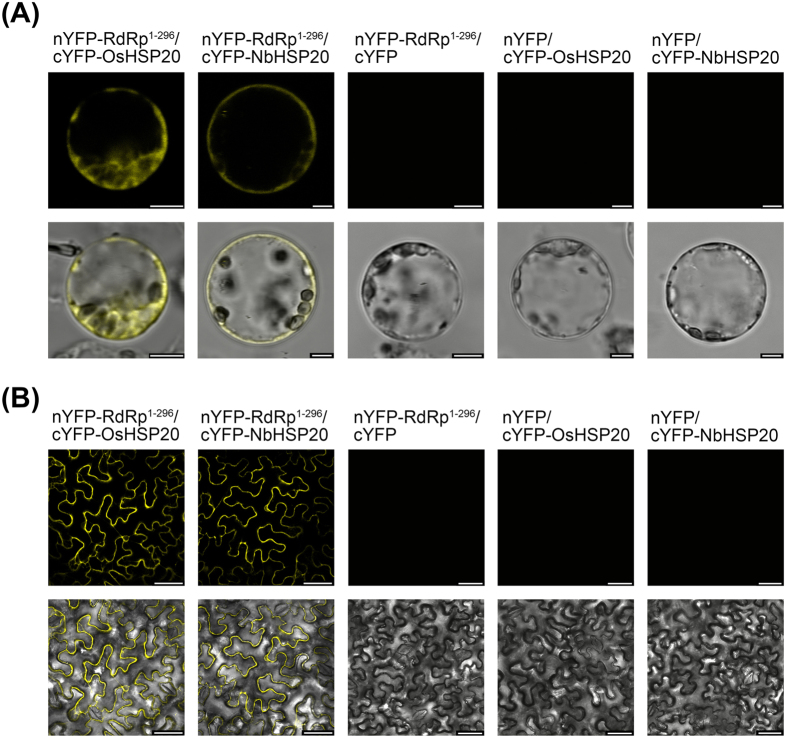
To verify the YTH results, we used bimolecular fluorescence complementation (BiFC) assays to test the in vivo interactions between the N-terminus (residues 1–296) of RSV RdRp and host HSP20 in living plant cells. The coding sequence of RdRp1–296 was cloned into the vector pCV-nYFP-C and that of OsHSP20 or NbHSP20 into pCV-cYFP-C to generate plasmids pCV-nYFP-RdRp1–296, pCV-cYFP-OsHSP20 and pCV-cYFP-NbHSP20, respectively. Rice protoplasts were transfected with pCV-nYFP-RdRp1–296/pCV-cYFP-OsHSP20 and pCV-nYFP-RdRp1–296/pCV-cYFP-NbHSP20, while the pairs pCV-nYFP-RdRp1–296/pCV-cYFP, pCV-nYFP/pCV-cYFP-OsHSP20 and pCV-nYFP/pCV-cYFP-OsHSP20 were used as the negative controls. N. benthamiana leaves were transformed by co-infiltration with A. tumefaciens C58C1 cells harbouring the same combinations. Samples were examined for YFP fluorescence using confocal laser scanning microscopy. As shown in Fig. 7, co-expression of pCV-nYFP-RdRp1–296/pCV-cYFP-OsHSP20 or pCV-nYFP-RdRp1–296/pCV-cYFP-NbHSP20 in both rice protoplasts (18 h after transfection) (Fig. 7A) and N. benthamiana leaf cells (48 h after infiltration) (Fig. 7B) resulted in a strong YFP fluorescence in the cytoplasm and nucleus, whereas no fluorescence was observed in the negative controls.
Figure 7. Interactions between the N-terminus (residues 1-296) of RSV RdRp and host HSP20 in living plant cells.
(A) Visualization of RdRp1–296-HSP20 interaction in rice protoplasts by BiFC assay. Rice protoplasts were co-transfected with recombinant BiFC vectors containing the constructs indicated above the images. The results were observed 18 h after transfection. Scale bar, 5 μm. (B) Visualization of RdRp1–296-HSP20 interaction in N. benthamiana epidermal cells by BiFC assay. N. benthamiana leaves were co-infiltrated with recombinant BiFC vectors containing the constructs indicated above the images. The results were observed 48 h after infiltration. Scale bar, 50 μm. The fluorescent and merged images are depicted in the upper and lower panels, respectively.
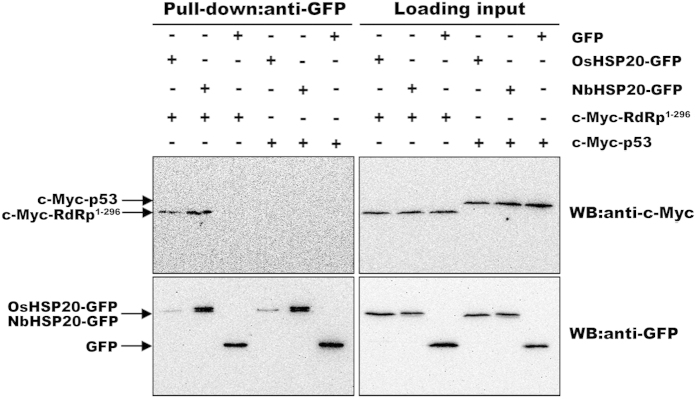
Interactions between the N-terminus of RSV RdRp and host HSP20 were further confirmed through in vitro pull-down assays. Equal amounts of in vitro translated c-Myc-RdRp1–296 and OsHSP20-GFP or NbHSP20-GFP were mixed and then pulled down with the GFP-Trap M beads followed by western blot assays using an anti-c-Myc or anti-GFP antibody. In these experiments, combinations of OsHSP20-GFP and c-Myc-p53, NbHSP20-GFP and c-Myc-p53, GFP and c-Myc-RdRp1–296, and GFP and c-Myc-p53 were used as controls. Immunoblot analyses using an anti-c-Myc antibody demonstrated that c-myc-RdRp1–296 was pulled down by OsHSP20-GFP and NbHSP20-GFP, but not by the control GFP (Fig. 8, top), although the anti-GFP antibody did pull-down OsHSP20-GFP, NbHSP20-GFP and control GFP proteins (Fig. 8, bottom). These experiments clearly demonstrated that RdRp1–296 bound to both OsHSP20-GFP and NbHSP20-GFP in vitro. Thus these results consistently indicated that the N-terminal part of RSV RdRp was responsible for its interactions with OsHSP20 or NbHSP20 in vivo and in vitro.
