Abstract
Purpose
Currently, there are no definitive immunomarkers for epithelial stem cells (corneal and conjunctival) or their poorly understood niche microenvironment. The H2B-GFP/K5tTA mouse enables visualization of label-retaining cells (LRCs), which exhibit the functional marker of stem cell quiescence. We used immunofluorescence tomography to evaluate putative stem cell markers and LRCs of the mouse ocular surface.
Methods
H2B-GFP/K5tTA mice were pulsed for 56 days and then chased with doxycycline to label LRCs. Limbus and eyelid tissue was 3-dimensionally (3-D) reconstructed using immunofluorescence tomography to identify and characterize LRCs using the putative stem cell markers sox9, keratin 19, lrig1, blimp1, and abcb5.
Results
After 28 days of chase, LRCs were localized to the entire limbus epithelium and, infrequently, the anterior limbal stroma. Label-retaining cells comprised 3% of limbal epithelial cells after 56 days of chase. Conjunctival LRCs were localized to the fornix and comprised 4% of the total fornix epithelial cells. No stem cell immunomarker was specific for ocular surface LRCs; however, blimp1 enriched for limbal basal epithelial cells and 100% of green fluorescent protein-positive (GFP+) cells at the limbus and fornix were found to be lrig1-positive.
Conclusions
Label-retaining cells represent a larger population of the mouse limbus than previously thought. They decrease in number with increased doxycycline chase, suggesting that LRC populations with different cell cycle lengths exist at the limbus. We conclude that current immunomarkers are unable to colocalize with the functional marker of epithelial stem cell quiescence; however, blimp1 may enrich for limbal epithelial basal cells.
Keywords: 3-D reconstruction, limbal epithelial stem cells, cornea, conjunctiva, H2B-GFP
Adult epithelial stem cells are slow-cycling, undifferentiated progenitor cells that have a critical role in self-renewal of epithelial tissues through asymmetric division.1–3 Like skin, renewal of the ocular surface epithelium also requires adult epithelial stem cells, and current evidence suggests that stem cells localized to specific protective niches give rise to the corneal and conjunctival epithelium.
Corneal epithelial stem cells and their progeny have been shown to reside at the limbus and renew the corneal epithelium via an X,Y,Z mechanism, where desquamated cells (Z) are replaced by basal cells (X) and cells that migrate from the periphery (Y).4 The limbal progenitors self-replicate and give rise to transient amplifying cells, which more frequently divide and generate differentiated cells to carry out the corneal epithelium's barrier function. Corneal epithelial stem cells reside in protective niches formed by their extracellular matrix microenvironment, which is thought to regulate their quiescence.3,5–7 While the junction between cornea and conjunctiva has long been known as the site of the corneal epithelial stem cell,8 the biology of adult epithelial stem cells and their surrounding niche is poorly understood.
In general, corneal epithelial cells purified from the limbal epithelium exhibit higher clonogenic capabilities in vitro than cells isolated from central cornea.9 While the exact number of limbal stem cells per cornea is unknown, it is thought that they represent only a small subpopulation estimated at approximately 1% of basal epithelial cells at the limbus.10 This extremely low population of limbal stem cells means that they are difficult to locate with conventional histology methods and, therefore, immunofluorescence tomography may be helpful in characterizing this stem cell population as it enables the identification and high-resolution, repetitive analysis of single cells in a large tissue volume.
A hallmark of epithelial stem cells is their slow-cycling nature, which can be identified using proliferative markers such as [3H]-thymidine or bromodeoxyuridine (BrdU). These cells can be pulse-chase labeled to allow dilution of fluorescent markers from rapidly dividing cells to uncover cells that retain label and cycle slowly, that is, label retaining cells (LRCs). Using this approach LRCs have been identified in the limbus and fornix of the ocular surface.11,12 However, it should be noted that these labeling approaches produce toxic effects to the epithelium, which restricts the length of the pulse phase.13 Labeling of BrdU is thought to be more toxic than [3H]-thymidine.14 With a limited pulse labeling time there is an uncertainty that all epithelial cells were labeled before chase. To increase the pulse phase and study quiescent epithelial cells using endogenous green fluorescent protein (GFP) labeling, the Fuchs lab developed the H2B-GFP/K5tTA mouse with GFP tagged to the histone H2B and controlled by the Tet(Off) system.15,16 When these bi-genic mice are chased with doxycycline, GFP fluorescence is diluted 2-fold with each epithelial cell division, while it is retained in LRCs.
Several candidate protein markers, such as sox9, keratin 19, abcb5, and lrig1, have been proposed to mark corneal stem cells selectively but a limbal stem cell marker remains elusive.1,17–19 Furthermore, the transcriptional repressor, blimp1, is known as a “master regulator” of hematopoietic stem cells20 that has a crucial role in photoreceptor development21 and is a putative epithelial stem cell marker involved in regulating sebaceous gland turnover.22 The role of blimp1 in corneal homeostasis is virtually unknown and its colocalization with limbal epithelial cells has not been evaluated until this point to our knowledge.
