Abstract
An outbreak of acute gastroenteritis occurred at a restaurant in Yokohama in December 2011. Because many of the customers had consumed raw sea snail, sea snail was suspected to be the source of this outbreak. To determine whether sea snail contains Norovirus (NoV) or Sapovirus (SaV), we analyzed 27 sea snail samples collected over 5 months (May, June, August, October, and December 2012) and 59.3 % were positive for NoV and/or SaV. The levels of NoV ranged from 1.5 × 103 to 1.5 × 105 copies/g tissue, and those of SaV from 1.5 × 102 to 1.3 × 103 copies/g tissue. The highest levels were observed in sea snails collected in December. A phylogenetic analysis of the NoVs showed that the viral strains were NoV genotypes GI.4, GI.6, GII.4, GII.12, GII.13, and GII.14, and the SaV strains were genotypes GI.2 and GI.3. The NoV GII.4 Sydney 2012 variants were only detected in December. This variant was a major source of gastroenteritis in Japan in the winter of 2012/2013. In contrast, the NoV GII.4 strains detected in May and June 2012 were not the Sydney 2012 variant. This study demonstrates that sea snail contains multiple genogroups and genotypes of NoV and SaV strains. We conclude that the sea snail presents a risk of gastroenteritis when consumed raw.
Keywords: Norovirus, Sapovirus, Sea snail, Phylogenetic analysis
Introduction
Acute gastroenteritis caused by Norovirus (NoV) or Sapovirus (SaV) is a common infectious disease. These viruses are classified as caliciviruses, and have single-stranded positive-sense 7.5-kb RNA genomes (Berke et al. 1997; Wilhelmi et al. 2003; Patel et al. 2009; Morillo and Timenetsky 2011). Both NoV and SaV are classified into I–VI genogroups. The NoVs responsible for human gastroenteritis occur in genogroup I (GI), GII, and GIV (Zheng et al. 2006; Wang et al. 2007; Miura et al. 2013). Of the many genotypes that exist, genotype GII.4 causes most human outbreaks (Takanashi et al. 2011). The characteristic incubation period for NoV and SaV infections is 1–2 days, and the major clinical symptoms are diarrhea, vomiting, nausea, and abdominal cramps (Patel et al. 2009). Oysters are a known vehicle of NoV transmission, and their raw consumption causes gastroenteritis outbreaks attributable to NoV infection (Baker et al. 2011).
Bivalves, such as oysters, filter large amounts of water, thus any human pathogen in sewage can bioaccumulate in their digestive tissues (Le Guyader et al. 2010; Schaeffer et al. 2013). Because contamination of shellfish is related to sewage (Wang and Deng 2012; Prato et al. 2013), consumption of raw bivalves is a potential cause of viral gastroenteritis (Prato et al. 2004).
In 2011, an outbreak of acute gastroenteritis at a restaurant was reported in the Public Health Center, Yokohama. Because plural NoVs and SaVs were detected from the stools of the patients, bivalves were suspected as the cause of the food-borne gastroenteritis. However, the restaurant had supplied raw sea snails instead of bivalves to prevent norovirus infection. In Japan, sea snail is sold at fish markets and is eaten raw or boiled, although it is rarely eaten raw in Japan or other countries. As far as we know, the detection of NoV or SaV in sea snail has not been reported. Bivalves such as the oyster are known to bioaccumulate pathogens that are major etiological agents of gastroenteritis. However, compared with bivalves, sea snail has been considered to present little risk of NoV infection. The aim of this study was to determine whether NoV or SaV is detectable in sea snails. We also investigated the concentrations of NoV and SaV contamination in the sea snail and constructed a phylogenetic tree to characterize these viruses.
