Abstract
Purpose
To assess retinal architectural alterations that occur following membrane peeling procedures and the impact of peel technique on these alterations utilizing intraoperative optical coherence tomography (iOCT).
Methods
This is a subanalysis of the prospective PIONEER iOCT study of eyes undergoing a membrane peeling for a vitreomacular interface (VMI) disorder. Intraoperative scanning was performed with a microscope-mounted OCT system. Macroarchitectural alterations (e.g., full-thickness retinal elevations) and microarchitectural alterations (e.g., relative layer thickness alterations) were analyzed. Video/iOCT correlation was performed to identify instrument-tissue manipulations resulting in macroarchitectural alterations.
Results
One hundred sixty-three eyes were included in the macroarchitectural analysis. Instrumentation utilized for membrane peeling included forceps alone for 73 eyes (45%), combined diamond-dusted membrane scraper (DDMS) and forceps for 87 eyes (53%), and other techniques in three eyes (2%). Focal retinal elevations were identified in 45 of 163 eyes (28%). Video/iOCT correlation identified 69% of alterations involved forceps compared to 26% due to DDMS. Sixteen percent of retinal alterations persisted 1 month following surgery. The microarchitectural analysis included 134 eyes. Immediately following membrane peeling, there was a significant increase in the ellipsoid zone to retinal pigment epithelium height (+20%, P < 0.00001) and the cone outer segment tips to retinal pigment epithelium height (+18%, P < 0.00001).
Conclusions
Significant subclinical retinal architectural changes occur during membrane peeling for VMI conditions. Differences in surgical instruments may impact these architectural alterations.
Keywords: intraoperative OCT, membrane peeling, vitreomacular interface
Membrane peeling is a common vitreoretinal procedure performed during the surgical management of vitreomacular interface disorders (e.g., epiretinal membranes [ERM], macular holes [MHs], and vitreomacular traction [VMT] syndrome). The acute impact of surgical maneuvers is generally unknown. In the postoperative period, internal limiting membrane (ILM) peeling has been associated with both edema and thinning of the retinal nerve fiber layers.1–4 These retinal changes have been noted to persist even after 3 years of the surgery.5 Until recently, visualizing the acute intraoperative impact of surgical maneuvers on retinal architecture was not possible. Recent advances in optical coherence tomography (OCT) technology have allowed the introduction of OCT into the operating room. Intraoperative OCT (iOCT) has been utilized to visualize the retinal architecture prior, during, and following membrane peeling.6–13 Small studies have described subtle alterations in the architecture of the retina, including expansion of the ellipsoid zone to retinal pigment epithelium (EZ-RPE).6,7,9,12 The postoperative impact of these changes and the intraoperative factors that contribute to these alterations remain unknown.
The PIONEER study is a large prospective multisurgeon study that examined the feasibility and utility of iOCT for ophthalmic surgery.6 In this report, we examine eyes from the PIONEER study that underwent membrane peeling for a vitreomacular interface disorder to better elucidate the subclinical alterations that occur following surgical manipulations. Retinal changes are longitudinally assessed for micro- and macroarchitectural alterations.
Methods
PIONEER is an institutional review board-approved prospective multisurgeon single-center study examining the use of iOCT in ophthalmic surgery.6 The study adhered to the tenets of the Declaration of Helsinki. The methods of the PIONEER study have been previously described.6 The PIONEER study included a standardized imaging protocol utilizing a microscope-mounted iOCT system. The study included a 2-year period of postoperative surveillance. The postoperative period visits and imaging were based on surgeon discretion and based on standard of care. This study represents a subanalysis of eyes that were identified from the PIONEER study that underwent pars plana vitrectomy (PPV) with membrane peeling for vitreomacular interface disorders, specifically MH, ERM, and VMT syndrome. Two major iOCT features were examined in a quantitative fashion: “microarchitectural” and “macroarchitectural” alterations. These categories are defined below.
Surgical Procedure
All patients underwent standard three-port small-gauge PPV (23- or 25-gauge) by one of six surgeons. Membrane peeling was performed based on the surgeon-preferred method. Two main techniques were utilized: (1) direct engagement with forceps and subsequent peeling with forceps (i.e., forceps only), (2) diamond-dusted membrane scraper (DDMS) initiation of peel and completion of peel with vitreoretinal forceps (i.e., combined DDMS/forceps). Indocyanine green (ICG) was applied to aid in visualization based on surgeon preference. Internal limiting membrane peeling was completed in all cases of MH and was attempted for all cases of ERM.
iOCT Scanning System and Clinic OCT System
A microscope-mounted portable iOCT system (Bioptigen Envisu SDOIS; Bioptigen, Research Triangle Park, NC, USA) was utilized for intraoperative imaging, as previously described.6 iOCT was performed at various surgical milestones including preincision and following membrane peeling (i.e., postpeel). A consistent image acquisition protocol was utilized, including cubic 10 × 10 mm volume scans (at 0 and 90 degrees), 10 × 5 mm volume scans with oversampling for averaging, and 10-mm radial volume scans. Each scan consisted of 100 B-scans distributed across the area with 1000 A-scans per B-scan. For the 10 × 10 mm cube scans, which translated to a scan density of one B-scan every 0.1 mm.
