Abstract
BACKGROUND
The Food and Drug Administration can set standards that reduce the nicotine content of cigarettes.
METHODS
We conducted a double-blind, parallel, randomized clinical trial between June 2013 and July 2014 at 10 sites. Eligibility criteria included an age of 18 years or older, smoking of five or more cigarettes per day, and no current interest in quitting smoking. Participants were randomly assigned to smoke for 6 weeks either their usual brand of cigarettes or one of six types of investigational cigarettes, provided free. The investigational cigarettes had nicotine content ranging from 15.8 mg per gram of tobacco (typical of commercial brands) to 0.4 mg per gram. The primary outcome was the number of cigarettes smoked per day during week 6.
RESULTS
A total of 840 participants underwent randomization, and 780 completed the 6-week study. During week 6, the average number of cigarettes smoked per day was lower for participants randomly assigned to cigarettes containing 2.4, 1.3, or 0.4 mg of nicotine per gram of tobacco (16.5, 16.3, and 14.9 cigarettes, respectively) than for participants randomly assigned to their usual brand or to cigarettes containing 15.8 mg per gram (22.2 and 21.3 cigarettes, respectively; P<0.001). Participants assigned to cigarettes with 5.2 mg per gram smoked an average of 20.8 cigarettes per day, which did not differ significantly from the average number among those who smoked control cigarettes. Cigarettes with lower nicotine content, as compared with control cigarettes, reduced exposure to and dependence on nicotine, as well as craving during abstinence from smoking, without significantly increasing the expired carbon monoxide level or total puff volume, suggesting minimal compensation. Adverse events were generally mild and similar among groups.
CONCLUSIONS
In this 6-week study, reduced-nicotine cigarettes versus standard-nicotine cigarettes reduced nicotine exposure and dependence and the number of cigarettes smoked. (Funded by the National Institute on Drug Abuse and the Food and Drug Administration Center for Tobacco Products; ClinicalTrials.gov number, NCT01681875.)
Twenty years ago, benowitz and Henningfield published a landmark commentary that coincided with initial attempts by the Food and Drug Administration (FDA) to regulate tobacco products.1 They reasoned that if the nicotine content of cigarettes were limited to approximately 0.5 mg per cigarette (approximately 0.7 mg per gram of tobacco), cigarettes would be rendered nonaddictive. Although a reduction in nicotine content was endorsed by representatives of the medical community,2 in 2000, the FDA lost its initial argument to regulate cigarettes in a hearing before the Supreme Court, and the proposal ultimately languished.3 In the past 8 years, the prospect of reducing the addictiveness of cigarettes has received renewed attention from numerous health organizations, including the Institute of Medicine,4 the World Health Organization (WHO),5 and the Office of the U.S. Surgeon General.6 This renewed attention paralleled changes in the regulatory oversight of tobacco products. The Tobacco Control Act, enacted in 2009, granted the FDA authority to reduce, but not completely eliminate, nicotine if such action is likely to benefit public health.7 Likewise, the WHO Framework Convention on Tobacco Control enables the development of guidelines for the regulation of the contents and emissions of tobacco products, including those related to dependence liability.8
The results of several relatively small studies suggest that cigarettes with very low nicotine content are associated with a desirable set of outcomes, including reduced smoking, reduced nicotine exposure, reduced nicotine dependence, increased abstinence, reduced exposure to toxicants, and few adverse events.9–14 Unlike “light” cigarettes, which reduce machine-generated nicotine yields by increasing ventilation but not by reducing the nicotine content of the tobacco, reduced-nicotine cigarettes appear to result in minimal and transient compensatory smoking.15–18 However, to our knowledge, no large-scale clinical trials of reduced-nicotine cigarettes have been conducted. Furthermore, little is known about the dose-related effects of reduced nicotine.9 Data derived from trials assessing a range of reduced-nicotine cigarettes are critical for providing an empirical basis for regulatory decisions pertaining to nicotine product standards. We evaluated the effects of smoking cigarettes that contained different levels of nicotine for 6 weeks in participants who were not interested in quitting smoking.
METHODS
STUDY DESIGN AND OVERSIGHT
We conducted a seven-group, double-blind, randomized trial at 10 sites between June 2013 and July 2014. After a 2-week baseline period, 840 smokers who were not planning to quit within the next 30 days were randomly assigned (in randomly permuted blocks of 7 and 14, stratified according to site) to smoke for 6 weeks one of seven types of cigarettes that varied in nicotine content (referred to herein as “study cigarettes”). Study cigarettes were the participant’s usual brand, an investigational cigarette (i.e., a cigarette developed for research purposes only) with nicotine content similar to that found in most commercial products (primary control cigarettes), or one of five investigational cigarettes with 2 to 33% of the nicotine in the primary control cigarettes. All study cigarettes were provided free of charge. Of the two types of investigational cigarettes with 0.4 mg of nicotine per gram of tobacco, one type had a higher tar yield than the other. The findings in the group of 123 participants assigned to the high-tar cigarettes were identified a priori as exploratory and are reported separately.
The study was approved by the institutional review board at each study site and was reviewed by the FDA Center for Tobacco Products. It was monitored by an independent data and safety monitoring board. The trial was conducted and reported with fidelity to the study protocol, which is available with the full text of this article at NEJM.org.