The second ocular surface stem cell is the conjunctival epithelial stem cell that has been demonstrated to belong to a separate and distinct lineage.23 The conjunctiva contributes to tear film stability and constitutes the palpebral conjunctiva attached to the eyelid and the bulbar conjunctiva, which forms the most superficial layer of epithelium across the front of the sclera. Evidence indicates that the fornix contains LRCs24 and highly clonogenic cells9 that may contribute to epithelial and secretory goblet cells, which form the conjunctiva. Goblet cell formation in cultures of transiently amplifying cells derived from a single conjunctival clone have been reported previously.9 The increased density of goblet cells in the fornix region may be a result of a bipotent conjunctival stem cell concentrated in this area, which gives rise to keratinocytes and goblet cells.25 Again, little is known about the biology of these cells and mechanisms regulating conjunctival self-renewal.
Recently, we used immunofluorescence tomography to characterize and quantify the meibomian gland adult epithelial stem cells26 using the H2B-GFP/K5tTA bi-genic mouse.27 In this study, immunofluorescence tomography was used to 3-dimensionally (3-D) reconstruct the limbus of the H2B-GFP/K5tTA mouse to colocalize putative stem cell immunomarkers, such as sox9, lrig1, and blimp1, with LRCs. Furthermore, LRCs with different rates of cell division may exist at the limbus as they decrease in number with increased doxycycline chase. The conjunctiva is likely to be renewed by quiescent cells at the fornix. Gene analysis of LRCs isolated from the ocular surface epithelial tissues, including cornea and conjunctiva, will shed light on the similarities and differences of gene expression in LRCs from separate tissues and is required to fully elucidate a unique protein marker of limbal stem cells.
Methods
H2B-GFP/K5tTA Transgenic Mouse Generation and Pulse-Chase Labeling
At all times mice were treated according to the ARVO Statement on the Use of Animals in Ophthalmic and Vision Research, and experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of California, Irvine (P.I. Jester, protocol# 2011-3002, approved September 8, 2011). To breed H2B-GFP/K5tTA mice, we crossed K5tTA transgenic mice generously provided by Stuart Yuspa, MD, at the National Cancer Institute (Bethesda, MD, USA)28 with TRE/H2B-GFP transgenic mice obtained from Jackson Laboratory (005104; Bar Harbor, ME, USA),16 and donated by the Fuchs lab, to generate bigenic H2B-GFP/K5tTA mice. K5tTA mice with a keratin 5 (K5) promoter driving the tTA transgene are crossed with transgenic mice expressing a tightly regulated tetracycline-responsive element (TRE) driving H2B-GFP expression. Intranuclear GFP expression within keratin 5+ epithelial cells was achieved using the “tet-off” strategy where histone H2B-GFP expression is dependent on the doxycycline-controlled transactivator protein (tTA). In the progeny of these mice, GFP fluorescence is expressed in epithelial cells of the ocular surface. When these mice are fed doxycycline in their diet and the chase phase is initiated, GFP fluorescence is diluted 2-fold with each division and GFP is retained in slow-cycling, putative stem cells only over long-term chase.
To ensure all epithelial cells are labeled, H2B-GFP/K5tTA mice were pulsed for 56 days at P0 before introducing a doxycycline diet (2 g/kg; Bio-Serv, Flemington, NJ, USA). After 0 to 56 days doxycycline chase mice were killed by carbon dioxide asphyxiation and cervical neck dislocation to evaluate label dilution and epithelial cell quiescence through GFP label retention. Low magnification fluorescent imaging was done using a Leica MZ 164A dissecting microscope (Leica Biosystems, Nussloch, Germany) and ×5/0.5 LWD objective.
Tissue Embedding, Sectioning, and Immunostaining
Mouse corneas were excised, fixed in 2% paraformaldehyde in PBS for at least 24 hours and embedded in low melting point 3% agarose necessary to orient the tissue appropriately. Tissues were increasingly dehydrated with ethanol (EtOH; 50–75–90–100% at 30-minute intervals) before resin infiltration with butyl methyl methacrylate (BMMA; Sigma-Aldrich Corp., St. Louis, MO, USA; 2:1; 1:1; 1:2; EtOH:BMMA). The BMMA-embedded blocks then were polymerized for a minimum of 8 hours using UV light at 4°C in a temperature-regulated ice cooler box (Ted Pella, Redding, CA, USA). Additionally, selected tissues were embedded in OCT and cryo-sections cut at 10 μm using a Leica cryostat. After drying, sections were labeled with 4′,6-diamidino-2-phenylendole (DAPI; BMMA; Sigma-Aldrich Corp.) which was added to the mounting agent (1:1 Glycerol:PBS) at a concentration of 1:15,000.
The BMMA plastic blocks of corneas were serially sectioned at 2 μm using a Leica EM UC7 Ultramicrotome equipped with a diamond knife (DiATOME, Nidau, Switzerland). The protocol for sequential immunostaining and image acquisition has been described previously.29 All immunostaining steps were done using a TedPella BioWave microwave (Ted Pella) for antigen retrieval as well as rapid and consistent staining under vacuum and at regulated temperatures.