Figure 8. In vitro pull-down analysis of interactions between the N-terminus of RSV RdRp and host HSP20.
In vitro translated c-Myc-RdRp1–296 or c-Myc-p53 fusion protein was incubated with equal amount of OsHSP20-GFP, NbHSP20-GFP or GFP, and accreted to GFP-Trap M beads. Beads were washed and analyzed by western blot assays using an anti-c-Myc antibody (upper) or an anti-GFP antibody (lower). The two right membranes show the inputs of in vitro translated proteins in pull-down assays. Black arrows indicate the corresponding bands of c-Myc-p53, c-Myc-RdRp1–296, OsHSP20-GFP, NbHSP20-GFP and GFP, respectively.
Both sub-cellular localization and distribution patterns of OsHSP20 and NbHSP20 were affected by the N-terminal part of RSV RdRp
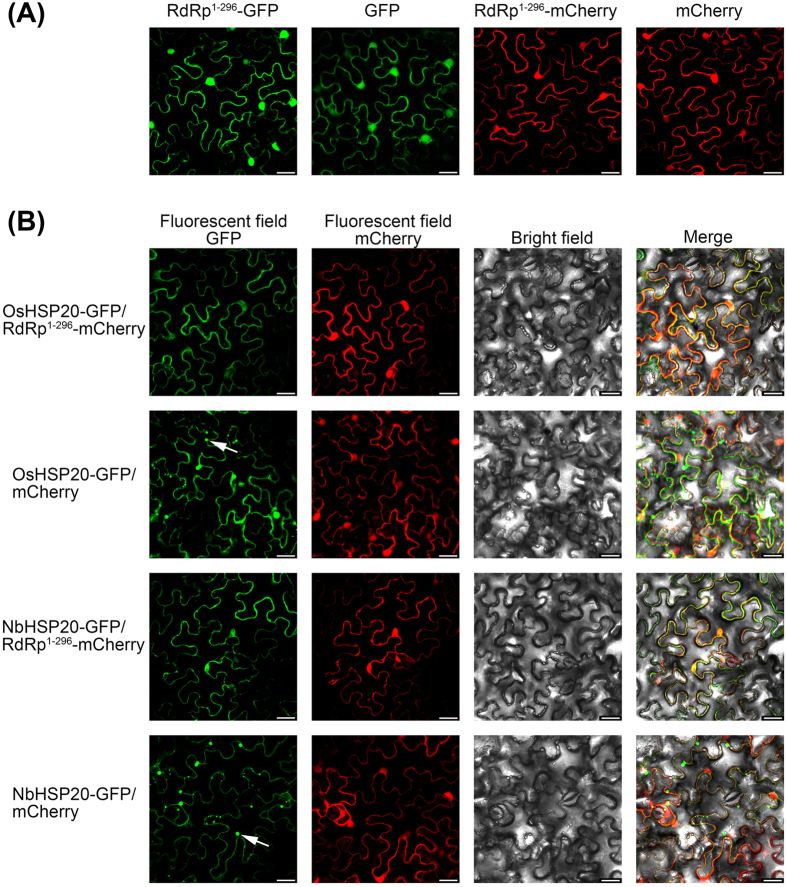
To determine whether the sub-cellular alteration of HSP20s was associated with that of RSV RdRp, plasmids expressing RdRp1–296-GFP and RdRp1–296-mCherry were first constructed and introduced into N. benthamiana epidermal cells by Agrobacterium infiltration. Fluorescence microscopy indicated that both RdRp1–296-GFP and RdRp1–296-mCherry resulted in a pattern of diffuse and uniform fluorescence in the cytoplasm and nucleus at 2 dpi (Fig. 9A). When RdRp1–296-mCherry was co-expressed with either OsHSP20-GFP or NbHSP20-GFP, the two proteins co-localized in a pattern identical to that formed by RdRp1–296-mCherry alone (Fig. 9B) and no GFP granules were seen in the cytoplasm. Control combinations ruled out the possibility that GFP or mCherry expression might have some aberrant effects on the distribution of RdRp1–296-mCherry, OsHSP20-GFP or NbHSP20-GFP. The co-localization of RdRp1–296 and HSP20 confirmed that the interaction between RSV RdRp and HSP20 mediated a dramatic effect on the distribution of HSP20. The sub-cellular distribution of GFP-fused OsHSP20 or GFP-fused NbHSP20 when co-expressed with RSV RdRp was very similar to that in RSV-infected cells, suggesting that RSV RdRp was responsible for both sub-cellular localization and distribution patterns of OsHSP20 and NbHSP20 in infected plants.
Figure 9. Localization of OsHSP20 or NbHSP20 was affected by expression of the N-terminus of RSV RdRp.
(A) Sub-cellular localization of RSV RdRp1–296 fused with GFP or mCherry in N. benthamiana leaf epidermal cells. (B) Epidermal cells of N. benthamiana transiently co-expressing RdRp1–296-mCherry and OsHSP20-GFP or NbHSP20-GFP. The white arrow points to a granule. The results were observed 48 h after infiltration. Scale bar, 25 μm.