PARTICIPANTS
Participants were recruited through flyers, direct mailings, television and radio announcements, and other advertisements. Eligibility criteria included an age of 18 years or older, at least five cigarettes smoked per day, and an expired carbon monoxide level of more than 8 ppm or a urinary cotinine level of more than 100 ng per milliliter. Exclusion criteria were the intention to quit smoking within the next 30 days; use of other tobacco products in addition to machine-manufactured cigarettes on more than 9 of the previous 30 days; a serious medical or psychiatric disorder or unstable condition; positive toxicologic screening for illicit drugs other than cannabis; pregnancy, a plan to become pregnant, or breast-feeding; and use of “roll your own” cigarettes exclusively. All participants provided written informed consent before enrollment. They were paid up to $835 for participating in the study.
STUDY ASSESSMENTS
The primary outcome, the average number of cigarettes smoked per day during week 6, was assessed with the use of an interactive voice-response system (InterVision Media), which telephoned participants and asked them to report the number of study and nonstudy cigarettes smoked the previous day; withdrawal symptoms were also assessed daily during the baseline period and the first week after randomization.
The following measures were administered during 1 or more of 10 in-person visits: the Fagerström Test for Nicotine Dependence (score range, 0 to 10, with higher values indicating greater dependence),19 the 37-item Wisconsin Inventory of Smoking Dependence Motives (score range, 11 to 77, with higher values indicating greater dependence),20 the 8-item Minnesota Nicotine Withdrawal Scale (score range, 0 to 32, with higher values indicating more severe withdrawal),21,22 the Center for Epidemiological Studies–Depression Scale (score range, 0 to 60, with higher values indicating greater depression and a score of ≥16 used as a common criterion for depression)23, and the 10-item Questionnaire on Smoking Urges (score range, 10 to 70, with higher values indicating greater craving).24 Biomarkers were assessed in urine samples from the first voiding in the morning (with a spot urine sample used if the participant forgot to obtain a sample of the first voiding). Samples were collected at randomization, week 2, and week 6. Participants smoked a single cigarette through a handheld device (Borgwaldt), which measures the number and volume of puffs, at baseline, week 2, and week 6. Participants were paid $90 for abstaining from the use of all nicotine and tobacco products for 1 day between week 6 and the abstinence-assessment visit (with abstinence defined as no smoking for ≥18 hours). The purpose of this assessment was to determine whether 6 weeks of use of reduced-nicotine cigarettes alters the effect of abstinence from smoking on withdrawal and craving. Abstinence assessments were conducted only if the expired carbon monoxide level was less than 50% of the value at week 6 or less than 6 ppm. Approximately 30 days after completion of the 6-week period, participants were contacted by telephone to assess smoking behavior.
CIGARETTES AND PRODUCT BLINDING
Investigational cigarettes were obtained from the National Institute on Drug Abuse. The study groups assigned to the investigational cigarettes were defined according to the nicotine content, averaged across menthol and nonmenthol products (which were assigned on the basis of the participant’s preference): 15.8, 5.2, 2.4, 1.3, and 0.4 mg of nicotine per gram of tobacco. Products also differed in the content or yield of minor alkaloids and nitrosamines and in the application of casings, including sugars (which were higher in the cigarettes with 15.8 mg of nicotine per gram of tobacco than in the reduced-nicotine cigarettes in order to balance the ratio of nicotine to sugar). Additional product information is provided in Tables S1 and S2 of the Supplementary Appendix, available at NEJM.org.
Administrative staff who had no contact with the study participants labeled each cigarette carton with a blind code. Participants, investigators, and study staff had no knowledge of which product was given to a participant or whether various participants received the same or different products (except in the case of participants assigned to their usual brand).
At each weekly visit during the study period, participants were provided with a 14-day supply of cigarettes (the number of baseline cigarettes per day × 14). A 14-day supply, rather than a 7-day supply, was provided to account for missed visits and to allow for increases in smoking relative to baseline (e.g., compensatory smoking). Participants were instructed to refrain from the use of other cigarettes; however, there was no incentive to use the study product and no penalty for the use of nonstudy cigarettes.
LABORATORY ANALYSES
We used liquid chromatography with tandem mass spectrometry for the following analyses: urinary total nicotine equivalents (a measure of nicotine exposure25); the salivary ratio of 3′-hydroxycotinine to cotinine (a measure of CYP2A6 metabolic activity, reflecting the rate of nicotine metabolism26); and urinary total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (total NNAL, a biomarker of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone [NNK] exposure).27–29
ADVERSE-EVENT REPORTING
Adverse events were recorded when participants reported any negative changes in their physical or mental health; medication changes; immediate medical care; hospitalization; emergency care; nonemergency care for illness, injury, or other medical condition; cold, influenza, or other respiratory illness; or possible depression. Licensed medical professionals reviewed all adverse events and provided follow-up actions.
STATISTICAL ANALYSIS
The primary analysis compared the average number of cigarettes smoked during week 6 in the group assigned to the primary control cigarettes (15.8 mg of nicotine per gram of tobacco) with the number smoked in the groups assigned to reduced-nicotine cigarettes (5.2, 2.4, 1.3, and 0.4 mg per gram), regardless of adherence. At each visit, the weekly average number of cigarettes per day was calculated, with all days since the previous visit included in the calculation. If a participant missed a visit, data from the 7 days after the previous visit were used.
Linear regression was used for the primary analysis, with adjustment for the baseline number of cigarettes smoked per day. A Bonferroni adjustment was made to account for the comparison of the four groups assigned to reduced-nicotine cigarettes with the group assigned to the control cigarettes in order to determine statistical significance (with the use of a two-tailed test at an alpha level of 0.0125), resulting in an overall type I error rate of 0.05. Analyses of secondary outcomes were performed with the same approach (a two-tailed test at an alpha level of 0.0125). Additional pairwise comparisons were performed for the reduced-nicotine cigarettes that differed significantly from the primary control cigarettes, with the alpha level determined according to the number of pairwise comparisons completed.