Materials and Methods
Sea Snail Samples
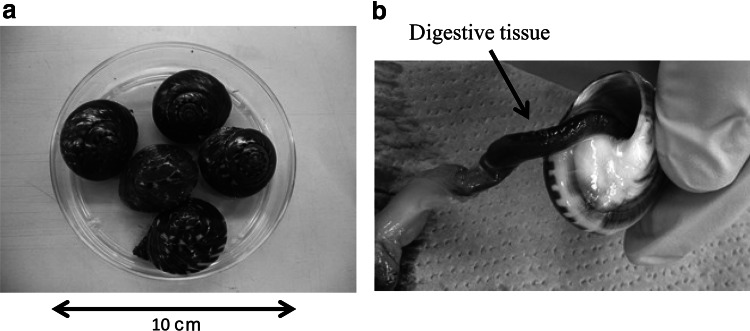
An outbreak of acute gastroenteritis occurred at a restaurant in Yokohama in December 2011. An investigation revealed that 71 (37.0 %) of 192 customers displayed symptoms of gastroenteritis. NoVs were detected in 24 (70.6 %) of the 34 stool specimens tested. Many of these customers had consumed raw sea snail Umbonium giganteum (Fig. 1a), which is a marine filter-feeding gastropod mollusk of the family Trochidae. Therefore, this sea snail was suspected of being the source of the outbreak. After the outbreak, 27 sea snail samples were collected in May, June, August, October, and December 2012 from Kanagawa Prefecture, in the coastal region from which the sea snails had been supplied to the restaurant. Five sea snails were obtained in May, June, August, and October, and seven in December. Although the weight of the sea snails differed depending on individuals or collection periods, each sea snail was approximately 10–20 g. The samples were transported to the laboratory about 1 day after their collection, where their digestive tissues (Fig. 1b), which were separated from other tissues, were cut into two portions. One of the two portions was weighed (5.0 × 10−2–6.0 × 10−1 g: average 2.5 × 10−1 g) and then homogenized separately with a Multi-beads Shocker® (Yasui Kikai, Osaka, Japan).
Fig. 1.
a Shells of the sea snail Umbonium giganteum. b Digestive tissue of the sea snail
RNA Extraction and cDNA Synthesis
Viral RNA was extracted from the homogenized sea snail samples with a Qiagen RNeasy Mini Kit (Qiagen, Hilden, Germany) in a final volume of 30 μl, according to the manufacturer’s instructions. The cDNA (50 μl) was synthesized with SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) and random hexamer primers (Takara Bio Inc., Shiga, Japan). Fifty μl of cDNA was synthesized from 5 μl of the extracted viral RNA in October, and from 10 μl of RNA in May, June, August, and December. The reverse transcription (RT) reaction was incubated at 42 °C for 60 min and then at 95 °C for 5 min to inactivate the enzyme. The amount of cDNA used for real-time PCR was 2.5 μl of the sample or control.
Real-Time PCR to Quantify NoV and SaV
Real-time PCR amplification of the NoV and SaV genomes was performed with a StepOne™ Real-Time PCR System (Applied Biosystems, Foster City, CA, USA), using a QuantiTect Probe PCR Kit (Qiagen). The optimized primers used for NoV GI were NV192 and NV193, and the probe was TM9 (Hoehne and Schreier 2006); for NoV GII, the primers were JJV2F and COG2R (Jothikumar et al. 2005), and the probe was GII (Hymas et al. 2007). The concentrations of the primers and probes were 900 and 300 nM, respectively. The primers and probes used for SaV were the same as described previously (Oka et al. 2006), and the concentrations of the primers and probes were 400 nM and 200 nM, respectively. The reaction mixture was subjected to the following amplification conditions: for NoV, 2 min at 50 °C, 15 min at 95 °C, 50 cycles of 15 s at 94 °C, and 60 s at 60 °C; for SaV, the annealing temperature was at 62 °C instead of 60 °C.
The samples were analyzed with real-time PCR using standard plasmid (pcDNA3.1/V5-His TOPO; Invitrogen) as the positive control to create the standard curve, and nuclease-free water as the negative control. Each viral genome copy number was determined by comparison with tenfold serial dilutions of a standard curve constructed with a standard plasmid. The average quantity of NoV or SaV in two wells was used to determine the number of viral genome copies/g tissue. Only those samples that displayed amplification in two wells were analyzed quantitatively, and the limit of quantification was 100 copies/g tissue (Manso and Romalde 2013).
In separate experiments, the presence of PCR inhibitors was evaluated by adding internal controls. Each volume of sample RNA extract (5–10 μl) was reverse transcribed with 2.5 μl containing NoV GI RNA as internal controls (a rather high concentration; 2.9 × 106 copies/2.5 μl) (Costafreda et al. 2006; Le Guyader et al. 2008). Nuclease-free water was used in the RT-reaction as the negative control. Efficiency of PCR was performed with the same conditions of real-time PCR for NoV GI.
PCR for NoV and SaV
Semi-nested PCR for NoV GI was performed using primers COG1F and G1-SKR for the first PCR, and G1-SKF and G1-SKR for the second PCR. For NoV GII, primers COG2F and G2-SKR were used for the first PCR, and G2-SKF and G2-SKR for the second PCR. These primers were used to amplify parts of the polymerase gene and the capsid region, respectively (Kojima et al. 2002; Kageyama et al. 2003). Nested PCR for SaV was performed using a mixture of forward primers (SV-F13 and SV-F14) and reverse primers (SV-R13 and SV-R14) for the first PCR, and forward primer SV-F22 and reverse primer SV-R2 for the second PCR (Okada et al. 2006).