Before immunofluorescence staining, GFP fluorescence was imaged to preserve endogenous signal. Sections then were treated with acetone for 10 minutes to remove BMMA and immunostained with fluorescent antibodies before being mounted with 1:1 Glycerol:PBS with 1:15,000 DAPI. Serial sections were sequentially immunolabeled with either sox9 (1:500; Millipore, Billerica, MA, USA), collagen IV (1:500; Abcam, Cambridge, UK), abcb5 (1:500; Abcam), α-smooth muscle actin (1:250; Sigma-Aldrich Corp.), blimp1 (1:500; Abcam), lrig1 (1:500; Abcam), and keratin 5 (1:1000; Abcam). The total epithelial cell, LRC, and immunostained LRC count from all epithelial layers of the 3-D reconstructed limbus, cornea and fornix conjunctiva was quantified through physical and computational counting with ImageJ (available in the public domain at http://imagej.nih.gov/ij/). Epithelial cells were segmented using Amira software (Visage Imaging, San Diego, CA, USA) and cells underneath the basement membrane were removed before quantification.
Immunofluorescence Tomography 3-D Reconstruction
Fluorescence imaging of GFP and AlexaFluor 546 tagged antibody markers was done using a Leica DMI6000B inverted epi-fluorescence microscope (Leica Biosystems) with an ASI automated stage driven by Metamorph software (Molecular Devices, Sunnyvale, CA, USA). Sections were mosaic imaged using a Leica ×20 0.75NA objective and a CCD camera capturing images with a pixel area of 0.46 μm2. Thus, the volume of each pixel (voxel) in the 3-D reconstructions is 0.46 × 0.46 × 2 μm. The 3-D reconstructions of the H2B-GFP/K5tTA mouse limbus comprised of 186 (sox9, 372 μm), 122 (lrig1, 244 μm), and 87 (blimp1, 174 μm) BMMA plastic sections.
Stitching of tiled mosaics was completed with a 10% overlap using Metamorph. The stitched mosaics that make up the image of each section were converted to 8-bit and contrast enhanced in ImageJ before semiautomated alignment and 3-D reconstruction using Amira software. The segmentation of basement membrane and vasculature from the collagen IV immunostaining of limbus also was done using Amira.
Results
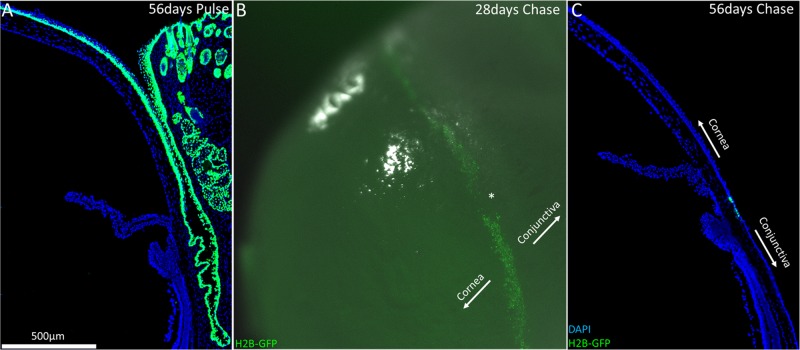
At P0, H2B-GFP/K5tTA mice were fed normal chow, without doxycycline, for 56 days, which constitutes the pulse phase. At 56 days pulse, the entire epithelial layer of the H2B-GFP/K5tTA mouse ocular surface, including cornea, conjunctiva, and meibomian gland, was labeled green due to the nuclear expression of histone H2B tagged with GFP (Fig. 1A). This extended pulse phase of 56 days ensures all keratin 5 epithelial cells express GFP and is not toxic to the mouse over long-term pulse-chase, unlike BrdU.15,30
Figure 1.
Slow-cycling limbal stem cells responsible for corneal epithelial cell renewal were localized to the limbus in the H2B-GFP/K5tTA mouse after >28 days doxycycline chase. (A) At 56 days pulse (no doxycycline), keratin 5+ cells express nuclear GFP, which includes all the basal keratinocytes of the ocular surface: the cornea, conjunctiva, and meibomian gland. (B) Low-magnification image of H2B-GFP/K5tTA mouse whole eye after 28 days doxycycline chase. At 56 days pulse – 28 days chase, LRCs were seen throughout the circumference of the corneal limbus, although regions devoid of LRCs were also observed (*). (C) After 56 days doxycycline chase, LRCs at the limbus are GFP+ because they divide infrequently compared to other epithelial cells which lose half of their GFP signal with every round of cell division.
Once H2B-GFP/K5tTA mice are fed doxycycline chow, GFP+ epithelial cells lose fluorescence signal by 50% with every cell division. After 28 day doxycycline chase, an abundance of LRCs were seen to occupy the entire peripheral corneal limbus of the H2B-GFP/K5tTA mouse eye by low magnification fluorescent imaging (Fig. 1B). By 56 days chase, GFP+ LRCs were markedly fewer and localized to the limbus region at the barrier between conjunctiva and corneal epithelium, as shown in a cross-section of frozen tissue (Fig. 1C). The decrease in LRC population with longer chase, and the GFP intensity differences among LRCs, suggests that distinct populations of LRCs exist at the limbus with varied cell cycle lengths.