For images obtained outside the operating room, preoperative and postoperative OCT images were imaged on the Cirrus 3D-OCT 1000 (Carl Zeiss Meditec, Dublin, CA, USA) using the 512 × 128 scan pattern and HD five-line raster pattern and were assessed preoperatively, in the acute postoperative period (1–10 days postoperation), and in the subacute postoperative period (11–45 days postoperation).
iOCT and Clinical OCT Image Analysis
Macroarchitectural changes were defined as focal disturbances in the retinal architecture that was readily apparent compared to preincision images (e.g., full-thickness retinal elevation, inner retinal elevation; Fig. 1). Microarchitectural retinal changes were defined as diffuse changes in retinal layer thickness following surgical manipulation that were not readily apparent as abnormalities without direct comparison and/or quantitative assessment (e.g., expansion of the EZ-RPE; Fig. 1). iOCT images were imported into an internally developed custom OCT analysis software, which was utilized for measurements and summed voxel projection (SVP) analysis.7 Automated analysis was not performed. All measurements were performed manually.
Figure 1.
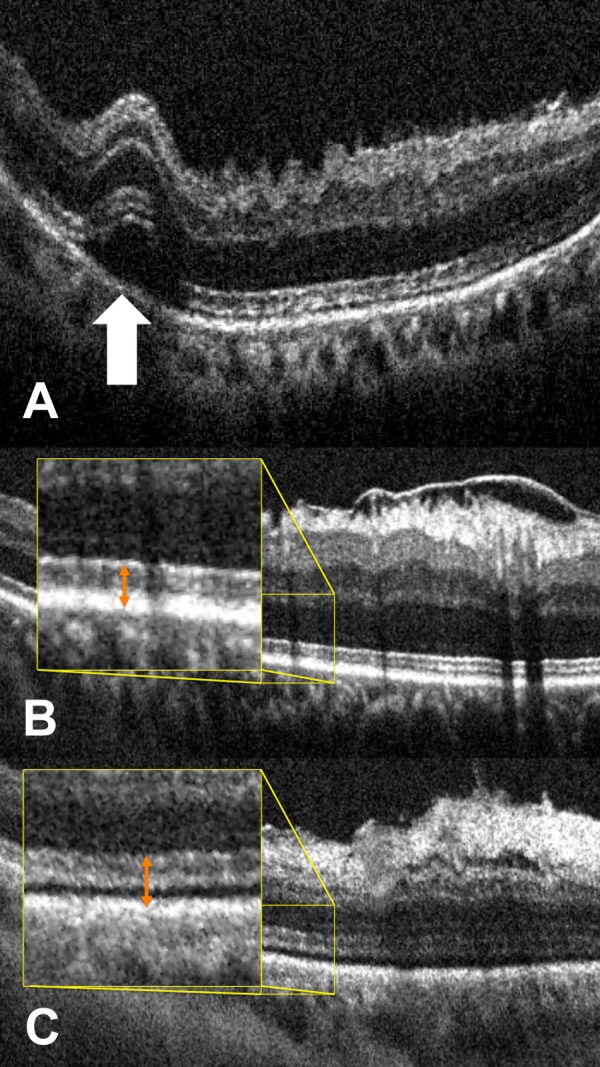
Macroarchitectural and microarchitectural alterations on iOCT following membrane peeling. (A) Postpeel iOCT B-scan revealing full-thickness retinal elevation at area of engagement demonstrating macroarchitectural change (white arrow). (B) Prepeel iOCT B-scan showing ERM and typical EZ-RPE height (inset, orange double arrow). (C) Postpeel iOCT demonstrating removal of ERM with associated microarchitectural alterations with expansion of the EZ-RPE (inset, double orange arrow).
For macroarchitectural alterations, sets of preincision and postpeel iOCT images were compared, and the retinal changes immediately following membrane peeling were noted. Exclusion criteria for macroarchitectural analysis included lack of preincision scans, eyes without postpeel scans, and scans of insufficient quality for qualitative interpretation. The iOCT findings of abrupt changes in the preretinal contour with associated absolute shadowing were interpreted to represent superficial hemorrhages and were excluded from the analysis. The OCT analysis software created a SVP of the iOCT-generated fundus map. Utilizing the OCT B-scans, the approximate fundus site was co-localized and marked on the SVP. Additionally, fundus landmarks (e.g., the optic nerve, fovea, and retinal vessels) were marked on the SVP with the software to facilitate video-iOCT correlation and manual co-registration (Fig. 2). These annotated images were then saved and exported for video iOCT correlation. Postoperative OCT analysis was performed using the Cirrus Review Software Version 6.5.0.772 (Carl Zeiss Meditec). The previous annotated SVP from the iOCT scan was utilized to co-localize the areas of interest from the iOCT scans to the postoperative scans, including the OCT B-scans and the thickness map. Comparison assessment of overall technique (e.g., forceps alone and combined technique) was examined for macroarchitectural changes. In addition, comparison of specific instruments resulting in alterations (e.g., forceps, DDMS) with video/iOCT correlation was performed when available.
