Abstract
The bacterial actin-like protein MreB is thought to form a continuous helical polymer at the membrane to confer rod shape. Two new studies now show that MreB forms discrete dynamic patches that travel circumferentially.
MreB is a prokaryotic actin homolog [1] present in most rod-shaped bacteria. Inactivation of MreB by mutation or drugs causes rod-shaped cells to round up, indicating that MreB is required for the maintenance of rod shape, as are a number of other components of the peptidoglycan elongation machinery, including the penicillin-binding proteins, RodA, RodZ, MreC and MreD [2].
It has been assumed for many years that MreB assembles into membrane-associated polymers that form a continuously helical cytoskeletal structure around a rod-shaped bacterium's long axis. These structures, visualized in whole cells either by GFP fusions or immunofluorescence, were first reported in Bacillus subtilis [3], and subsequently observed in Escherichia coli and other bacteria. MreB structures are dynamic and relocalize transiently to the site of cytokinesis [4]. Cytoplasmic MreB interacts directly or indirectly with the transmembrane components of the peptidoglycan elongation machinery [5], leading to the idea that MreB cables provide the organizational and mechanical support to direct the peptidoglycan elongation machinery and guide cell shape [6]. Two new reports [7,8] now challenge the existence of long-range helical MreB cables.
The first doubts about the presence of continuous MreB cables came from direct visualization of peptidoglycan strands in Bacillus subtilis by atomic force microscopy [9] and Caulobacter crescentus by electron cryotomography [10]. These studies concluded that the peptidoglycan strands are arranged in loosely oriented radial hoops, roughly perpendicular to the long axis of a rod-shaped cell. It was not obvious how helical MreB polymers could guide the synthesisis of peptidoglycan to make this pattern, although staining with fluorescent vancomycin, which labels sites of nascent peptidoglycan, appeared to be helical [11]. The idea of long-range MreB helical polymers was further undermined by a cryoelectron tomography study of several rod-shaped bacterial species that specifically searched for continuous MreB polymers/cables in intact cells. Although long MreB polymers were detected in cells overproducing MreB, normal cells showed no signs of MreB polymers, at least longer than ∼80 nm [12]. These results were also consistent with a single molecule study of MreB in C. crescentus [13], which presaged the present reports by showing that a subset of MreB molecules moved in meandering circumferential paths instead of fixed helical paths.
Using advanced high-resolution fluorescence imaging of live B. subtilis cells, the two new reports [7,8] demonstrate that neither MreB nor its two paralogs form a long-range continuous helix, and suggest that previous data showing helices may have been a result of incorrect protein levels, interference from GFP tags, or optical artifacts. Instead, MreB forms discrete complexes that move around the circumference of the rod-shaped cell. Most importantly, the mobility of individual MreB complexes is bidirectional, independent of other complexes, yet totally dependent on peptidoglycan elongation machinery activity. This changes our view of MreB from a global cytoskeletal director of wall growth to a combination of director and nimble, localized responder.
Garner et al. [7] observed that, in B. subtilis, functional GFP fusions to three MreB paralogs, MreB, Mbl and MreBH, formed independent patches that moved processively but often reversibly around the circumference of the cell, roughly perpendicular to the cell's long axis. Mutational inactivation of MreB's ATPase activity did not affect the circumferential movement. As ATP is not required for assembly of B. subtilis MreB into polymers in vitro [14], polymerization dynamics are unlikely to be important for MreB mobility. Depletion of peptidoglycan elongation machinery proteins RodA, RodZ and Pbp2A as well as treatment with antibiotics targeting different steps in peptidoglycan biosynthesis rapidly halted patch motion, indicating that continuous peptidoglycan synthesis is required to maintain mobility of the complexes. Interestingly, intermediate depletion levels stopped some patches but not others, suggesting that the patches operate independently from each other.