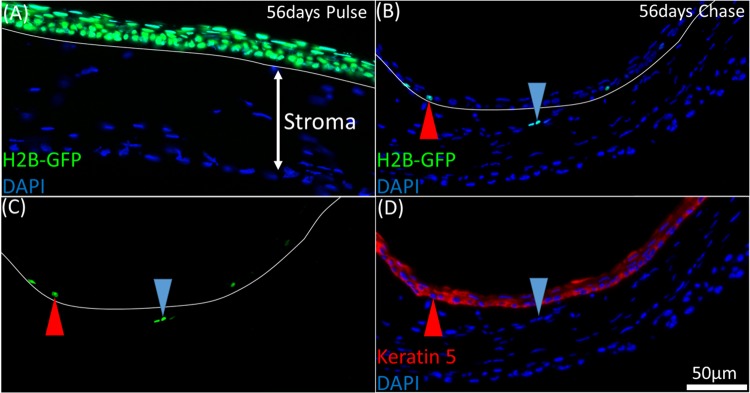
Immunofluorescence tomography 3-D reconstructions captured infrequent events at high-resolution in a fixed tissue through serial sectioning of volumes >100 μm at a section thickness of 2 μm. Basal corneal epithelial cells fluoresce green at 56 days pulse because of the expression of the H2B-GFP fusion protein in the nucleus, which is driven by the keratin 5 promoter (Fig. 2A). At 56 days doxycycline chase, rare GFP+ LRCs were identified in the limbal epithelium (Figs. 2B–D, red arrow) and extremely rarely in the limbal stroma (Figs. 2B–D, blue arrow). Keratin 5 immunostaining on the same sections revealed that while the limbal epithelial LRC were K5+, stromal LRCs did not stain for K5, implying that they may have migrated from the limbal epithelium within the 56-day chase period without enough proliferation to completely dilute the GFP label. In Figure 2C, three GFP+ cells (blue arrow) in the stroma display different green fluorescence intensities, suggesting that these cells have, in fact, divided, albeit infrequently. Another possibility here is ectopic expression of K5 in these cells before the doxycycline chase phase; however, only epithelial cells were seen to be GFP+ at the end of the pulse phase, as shown in Figure 2A.
Figure 2.
LRCs in the H2B-GFP/K5tTA mouse were localized to the limbus epithelium and infrequently, the anterior limbal stroma. (A) At 56 days pulse, all keratin 5+ corneal epithelial cells express nuclear GFP tagged to histone H2B. (B, C) A 2 μm BMMA cross-section of the H2B-GFP/K5tTA mouse limbus after 56 days doxycycline chase. GFP+ cells were primarily localized to the limbus epithelium (red arrow); however, on rare occasion, they were observed in the limbus stroma with varying fluorescence intensities (blue arrow). (D) Keratin 5 staining (B, C) shows that the GFP+ stromal cells are Keratin 5−. This suggests that migration of limbal epithelial cells may occur or there is ectopic expression of keratin 5.
In the 3-D reconstructions generated from immunostained and aligned BMMA serial sections of the H2B-GFP/K5tTA mouse limbus (Fig. 3), LRCs were few in number compared to sox9+ cells, which were present in a large fraction of cornea and conjunctiva basal cells (Fig. 3A). Blimp1+ cells were found more frequently in the limbus and conjunctiva rather than the corneal epithelium (Fig. 3B). Segmenting the vasculature and basement membrane from 3-D reconstructions generated through collagen IV immunostaining (Fig. 3C), we observed a flat continuous basement membrane in the mouse, which was well-vascularized but devoid of any crypt-like structures like the palisades of Vogt seen in human limbus tissue.
Figure 3.
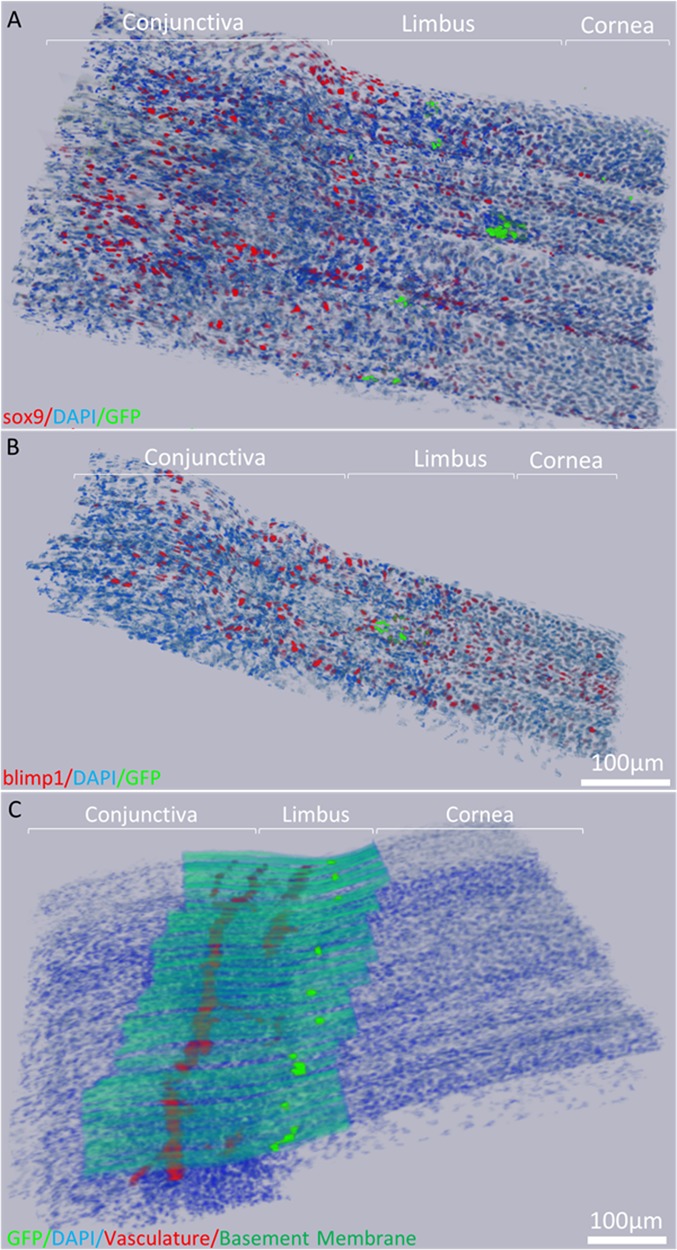
Immunofluorescence tomography 3-D reconstruction of the H2B-GFP/K5tTA mouse limbus. (A) Reconstruction of sox9 immunostaining and LRCs after 56 days doxycycline chase using 186, 2-μm BMMA serial sections (372 μm). (B) 3-D Reconstruction of blimp1 immunostaining and LRCs in the mouse limbus using 87 serial sections (174 μm). (C) 3-D reconstruction of the basement membrane and vasculature network beneath the limbus epithelium which harbors LRCs.