To reduce the risk of contamination, we used separate working areas to prepare the PCR mixes, to add the templates, and to perform the PCR reactions. We also included a no-template control in all PCR reaction series to confirm the absence of contamination.
Sequencing and Phylogenetic Analyses
The PCR products were purified for sequence analysis with a Montage DNA Gel Extraction Kit (Merck Millipore, Darmstadt, Germany), according to the manufacturer’s instructions. The nucleotide sequences of the purified products (QIAquick PCR Purification Kit, Qiagen) were determined with a BigDye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems), Centri-Sep™ Spin Columns (Princeton Separations, Adelphia, NJ, USA), and a Genetic Analyzer 3130 (Applied Biosystems). Multisequence alignments were generated with ClustalW (Thompson et al. 1994), and a neighbor-joining phylogenetic tree was constructed with MEGA 5, with 1000 bootstrap replicates.
NoV genotyping was performed as previously described (Kroneman et al. 2013), and genotypes were assigned using a publicly accessible typing tool (http://www.rivm.nl/mpf/norovirus/typingtool) (Kroneman et al. 2011).
The GenBank accession numbers for the reference strains are as follows: AB042808 (Chiba 407), AF093797 (BS5), X76716 (Bristol), AJ277618 (Wortley), AY113106 (Fayetteville), AY130761 (M7), U65427 (Sapporo), U73124 (Parkville), AF194182 (Stockholm/318), AJ412800 (Chiba/000496F), and DQ366345 (Ehime643).
Nucleotide Sequence Accession Numbers
The nucleotide sequences determined in this study were deposited in the GenBank database under accession numbers AB934018–AB934035. The reference strains used in this study are described on each phylogenetic tree.
Results
Virus Detection
A total of 27 sea snail samples were collected in Kanagawa Prefecture in May–December 2012. A summary of the detection and quantification of NoV and SaV with real-time PCR and conventional PCR is shown in Table 1. Sixteen samples (59.3 %) contained at least one of NoV or SaV. Mixed contamination was present in 11 samples (40.7 %). NoV and SaV were detected in 15 (55.6 %) and 12 (44.4 %) samples, respectively (Table 1). NoV was detected in sea snails in May, June, October, and December. SaV was detected in sea snails in May, October, and December. The proportion of sea snails carrying NoV or SaV was highest in December.
Table 1.
Estimated concentrations of noro- and sapovirus, and detected genotypes in sea snail samples
| Month | Sample name | NoV GI | NoV GII | SaV | Efficiency rate (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Real-time PCRa (Copies/g) | Conventional PCR | Genotype | Real-time PCRa(Copies/g) | Conventional PCR | Genotype | Real-time PCRa(Copies/g) | Conventional PCR | Genotype | |||
| May-2012 | nagarami A | 4.0 × 102 | − | − | 7.9 × 103 | − | − | + | + | GI.3 | 42.7 |
| nagarami B | − | − | − | 1.5 × 103 | − | − | − | + | GI.2 | 38.7 | |
| nagarami C | − | − | − | − | − | − | + | + | GI.3 | 58.9 | |
| nagarami D | − | + | GI.4 | 4.0 × 103 | + | GII.14 | 1.5 × 102 | − | − | 100 | |
| nagarami E | − | − | − | 3.3 × 103 | + | GII.4 | + | − | − | 100 | |
| Jun-2012 | nagarami F | − | − | − | − | + | GII.4 | + | − | − | 100 |
| nagarami G | − | − | − | − | − | − | − | − | − | 76.7 | |
| nagarami H | − | − | − | − | − | − | − | − | − | 51.5 | |
| nagarami I | − | − | − | − | − | − | − | − | − | 72.8 | |
| nagarami J | − | − | − | − | − | − | − | − | − | 74.9 | |
| Aug-2012 | nagarami K | − | − | − | − | − | − | − | − | − | 56.1 |