Discussion
sHSPs, defined by possessing a conserved α-crystallin domain (ACD), are the most abundant and complex subset of HSPs in plants41. A key function of the sHSPs is to prevent aggregation of denatured proteins. By forming a soluble complex with substrate proteins, they can create a transient reservoir of substrates for subsequent refolding by ATP-dependent chaperone systems6,8,10,11. Rice has a total of 14 sHSPs subfamilies, which are predicted to locate to various cellular organelles, including the cytosol, nucleus, chloroplasts, mitochondria, endoplasmic reticulum, and peroxisomes42. Organelle-targeted sHSPs are unique to plants and their diverse functions are thought to be associated with their sub-cellular localization43 although little is known about their distinct functions41. Here both OsHSP20 and NbHSP20 were shown to be localized within the cytosol (Fig. 3), which was consistent with the predicted localization of the Class I (CI) subfamily of sHSPs42, the subfamily of which both HSP20s appear to be members. Sequence analysis showed that such HSP20s from a variety of plant species contained a highly conserved motif in the N-terminal part that has not previously been described in addition to the characteristic ACD domain of sHSPs43. The novel conserved motif “DPFSLDVWDPF” may act as a signal involved in their localization. Within the cytosol, both GFP-fused OsHSP20 and NbHSP20 formed fluorescent granules of different sizes and shapes when expressed alone in N. benthamiana epidermal cells or rice protoplasts (Fig. 3) and strong self-interactions were always observed in yeast colonies co-expressing BD-OsHSP20/AD-OsHSP20, or BD-NbHSP20/AD-NbHSP20 (Fig. 2), suggesting that both HSP20 proteins could function in vivo as dimers or larger oligomers. This was consistent with many previous reports showing that some sHSPs formed large oligomers in vitro from multiple subunits41,44,45. Interestingly, the fluorescent granules formed by GFP-fused OsHSP20 and NbHSP20 were able to move within the cytosol, suggesting that they might interact with other components of the plants. Most likely, these cytoplasmic granules were aggregates consisting mainly of oligomerized sHSPs, which could be, to a degree, similar in structure and composition to those of heat shock granules formed by accumulation of HSPs under hyper-thermic condition9. Further work is needed to identify those components and help understand the significance of these granules.
Interactions between host plant proteins and viral components are presumed to play an important role in the RSV life cycle or in viral pathogenicity. In addition to interactions between RSV or specific RSV gene products and host proteins that have already been reported32,37,38, we have now shown that the expression pattern and sub-cellular distribution of an OsHSP20 gene was regulated by RSV infection. This is the first report that sHSPs could be manipulated by a plant virus. A series of in vivo and in vitro protein-protein interaction assays further showed that the HSP20 interacted with the RSV RdRp and is the first report of a sHSP-plant viral RdRp interaction.
In the YTH assays, host HSP20 proteins interacted specifically with the N-terminus (residues 1–296) of RSV RdRp (Fig. 6). This segment contains a viral ovarian tumour (OTU) domain near its N-terminus which is thought to have deubiquitination activity40,46. The deubiquitylating function of viral RdRp has been suggested to provide a way to interfere with the proteolytic pathway in host plants during viral infection40,46. This might be a novel strategy of viral pathogenicity considering that sHSPs function as important players in regulating cellular proteostasis47. A previous study reported that RSV pc4 interacted with a small heat shock protein (EU325986) from Wuyujing 3, a susceptible japonica rice cultivar48, in yeast cells. However, in our YTH assays, only interaction between the OsHSP20 and viral RdRp was detected. To determine whether the HSP20 interacted with RSV pc4, we also investigated the pc4-OsHSP20 or pc4-NbHSP20 interaction by YTH assays, but no interaction was detected between the two proteins (Figure S3). Nucleotide sequence analysis indicated that pc4 gene sequences varied among RSV isolates from different geographical origins49, which might explain why pc4 did not interact with OsHSP20 or NbHSP20 in our experiments.
In transient expression systems, GFP fluorescence appeared as granular structures in the cytoplasm when HSP20 was expressed alone in N. benthamiana epidermal cells or rice protoplasts (Fig. 3). However, no GFP granules or reconstituted YFP-fluorescent granules were detected when the N-terminus of RSV RdRp and HSP20 were co-expressed in the leaves of N. benthamiana or in rice protoplasts (Figs 7 and 9), and the RdRp1–296/HSP20 complex had a diffuse distribution pattern in the cytoplasm and nucleus (Fig. 7), similar to that in N. benthamiana epidermal cells co-expressing RdRp1–296-mCherry/OsHSP20-GFP or RdRp1–296-mCherry/NbHSP20-GFP (Fig. 9). These results consistently suggested that the strong interactions between the N-terminus of RSV RdRp and HSP20 significantly affected the sub-cellular localization and distribution pattern of host HSP20 proteins. We hypothesize that the hetero-interaction between the HSP20 and viral RdRp destroyed the self-interaction of HSP20, leading to the disappearance of fluorescent granules and the diffuse distribution pattern of HSP20. If sub-cellular localization and self-interaction in vivo is important for its chaperone activities, it would be expected that this distributional change would affect the function of HSP20. In addition, RSV infection also affected the sub-cellular distribution of host HSP20 proteins. The GFP granules were not present if OsHSP20-GFP or NbHSP20-GFP were expressed in the cytoplasm of RSV-infected N. benthamiana epidermal cells (Fig. 4).
Previous research has revealed that some plant HSPs (especially HSP70s) were involved in the viral life cycle, including cell entry, virion assembly, the transfer of the viral genome segments into the nucleus, replication of the viral genome, morphogenesis of the virion particles and transformation of the cell4,50. However, the biological function of plant sHSPs in viral infection has been unknown. We have now demonstrated that the host HSP20 proteins interacted with the viral RdRp of RSV and that the N-terminal part of the RdRp was crucial for this interaction. Further experimental work will be needed to understand the significance of this interaction but the fact that the viral RdRp interferes with the self-interaction of the host HSP20 suggested that the virus might suppress the formation of stress granules that could potentially be an anti-viral response. Stress granules are initially induced but then dispersed in animal cells infected by poliovirus51 and this could be a broadly similar effect. Alternatively (or additionally), it is interesting that a previous study showed that HSP70 is necessary for RSV infection and that HSP70 also interacts with the N-terminal part of RSV RdRp. This may suggest that interactions between various HSPs and the viral RdRp play an important role in viral replication. These findings are therefore a step forward in understanding a virus that causes a seriously damaging disease of one of the most important crop plants in the world.