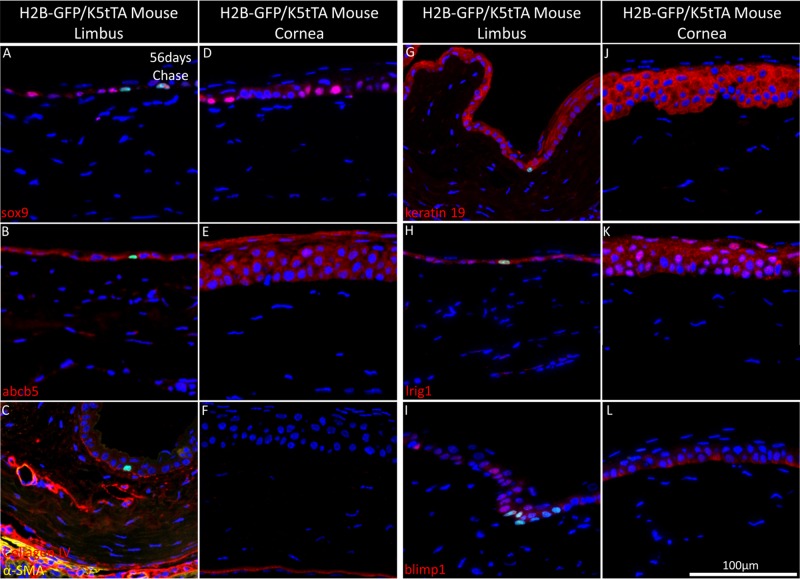
The BMMA serial sections of the H2B-GFP/K5tTA mouse limbus and central cornea were cut at 2 μm and immunostained with putative markers of stem cells and their surrounding niche, including sox9 (Figs. 4A, 5D), abcb5 (Figs. 4B, 5E), collagen IV and α-SMA (Figs. 4C, 4F), keratin 19 (Figs. 4G, 4J), lrig1 (Figs. 4H, 4K), and blimp1 (Figs. 4I, 4L). Label-retaining cells were observed to express sox9 (Fig. 4A), abcb5 (Fig. 4B), lrig1 (Fig. 4H), and blimp1 (Fig. 4I), but these proteins also were found in basal cells that had lost GFP label through multiple divisions and were not specific for long-term chased LRCs. Collagen IV highlights the well-vascularized basement membrane and extracellular matrix that lies underneath the limbal epithelial stem cells (Fig. 4C). No immunomarkers were found here to exclusively colocalize with LRCs and the slow-cycling functional marker of stem cell quiescence (Figs. 4A–L).
Figure 4.
Immunohistochemistry staining of the H2B-GFP/K5tTA mouse limbus and central cornea with putative stem cell markers at 56 days doxycycline chase. The 2 μm BMMA sections of the H2B-GFP/K5tTA mouse limbus (A–C, G–I) and central cornea (D–F, J–L) after 56 days doxycycline chase were immunostained for (A, D) sox9, (B, E), abcb5, (C, F) α-smooth muscle actin, and collagen IV, (G, I) keratin 19, (H, K) lrig1, (I, L) blimp1. Sox9 is localized to LRCs and dividing basal cells of the ocular surface while collagen IV is expressed in the basement membrane and the vasculature network, which terminates at the limbus. Keratin 19 is found in the basal and suprabasal layers of the ocular surface, while the putative stem cell markers lrig1 and blimp1 are found expressed in LRCs and other basal cells of the limbus and the cornea.