| nagarami L | − | − | − | − | − | − | − | − | − | 39.1 | |
| nagarami M | − | − | − | − | − | − | − | − | − | 61.7 | |
| nagarami N | − | − | − | − | − | − | − | − | − | 24.9 | |
| nagarami O | − | − | − | − | − | − | − | − | − | 38.8 | |
| Oct-2012 | nagarami P | + | − | − | − | − | − | − | − | − | 100 |
| nagarami Q | + | − | − | − | − | − | − | − | − | 78.7 | |
| nagarami R | − | − | − | − | − | − | − | − | − | 85.3 | |
| nagarami S | − | − | − | − | − | − | − | − | − | 63.0 | |
| nagarami T | − | − | − | 4.7 × 103 | − | − | 5.8 × 102 | − | − | 75.7 | |
| Dec-2012 | nagarami U | 4.5 × 103 | + | GI.4 | 6.9 × 104 | + | GII.4 | 2.8 × 102 | − | − | 100 |
| nagarami V | 2.7 × 102 | − | − | 2.2 × 104 | + | GII.4 | + | − | − | 100 | |
| nagarami W | − | + | GI.6 | 3.2 × 104 | + | GII.13 | − | + | GI.2 | 53.0 | |
| nagarami X | + | − | − | − | + | GII.4 | − | − | − | 50.3 | |
| nagarami Y | 1.7 × 103 | − | − | 5.6 × 104 | + | GII.12 | − | − | − | 91.5 | |
| nagarami Z | 4.7 × 103 | + | GI.6 | 7.6 × 104 | + | GII.4 | 3.7 × 102 | − | − | 39.1 | |
| nagarami AA | 2.8 × 103 | − | – | 1.4 × 105 | + | GII.4 | 1.3 × 103 | − | − | 49.7 | |
aIn real-time PCR, Numbers represent copy numbers that displayed amplification in two wells and more than the limit of quantification. + indicated less than the limit of quantification
Virus Quantification
Six, 11, and five samples of NoV GI, NoV GII, and SaV, respectively, were quantifiable, and were therefore available for quantification. The concentrations of total NoV (GI plus GII) in the sea snails ranged from 1.5 × 103 to 1.5 × 105 copies/g tissue during the period of investigation. The highest quantities of total NoV and SaV were recorded in December, and no NoV was detected in August. Thus, the NoV concentrations in sea snails demonstrated a strong seasonal trend. The quantities of NoV GI ranged from 2.7 × 102 to 4.7 × 103 copies/g tissue; GII ranged from 1.5 × 103 to 1.4 × 105 copies/g tissue; and SaV ranged from 1.5 × 102 to 1.3 × 103 copies/g tissue (Table 1).
Efficiency of PCR was more than 24.9 % in all the samples and was more than 38.7 % in the quantitative samples (Table 1). An RT-negative control and a no-template control produced negative results. The amplification of PCR indicated no conspicuous inhibition.
Phylogenetic Analysis of Sea Snail Samples
Thirteen sea snail samples were NoV- or SaV-positive, as determined with nested PCR, and were confirmed with a sequencing analysis. Phylogenetic analyses of NoV and SaV showed that several viral strains were present during each month. In detail, these genetic types were NoV GI.4 and GI.6 (Fig. 2), NoV GII.4, GII.12, GII.13, and GII.14 (Fig. 3), and SaV GI.2 and GI.3 (Fig. 4). Of the two NoV GI.4 strains, one was collected in May and the other in December. The nucleotide sequence identities of the two NoV GI.4 strains were 98.6 %. The two NoV GI strains detected in December 2012 had 100 % identical sequence and were classified as NoV GI.6. The five NoV GII.4 strains detected in December 2012 were classified as the NoV GII.4 Sydney 2012 variant and showed 99.3–100 % similarity to the NoV GII.4 Sydney 2012 variant reference sequence (JX459908). The other NoV GII.4 strain detected in June was closely related to the NoV GII.4 New Orleans 2009 variant reference sequence (GU445325). The NoV GII.4 Sydney 2012 variants were only detected in December. The NoV GII.4 strain detected in May was not a New Orleans 2009 or Sydney 2012 variant.
Fig. 2.