Materials and Methods
Plant materials and growth conditions
Nicotiana benthamiana plants were grown in 10-cm pots filled with a mixture of 60% vermiculite and 40% meadow soil and mechanically inoculated at the six-leaf stage with crude extracts from RSV-infected O. sativa leaves, as described previously52. Rice (O. sativa) was grown by germinating seeds on mesh supported in plastic containers containing ½ strength Murashige and Skoog (MS) nutrient solution. All plants used in this study were grown in a growth chamber at 25 °C with 16 h light/8 h dark and 70% r.h.
Cloning and sequencing of a rice sHSP and its homolog in Nicotiana benthamiana
The coding sequence of intact OsHSP20 was amplified by PCR with the primer pair Os20F/Os20R (Table 1) from a leaf cDNA library of rice cv. Nipponbare plants, ligated into the pGEM-T vector (Promega, Madison, WI, USA), and then transformed into competent Escherichia coli DH5α. The recombinant plasmid DNA (pGEM-OsHSP20), used for sequencing was prepared using the QIAprep spin mini prep kit (Qiagen), and the inserts were sequenced entirely on both strands using the BigDye Terminator v3.1 Cycle Sequencing Kit (Perkin Elmer Applied Biosystems, Foster, USA) on an ABI PRISM 3730 DNA Sequencer (Perkin Elmer Applied Biosystems, Foster, USA) with universal primers T7 and SP6. The sequence of HSP20 gene from N. benthamiana was searched on the website of the SOL genomic network (http://solgenomics.net). The full-length coding sequence of a homologue of OsHSP20 in N. benthamiana (Niben.v0.4.2.Scf54613, named as NbHSP20 in this study) was cloned with the primer pair Nb20F/Nb20R (Table 1) from N. benthamiana leaf cDNA and the resultant recombinant plasmid DNA (pGEM-NbHSP20) was sequenced as described above. Sequence assembly and analysis was performed using the DNAman version 6.0 program (Lynnon BioSoft, Quebec, Canada).
Yeast two-hybrid (YTH) assays
The yeast GAL4 binding domain vector pGBKT7 and GAL4 activation domain vector pGADT7 (Clontech, Palo Alto, CA) were used for yeast two hybrid (YTH) assays. RSV- Zhejiang isolate and its cDNA library were from our laboratory40. To construct plasmids for YTH analysis, the coding sequence of OsHSP20 was digested from the recombinant plasmids pGEM-OsHSP20 with the restriction enzymes NdeI and BamHI and then inserted into the NdeI/BamHI sites of yeast GAL4 binding domain pGBKT7 and activation domain pGADT7 vectors, creating the recombinant bait plasmid BD-OsHSP20 and prey plasmid AD-OsHSP20, respectively. The coding sequence of NbHSP20 was digested from the recombinant plasmid pGEM-NbHSP20 with restriction enzymes NdeI and EcoRI, and then inserted into the NdeI/EcoRI sites of pGBKT7 and pGADT7 vectors, creating the recombinant plasmids BD-NbHSP20 (bait) and AD-NbHSP20 (prey).
In further YTH assays, the coding sequences of five fragments of RSV RdRp (1–296, 297–987, 988–1810, 1811–2387 and 2388–2920) were amplified separately from RSV cDNA using primer pairs R1-1F/R1-1R, R1-2F/R1-2R, R1-3F/R1-3R, R1-4F/R1-4R and R1-5F/R1-5R (Table 1), respectively. The products were then inserted into the NdeI/BamHI, NdeI/EcoRI or the unique BamHI site of pGBKT7 and pGADT7, creating the recombinant bait and prey plasmids, BD- and AD-RdRp1–296, -RdRp297–987, -RdRp988–1810, -RdRp1811–2387 and -RdRp2388–2920, respectively.
Yeast transformation and library screening were conducted in accordance with the recommended procedures (Matchmaker Gold Yeast Two-Hybrid System; Yeastmaker Yeast Transformation System 2, Clontech). Briefly, the RSV cDNA library was screened using BD-OsHSP20 and -NbHSP20 as the baits in Saccharomyces cerevisiae strain Y2HGold (Clontech). Yeast transformants were selected on a synthetic defined medium lacking Ade, His, Leu, and Trp (SD/-Ade/-His/-Leu/-Trp) and transferred onto synthetic defined medium lacking Ade, His, Leu, and Trp with 40 μg ml−1 X-α-Gal and 70 ng ml−1 AbA (SD/-Ade/-His/-Leu/-Trp/X-α-Gal/AbA). The positive candidate plasmids containing the viral cDNAs were isolated and determined by sequencing. Protein-protein interactions were confirmed in yeast by co-transformation into S. cerevisiae strain Y2HGold with bait and prey plasmids53. Co-transformants were first plated on SD/-Ade/-His/-Leu/-Trp medium, and positive yeast colonies that grew on the auxotrophic medium were then tested for α-galactosidase activity on SD/-Ade/-His/-Leu/-Trp/X-α-Gal/AbA medium. BD-53 and AD-T were also co-transformed as a positive control, while BD-Lam and AD-T were co-transformed as a negative control. Three independent experiments were performed to confirm the results.