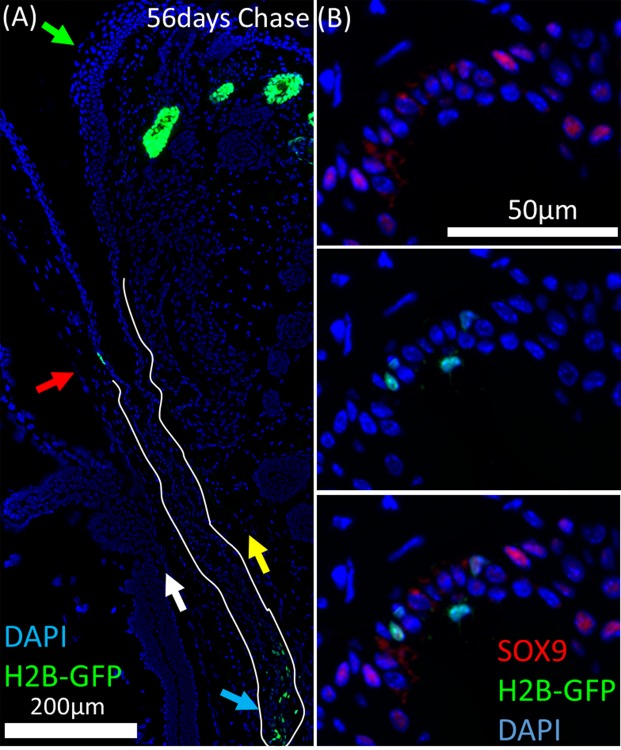
Figure 5.
Label-retaining cells in the H2B-GFP/K5tTA mouse conjunctiva were localized to the fornix region and are most likely responsible for the renewal of palpebral and bulbar conjunctiva epithelium. (A) After 56 days doxycycline chase, GFP+ LRCs in the cornea were localized to the limbus (red arrow) whereas conjunctival LRCs were localized to the fornix (blue arrow). The daughter cells from LRCs in the fornix conjunctiva are likely to repopulate the bulbar (white arrow) and palpebral (yellow arrow) conjunctiva with epithelial cells. No LRCs were observed at the eyelid margin (green arrow). (B) Sox9 immunostaining shows LRCs and dividing cells of the fornix conjunctiva are sox9+ or sox9−.
No LRCs were observed in the central corneal epithelium or the bulbar/palpebral conjunctival epithelium, lending support to the theory of specialized “niches” of epithelial stem cells. In the H2B-GFP/K5tTA mouse ocular surface after 56 days chase, LRCs were found only in the limbus epithelium and fornix conjunctiva, as shown in Figure 5A (blue arrow) favoring the hypothesis that renewal of the bulbar and palpebral conjunctiva is directed from slow-cycling stem cells at the fornix. Furthermore, no LRCs were observed at the eyelid margin. Label-retaining cells of the fornix conjunctiva were either sox9+ or sox9− (Fig. 5B).
To quantify LRCs and their expression of the above markers, the 3-D objects counter in ImageJ was used on the 3-D reconstructions and the percentage of sox9+, lrig1+, and blimp1+ cells of the total epithelial cell nuclei and LRC count were evaluated for the limbus, cornea, and fornix conjunctiva epithelium (Fig. 6). We found that these markers are not specific for LRCs; however, blimp1 is enriched in limbal basal epithelial cells and lrig1 is expressed in 100% of LRCs found at the limbus and fornix. Lrig1 is expressed in the majority of ocular surface epithelial cells according to our immunostaining.
Figure 6.

Three-dimensional quantification of sox9+, lrig1+, and blimp1+ epithelial cells and LRCs across the ocular surface epithelium. Using the 3-D objects counter in ImageJ, the co-localization of sox9, lrig1, and blimp1 with epithelial cells and LRCs was quantified in the (A) limbus (n = 1987 average nuclei), (B) cornea (n = 3407 average nuclei), and (C) fornix conjunctiva epithelium (n = 2386 average nuclei) of the H2B-GFP/K5tTA mouse by immunofluorescence tomography. The limbus and fornix conjunctiva exhibited lrig1+ LRCs and blimp1± or sox9± LRCs. No LRCs were observed in the central corneal epithelium.
Discussion
Slow-cycling limbal stem cells necessary for the maintenance of corneal epithelial cell turnover surround the entire periphery of the H2B-GFP/K5tTA mouse cornea. In 3-D reconstructions generated at 56 days chase, foci of LRCs are observed in much lower populations than those seen at 28 days chase, where low magnification imaging reveals a large band of GFP+ LRCs at the peripheral limbus. Through 3-D quantification, we determined that approximately 3% of limbal epithelial cells were LRCs at 56 days doxycycline chase. This raises the possibility that stem and/or progenitor epithelial cells exist with different rates of cell division at the limbus and questions the current theory of transient amplification and asymmetric division of epithelial stem cells. Lineage tracing experiments in the cornea have shown cell renewal from the limbus involves proliferation and homogeneous migration of clusters of cells from the limbus to the central cornea.31
The limbal stromal translocation of LRCs is a new finding that raises important questions about epithelial-mesenchymal transition (EMT) and stromal migration of limbal epithelial cells. It has been shown previously that limbal epithelial cells invade the stroma by EMT after air exposure through in vitro culture.32 Our data support these findings and suggest that limbal epithelial cells may undergo EMT in vivo. Isolation of LRCs and further characterization by immunofluorescence tomography and in vitro analysis in 3-D cultures will help to determine their differentiation potential and capacity to undergo EMT.