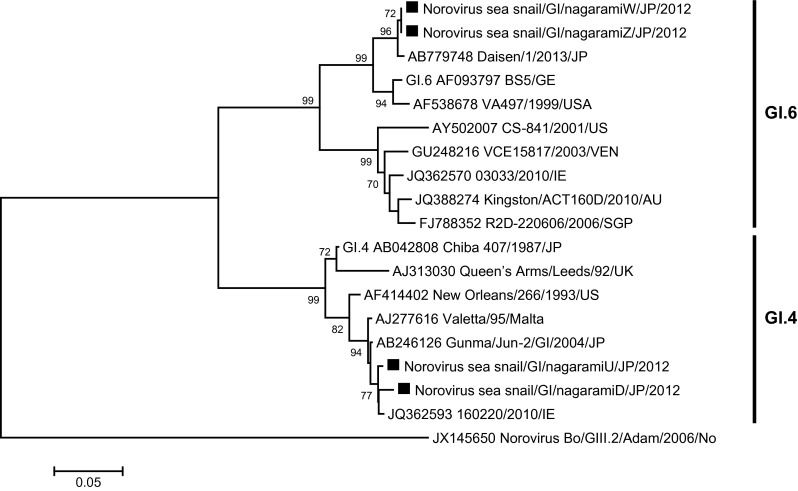
Phylogenetic analysis of Norovirus GI, capsid N/S region. Phylogenetic tree of Norovirus GI strains and reference strains based on the partial capsid sequence, constructed with the neighbor-joining method. The numbers on the branches show only the bootstrap values above 70 %. Filled square indicates Norovirus GI strains isolated in this study
Fig. 3.
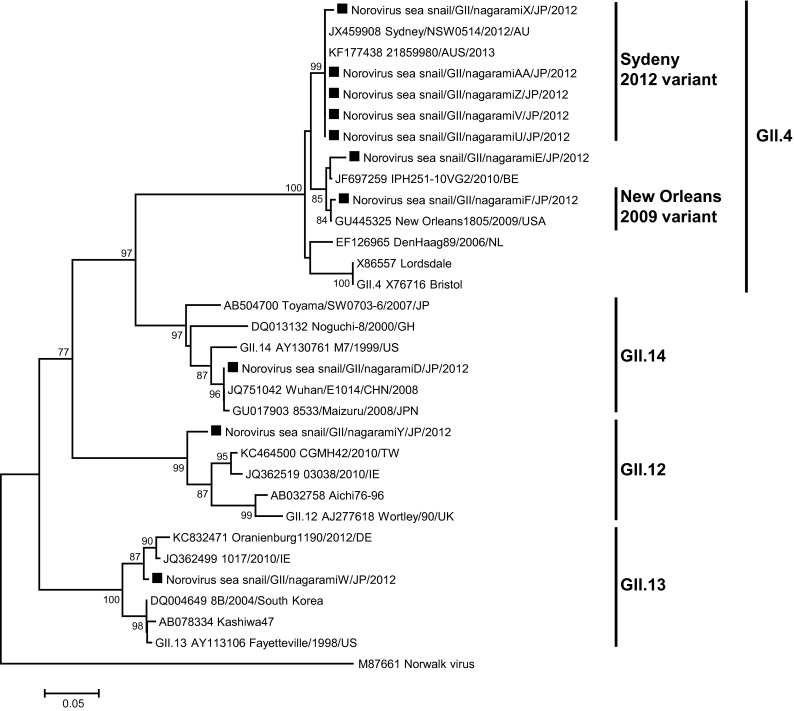
Phylogenetic analysis of Norovirus GII, capsid N/S region. Phylogenetic tree of the Norovirus GII strains and reference strains based on the partial capsid sequence, constructed with the neighbor-joining method. The numbers on the branches show only the bootstrap values above 70 %. Filled square indicates Norovirus GII strains isolated in this study
Fig. 4.
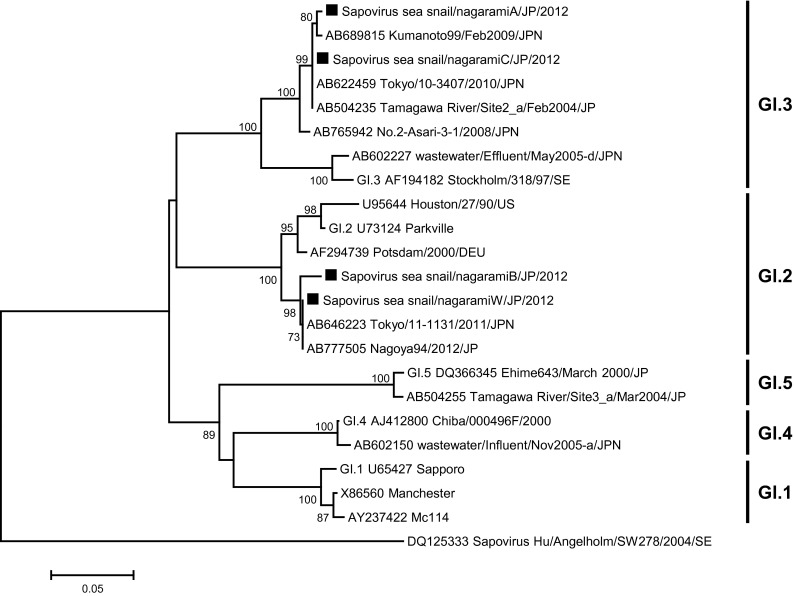
Phylogenetic analysis of Sapovirus, capsid region. Phylogenetic tree of Sapovirus strains and reference strains based on the partial capsid sequence, constructed with the neighbor-joining method. The numbers on the branches show only the bootstrap values above 70 %. Filled square indicates Sapovirus strains isolated in this study
The nucleotide sequence identity of the two SaV GI.2 strains was 98.8 %, and that of the two SaV GI.3 strains detected in May was 99.3 %. One of the two SaV GI.2 strains was collected in May and the other in December.