Secondary analyses included linear regression with adjustment for the baseline number of cigarettes, age, sex, race (white, black, or other), and nicotine metabolite ratio; repeated-measures analysis with the use of a linear mixed model; and analyses in which the participants assigned to their usual brand of cigarettes served as the reference group. Participants who did not complete the study were not included in the regression analyses but were included in the mixed models. The number of adverse events was compared across groups with the use of zero-inflated negative binomial regression.
RESULTS
STUDY PARTICIPANTS
Data were collected from 840 participants randomly assigned to a study group, 839 of whom were included in the analyses; 1 participant, who was determined to be ineligible after randomization, was excluded. Retention at week 6 exceeded 92% (780 participants) and did not differ significantly according to group (see Fig. S1 in the Supplementary Appendix). Baseline characteristics are shown in Table 1, and in Table S3 in the Supplementary Appendix. The only significant omnibus group effect was for the expired carbon monoxide level; none of the reduced-nicotine groups differed significantly from the group assigned to 15.8 mg per gram for any of the other baseline variables. The 59 participants who did not complete all 6 weeks of the intervention tended to be slightly younger and less educated and were more likely to be male and white than those who completed the intervention, but variables related to smoking did not differ significantly between participants who did not complete the study and those who did (see Table S3 in the Supplementary Appendix).
Table 1.
Demographic and Smoking Characteristics of Study Participants before Randomization, Overall and According to Assigned Group.*
| Characteristic | Overall (N = 839) | Usual Brand of Cigarettes (N = 118) | Investigational Cigarettes | |||||
|---|---|---|---|---|---|---|---|---|
| 15.8 mg/g (N = 119) | 5.2 mg/g (N = 122) | 2.4 mg/g (N = 119) | 1.3 mg/g (N = 119) | 0.4 mg/g (N = 119) | 0.4 mg/g, high tar (N = 123) | |||
| Age — yr | 41.7±13.2 | 41.5±13 | 42.2±13.7 | 42.0±13.0 | 42.2±12.1 | 41.4±14 | 42.9±13.6 | 39.6±13 |
| Male sex — no. (%) | 481 (57.3) | 72 (61.0) | 71 (59.7) | 68 (55.7) | 77 (64.7) | 62 (52.1) | 63 (52.9) | 68 (55.3) |
| Race — no. (%) | ||||||||
| White | 428 (51.0) | 58 (49.2) | 58 (48.7) | 58 (47.5) | 59 (49.6) | 61 (51.3) | 66 (55.5) | 68 (55.3) |
| Black | 321 (38.3) | 51 (43.2) | 48 (40.3) | 51 (41.8) | 46 (38.7) | 44 (37.0) | 44 (37.0) | 37 (30.1) |
| Hispanic ethnic group — no. (%) | 42 (5.0) | 2 (1.7) | 7 (5.9) | 8 (6.6) | 6 (5.0) | 7 (5.9) | 6 (5.0) | 6 (4.9) |
| Attended college — no. (%) | 471 (56.1) | 59 (50.0) | 66 (55.5) | 67 (54.9) | 70 (58.8) | 63 (52.9) | 70 (58.8) | 76 (61.8) |
| Use of menthol cigarettes — no. (%) | 481 (57.3) | 66 (55.9) | 74 (62.2) | 68 (55.7) | 72 (60.5) | 68 (57.1) | 61 (51.3) | 72 (58.5) |
| Cigarettes/day — no.† | 15.6±7.6 | 15.1±7.5 | 15.7±8.3 | 16.1±7.7 | 15.3±7.2 | 15.4±7.5 | 15.4±7.6 | 15.8±7.5 |
| Total nicotine equivalents — geometric mean (range)‡ | 42.1 (0.1–325.7) | 40.6 (2.0–184.6) | 41.8 (0.1–222.3) | 43.2 (3.2–229.8) | 42.1 (0.7–176.2) | 46.1 (1.2–196.5) | 38.2 (0.2–325.7) | 42.8 (1.6–223.2) |
| Expired carbon monoxide — ppm§ | 15.1±8.0 | 13.1±6.9 | 14.9±8.3 | 14.9±8.2 | 16.7±8.3 | 16.9±8.8 | 14±7.2 | 15.3±7.4 |
| Use of other tobacco product in past 30 days — no. (%) | 202 (24.1) | 34 (28.8) | 31 (26.1) | 32 (26.2) | 25 (21.0) | 26 (21.8) | 26 (21.8) | 28 (22.8) |
| Total score on Fagerström Test for Nicotine Dependence | 5.1±2.2 | 5.0±2.2 | 5.1±2.3 | 5.3±2.2 | 5.0 ±2.1 | 5.0±2.4 | 5.2±2.2 | 5.3±2.1 |
Nicotine content is reported in milligrams per gram of tobacco filler. Plus–minus values are means ±SD. Values for total nicotine equivalents are geometric means and ranges. Demographic characteristics, use of other tobacco products, and the score on the Fagerström Test for Nicotine Dependence (ranging from 0 to 10, with higher scores indicating greater dependence) were assessed during the in-person screening on the basis of self-report. Cigarette type, expired carbon monoxide level, and total nicotine equivalents were assessed on the day of randomization. There were no significant between-group differences in baseline characteristics except as noted for expired carbon monoxide levels.