From the LRC data obtained from the H2B-GFP/K5tTA mouse limbus, it is reasonable to suggest that a higher proportion of mouse limbal basal cells than those isolated from human tissue by the ATP-binding cassette ABCG233 or by the high nuclear:cytoplasm side-population34 are, in fact, corneal epithelium progenitors. Purification of human corneal epithelial cells with cell surface markers, such as ABCG2, ABCB5, and β4-integrin, does provide cells with high clonogenic potential and, therefore, is a good method to enrich basal limbal epithelial cells and progenitors. However, in this study we found no immunomarker that colocalizes with the functional marker of epithelial stem cell quiescence. Sox9 expression in >20% of epithelial cells of ocular surface tissues, including the cornea and conjunctiva, suggests that sox9 is not a candidate marker of epithelial progenitor cells. While the SOX9 gene has been suggested to be an essential adult stem cell gene,35 its role in regulating limbal epithelial stem cell function remains unclear. Lrig1 was found in 100% of LRCs quantified, although it did not exclusively colocalize with LRCs as non-LRCs also exhibited lrig1 expression. Interestingly, blimp1 was found in most LRCs (66%) and enriched in the nucleus of basal limbal epithelial cells, whereas the low expression of blimp1 in the corneal epithelium was mostly cytoplasmic.
The LRCs that most likely represent a stem cell population for the bulbar and palpebral conjunctiva are localized to the fornix conjunctiva. In the H2B-GFP/K5tTA mouse conjunctiva after long-term chase of 56 days, the bulbar and palpebral conjunctiva regions do not exhibit label-retaining epithelial cells. The lid margin has been postulated previously to be a niche zone for quiescent epithelial cells,36 but no label-retention was seen at the lid margin in 3-D reconstructions of the conjunctiva after 56 days doxycycline chase. This is supported by a previous study that documented the LRCs in the eyelid and meibomian gland using immunofluorescence tomography.37 The localization of the quiescent epithelial cells to the fornix further supports the hypothesis that the fornix is the region of progenitor or adult stem cells in the conjunctiva.9,24,38
In conclusion, the quiescent epithelial cell population of the cornea occupies almost the entire peripheral limbus of the mouse cornea at 28 days chase; however, only 3% of limbal epithelial cells are LRCs at 56 days chase. This suggests that two LRC populations with different cell cycle lengths exist, with the more quiescent cells likely to represent the true limbal epithelial stem cell population. Immunostaining with putative limbal stem cell immunomarkers confirms the absence of a definitive protein marker that colocalizes with the functional marker of slow-cycling LRCs. Although, this work indicates that blimp1 is enriched in limbal epithelial cells and may be involved in the regulation of limbal stem cell cycle. In the future, isolation and functional analysis in vitro of these LRCs at different chase time-points will enable us to characterize their gene expression, reveal new potential markers, and help determine their clonogenic capacity and multipotency.
Acknowledgments
Supported by NEI EY021510 and the Skirball Program in Molecular Ophthalmology.
Disclosure: G.J. Parfitt, None; B. Kavianpour, None; K.L. Wu, None; Y. Xie, None; D.J. Brown, None; J.V. Jester, None
References
- 1. Stepp MA,, Zieske JD. The corneal epithelial stem cell niche. Ocul Surf. 2005; 3: 15–26. [DOI] [PubMed] [Google Scholar]
- 2. Alonso L,, Fuchs E. Stem cells of the skin epithelium. Proc Natl Acad Sci U S A. 2003; 100 (suppl 1): 11830–11835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Slack JM. Stem cells in epithelial tissues. Science. 2000. ; 287: 1431–1433. [DOI] [PubMed] [Google Scholar]
- 4. Thoft RA,, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983; 24: 1442–1443. [PubMed] [Google Scholar]
- 5. Li W,, Hayashida Y,, Chen YT,, Tseng SC. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007; 17: 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Watt FM,, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000. ; 287: 1427–1430. [DOI] [PubMed] [Google Scholar]
- 7. Lavker RM,, Sun TT. Epidermal stem cells: properties, markers, and location. Proc Natl Acad Sci U S A. 2000; 97: 13473–13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schermer A,, Galvin S,, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986; 103: 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pellegrini G,, Golisano O,, Paterna P,, et al. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol. 1999; 145: 769–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Budak MT,, Alpdogan OS,, Zhou M,, Lavker RM,, Akinci MA,, Wolosin JM. Ocular surface epithelia contain ABCG2-dependent side population cells exhibiting features associated with stem cells. J Cell Sci. 2005; 118: 1715–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cotsarelis G,, Cheng SZ,, Dong G,, Sun TT,, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989. ; 57: 201–209. [DOI] [PubMed] [Google Scholar]
- 12. Zhao J,, Mo V,, Nagasaki T. Distribution of label-retaining cells in the limbal epithelium of a mouse eye. J Histochem Cytochem. 2009; 57: 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuwagata M,, Ogawa T,, Nagata T,, Shioda S. The evaluation of early embryonic neurogenesis after exposure to the genotoxic agent 5-bromo-2′-deoxyuridine in mice. Neurotoxicology. 2007. ; 28: 780–789. [DOI] [PubMed] [Google Scholar]