Figure 2.
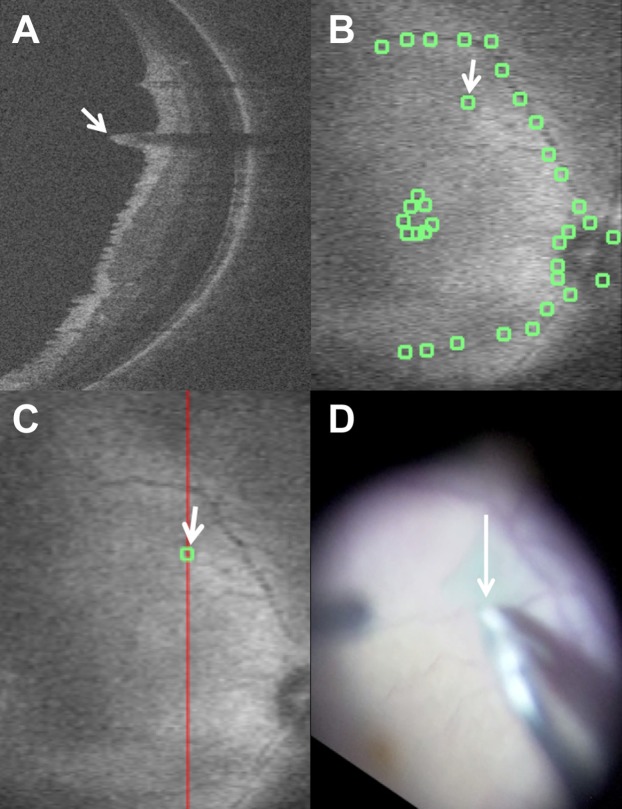
Correlation of macroarchitectural alterations with intraoperative maneuvers. (A) Intraoperative optical coherence tomography following membrane peeling with macroarchitectural alteration identified as inner retinal elevation (white arrow). Shadowing is noted secondary to the associated elevated retinal vessel associated with the inner retinal elevation. (B) Summed voxel projection with manual landmark identification and architectural alteration identification (white arrow). (C) Summed voxel projection with corresponding B-scan line (red line) for (A) and alteration site (white arrow). (D) Video correlation of peel initiation site with forceps (white arrow) that co-localizes to area of macroarchitectural alteration.
For microarchitectural alterations, quantitative assessment was performed of retinal layer thickness at each time point. Exclusion criteria for microarchitectural analysis included lack of preincision scans, lack of postpeel scans, scans of insufficient quality for quantitative assessment of retinal layer thickness, and extensive retinal architectural distortion precluding intraretinal layer measurement. Retinal zone measurements were performed for each time point including inner retinal thickness (i.e., nerve fiber layer), middle retinal thickness (ganglion cell layer, inner plexiform layer, outer plexiform layer), and outer retinal thickness (i.e., outer nuclear layer, EZ, RPE). Each of these zones were measured 1.2 mm nasal, temporal, superior, and inferior to the retina, as previously reported.14 In addition to these zones, specific layer analysis was also performed EZ to RPE distance (i.e., subretinal hyporeflectivity), cone outer segment tips (COSTs) to RPE distance also 1.2 mm away from the fovea. All iOCT measurements were performed with custom OCT analysis software as previously described.7,14 Preoperative and postoperative microarchitectural analysis was performed utilizing the Cirrus Review software. Preoperative measurements were compared between the Cirrus Reader software and the custom OCT analysis software. No significant differences were found in the measurements between the software systems. The measurements outlined above were repeated at each postoperative time point for each Cirrus scan. Comparison assessment of overall technique (e.g., forceps alone and combined technique) was examined for differences in microarchitectural changes. Due to the diffuse nature and definition of microarchitectural changes, specific instruments utilized during various maneuvers were unable to be compared.
Surgical Video/iOCT Correlation
Surgical videos were analyzed using VLC Media Player Software Version 2.1.5 (VideoLAN, Paris, France). The images of the videos were rotated to precisely orient the video images of the retina with the SVP fundus maps labeled with locations of the postpeel retinal alterations. Subsequently, surgical dynamics (e.g., tissue-instrument interaction, indirect traction) were identified at the areas of interest based on co-localization of the labeled SVP fundus map and the surgical video.