Discussion
It is well known that bivalves, including oysters, cause acute gastroenteritis. When an oyster grows in contaminated water, it can bioaccumulate multiple pathogens in its digestive tissues, and several viruses have been detected in oyster tissues. Therefore, eating raw shellfish, such as oysters, entails a risk of acute gastroenteritis (Baker et al. 2011). Oyster-associated outbreaks can involve different genetic types of NoV in the same individual (Sugieda et al. 1996), and multiple NoV strains have been detected in oysters (Le Guyader et al. 2008). Another six kinds of bivalves (Manila clams, clams, mussels, razor clams, blood clams, and scallops) can be contaminated with NoVs or SaVs (Iizuka et al. 2010; Xia Ming et al. 2013), and multiple gastroenteric viruses or viral strains have been detected in fecally contaminated shellfish (Gallimore et al. 2005). When an outbreak occurred in Yokohama in 2011, several NoV strains were detected. Although the patients had not eaten bivalves, many of them had eaten raw sea snail. The raw sea snail was pickled in Shochu (Japanese spirit distilled from rice) and eaten, but because NoV is resistant to ethanol, the viruses in the sea snail digestive tissues remained infectious.
The aim of this study was to clarify whether sea snails carry NoV and SaV in their digestive tissues. The sea snail samples examined in this study were collected in May, June, August, October, and December 2012. We could not obtain samples in the other months because sea snail is not harvested from January to April.
Digestive tissue can be a PCR inhibitor and cause false negatives. The presence of PCR inhibitors was evaluated in this study, but evaluation of nucleic extraction efficiencies by adding another external virus as a control was not performed. There is a possibility of underestimating the quantitative results by loss of nucleic extraction or other inhibitors. We used separate working areas and negative controls to avoid the risk of false positives. Our report showed the estimated minimum values of viral contamination in sea snails.
A previous study reported that the 50 % infectious dose of NoV GII was 103 genome copies according to the dose–response relationship in the secretor phenotype population (Teunis et al. 2008). We did not investigate whether the detected viruses were infectious. The NoV GII concentration was higher than the NoV GI concentration, and exceeded 103 copies/g tissue during the investigation period. Therefore, our data show that the NoV GII detected in the sea snail exceeded the infectious dose of NoV for humans. The seasonal distribution indicated that NoV contamination in sea snails is high in December.
A phylogenetic analysis demonstrated that several strains of these viruses were present. The most frequently identified genotype in the sea snail was NoV GII.4. Interestingly, the five NoV GII.4 strains detected in December were the Sydney 2012 variant, which was a major source of the gastroenteritis outbreaks in Japan in the winter of 2012/2013 (Thongprachum et al. 2014). The strains in the other NoV GII.4-positive samples, which were collected in May and June, were not closely related to the Sydney 2012 variant. Mixed contaminations were observed in 11 sea snail samples in this study. These results suggest that this sea snail can bioaccumulate and retain viral pathogens.
In conclusion, we detected both NoV and SaV in sea snail samples. Bivalves are recognized as typical vehicles of food-borne NoV. We conclude that the sea snail presents a risk of gastroenteritis and is a newly recognized vehicle of food-borne NoV and SaV.
Acknowledgments
This work was supported by all the staff at the Public Health Center, Yokohama, Japan, who collected the specimens and provided data. We thank Yuriko Murai and Naoko Ohshima for their helpful advice.