Garner et al. [7] used total internal reflection fluorescence microscopy (TIRFM) to track dynamics of individual particles on the cell surface. When the same three MreB paralogs along with the peptidoglycan elongation machinery components MreC, MreD and Pbp2A were tracked, all six proteins moved with approximately equal speeds (20–30 nm per second) in linear, non-helical paths across the cell width, suggesting that they function together as a macromolecular unit. Addition of vancomycin, a drug that blocks the initial step of peptidoglycan synthesis, rapidly stopped them. Particle tracking also confirmed that these units are randomly distributed throughout the cell length, are independent from each other, discontinuous, and can reverse direction. The likely result of this type of movement is that new peptidoglycan is sewn together in circumferential hoops.
In a complementary study, Dominguez-Escobar et al. [8] also used TIRFM to study the localization and dynamics of GFP fusions to the same three MreB paralogs. Like Garner et al. [7], they observed patches containing the MreB paralogs moving bidirectionally along linear, evenly spaced paths that were roughly perpendicular to the cell longitudinal axis and had an average separation of 0.5 μm. Other peptidoglycan elongation machinery components, such as MreC, MreD and PbpH/2a, moved in similar patches. Importantly, this behavior seemsto be universal, as similar results were observed in Escherichia coli and C. crescentus cells.
Previous studies suggested that MreB polymers move by treadmilling [13,15]. The photobleaching recovery studies by Dominguez-Escobar et al. [8], however, showed that the patches could reverse direction, fuse, and split, behaviors at odds with treadmilling. When the authors blocked peptidoglycan incorporation and precursor synthesis with vancomycin and phosphomycin, respectively, or disrupted the peptidoglycan backbone with lysozyme treatment, the MreB, Mbl, and PbpH patches all stopped moving. Interestingly, movement of the patches slowed significantly in mutants of peptidoglycan transpeptidases (PbpH and Pbp2A) or after a partial block of peptidoglycan synthesis using low concentrations of phosphomycin.
The data from both papers [7,8] make a convincing case that MreB is not assembled in continuous cables, that peptidoglycan synthesis itself drives the motion and polymerization dynamics of the MreB patches, and that MreB is co-complexed with transmembrane components of the peptidoglycan elongation machinery as implicated by previous studies [5,16] (Figure 1). By challenging the prevailing model, these studies raise a number of important questions. For example, why is MreB required for rod shape if it is responding to the peptidoglycan elongation machinery instead of driving it? One possibility is that short MreB polymers, attached to the membrane by contacts with the peptidoglycan elongation machinery, respond to the tracks made by the newly synthesized peptidoglycan strands and reinforce their radial directionality. This could explain how multiple independent peptidoglycan elongation machinery complexes can ply roughly parallel paths, and would permit more flexibility and rapid responsiveness to physiological needs than a fixed cytoskeleton. Point mutations in MreB can cause significant changes in cell shape [17] and round cells with inactivated MreB can recover back to rod shape upon reactivation [18], suggesting that MreB filaments intrinsically can constrain individual mobile complexes to achieve a defined overall cell wall geometry.
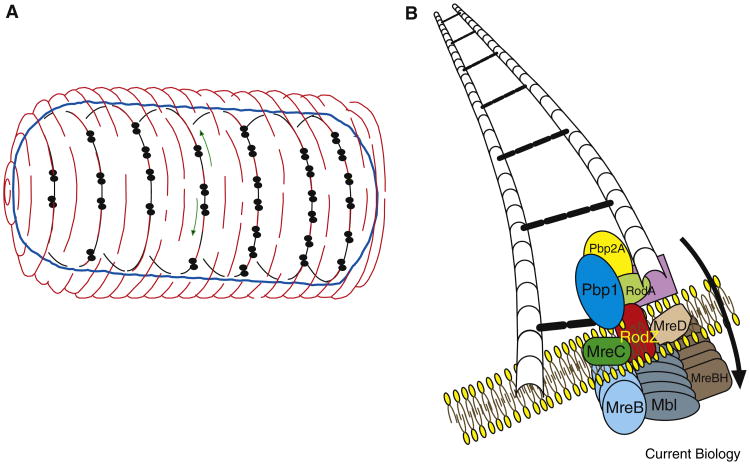
Figure 1.