Statistical Analysis
All statistical analysis was performed with JMP Software Version 10.0.2 (SAS Institute, Cary, NC, USA) or Excel (Microsoft, Redmond, WA, USA). A P value of <0.05 was considered significant for the macroarchitectural analysis and a value of <0.01 was considered significant for the microarchitectural changes. χ2 test was used to assess the association between clinical and surgical variables and the presence of iOCT findings of the postpeel retinal changes. Quantitative assessment variables were compared with paired t-test.
Results
Clinical Characteristics and Demographics
One hundred eighty-nine eyes were initially identified from the PIONEER study that underwent membrane peeling for vitreomacular interface disorders. Eighteen eyes were eliminated from additional analysis due to poor quality of the iOCT images or lack of postpeel iOCT scan, precluding both macroarchitectural and microarchitectural analysis. Of the remaining 163 eyes, the mean age was 66.6 years (SD ± 10.0, range, 29–89 years). There were 75 males (46%) and 88 females (54%). The mean preoperative visual acuity (VA) was 20/98 (range, 20/25 to counting fingers). Ninety-seven eyes were treated for ERM (60%), 60 eyes were treated for MH (37%), and six eyes were treated for VMT (4%). Twenty-five eyes (15%) underwent 25-gauge PPV while the remaining 138 eyes (85%) underwent 23-gauge PPV. Instrumentation utilized for membrane peeling included forceps alone for 73 eyes (45%), combined DDMS and forceps for 87 eyes (53%), and combined forceps and microvitreoretinal blade in three eyes (2%). No intraoperative surgical complications were noted. The mean postoperative VA at 1 month improved to 20/68 (P < 0.0001).
iOCT Macroarchitectural Analysis and Video Correlation
In 148 of 163 (91%) eyes, iOCT confirmed complete membrane removal following initial peeling. In 15 of 163 (9%) of cases, iOCT identified residual membranes that required additional membrane peeling. Following membrane peeling, macroarchitectural alterations (i.e., focal areas of retinal elevations) were seen in 45 eyes (28%). Thirty-eight eyes (23%) had inner retinal elevations (Figs. 2, 3), and eight eyes (5%) had full-thickness retinal elevations (Fig. 1). All retinal elevations were outside the fovea. Of the 45 eyes with postpeel retinal elevations, 30 eyes (67%) had only one elevation, eight eyes (18%) had two elevations, and seven eyes (15%) had three or more retinal elevations. When examining overall peel technique, forceps peeling alone was associated with 23 of the 45 cases. This constitutes 23 of 73 total forceps cases. Combined DDMS/forceps was identified in 18 of the 45 cases. This constitutes 18 of 87 total combined cases. There was no significant difference between the two overall techniques and the incidence of macroarchitectural changes (P = 0.36). Architectural changes were noted across surgeons and did not appear to be associated with any specific surgeon(s).
Figure 3.
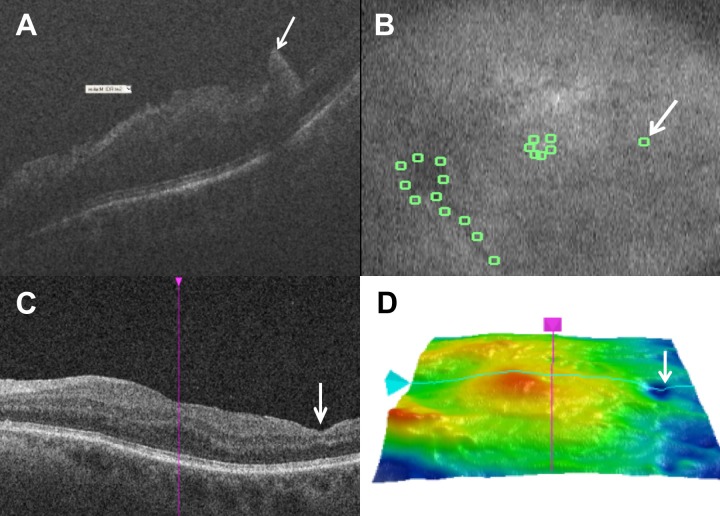
Longitudinal assessment of retinal anatomy associated with macroarchitectural alterations. (A) Intraoperative optical coherence tomography following membrane peeling with macroarchitectural alteration identified as a possible full-thickness retinal elevation (white arrow). (B) Summed voxel projection with manual landmark identification and corresponding architectural alteration identification (white arrow). (C) Postoperative optical coherence tomography with inner retinal thinning in the area corresponding to previous retinal elevation. (D) Postoperative tomographic retinal thickness map with focal area of thinning (white arrow) in the temporal retina corresponding to area of intraoperative macroarchitectural changes.