The number of cigarettes smoked per day was calculated as the weekly average during the baseline period with the use of data collected by means of an interactive voice-response system.
As a measure of nicotine exposure, urinary total nicotine equivalents are presented in nanomoles per milligram of creatinine.
P<0.001 for the comparison across groups.
CIGARETTE USE
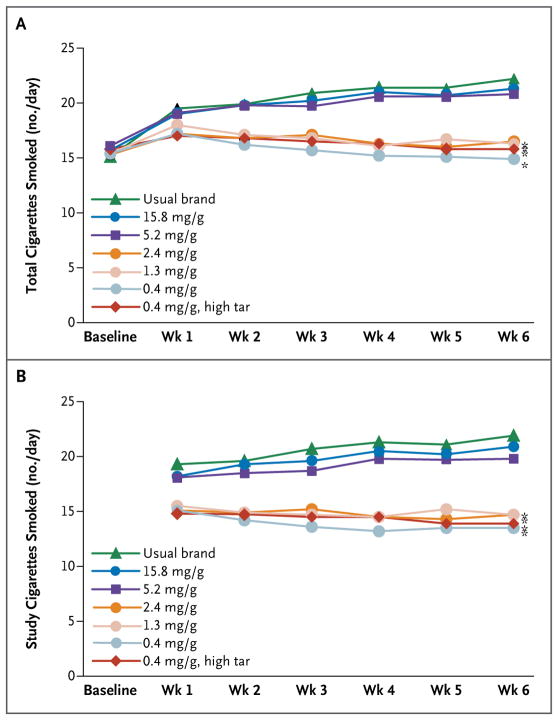
Significant differences were observed among study groups for the total number of cigarettes (study and nonstudy) smoked per day at week 6 (Fig. 1A). Participants assigned to their usual brand and those assigned to control cigarettes (15.8 mg per gram of tobacco) smoked 22.2 and 21.3 cigarettes per day, respectively — significantly more than those assigned to cigarettes containing 2.4, 1.3, and 0.4 mg of nicotine per gram (16.5, 16.3, and 14.9 cigarettes per day, respectively; P<0.001). The group assigned to cigarettes with 5.2 mg of nicotine per gram smoked 20.8 cigarettes per day, which did not differ significantly from the number of cigarettes smoked in the group assigned to cigarettes containing 15.8 mg of nicotine per gram. Similar results were observed when the analysis was restricted to the number of study cigarettes smoked per day (Fig. 1B). Subgroup analyses revealed a significant interaction (P=0.002) with menthol for total cigarettes smoked per day only for cigarettes with 5.2 mg of nicotine per gram (as compared with cigarettes containing 15.8 mg per gram); the number of cigarettes smoked per day was reduced significantly among participants who smoked menthol cigarettes (P=0.001) but not among those who smoked nonmenthol cigarettes (P = 0.16) (Fig. S2 in the Supplementary Appendix).
Figure 1. Number of Cigarettes Smoked per Day According to Nicotine Content.
The mean number of cigarettes smoked per day was based on the number reported by participants with the use of an interactive voice-response system. Panel A shows the mean total number of cigarettes smoked per day, including both study and nonstudy cigarettes. Panel B shows the mean number of study cigarettes smoked per day. All analyses were adjusted for the baseline number of cigarettes smoked per day. An asterisk indicates P<0.001 for the comparison at week 6 with the primary control cigarettes (those with 15.8 mg of nicotine per gram of tobacco).
Participants assigned to cigarettes with 5.2 mg of nicotine or less per gram were more likely to report smoking at least one nonstudy cigarette during the study than those assigned to cigarettes with 15.8 mg of nicotine per gram (73 to 81% vs. 57%, P<0.005). Reduced-nicotine cigarettes were associated with a higher percentage of days on which nonstudy cigarettes were smoked (15% vs. 24 to 35%) but had little effect on the median number of nonstudy cigarettes smoked on those days (two vs. three or four).
Eighty-one percent of participants completed the telephone follow-up 30 days after the 6-week randomized smoking phase. Participants assigned to cigarettes with 0.4 mg of nicotine per gram were more likely to report attempts to quit than were those assigned to cigarettes with 15.8 mg per gram (34.7% vs. 17%, P = 0.005) (Fig. S3 in the Supplementary Appendix). Other groups assigned to reduced-nicotine cigarettes did not differ significantly from the group assigned to the primary control cigarettes with respect to reported attempts to quit, and no significant differences in attempts were observed in comparisons with the group of participants assigned to their usual brand (Table S12 in the Supplementary Appendix). In comparisons with the group of participants assigned cigarettes with 15.8 mg of nicotine per gram, the number of cigarettes smoked per day at follow-up was significantly lower among participants assigned to cigarettes with 1.3 mg per gram (P = 0.007) or 0.4 mg per gram (P<0.001) (Fig. S3 in the Supplementary Appendix).