In vitro pull-down assay and western blot analysis
For the in vitro pull-down assay, the fusion gene c-Myc-RdRp1–296 was amplified by PCR with primer pair P1-1F/P1-1R (Table 1) using BD-RdRp1–296 as template and was digested with KpnI/NotI and cloned into the same sites of the pCMVTNT expression vector (Promega, Madison, WI, USA), producing pCMV:c-Myc-RdRp1–296. The fusion genes OsHSP20-GFP and NbHSP20-GFP were amplified by PCR with primer pairs POs20F/POs20R and PNb20F/PNb20R (Table 1) using pCV-OsHSP20-GFP and pCV-NbHSP20-GFP as templates, respectively. The amplified PCR products were digested with EcoRI/KpnI or EcoRI/NotI, and cloned into the same sites of pCMVTNT, producing pCMV:OsHSP20-GFP and pCMV:NbHSP20-GFP, respectively. GFP and c-Myc-p53 sequences were amplified from pCV-GFP-N1 and pGBKT7–53 (Clontech) using primer pairs GFPF/POs20R and P53F/P53R (Table 1), respectively. The PCR products were then inserted into the pCMVTNT vector via the EcoRI/KpnI or SalRI/NotI sites, creating the control plasmids pCMV:GFP and pCMV:p53, respectively.
These PCR amplifications used LA Taq polymerase (TaKaRa Bio, Dalian, China), and the PCR amplification program as follows: preheating for one cycle of 3 min at 94 °C; 30 cycles of 30 s at 94 °C, 40 s at 58 °C, 1–3 min at 72 °C; and a final extension at 72 °C for 10 min. All clones derived from the PCR products were verified by sequencing, and the recombinant plasmids were confirmed by restriction analyses.
The in vitro pull-down assay was performed using a TNT Coupled Wheat Germ Extract System following the manufacturer’s recommendations (Promega). The plasmids for the generation of c-Myc-p53 fusion protein and GFP protein were used as negative controls in the experiments. In vitro translated c-Myc-RdRp1–296 (15 μL) and OsHSP20-GFP (15 μL) or NbHSP20-GFP (15 μL) were mixed and incubated at 4 °C overnight, then 20 μL of GFP-Trap M beads (ChromoTek, Martinsried, Germany) were added and incubated for another 6 h. The beads were washed four times with 0.5 ml washing buffer (0.05 M Tris-HCl, 0.15 M NaCl) and the bound complexes were eluted by boiling with 1 × protein loading buffer for 5 min. Samples were analyzed by western blotting as described elsewhere38. Briefly, Protein samples were separated by 12% SDS-PAGE gel electrophoresis and transferred by electroblotting to PVDF membrane (Millipore, Bedford, MA, USA). Transferred proteins were detected with anti-c-Myc or anti-GFP (monoclonal antibody; 1:2,000 dilution; Quanshijin, Beijing, China) primary antibodies and an anti-mouse horseradish peroxidase-conjugated secondary antibody (1:5,000 dilution; Kangweishiji, Beijing, China).
Rice protoplast isolation and transfection
Rice protoplasts were isolated from 2-week-old seedlings as described54 with minor modifications. Briefly, young leaves and sheaths were chopped and dipped in enzyme solution (0.5 M mannitol, 1.5% cellulose RS (Yakult Honsa, Tokyo, Japan), 0.75% macerozyme R10 (Yakult Honsa), 1 mM CaCl2, and 0.1% BSA). This mixture was incubated on a shaking incubator (60 rpm) for 4 to 5 h at room temperature in the dark then filtered through Miracloth. Protoplasts were pelleted by centrifugation for 5 min at 200 g and resuspended in an equal volume of W5 solution (154 mM NaCl, 125 mM CaCl2, 5 mM KCl, and 1.5 mM MES, adjusted to pH 5.7). Protoplasts were centrifuged and re-suspended in MMG solution (0.4 M mannitol, 15 mM MgCl2, and 4.7 mM MES, adjusted to pH 5.7). Plasmid DNA (10 or 20 μg) was added to the protoplast solution and transfected with 40% polyethylene glycol (PEG) solution (40% PEG 4000, 0.4 M mannitol, and 100 mM Ca (NO3)2) for 20min at room temperature. W5 solution was added stepwise to dilute the PEG solution and discarded. Transfected protoplasts were incubated overnight at room temperature and then observed under confocal microscopy.
Sub-cellular localization and BiFC assay
The binary expression vectors pCV-GFP-N1 and pCV-mCherry-N1, and the BiFC vectors pCV-nYFP-C and pCV-cYFP-C (for split YFP N-terminal/C-terminal fragment expression) were previously constructed in our laboratory55. For BiFC assays, the coding sequence of the N-terminal fragment (residues 1–296) of RSV RdRp was amplified using primer pair B1–1F/B1–1R (Table 1) and cloned into pCV-nYFP-C as a fusion with the N-terminal fragment of YFP via the BamHI/SacI sites, forming pCV-nYFP-RdRp1–296. The full-length coding sequences of OsHSP20 and NbHSP20 were amplified by PCR using primer pairs BOs20F/BOs20R and BNb20F/BNb20R (Table 1), respectively. PCR products were cloned into the BamHI/SacI or KpnI/SacI sites of pCV-cYFP-C as a fusion with the C-terminal fragment of YFP, resulting in pCV-cYFP-OsHSP20 and pCV-cYFP-NbHSP20, respectively.
For sub-cellular localization studies, the coding sequence of the N-terminal fragment (residues 1–296) of RSV RdRp was amplified using primer pair B1–1F/G1–1R (Table 1) and cloned into pCV-GFP-N1 and pCV-mCherry-N1 via the BamHI/SalI sites, forming pCV-RdRp1–296-GFP and pCV-RdRp1–296-mCherry, respectively. The full-length coding sequences of OsHSP20 and NbHSP20 were amplified by PCR with primer pairs BOs20F/GOs20R and BNb20F/GNb20R (Table 1), respectively. The products were subsequently digested with BamHI/SalI or KpnI and ligated into the corresponding sites of pCV-GFP-N1, generating recombinant plasmids pCV-OsHSP20-GFP and pCV-NbHSP20-GFP, respectively.