Of the 45 eyes with postpeel macroarchitectural changes, 28 eyes had the corresponding surgical videos for review. Specific instrument use was also assessed. Of the 28 eyes, 13 (46%) were from forceps only cases, 14 (50%) were from combined DDMS/forceps cases, and one was from a microvitreoretinal blade/forceps case. On review of the iOCT scans of the 28 eyes with video available, 42 independent retinal elevations were noted on iOCT. Of these 42 retinal elevations, 40 retinal elevations (95%) were correlated with instrument-tissue interaction. Clear traction with significant membrane adherence was noted in five eyes (18%) during peeling and not related to initiating a peel with retina/instrument interaction. Of those eyes with macroarchitectural alterations, 29 of 42 (69%) elevations were specifically associated with forceps/tissue interaction (Fig. 2), whereas 11 of 42 (26%) were associated with the DDMS tissue interaction.
iOCT Microarchitectural Analysis
Microarchitectural analysis was performed on 134 eyes. Of the initial 189 scans, 44 eyes were excluded due to poor scan quality or lack of postpeel scans, and 12 scans were unable to be measured due to significant pre-existing distortions in retinal architecture. There were differences in retinal layer measurements between the preoperative and preincision scans. Immediately following membrane peeling, there was a significant decrease in inner retinal thickness (−8%, P < 0.003). Additionally, there was a significant increase in the outer retinal thickness (+6%, P < 0.007), EZ-RPE thickness (+20%, P < 0.00001), and the COST-RPE thickness (+18%, P < 0.00001; Fig. 1). Scans during the acute postoperative period (i.e., 1–10 days postoperation) revealed significant changes in retinal layer thicknesses compared to postpeel scans. Increased inner retinal thickness (+18%) as well as a decrease in EZ-RPE (−20%) and COST-RPE height (−20%, P < 0.0001, for all comparisons). In the subacute postoperative period (11–45 days postoperation) the retinal layer changes had returned to baseline values with the exception of a decrease in inner retinal thickness (−11%, P < 0.003) and a reduction in the COST-RPE height (−6%, P < 0.008) compared to presurgery levels.
In examining peel technique, there were no significant differences in microarchitectural alterations when comparing forceps alone and the combined DDMS/forceps approach. There was a trend toward increased outer retinal expansion with forceps only peeling compared to the combined technique (+10.7 μm versus 4.5 μm, P = 0.07).
Longitudinal Macroarchitectural and Microarchitectural OCT Analysis
From the 45 eyes with macroarchitectural alterations on iOCT, 63 inner retinal elevations and 11 full-thickness retinal elevations were noted on the postpeel scan. At 1-week postoperative follow-up, 12 of 63 (19%) postpeel inner retinal elevations exhibited persistent retinal alterations. Of the 11 full-thickness retinal elevations noted on iOCT, two (18%) persisted as inner retinal alterations at 1 week, but none persisted as full-thickness retinal changes. Five of 14 persistent retinal changes were identified as inner retinal swelling and 9 of 14 were noted as inner retinal thinning. At 1-month, three of the five inner retinal swellings had resolved, while all nine of the postoperative inner retinal thinning persisted (Fig. 3).
Retinal layer analysis of eyes within 1 week following surgical intervention showed that there was a significant decrease in the parafoveal EZ-RPE and COST-RPE height compared to the postpeel scan (−20%, −15% respectively, P = 0.007, < 0.001). There was an increase in the parafoveal inner retinal thickness of 32% (P < 0.001). There was no significant change in central foveal thickness.
At 1-month follow-up, there was a significant reduction in central foveal thickness of 5% compared to baseline (P = 0.001). There was a reduction in the parafoveal inner retinal thickness of 6% compared to baseline (P = 0.001). The parafoveal EZ-RPE alterations returned to baseline at 1 month. The parafoveal COST-RPE height remained slightly elevated with a 9% increase over baseline (P = 0.016).
Although there was no direct differences in peel technique for microarchitectural alterations, the duration of the changes appeared to be potentially different. In the combined DDMS/forceps group, all retinal layer changes had returned to baseline by 1 week postoperatively. In the forceps group, there was a significant reduction in the COST-RPE distance compared to baseline at 1 month (P = 0.003). Additionally, the acute perioperative increase in inner retinal thickness trended toward greater magnitude in the DDMS/forceps group compared to the forceps group (P = 0.22). However, by 1 week the combined DDMS/forceps group had returned to postpeel thickness, whereas the inner retinal thickening following initial peeling in the forceps groups did not return to postpeel levels until 1 month.