- 14. Duque A,, Rakic P. Different effects of bromodeoxyuridine and [3H]thymidine incorporation into DNA on cell proliferation, position, and fate. J Neurosci. 2011; 31: 15205–15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fuchs E,, Horsley V. Ferreting out stem cells from their niches. Nat Cell Biol. 2011. ; 13: 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tumbar T,, Guasch G,, Greco V,, et al. Defining the epithelial stem cell niche in skin. Science. 2004. ; 303: 359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pajoohesh-Ganji A,, Stepp MA. In search of markers for the stem cells of the corneal epithelium. Biol Cell. 2005; 97: 265–276. [DOI] [PubMed] [Google Scholar]
- 18. Chen Z,, de Paiva CS,, Luo L,, Kretzer FL,, Pflugfelder SC,, Li DQ. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004; 22: 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schlotzer-Schrehardt U,, Kruse FE. Identification and characterization of limbal stem cells. Exp Eye Res. 2005; 81: 247–264. [DOI] [PubMed] [Google Scholar]
- 20. Turner CA,, Jr, Mack DH,, Davis MM. Blimp-1 a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell. 1994; 77: 297–306. [DOI] [PubMed] [Google Scholar]
- 21. Katoh K,, Omori Y,, Onishi A,, Sato S,, Kondo M,, Furukawa T. Blimp1 suppresses Chx10 expression in differentiating retinal photoreceptor precursors to ensure proper photoreceptor development. J Neurosci. 2010; 30: 6515–6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Horsley V,, O'Carroll D,, Tooze R,, et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006. ; 126: 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wei ZG,, Sun TT,, Lavker RM. Rabbit conjunctival and corneal epithelial cells belong to two separate lineages. Invest Ophthalmol Vis Sci. 1996; 37: 523–533. [PubMed] [Google Scholar]
- 24. Wei ZG,, Cotsarelis G,, Sun TT,, Lavker RM. Label-retaining cells are preferentially located in fornical epithelium: implications on conjunctival epithelial homeostasis. Invest Ophthalmol Vis Sci. 1995; 36: 236–246. [PubMed] [Google Scholar]
- 25. Wei ZG,, Lin T,, Sun TT,, Lavker RM. Clonal analysis of the in vivo differentiation potential of keratinocytes. Invest Ophthalmol Vis Sci. 1997; 38: 753–761. [PubMed] [Google Scholar]
- 26. Parfitt GJ,, Geyfman M,, Xie Y,, Jester JV. Characterization of quiescent epithelial cells in mouse meibomian glands and hair follicle/sebaceous glands by immunofluorescence tomography. J Invest Dermatol. 2015; 135: 1175–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parfitt GJ,, Xie Y,, Geyfman M,, Brown DJ,, Jester JV. Absence of ductal hyper-keratinization in mouse age-related meibomian gland dysfunction (ARMGD). Aging. 2013. ; 5: 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diamond I,, Owolabi T,, Marco M,, Lam C,, Glick A. Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol. 2000; 115: 788–794. [DOI] [PubMed] [Google Scholar]
- 29. Parfitt GJ,, Xie Y,, Reid KM,, Dervillez X,, Brown DJ,, Jester JV. A novel immunofluorescent computed tomography (ICT) method to localise and quantify multiple antigens in large tissue volumes at high resolution. PLoS One. 2012; 7: e53245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fuchs E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell. 2009. ; 137: 811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Di Girolamo N,, Bobba S,, Raviraj V,, et al. Tracing the fate of limbal epithelial progenitor cells in the murine cornea. Stem Cells. 2015; 33: 157–169. [DOI] [PubMed] [Google Scholar]
- 32. Kawakita T,, Espana EM,, He H,, Li W,, Liu CY,, Tseng SC. Intrastromal invasion by limbal epithelial cells is mediated by epithelial-mesenchymal transition activated by air exposure. Am J Path. 2005; 167: 381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Paiva CS,, Chen Z,, Corrales RM,, Pflugfelder SC,, Li DQ. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells. 2005; 23: 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Epstein SP,, Wolosin JM,, Asbell PA. P63 expression levels in side population and low light scattering ocular surface epithelial cells. Trans Am Ophthalmol Soc. 2005; 103: 187–199 discussion 199. [PMC free article] [PubMed] [Google Scholar]
- 35. Nowak JA,, Polak L,, Pasolli HA,, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008; 3: 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wirtschafter JD,, Ketcham JM,, Weinstock RJ,, Tabesh T,, McLoon LK. Mucocutaneous junction as the major source of replacement palpebral conjunctival epithelial cells. Invest Ophthalmol Vis Sci. 1999; 40: 3138–3146. [PubMed] [Google Scholar]
- 37. Parfitt GJ,, Geyfman M,, Xie Y,, Jester JV. Characterization of quiescent epithelial cells in mouse meibomian glands and hair follicle/sebaceous glands by immunofluorescence tomography. J Invest Derm. 2015; 135: 1175–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wei ZG,, Wu RL,, Lavker RM,, Sun TT. In vitro growth and differentiation of rabbit bulbar, fornix, and palpebral conjunctival epithelia. Implications on conjunctival epithelial transdifferentiation and stem cells. Invest Ophthalmol Vis Sci. 1993; 34: 1814–1828. [PubMed] [Google Scholar]