Conflict of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
References
- Baker K, Morris J, McCarthy N, Saldana L, Lowther J, Collinson A, et al. An outbreak of norovirus infection linked to oyster consumption at a UK restaurant, February 2010. Journal of Public Health: Oxford Journals. 2011;33(2):205–211. doi: 10.1093/pubmed/fdq089. [DOI] [PubMed] [Google Scholar]
- Berke T, Golding B, Jiang X, Cubitt DW, Wolfaardt M, Smith AW, et al. Phylogenetic analysis of the caliciviruses. Journal of Medical Virology. 1997;52(4):419–424. doi: 10.1002/(SICI)1096-9071(199708)52:4<419::AID-JMV13>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Costafreda MI, Bosch A, Pintó RM. Development, evaluation, and standardization of a real-time TaqMan reverse transcription-PCR assay for quantification of hepatitis A virus in clinical and shellfish samples. Applied and Environmental Microbiology. 2006;72(6):3846–3855. doi: 10.1128/AEM.02660-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallimore CI, Cheesbrough JS, Lamden K, Bingham C, Gray JJ. Multiple norovirus genotypes characterised from an oyster-associated outbreak of gastroenteritis. International Journal of Food Microbiology. 2005;103(3):323–330. doi: 10.1016/j.ijfoodmicro.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Hoehne M, Schreier E. Detection of Norovirus genogroup I and II by multiplex real-time RT–PCR using a 3′-minor groove binder-DNA probe. BMC Infectious Diseases. 2006;6:69. doi: 10.1186/1471-2334-6-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hymas W, Atkinson A, Stevenson J, Hillyard D. Use of modified oligonucleotides to compensate for sequence polymorphisms in the real-time detection of norovirus. Journal of Virological Methods. 2007;142(1–2):10–14. doi: 10.1016/j.jviromet.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Iizuka S, Oka T, Tabara K, Omura T, Katayama K, Takeda N, et al. Detection of sapoviruses and noroviruses in an outbreak of gastroenteritis linked genetically to shellfish. Journal of Medical Virology. 2010;82(7):1247–1254. doi: 10.1002/jmv.21791. [DOI] [PubMed] [Google Scholar]
- Jothikumar N, Lowther JA, Henshilwood K, Lees DN, Hill VR, Vinjé J. Rapid and sensitive detection of noroviruses by using Taqman-based one-step reverse transcription-PCR assays and application to naturally contaminated shellfish samples. Applied and Environmental Microbiology. 2005;71(4):1870–1875. doi: 10.1128/AEM.71.4.1870-1875.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama T, Kojima S, Shinohara M, Uchida K, Fukushi S, Hoshino FB, et al. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription–PCR. Journal of Clinical Microbiology. 2003;41(4):1548–1557. doi: 10.1128/JCM.41.4.1548-1557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Kageyama T, Fukushi S, Hoshino FB, Shinohara M, Uchida K, et al. Genogroup-specific PCR primers for detection of Norwalk-like viruses. Journal of Virological Methods. 2002;100(1–2):107–114. doi: 10.1016/S0166-0934(01)00404-9. [DOI] [PubMed] [Google Scholar]
- Kroneman A, Vega E, Vennema H, Vinjé J, White PA, Hansman G, et al. Proposal for a unified norovirus nomenclature and genotyping. Archives of Virology. 2013;158(10):2059–2068. doi: 10.1007/s00705-013-1708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroneman A, Vennema H, Deforche K, Avoort HVD, Peñaranda S, Oberste MS, et al. An automated genotyping tool for enteroviruses and noroviruses. Journal of Clinical Virology. 2011;51(2):121–125. doi: 10.1016/j.jcv.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Le Guyader FS, Krol J, Ambert-Balay K, Ruvoen-Clouet N, Desaubliaux B, Parnaudeau S, et al. Comprehensive analysis of a norovirus-associated gastroenteritis outbreak, from the environment to the consumer. Journal of Clinical Microbiology. 2010;48(3):915–920. doi: 10.1128/JCM.01664-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guyader FS, Le Saux JC, Ambert-Balay K, Krol J, Serais O, Parnaudeau S, et al. Aichi virus, norovirus, astrovirus, enterovirus, and rotavirus involved in clinical cases from a French oyster-related gastroenteritis outbreak. Journal of Clinical Microbiology. 2008;46(12):4011–4017. doi: 10.1128/JCM.01044-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manso CF, Romalde JL. Detection and characterization of Hepatitis A virus and Norovirus in mussels from Galicia (NW Spain) Food and Environmental Virology. 2013;5(2):110–118. doi: 10.1007/s12560-013-9108-2. [DOI] [PubMed] [Google Scholar]