Discussion
Over the last decade, clinical management of vitreoretinal diseases has been transformed through the use of OCT. It is clear that the clinician's en face assessment may not pick up subtle clinical abnormalities that are functionally and structurally important, such as subtle intraretinal fluid. OCT has power the discriminate these micron-level perturbations. Similarly, visualizing changes in the photoreceptor layers are not possible with slit-lamp biomicroscopy, but with spectral-domain OCT (SD-OCT) the clinician can easily visualize alterations. One important example of this is with the approval of ocriplasmin. In a recent retrospective study, quantitative assessment of retinal structural changes in eyes following ocriplasmin injection with SD-OCT was performed.14 In select eyes, EZ-RPE height was significantly decreased 1 week following injection. This EZ-RPE height loss gradually recovered with time. Three months following injection, outer retinal thickness recovered to baseline.14 These changes were not identifiable during the phase 3 trials, as time-domain OCT was utilized in those studies.15
As OCT has transformed the diagnostic capability for clinical management of vitreoretinal conditions, the use of OCT in the operating room has similar potential to enhance our understanding of the subclinical architectural environment and provide new feedback to surgeons on the impact of surgical maneuvers on the retinal architecture. Previous smaller studies have suggested that iOCT may identify subclinical alterations in the architecture of the retina, including in retinal detachment repair, ERM peeling, and MH repair.9,12,16,17 Subtle changes appear to be common in the photoreceptor layers following membrane peeling, particularly an expansion of the EZ-RPE heights.9,12,18 The actual anatomic configuration that these OCT changes represent remains unknown. The expansion of the EZ-RPE may reflect subtle subretinal fluid, outer segment stretching, or perhaps partial disinsertion from the RPE. These findings may have implications for the current classification of the interdigitation zone. Interestingly, there does not appear to be any significant changes in the brightness or thickness of this layer following separation from the RPE band. This deserves additional analysis but is beyond the scope of this report.
The overall anatomic/functional significance also remains unclear. A recent report from the PIONEER study supported the potential anatomic importance of these changes. In that report, alterations in the EZ-RPE appeared to have important implications for anatomic normalization following MH repair. In fact, increased EZ-RPE height following ILM peeling in MH cases correlated with more rapid normalization of the fovea with decrease in persistent subfoveal fluid formation following repair, which resulted in faster visual recovery following repair.7
In this report, macroarchitectural alterations with video-iOCT correlation were performed with longitudinal assessment of alterations to 1 month postoperatively in all eyes. Generally, isolated inner retinal alterations appeared to be at more risk for long-term retinal alterations compared to full-thickness retinal alterations. In the acute postoperative period, both focal inner retinal swelling and inner retinal thinning were identified. During the subacute period, the inner retinal thinning was the more frequent persistent abnormality. The functional implications of these changes still remain unclear. As most of these changes are extrafoveal, VA may not be the best metric for assessing any functional implications. Future studies should potentially include assessment of microperimetry, dissociated optic nerve fiber layer (DONFL) incidence/severity, contrast sensitivity, and other measures of visual quality. The long-term implications of these changes are also of interest and these follow-up studies are underway.
There was a strong correlation between formation of these macroarchitectural alterations and instrument–tissue interaction. There have been several reports of the postoperative inner retinal swelling following the ILM peeling.3,19 Several authors have conjectured that the trauma from the ILM peeling spares the ganglion cells and is limited to the Müller cell end plates, which have the inner processes between the nerve fiber layer and the ILM.3,4 In our study, we found that the inner retinal elevations (23%) as well as the full-thickness retinal elevations (5%) can develop immediately after the ILM peeling. The majority of these retinal elevations were limited to one or two retinal elevations per the affected eye (85%). The iOCT findings of a full-thickness retinal elevation in our study may represent a transient phenomenon that has not been previously reported. Previous reports have suggested that Müller cells were injured from the direct contact with the surgical instruments.3,4 In our study, 95% of the iOCT macroarchitectural alterations were correlated with instrument utilization. Additionally, we found that the retinal changes were not just associated with direct initiation, but also indirect traction during membrane removal.
In regards to the role of specific surgical instruments architectural alterations, Steel et al.20 compared the forceps-only group (41 eyes) versus DDMS-only group (16 eyes) and concluded that the DDMS was associated with increased postoperative retinal changes. Importantly, no iOCT data were available in this study. In contrast, our study compared the forceps-only group (73 eyes) versus the combined forceps and DDMS group (87 eyes); we found that the forceps were involved in 62% of macroarchitectural alterations compared to the DDMS in 23%. However, the previous study focus was on the degree of DONFL defect rather than acute changes.
Several reports have described postoperative swelling of the nerve fiber layer that may occur following ILM peeling and that the swelling typically subsides after a month.3,19 On the other hand, inner retinal thinning, also described as DONFL, is a more persistent and long-term postoperative OCT finding.2 The focus of this report was on the intraoperative and early postoperative changes. Long-term assessment in this cohort is underway to examine the incidence of DONFL and its correlation with intraoperative architectural alterations. The findings in this study are consistent with previous reports suggesting that inner retinal swelling predominantly resolves by 1 month.