For the BiFC assay in rice protoplasts, transient transfection of rice protoplast cultures with the combinations pCV-nYFP-RdRp1–296/pCV-cYFP-OsHSP20 and pCV-nYFP-RdRp1–296/pCV-cYFP-NbHSP20 were performed according to the protocol above, while the combinations pCV-nYFP-RdRp1–296/pCV-cYFP, pCV-nYFP/pCV-cYFP-OsHSP20 and pCV-nYFP/pCV-cYFP-NbHSP20 were used as the negative controls. For sub-cellular localization in rice protoplasts, the recombinant plasmids pCV-OsHSP20-GFP and pCV-NbHSP20-GFP were transfected into rice protoplasts, respectively. Fluorescence was detected in rice protoplasts 16–20 h after transfection.
The recombinant binary constructs above were introduced into Agrobacterium tumefaciens strain C58C1 by electroporation (Bio-Rad Gene Pulser, 0.2 cm cuvettes, 25 microF, >2.5 kV). Agroinfiltration was done as described56 with a few modifications. Briefly, cultures of C58C1 harbouring a relevant binary plasmid were grown in YEP medium containing rifampicin (50 μg ml−1) and kanamycin (100 μg ml−1) at 28 °C for 16 h. For the BiFC assay, C58C1 strains containing the BiFC plasmids were re-suspended and adjusted to an OD600 of 0.8:0.8 with infiltration medium (10 mM MES, pH 5.6, 10 mM MgCl2, 200 mM acetosyringone) before leaf infiltration. For sub-cellular localization, Agrobacterium cultures containing the pCV derivatives were re-suspended and diluted to an OD600 of 0.6 before leaf infiltration. The cell suspensions were incubated at room temperature for 2 to 4 h and then used to infiltrate 5- to 6-week-old N. benthamiana leaves. Expression of fluorescent proteins was examined at 48 h post agroinfiltration57.
Confocal microscopy
Fluorescence analysis was performed using a Leica TCS SP5 confocal laser scanning microscope (Leica Microsystems, Heidelberg, Germany). GFP was excited at 488 nm and the emitted light captured between 500–550 nm, YFP was excited at 514 nm and the emitted light captured between 530–600 nm, and mCherry was excited at 561 nm and emission light captured between 570–630 nm. For analysis of co-localization assays, multi-tracking was used to prevent emission cross-talk between the channels. Images were captured digitally and handled using the Leica TCS software. Post-acquisition image processing was done with Adobe Photoshop version 7.0 software (Adobe Systems Inc., San Jose, CA, USA).
Additional Information
How to cite this article: Li, J. et al. Interaction of HSP20 with a viral RdRp changes its sub-cellular localization and distribution pattern in plants. Sci. Rep. 5, 14016; doi: 10.1038/srep14016 (2015).
Supplementary Material
Acknowledgments
This work was funded by National Science and Technology Support Program (2012BAD19B03), the China 973 Program (2010CB126203), 863 program (2007AA10Z414), and the Zhejiang Provincial Foundation for Natural Science (LQ14C140003 and Y3090657). We thank Professor M.J. Adams, Stevenage, UK for help in correcting the English of the manuscript.
Footnotes
Author Contributions H.Z. and J.C. were responsible for study conception, design, and coordination; J.L. and C.X. carried out the experiments for vector construction, yeast two hybridization, agro-infiltration, rice protoplast transformation, and fluorescence assays; J.L., C.X. and J.Y. performed the experiments for gene expression. H.Z., J.L. and J.C. were responsible for data collection and analysis and drafted the manuscript. All authors read and approved the final manuscript.
References
- Ahuja I., de Vos R. C., Bones A. M. & Hall R. D. Plant molecular stress responses face climate change. Trends Plant Sci. 15, 664–674, 10.1016/j.tplants.2010.08.002 (2010). [DOI] [PubMed] [Google Scholar]
- Timperio A. M., Egidi M. G. & Zolla L. Proteomics applied on plant abiotic stresses: role of heat shock proteins (HSP). J. Proteomics 71, 391–411, 10.1016/j.jprot.2008.07.005 (2008). [DOI] [PubMed] [Google Scholar]
- Wang W., Vinocur B., Shoseyov O. & Altman A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 9, 244–252, 10.1016/j.tplants.2004.03.006 (2004). [DOI] [PubMed] [Google Scholar]
- Mayer M. P. Recruitment of Hsp70 chaperones: a crucial part of viral survival strategies. Rev. Physiol. Biochem. Pharmacol. 153, 1–46, 10.1007/s10254-004-0025-5 (2005). [DOI] [PubMed] [Google Scholar]
- Mayer M. P. & Bukau B. Hsp70 chaperone systems: diversity of cellular functions and mechanism of action. Biol. Chem. 379, 261–268 (1998). [PubMed] [Google Scholar]
- Haslbeck M., Franzmann T., Weinfurtner D. & Buchner J. Some like it hot: The structure and function of small heat shock proteins. Nat. Struct. Mol. Biol. 12, 842–846, 10.1038/nsmb993 (2005). [DOI] [PubMed] [Google Scholar]
- Sun W., Van Montagu M. & Verbruggen N. Small heat shock proteins and stress tolerance in plants. Biochim. Biophys. Acta 1577, 1–9, 10.1016/S0167-4781(02)00417-7 (2002). [DOI] [PubMed] [Google Scholar]