ASSESSMENTS OF EXPOSURE
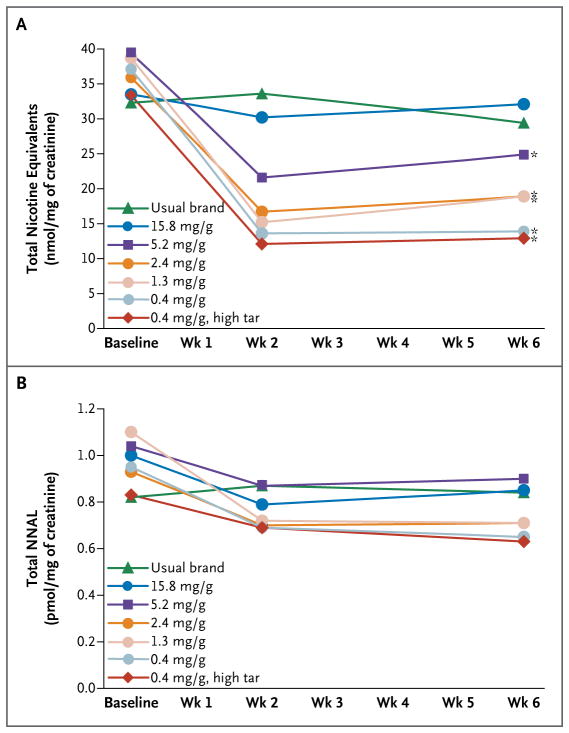
Smokers assigned to cigarettes with 5.2 mg of nicotine or less per gram had significantly lower urinary total nicotine equivalents than those assigned to cigarettes with 15.8 mg per gram (P≤0.01) (Fig. 2A); cotinine levels are shown in Fig. S4 in the Supplementary Appendix. With a prespecified criterion of P<0.0125 for the primary analysis, the urinary total NNAL level was not significantly lower among participants who smoked cigarettes with 0.4 mg of nicotine per gram than among those who smoked control cigarettes (unadjusted model, P=0.02; adjusted model, P=0.009) (Fig. 2B). Groups did not differ significantly with respect to the expired carbon monoxide level (Fig. S5 in the Supplementary Appendix), the interval since the most recent cigarette smoked, or the number of cigarettes smoked before the carbon monoxide assessment.
Figure 2. Biomarkers of Exposure to Nicotine and 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanone (NNK) According to the Nicotine Content of Cigarettes.
Total nicotine equivalents is a measure of nicotine exposure. Total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) is a measure of NNK exposure. Urinary total nicotine and NNAL values are presented as geometric means adjusted for creatinine. Urine samples were collected at baseline, week 2, and week 6. All analyses were adjusted for baseline values. An asterisk indicates P≤0.01 for the comparison at week 6 with cigarettes containing 15.8 mg of nicotine per gram. Additional pairwise analyses were conducted among the reduced-nicotine groups. The group assigned to cigarettes containing 0.4 mg of nicotine per gram differed significantly from the group assigned to 5.2 mg per gram at week 6 (P = 0.001). The group assigned to 0.4 mg per gram (high tar) differed significantly from both the group assigned to 2.4 mg per gram and the group assigned to 5.2 mg per gram at week 6 (P≤0.01).
The total puff volume at week 6 was significantly lower in the group that smoked cigarettes with 0.4 mg of nicotine per gram than in the group that smoked cigarettes with 15.8 mg per gram (Fig. S6 in the Supplementary Appendix). The increase in the expired carbon monoxide level after smoking was not significantly related to the group assignment (Fig. S6 in the Supplementary Appendix).
SUBJECTIVE EFFECTS
When asked to estimate the nicotine level in the assigned study cigarettes, participants smoking cigarettes with 2.4 mg of nicotine or less per gram provided an estimate that was significantly lower than the estimate provided by participants smoking cigarettes with 15.8 mg per gram (P<0.005) (Fig. S7 in the Supplementary Appendix).
When participants estimated the number of cigarettes that they would smoke if the cigarettes cost $6 per pack, participants assigned to cigarettes with 2.4 mg of nicotine or less per gram predicted that they would smoke fewer than 11 cigarettes per day on average, whereas those assigned to cigarettes with 15.8 mg per gram predicted that they would smoke 17 cigarettes per day (P<0.001) (Fig. S8 in the Supplementary Appendix).
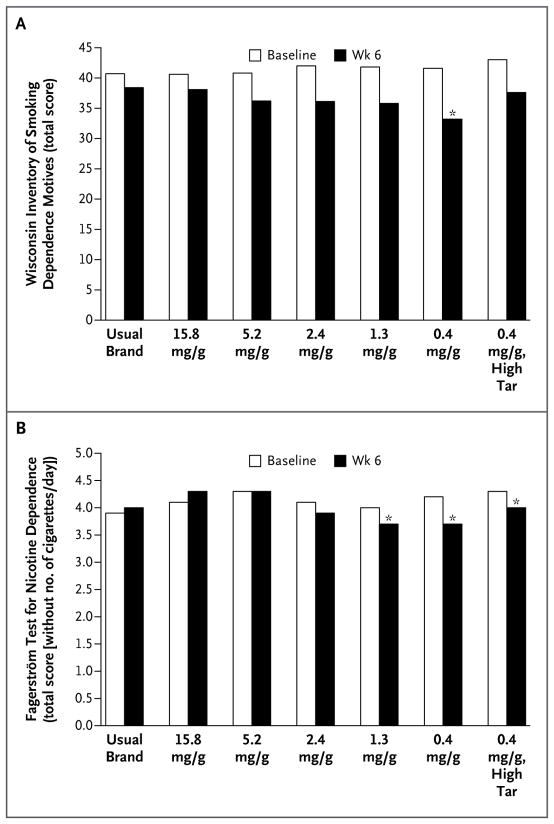
Dependence, as assessed on the basis of the total score on the Wisconsin Inventory of Smoking Dependence Motives, was significantly lower at week 6 among participants smoking cigarettes with 0.4 mg of nicotine per gram than among those smoking cigarettes with 15.8 mg of nicotine per gram (P = 0.001) (Fig. 3A). The score on the Fagerström Test for Nicotine Dependence at week 6 was lower among smokers assigned to cigarettes with 2.4 mg of nicotine or less per gram than among those assigned to cigarettes with 15.8 mg per gram (P≤0.001) (Fig. S9). An analysis that excluded the item assessing the number of cigarettes smoked per day had a similar pattern of results (Fig. 3B).