This study confirms previous findings on large-scale of previous smaller reports that diffuse alterations occur following membrane peeling in retinal layer thickness. Immediately following membrane peeling, there was a significant decrease in inner retinal thickness as well as an increase in the EZ-RPE and COST-RPE heights. The outer retinal changes had predominantly resolved in the early postoperative period. Interestingly, there was a significant transient increase in inner retinal thickness that occurred in the first postoperative week. This may correspond to direct trauma related to membrane peeling, similar to those changes previously described.3,19 The early alterations in the inner retina trended toward a greater magnitude in the membrane scraper group, but resolved quicker than the forceps group. Although the increase in height of the photoreceptor layers resolved, a persistent thinning was noted in the COST-RPE distance at final follow-up compared to baseline. The significance of these findings is unclear from both a functional and anatomic standpoint. In MH cases, these alterations appear to have important positive prognostic significance for speed of visual recovery.7
Integrative technology for iOCT is constantly improving. In this study, a microscope-mounted iOCT system was utilized. This does not allow for direct visualization of instrument-tissue interactions. New generation microscope-integrated systems will allow the surgeon to assess tractional forces during membrane engagement with instruments and the immediate repercussions of these interactions.8,10,21–23 In the future, studies examining true real-time interactions of the tissue and instruments may be illustrative regarding the impact of specifics of peeling (e.g., angle of tension) on the retinal changes.
There are several limitations to this study including the lack of randomization and limited follow-up period. Technical limitations include the lack of tracking and co-localization during imaging sequences, limiting the ability to precisely correlate the preincision and postpeel iOCT images to identical cross-section. Furthermore, variations in image quality may affect the interpretation of the iOCT images. These factors limit the ability to identify particularly subtle changes with direct correlation. However, utilizing a combination of the OCT en face fundus image and video correlation, many of these challenges were overcome. Future advances in microscope-OCT integration with on-board video registration will provide a more ideal platform for optimal correlation.
Additional study design limitation must also be considered. Although PIONEER was a prospective clinical study, the postoperative surveillance following study exit was based on standard of care at investigator discretion. In addition, this study was a subanalysis of the PIONEER study that was not preplanned and must be considered in this context. Finally, the comparative assessment of instrument utilizing poses unique challenges. Although the video/OCT correlation allowed for specific visualization of which instrument was utilized in an area of a macroarchitectural change, the assessment of a specific instrument manipulation related to microarchitectural changes was not possible. Instead, the overall technique was compared (e.g., DDMS/forceps versus forceps alone).
Even with these limitations, this study presents strong and unique new data to the literature on the impact of surgical manipulations on the retinal architecture. In this study, iOCT demonstrates significant microarchitectural and macroarchitectural alterations following surgical interventions for vitreomacular interface diseases. Macroarchitectural alterations appear to be more common with forceps than with the DDMS. The retinal layer alterations that occur go through a dynamic process from immediately following membrane peeling throughout the early healing process. As technology improves and long-term follow-up is available to better understand the implications of these changes, surgeons may be able to tailor the surgical approach to diseases to minimize alterations that have negative prognostic implications and potentially induce those changes that may have positive prognostic value.
Acknowledgments
Supported by Grant NIH/NEI K23-EY022947-01A1 (JPE), Ohio Department of Development TECH-13-059 (JPE, SKS), Research to Prevent Blindness (Cole Eye Institutional), and Thrombogenics THROM1403JE (JPE).
Disclosure: J.P. Ehlers, Bioptigen (C), Thrombogenics (C, R), Genentech (R), Leica (C), Zeiss (C), Alcon (C), P; J. Han, None; D. Petkovsek, None; P.K. Kaiser, Zeiss (C), Topcon (C), Alcon (C), Novartis (C), Bausch and Lomb (C); R.P. Singh, Zeiss (C); S.K. Srivastava, Bausch and Lomb (C, R); Allergan (R); Leica (C), Carl Zeiss Meditec (C), P
References
- 1. Lee SB,, Shin YI,, Jo YJ,, Kim JY. Longitudinal changes in retinal nerve fiber layer thickness after vitrectomy for epiretinal membrane. Invest Ophthalmol Vis Sci. 2014; 55: 6607–6611. [DOI] [PubMed] [Google Scholar]
- 2. Nukada K,, Hangai M,, Ooto S,, Yoshikawa M,, Yoshimura N. Tomographic features of macula after successful macular hole surgery. Invest Ophthalmol Vis Sci. 2013. ; 54: 2417–2428. [DOI] [PubMed] [Google Scholar]