- Miura T, Parnaudeau S, Grodzki M, Okabe S, Atmar RL, Le Guyader FS. Environmental detection of genogroup I, II, and IV noroviruses by using a generic real-time reverse transcription-PCR assay. Applied and Environmental Microbiology. 2013;79(21):6585–6592. doi: 10.1128/AEM.02112-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillo SG, Timenetsky Mdo C. Norovirus: An overview. Revista da Associação Medica Brasileira. 2011;57(4):453–458. doi: 10.1590/s0104-42302011000400023. [DOI] [PubMed] [Google Scholar]
- Oka T, Katayama K, Hansman GS, Kageyama T, Ogawa S, Wu FT, et al. Detection of human sapovirus by real-time reverse transcription-polymerase chain reaction. Journal of Medical Virology. 2006;78(10):1347–1353. doi: 10.1002/jmv.20699. [DOI] [PubMed] [Google Scholar]
- Okada M, Yamashita Y, Oseto M, Shinozaki K. The detection of human sapoviruses with universal and genogroup-specific primers. Archives of Virology. 2006;151(12):2503–2509. doi: 10.1007/s00705-006-0820-1. [DOI] [PubMed] [Google Scholar]
- Patel MM, Hall AJ, Vinjé J, Parashar UD. Noroviruses: A comprehensive review. Journal of Clinical Virology. 2009;44(1):1–8. doi: 10.1016/j.jcv.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Prato R, Lopalco PL, Chironna M, Barbuti G, Germinario C, Quarto M. Norovirus gastroenteritis general outbreak associated with raw shellfish consumption in South Italy. BMC Infectious Diseases. 2004;4:37. doi: 10.1186/1471-2334-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prato R, Martinelli D, Tafuri S, Barbuti G, Quarto M, Germinario CA, et al. Safety of shellfish and epidemiological pattern of enterically transmitted diseases in Italy. International Journal of Food Microbiology. 2013;162(2):125–128. doi: 10.1016/j.ijfoodmicro.2012.12.025. [DOI] [PubMed] [Google Scholar]
- Schaeffer J, Le Saux JC, Lora M, Atmar RL, Le Guyader FS. Norovirus contamination on French marketed oysters. International Journal of Food Microbiology. 2013;166(2):244–248. doi: 10.1016/j.ijfoodmicro.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugieda M, Nakajima K, Nakajima S. Outbreaks of Norwalk-like virus-associated gastroenteritis traced to shellfish: coexistence of two genotypes in one specimen. Epidemiology and Infection. 1996;116(3):339–346. doi: 10.1017/S0950268800052663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanashi S, Wang Q, Chen N, Shen Q, Jung K, Zhang Z, et al. Characterization of emerging GII.g/GII.12 noroviruses from a gastroenteritis outbreak in the United States in 2010. Journal of Clinical Microbiology. 2011;49(9):3234–3244. doi: 10.1128/JCM.00305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunis PF, Moe CL, Liu P, Miller SE, Lindesmith L, Baric RS, et al. Norwalk virus: How infectious is it? Journal of Medical Virology. 2008;80(8):1468–1476. doi: 10.1002/jmv.21237. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTALW: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongprachum A, Chan-it W, Khamrin P, Saparpakorn P, Okitsu S, Takanashi S, et al. Molecular epidemiology of norovirus associated with gastroenteritis and emergence of norovirus GII.4 variant 2012 in Japanese pediatric patients. Infection, Genetics and Evolution. 2014;23:65–73. doi: 10.1016/j.meegid.2014.01.030. [DOI] [PubMed] [Google Scholar]
- Wang QH, Costantini V, Saif LJ. Porcine enteric caliciviruses: Genetic and antigenic relatedness to human caliciviruses, diagnosis and epidemiology. Vaccine. 2007;25(30):5453–5466. doi: 10.1016/j.vaccine.2006.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Deng Z. Detection and forecasting of oyster norovirus outbreaks: Recent advances and future perspectives. Marine Environmental Research. 2012;80:62–69. doi: 10.1016/j.marenvres.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Wilhelmi I, Roman E, Sánchez-Fauquier A. Viruses causing gastroenteritis. Clinical Microbiology & Infection. 2003;9(4):247–262. doi: 10.1046/j.1469-0691.2003.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Ming H, Feng Fan J, Jun Wu L, Bo Liang Y. Prevalence of human enteric viruses and a potential indicator of contamination in shellfish in China. Journal of Food Safety. 2013;33(2):209–214. doi: 10.1111/jfs.12041. [DOI] [Google Scholar]
- Zheng DP, Ando T, Fankhauser RL, Beard RS, Glass RI, Monroe SS. Norovirus classification and proposed strain nomenclature. Virology. 2006;346(2):312–323. doi: 10.1016/j.virol.2005.11.015. [DOI] [PubMed] [Google Scholar]