Figure 3. Nicotine Dependence According to the Nicotine Content of Cigarettes.
Panel A shows total scores on the Wisconsin Inventory of Smoking Dependence Motives (score range, 11 to 77, with higher values indicating greater dependence), which is a multifactorial scale of nicotine dependence. Panel B shows total scores on the Fagerström Test for Nicotine Dependence, with the item concerning number of cigarettes smoked per day excluded (score range after exclusion of that item, 0 to 7, with higher values indicating greater dependence). All analyses were adjusted for the baseline score. An asterisk indicates P≤0.002 for the comparison at week 6 with cigarettes containing 15.8 mg of nicotine per gram.
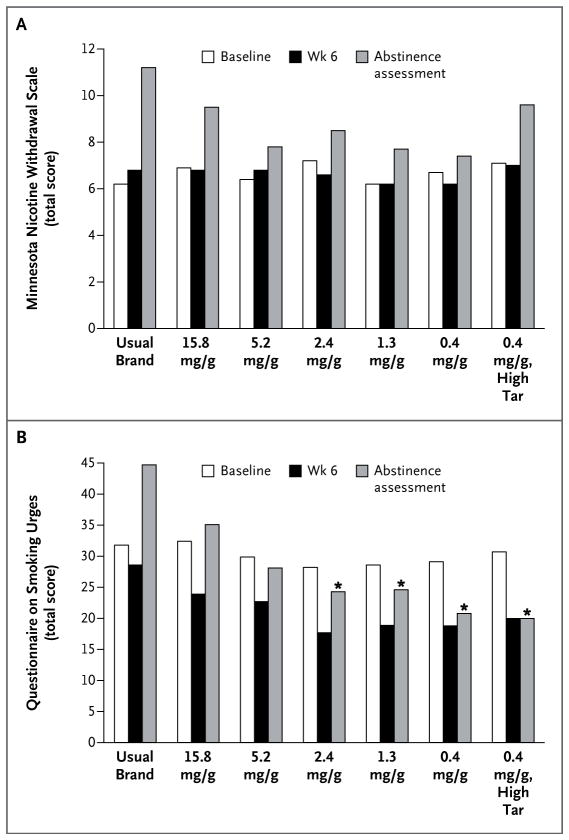
As compared with cigarettes containing 15.8 mg of nicotine per gram, cigarettes with 5.2 mg or less per gram did not significantly increase peak daily withdrawal during week 1 or withdrawal during week 6 (Fig. 4A, and Fig. S10 in the Supplementary Appendix). Less craving was observed at week 6 with cigarettes containing 2.4 or 0.4 mg of nicotine per gram than with the control cigarettes (P≤0.01) (Fig. S11 in the Supplementary Appendix).
Figure 4. Withdrawal and Craving According to the Nicotine Content of Cigarettes.
Panel A shows total scores on the 8-item Minnesota Nicotine Withdrawal Scale (score range, 0 to 32, with higher values indicating more severe withdrawal), and Panel B shows total scores on the 10-item Questionnaire on Smoking Urges (score range, 10 to 70, with higher values indicating greater craving) at baseline, week 6, and after 18 or more hours of abstinence. The Minnesota Nicotine Withdrawal Scale ratings were provided with reference to how participants felt in general since their most recent scheduled visit. The Questionnaire on Smoking Urges ratings were provided in reference to how the participant felt “right now” for the product currently being used (usual brand at baseline; randomized condition at week 6 and during the abstinence assessment). All analyses were adjusted for the baseline score. An asterisk indicates P≤0.003 for the comparison at the same time point with cigarettes containing 15.8 mg of nicotine per gram.
The groups did not differ significantly in the likelihood of completing the abstinence assessment; overall, 76% of participants who were randomly assigned to a study group completed the assessment. Withdrawal during the abstinence session, as assessed on the basis of the score on the Minnesota Nicotine Withdrawal Scale, did not differ significantly between the participants who smoked cigarettes with 15.8 mg of nicotine per gram and those who smoked cigarettes with reduced levels of nicotine (Fig. 4A). Scores for craving during abstinence were significantly reduced among participants who smoked cigarettes with 2.4 mg of nicotine or less per gram (P≤0.001) (Fig. 4B, and Fig. S12 in the Supplementary Appendix).
ADVERSE EVENTS, SELF-REPORTED HEALTH STATUS, AND SAFETY
None of the serious adverse events were judged to be related or possibly related to the study assignment. Participants’ ratings of overall health, respiratory health, and depression did not vary significantly according to the nicotine content of the cigarettes they smoked. Expired carbon monoxide levels exceeded 70 ppm in four participants (two assigned to cigarettes with 5.2 mg of nicotine per gram, and one each assigned to cigarettes with 2.4 mg per gram and cigarettes with 1.3 mg per gram). One participant assigned to cigarettes with 1.3 mg of nicotine per gram was withdrawn at week 2 because of an expired carbon monoxide level that exceeded 100 ppm (116 ppm; baseline level, 37 ppm); this participant also reported recent cannabis use, which may have contributed to the high carbon monoxide level. Additional information on adverse events is provided in Tables S46 through S49 in the Supplementary Appendix.