- Sun Y. & MacRae T. H. Small heat shock proteins: Molecular structure and chaperone function. Cell. Mol. Life Sci. 62, 2460–2476, 10.1007/s00018-005-5190-4 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover L., Scharf K. D. & Neumann D. Cytoplasmic heat shock granules are formed from precursor particles and are associated with a specific set of mRNAs. Mol. Cell. Biol. 9, 1298–1308, 10.1128/MCB.9.3.1298 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G. J., Roseman A. M., Saibil H. R. & Vierling E. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 16, 659–671, 10.1093/emboj/16.3.659 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHaourab H. S., Godar J. A. & Stewart P. L. Structure and mechanism of protein stability sensors: Chaperone activity of small heat shock proteins. Biochem. 48, 3828–3837, 10.1021/bi900212j (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimbo M., Ohnishi K., Hikichi Y., Yoshioka H. & Kiba A. Induction of a Small Heat Shock Protein and its functional roles in Nicotiana plants in the defense response against Ralstonia solanacearum. Plant Physiol. 145, 1588–1599, 10.1104/pp.107.105353 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowska J. et al. Stable formation of compositionally unique stress granules in virus-infected cells. J. Virol. 84, 3654–3665, 10.1128/JVI.01320-09 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovits R., Moshe A., Ghanim M. & Czosnek H. Recruitment of the host plant heat shock protein 70 by tomato yellow leaf curl virus coat protein is required for virus infection. PLoS ONE 8, e70280, 10.1371/journal.pone.0070280 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenz B., Windeisen V., Wege C., Jeske H. & Kleinow T. A plastid-targeted heat shock cognate 70 kDa protein interacts with the abutilon mosaic virus movement protein. Virology 401, 6–17, 10.1016/j.virol.2010.02.011 (2010). [DOI] [PubMed] [Google Scholar]
- Dufresne P. J. et al. Heat shock 70 protein interaction with turnip mosaic virus RNA-dependent RNA polymerase within virus-induced membrane vesicles. Virology 374, 217–227, 10.1016/j.virol.2007.12.014 (2008). [DOI] [PubMed] [Google Scholar]
- Hibino H. Biology and epidemiology of rice viruses. Annu. Rev. Phytopathol. 34, 249–274, 10.1146/annurev.phyto.34.1.249 (1996). [DOI] [PubMed] [Google Scholar]
- Park H. M. et al. Suppression of NS3 and MP is important for the stable inheritance of RNAi-mediated Rice stripe virus (RSV) resistance obtained by targeting the fully complementary RSV-CP gene. Mol. Cells 33, 43–51, 10.1007/s10059-012-2185-5 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. D. et al. Recent Rice stripe virus epidemics in Zhejiang province, China, and experiments on sowing date, disease–yield loss relationships, and seedling susceptibility. Plant Dis. 92, 1190–1196, 10.1094/PDIS-92-8-1190 (2008). [DOI] [PubMed] [Google Scholar]
- Satoh K. et al. Selective modification of rice (Oryza sativa) gene expression by rice stripe virus infection. J. Gen. Virol. 91, 294–305, 10.1099/vir.0.015990-0 (2010). [DOI] [PubMed] [Google Scholar]
- Toriyama S. Rice stripe virus. Descriptions of plant Viruses no. 375. http://www.dpvweb.net/dpv/showdpv.php?dpvno=375 (2000). 15/06/2000.
- Falk B. W. & Tsai J. H. Biology and molecular biology of viruses in the genus Tenuivirus. Annu. Rev. Phytopathol. 36, 139–163, 10.1146/annurev.phyto.36.1.139 (1998). [DOI] [PubMed] [Google Scholar]
- Li S., Xiong R., Wang X. & Zhou Y. Five proteins of Laodelphaxstriatellus are potentially involved in the interactions between Rice stripe virus and vector. PLoS ONE 6, e26585, 10.1371/journal.pone.0026585 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong R., Wu J., Zhou Y. & Zhou X. Identification of a movement protein of the tenuivirus rice stripe virus. J. Virol. 82, 12304–12311, 10.1128/JVI.01696-08 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. et al. The rice stripe virus pc4 functions in movement and foliar necrosis expression in Nicotiana benthamiana. Virology 425, 113–121, 10.1016/j.virol.2012.01.007 (2012). [DOI] [PubMed] [Google Scholar]
- Ramirez B. C. & Haenni A. L. Molecular biology of tenuiviruses, a remarkable group of plant viruses. J. Gen. Virol. 75, 467–475, 10.1099/0022-1317-75-3-467 (1994). [DOI] [PubMed] [Google Scholar]
- Barbier P., Takahashi M., Nakamura I., Toriyama S. & Ishihama A. Solubilization and promoter analysis of RNA polymerase from Rice stripe virus. J. Virol. 66, 6171–6174 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toriyama S., Takahashi M., Sano Y., Shimizu T. & Ishihama A. Nucleotide sequence of RNA 1, the largest genomic segment of Rice stripe virus, the prototype of the tenuiviruses. J. Gen. Virol. 75, 3569–3579, 10.1099/0022-1317-75-12-3569 (1994). [DOI] [PubMed] [Google Scholar]
- Takahashi M., Toriyama S., Hamamatsu C. & Ishihama A. Nucleotide sequence and possible ambisense coding strategy of Rice stripe virus RNA segment 2. J. Gen. Virol. 74, 769–773, 10.1099/0022-1317-74-4-769 (1993). [DOI] [PubMed] [Google Scholar]
- Zhu Y., Hayakawa T., Toriyama S. & Takahashi M. Complete nucleotide sequence of RNA 3 of Rice stripe virus: an ambisense coding strategy. J. Gen. Virol. 72, 763–767, 10.1099/0022-1317-72-4-763 (1991). [DOI] [PubMed] [Google Scholar]
- Zhu Y., Hayakawa T. & Toriyama S. Complete nucleotide sequence of RNA 4 of Rice stripe virus isolate T, and comparison with another isolate and with Maize stripe virus. J. Gen. Virol. 73, 1309–1312, 10.1099/0022-1317-73-5-1309 (1992). [DOI] [PubMed] [Google Scholar]