- 3. Pichi F,, Lembo A,, Morara M,, Veronese M,, Nucci P,, Ciardella AP. Early and late inner retinal changes after inner limiting membrane peeling. Int J Ophthalmol. 2014. ; 34: 437–446. [DOI] [PubMed] [Google Scholar]
- 4. Tadayoni R,, Paques M,, Massin P,, Mouki-Benani S,, Mikol J,, Gaudric A. Dissociated optic nerve fiber layer appearance of the fundus after idiopathic epiretinal membrane removal. Ophthalmology. 2001. ; 108: 2279–2283. [DOI] [PubMed] [Google Scholar]
- 5. Hisatomi T,, Notomi S,, Tachibana T,, et al. Ultrastructural changes of the vitreoretinal interface during long-term follow-up after removal of the internal limiting membrane. Am J Ophthalmol. 2014. ; 158: 550–556. [DOI] [PubMed] [Google Scholar]
- 6. Ehlers JP,, Dupps WJ,, Kaiser PK,, et al. The Prospective Intraoperative and Perioperative Ophthalmic Imaging With Optical Coherence Tomography (PIONEER) study: 2-year results. Am J Ophthalmol. 2014. ; 158: 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ehlers JP,, Itoh Y,, Xu L,, Kaiser PK,, Singh RP,, Srivastava SK. Factors associated with persistent subfoveal fluid and complete macular hole closure in the PIONEER study. Invest Ophthalmol Vis Sci. 2014; 56: 1141–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ehlers JP,, Kaiser PK,, Srivastava SK. Intraoperative optical coherence tomography using the RESCAN 700: preliminary results from the DISCOVER study. Br J Ophthalmol. 2014. ; 98: 1329–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ehlers JP,, Xu D,, Kaiser PK,, Singh RP,, Srivastava SK. Intrasurgical dynamics of macular hole surgery: an assessment of surgery-induced ultrastructural alterations with intraoperative optical coherence tomography. Retina. 2014. ; 34: 213–221. [DOI] [PubMed] [Google Scholar]
- 10. Binder S,, Falkner-Radler CI,, Hauger C,, Matz H,, Glittenberg C. Feasibility of intrasurgical spectral-domain optical coherence tomography. Retina. 2011. ; 31: 1332–1336. [DOI] [PubMed] [Google Scholar]
- 11. Dayani PN,, Maldonado R,, Farsiu S,, Toth CA. Intraoperative use of handheld spectral domain optical coherence tomography imaging in macular surgery. Retina. 2009. ; 29: 1457–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ray R,, Baranano DE,, Fortun JA,, et al. Intraoperative microscope-mounted spectral domain optical coherence tomography for evaluation of retinal anatomy during macular surgery. Ophthalmology. 2011. ; 118: 2212–2217. [DOI] [PubMed] [Google Scholar]
- 13. Pichi F,, Alkabes M,, Nucci P,, Ciardella AP. Intraoperative SD-OCT in macular surgery. Ophthalmic Surg Lasers Imaging. 2012. ; 43: S54 –S. [DOI] [PubMed] [Google Scholar]
- 14. Itoh Y,, Kaiser PK,, Singh RP,, Srivastava SK,, Ehlers JP. Assessment of retinal alterations following intravitreal ocriplasmin with SD-OCT. Ophthalmology. 2014; 121: 2506–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stalmans P,, Benz MS,, Gandorfer A,, et al. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N Eng J Med. 2012. ; 367: 606–615. [DOI] [PubMed] [Google Scholar]
- 16. Ehlers JP,, Ohr MP,, Kaiser PK,, Srivastava SK. Novel microarchitectural dynamics in rhegmatogenous retinal detachments identified with intraoperative optical coherence tomography. Retina. 2013. ; 33: 1428–1434. [DOI] [PubMed] [Google Scholar]
- 17. Lee LB,, Srivastava SK. Intraoperative spectral-domain optical coherence tomography during complex retinal detachment repair. Ophthalmic Surg Lasers Imaging. 2011. ; 42:e71-74. [DOI] [PubMed]
- 18. Ehlers JP,, Tam T,, Kaiser PK,, Martin DF,, Smith GM,, Srivastava SK. Utility of intraoperative optical coherence tomography during vitrectomy surgery for vitreomacular traction syndrome. Retina. 2014. ; 34: 1341–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diaz R,, Randolph J,, Sigler E,, Calzada J. Intraoperative grasp site correlation with morphologic changes in retinal nerve fiber layer after internal limiting membrane peeling. Ophthalmic Surg Lasers Imaging Retina. 2014. ; 45: 45–49. [DOI] [PubMed] [Google Scholar]
- 20. Steel DH,, Dinah C,, Habib M,, White K. ILM peeling techniques influences the degree of a dissociated optic nerve fibre layer appearance after macular hole surgery. Graefes Arch Clin Exp Ophthalmol 2015; 253: 691–698. [DOI] [PubMed] [Google Scholar]
- 21. Ehlers JP,, Tao YK,, Farsiu S,, Maldonado R,, Izatt JA,, Toth CA. Integration of a spectral domain optical coherence tomography system into a surgical microscope for intraoperative imaging. Invest Ophthalmol Vis Sci. 2011. ; 52: 3153–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ehlers JP,, Srivastava SK,, Feiler D,, Noonan AI,, Rollins AM,, Tao YK. Integrative advances for OCT-guided ophthalmic surgery and intraoperative OCT: microscope integration, surgical instrumentation, and heads-up display surgeon feedback. PLoS One. 2014. ; 9: e105224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ehlers JP,, Tao YK,, Farsiu S,, Maldonado R,, Izatt JA,, Toth CA. Visualization of real-time intraoperative maneuvers with a microscope-mounted spectral domain optical coherence tomography system. Retina. 2013. ; 33: 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]