EFFECT OF TAR YIELD
In comparisons with cigarettes containing 15.8 mg of nicotine per gram, the high-tar and standard-tar versions of the cigarettes with 0.4 mg per gram had similar effects with respect to the number of cigarettes smoked per day (Fig. 1), nicotine and NNK exposure (Fig. 2), retention, adherence, the score on the Fagerström Test for Nicotine Dependence (Fig. 3), and attempts to quit. The most notable difference was for the score on the Wisconsin Inventory of Smoking Dependence Motives (significantly reduced with the standard-tar version but not the high-tar version [Fig. 3A]).
DISCUSSION
Tobacco use is sustained by nicotine.6 Product standards that reduce the nicotine content of combusted tobacco could improve public health by preventing the initiation of daily smoking among nonsmokers and reducing the rate or prevalence of smoking, or both, among current smokers.30 In this 6-week study, participants assigned to cigarettes with 2.4 mg of nicotine or less per gram smoked 23 to 30% fewer cigarettes per day at week 6 than did participants assigned to cigarettes with 15.8 mg per gram. Reduced-nicotine cigarettes led to a reduction in nicotine exposure. NNAL levels did not differ significantly according to the nicotine content of the study cigarettes. The cigarettes with the lowest nicotine content (0.4 mg per gram) reduced dependence according to both measures used in this study. Neither the self-reported number of cigarettes smoked per day nor the expired carbon monoxide level indicated that smokers compensated for the reduction in nicotine by increasing smoking behavior. Use of reduced-nicotine cigarettes resulted in minimal evidence of withdrawal-related discomfort or safety concerns. In summary, these data suggest that if nicotine content is adequately reduced, smokers may benefit by smoking fewer cigarettes and experiencing less nicotine dependence, with few negative consequences. If confirmed in longer-term studies, these findings suggest that, when combined with other tobacco-control policies (e.g., taxation and expanded access to treatment), limiting the nicotine content of cigarettes in order to reduce cigarette use and nicotine dependence and facilitate efforts to quit smoking could improve public health.
Several factors should be considered in interpreting these data. First, use of nonstudy cigarettes was common, which probably attenuated the reduction in nicotine exposure relative to nicotine content and may have minimized the effects of nicotine reduction.31 Second, providing free cigarettes probably inflated the number of cigarettes smoked per day, since cigarette consumption decreases with increases in price.32 Third, the duration of use was limited to 6 weeks, which probably minimized decreases in the rate of smoking and nicotine dependence. A longer trial is currently under way (ClinicalTrials.gov number, NCT02139930). Fourth, the weekly expired carbon monoxide level was not reduced in parallel with the number of cigarettes smoked per day, suggesting possible compensation. However, the total puff volume from cigarettes smoked in the laboratory was reduced in the group assigned to cigarettes with 0.4 mg of nicotine per gram, and the consequent increase in the expired carbon monoxide level was not significantly related to the group assignment. The absence of between-group differences in weekly levels of expired carbon monoxide may be a consequence of its short half-life (2 to 6 hours) and the absence of differences in the interval since the last cigarette was smoked. Fifth, participants assigned to reduced-nicotine cigarettes estimated lower nicotine levels than participants assigned to control cigarettes, a finding that is consistent with the sensory effects of nicotine.33 Finally, the sample, although diverse with respect to race and educational level, was not nationally representative and excluded nondaily smokers and smokers with clinically significant or unstable psychiatric and medical conditions.34
Both the Tobacco Control Act and the Framework Convention on Tobacco Control enable regulators to directly address the addictiveness of tobacco products through product standards. This study provides preliminary short-term data suggesting that as compared with the nicotine content of conventional cigarettes, a substantial reduction in nicotine content is associated with reductions in smoking, nicotine exposure, and nicotine dependence, with minimal evidence of nicotine withdrawal, compensatory smoking, or serious adverse events.
Supplementary Material
Acknowledgments
Supported by a grant from the National Institute on Drug Abuse and the Food and Drug Administration Center for Tobacco Products (U54 DA031659).
We thank all the students, fellows, and staff members at the Center for the Evaluation of Nicotine in Cigarettes who were involved in this study and those who manufactured and characterized the investigational tobacco products used in the study. A full list of acknowledgments is provided in the Supplementary Appendix.
Footnotes
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction: the implications for tobacco regulation. N Engl J Med. 1994;331:123–5. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- 2.Henningfield JE, Benowitz NL, Slade J, Houston TP, Davis RM, Deitchman SD. Reducing the addictiveness of cigarettes. Tob Control. 1998;7:281–93. doi: 10.1136/tc.7.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FDA v. Brown & Williamson Tobacco Corp. 529 U.S. 120 (2000).
- 4.Institute of Medicine. Ending the tobacco problem: a blueprint for the nation. Washington, DC: National Academies Press; 2007. [Google Scholar]
- 5.WHO Study Group on Tobacco Product Regulation. Report on the scientific basis of tobacco product regulation: third report of a WHO study group. Geneva: World Health Organization; 2009. [PubMed] [Google Scholar]
- 6.The health consequences of smoking — 50 years of progress: a report of the Surgeon General: executive summary. Washington, DC: Department of Health and Human Services; 2014. ( http://www.surgeongeneral.gov/library/reports/50-years-of-progress/exec-summary.pdf) [Google Scholar]
- 7.Family Smoking Prevention and Tobacco Control Act (H.R. 1256).