- Du Z. et al. p2 of Rice stripe virus (RSV) interacts with OsSGS3 and is a silencing suppressor. Mol. Plant Pathol. 12, 808–814, 10.1111/j.1364-3703.2011.00716.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S. L., Dai X. J., Liang J. S. & Liang C. Y. Surface display of rice stripe virus NSvc2 and analysis of its membrane fusion activity. Virol. Sin. 27, 100–108, 10.1007/s12250-012-3237-x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. et al. Rice Stripe Tenuivirus NSvc2 Glycoproteins Targeted to the Golgi Body by the N-Terminal Transmembrane Domain and Adjacent Cytosolic 24 Amino Acids via the COP I- and COP II-Dependent Secretion Pathway. J. Virol. 88, 3223–3234, 10.1128/JVI.03006-13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong R., Wu J., Zhou Y. & Zhou X. Characterization and sub-cellular localization of an RNA silencing suppressor encoded by rice stripe tenuivirus. Virology 387, 29–40, 10.1016/j.virol.2009.01.045 (2009). [DOI] [PubMed] [Google Scholar]
- Toriyama S. Rice stripe virus: prototype of a new group of viruses that replicate in plants and insects. Microbiol Sci. 3, 347–351 (1986). [PubMed] [Google Scholar]
- Kong L., Wu J., Lu L., Xu Y. & Zhou X. Interaction between rice stripe virus disease-specific protein and host PsbP enhances virus symptoms. Mol. Plant. 7, 691–708, 10.1093/mp/sst158 (2014). [DOI] [PubMed] [Google Scholar]
- Jiang S. et al. Heat shock protein 70 is necessary for Rice stripe virus infection in plants. Mol. Plant Pathol. 15, 907–917, 10.1111/mpp.12153 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. T., Xie L., Lin Q. Y., Wu Z. J. & Xie L. H. Transcriptional profiling in rice seedlings infected by rice stripe virus. Acta Laser Biol. Sinica 5, 620–629 (2008). [Google Scholar]
- Zhang H. M. et al. Genomic analysis of rice stripe virus Zhejiang isolate shows the presence of an OTU-like domain in the protein encoded by RNA1 and a novel sequence motif conserved within the intergenic regions of ambisense segments of tenuiviruses. Arch. Virol. 152, 1917–1923, 10.1007/s00705-007-1013-2 (2007). [DOI] [PubMed] [Google Scholar]
- Zhong L. et al. Chloroplast Small Heat Shock Protein HSP21 Interacts with Plastid Nucleoid Protein pTAC5 and Is Essential for Chloroplast Development in Arabidopsis under Heat Stress. Plant Cell 25, 2925–2943, 10.1105/tpc.113.111229 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar N. K., Kim Y. K. & Grover A. Rice sHsp genes: genomic organization and expression profiling under stress and development. BMC Genomics 10, 393, 10.1186/1471-2164-10-393 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters E. R. The evolution, function, structure, and expression of the plant sHSPs. J. Exp. Bot. 64, 391–403, 10.1093/jxb/ers355 (2013). [DOI] [PubMed] [Google Scholar]
- Lambert W. et al. Subunit arrangement in the dodecameric chloroplast small heat shock protein Hsp21. Protein Sci. 20, 291–301, 10.1002/pro.560 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basha E., O’Neill H. & Vierling E. Small heat shock proteins and a-crystallins: Dynamic proteins with flexible functions. Trends Biochem. Sci. 37, 106–117, 10.1016/j.tibs.2011.11.005 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann M. J. & Kessler B. M. Ubiquitin and ubiquitin-like specific proteases targeted by infectious pathogens: Emerging patterns and molecular principles. Biochim. Biophys. Acta 1782, 809–816, 10.1016/j.bbadis.2008.08.010 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treweek T. M., Meehan S., Ecroyd H. & Carver J. A. Small heat-shock proteins: important players in regulating cellular proteostasis. Cell. Mol. Life Sci. 72, 429–451, 10.1007/s00018-014-1754-5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L. et al. Pc4, a putative movement protein of rice stripe virus, interacts with a type I DnaJ protein and a small Hsp of rice. Virus Genes 38, 320–327, 10.1007/s11262-008-0324-z (2009). [DOI] [PubMed] [Google Scholar]
- Wei T. Y. et al. Genetic diversity and population structure of Rice stripe virus in China. J. Gen. Virol. 90, 1025–1034, 10.1099/vir.0.006858-0 (2009). [DOI] [PubMed] [Google Scholar]
- Nagy P. D., Wang R. Y., Pogany J., Hafren A. & Makinen K. Emerging picture of host chaperone and cyclophilin roles in RNA virus replication. Virology 411, 374–382, 10.1016/j.virol.2010.12.061 (2011). [DOI] [PubMed] [Google Scholar]
- White J. P., Cardenas A. M., Marissen W. E. & Lloyd R. E. Inhibition of cytoplasmic mRNA stress granule formation by a viral proteinase. Cell Host Microbe 2, 295–305, 10.1016/j.chom.2007.08.006 (2007). [DOI] [PubMed] [Google Scholar]
- Wu W. Q. et al. Simultaneous detection and survey of three rice viruses in China. Plant Dis. 97, 1181–1186, 10.1094/PDIS-02-12-0207-RE (2013). [DOI] [PubMed] [Google Scholar]
- Gietz R. D. & Woods R. A. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 350, 87–96, 10.1016/S0076-6879(02)50957-5 (2002). [DOI] [PubMed] [Google Scholar]
- Kim H. et al. A rice orthologue of the ABA receptor, OsPYL/RCAR5, is a positive regulator of the ABA signal transduction pathway in seed germination and early seedling growth. J. Exp. Bot. 63, 1013–1024, 10.1093/jxb/err338 (2012). [DOI] [PubMed] [Google Scholar]
- Lu Y. et al. Garlic virus X 11-kDa protein granules move within the cytoplasm and traffic a host protein normally found in the nucleolus. Mol. Plant Pathol. 12, 666–676, 10.1111/j.1364-3703.2010.00699.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkes I. A., Runions J., Kearns A. & Hawes C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nature Protocols 1, 2019–2025, 10.1038/nprot.2006.286 (2006). [DOI] [PubMed] [Google Scholar]
- Walter M. et al. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40, 428–438, 10.1111/j.1365-313X.2004.02219.x (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.