- 8.WHO Framework Convention on tobacco Control. Geneva: World Health Organization; 2003. [Google Scholar]
- 9.Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction. 2007;102:324–34. doi: 10.1111/j.1360-0443.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- 10.Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P., III Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiol Biomarkers Prev. 2007;16:2479–85. doi: 10.1158/1055-9965.EPI-07-0393. [DOI] [PubMed] [Google Scholar]
- 11.Benowitz NL, Dains KM, Dempsey D, Herrera B, Yu L, Jacob P., III Urine nicotine metabolite concentrations in relation to plasma cotinine during low-level nicotine exposure. Nicotine Tob Res. 2009;11:954–60. doi: 10.1093/ntr/ntp092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benowitz NL, Dains KM, Hall SM, et al. Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev. 2012;21:761–9. doi: 10.1158/1055-9965.EPI-11-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatsukami DK, Hertsgaard LA, Vogel RI, et al. Reduced nicotine content cigarettes and nicotine patch. Cancer Epidemiol Biomarkers Prev. 2013;22:1015–24. doi: 10.1158/1055-9965.EPI-12-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatsukami DK, Kotlyar M, Hertsgaard LA, et al. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2010;105:343–55. doi: 10.1111/j.1360-0443.2009.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozlowski LT, O’Connor RJ. Cigarette filter ventilation is a defective design because of misleading taste, bigger puffs, and blocked vents. Tob Control. 2002;11(Suppl 1):I40–I50. doi: 10.1136/tc.11.suppl_1.i40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benowitz NL, Henningfield JE. Reducing the nicotine content to make cigarettes less addictive. Tob Control. 2013;22(Suppl 1):i14–i17. doi: 10.1136/tobaccocontrol-2012-050860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macqueen DA, Heckman BW, Blank MD, Janse Van Rensburg K, Evans DE, Drobes DJ. Transient compensatory smoking in response to placebo cigarettes. Psychopharmacology (Berl) 2012;223:47–54. doi: 10.1007/s00213-012-2685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatsukami DK, Donny EC, Koopmeiners JS, Benowitz NL. Compensatory smoking from gradual and immediate reduction in cigarette nicotine content. Cancer Epidemiol Biomarkers Prev. 2015;24:472–6. doi: 10.1158/1055-9965.EPI-14-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 20.Smith SS, Piper ME, Bolt DM, et al. Development of the Brief Wisconsin Inventory of Smoking Dependence Motives. Nicotine Tob Res. 2010;12:489–99. doi: 10.1093/ntr/ntq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 22.Toll BA, O’Malley SS, McKee SA, Salovey P, Krishnan-Sarin S. Confirmatory factor analysis of the Minnesota Nicotine Withdrawal Scale. Psychol Addict Behav. 2007;21:216–25. doi: 10.1037/0893-164X.21.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 24.Cox LS, Tiffany ST, Christen AG. Evaluation of the Brief Questionnaire of Smoking Urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 25.Scherer G, Engl J, Urban M, Gilch G, Janket D, Riedel K. Relationship between machine-derived smoke yields and biomarkers in cigarette smokers in Germany. Regul Toxicol Pharmacol. 2007;47:171–83. doi: 10.1016/j.yrtph.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Hukkanen J, Jacob P, III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 27.Murphy SE, Wickham KM, Lindgren BR, Spector LG, Joseph A. Cotinine and trans 3′-hydroxycotinine in dried blood spots as biomarkers of tobacco exposure and nicotine metabolism. J Expo Sci Environ Epidemiol. 2013;23:513–8. doi: 10.1038/jes.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy SE, Park SS, Thompson EF, et al. Nicotine N-glucuronidation relative to N-oxidation and C-oxidation and UGT2B10 genotype in five ethnic/racial groups. Carcinogenesis. 2014;35:2526–33. doi: 10.1093/carcin/bgu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carmella SG, Ming X, Olvera N, Brookmeyer C, Yoder A, Hecht SS. High throughput liquid and gas chromatography-tandem mass spectrometry assays for tobacco-specific nitrosamine and polycyclic aromatic hydrocarbon metabolites associated with lung cancer in smokers. Chem Res Toxicol. 2013;26:1209–17. doi: 10.1021/tx400121n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tengs TO, Ahmad S, Savage JM, Moore R, Gage E. The AMA proposal to mandate nicotine reduction in cigarettes: a simulation of the population health impacts. Prev Med. 2005;40:170–80. doi: 10.1016/j.ypmed.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 31.Dermody SS, Donny EC, Hertsgaard LA, Hatsukami DK. Greater reductions in nicotine exposure while smoking very low nicotine content cigarettes predict smoking cessation. Tob Control. 2014 Sep 5; doi: 10.1136/tobaccocontrol-2014-051797. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jha P, Chaloupka FJ. Curbing the epidemic: governments and the economics of tobacco control. Washington, DC: World Bank; 1999. [Google Scholar]
- 33.Thuerauf N, Kaegler M, Dietz R, Barocka A, Kobal G. Dose-dependent stereoselective activation of the trigeminal sensory system by nicotine in man. Psychopharmacology (Berl) 1999;142:236–43. doi: 10.1007/s002130050885. [DOI] [PubMed] [Google Scholar]
- 34.Donny EC, Hatsukami DK, Benowitz NL, Sved AF, Tidey JW, Cassidy RN. Reduced nicotine product standards for combustible tobacco: building an empirical basis for effective regulation. Prev Med. 2014;68:17–22. doi: 10.1016/j.ypmed.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.