Schematic representation of mobile patches of peptidoglycan elongation machinery/MreB.
(A) Whole cell with glycan strands represented in red, the cytoplasmic membrane in blue, and the circumferential paths followed by peptidoglycan elongation machinery/MreB paralog complexes in black. Traveling macromolecular complexes are depicted as double-dotted structures, and green arrows depict opposite directionality of two complexes. (B) Expanded view of one processive peptidoglycan elongation machinery/MreB complex synthesizing a new glycan strand. MreB, Mbl and MreBH form polymers along the patch, and localize at the inside edge of the membrane, represented by phospholipids. The peptidoglycan elongation machinery proteins interact with the MreB homologs. An old glycan strand serves as template for synthesis of the new strand. The peptide cross-links between the glycan strands are represented by black ovals. The arrow indicates the traveling direction.
Another key question is what determines initiation and reversal points for new peptidoglycan biosynthesis. If peptidoglycan biosynthesis occurs by the “make-before-break” strategy [19], the existing hoops of peptidoglycan would be required as templates for new peptidoglycan synthesis. Such a template-based sewing mechanism lacking a cytoskeletal lattice for structure might tend to magnify errors in template alignment over time, which would explain why the hoops of peptidoglycan are not precisely oriented with respect to each other but arranged more like a loosely knit sweater. Yet something keeps the general radial hoop structure roughly in line. Perhaps short MreB polymers assemble optimally on negatively curved membranes, which would guide the paths in the proper radial orientation. Consistent with this idea, weakening of MreB activity resulted in more random directionality of patch movement [8]. Patch reversal or splitting may be governed by the local availability of peptidoglycan template or peptidoglycan precursors. For example, a processively moving patch may reverse when it bumps up against another patch synthesizing new peptidoglycan, and proceed to fill in a gap behind it from an adjacent strand of existing peptidoglycan, perhaps what it just synthesized.
The patches represent rapidly moving molecular machines that span the cytoplasmic membrane. It will be important to understand how energy from the expanding wall is harnessed to push these complexes through the membrane, and if the complexes in turn regulate mechanical forces on the membrane and wall. Localized mechanical perturbation of peptidoglycan synthesis can have large effects on cell morphology; membrane-associated protein polymers, for example, can inhibit peptidoglycan synthesis on only one side of the cell, resulting in highly curved cells [20]. Furthermore, the rapid movement of such large complexes along the membrane means that the protein–protein interactions within them must be sufficiently strong to maintain the integrity and processivity of the complexes while allowing for regulatory feedback. Perhaps these complexes are analogous to actin–myosin motors, but with the peptidoglycan elongation machinery synthesis providing the energy instead of myosin, riding on short actin filaments. It is safe to say that we will be in for a fascinating ride the more we understand this system at the molecular level.
References
- 1.van den Ent F, Amos LA, Lowe J. Prokaryotic origin of the actin cytoskeleton. Nature. 2001;413:39–44. doi: 10.1038/35092500. [DOI] [PubMed] [Google Scholar]
- 2.Margolin W. Sculpting the bacterial cell. Curr Biol. 2009;19:R812–R822. doi: 10.1016/j.cub.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones LJ, Carballido-Lopez R, Errington J. Control of cell shape in bacteria: helical, actin-like filaments in Bacillus subtilis. Cell. 2001;104:913–922. doi: 10.1016/s0092-8674(01)00287-2. [DOI] [PubMed] [Google Scholar]
- 4.Graumann PL. Dynamics of bacterial cytoskeletal elements. Cell Motil Cytoskel. 2009;66:909–914. doi: 10.1002/cm.20381. [DOI] [PubMed] [Google Scholar]
- 5.Mattei PJ, Neves D, Dessen A. Bridging cell wall biosynthesis and bacterial morphogenesis. Curr Opin Struct Biol. 2010;20:749–755. doi: 10.1016/j.sbi.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Wang S, Arellano-Santoyo H, Combs PA, Shaevitz JW. Actin-like cytoskeleton filaments contribute to cell mechanics in bacteria. Proc Natl Acad Sci USA. 2010;107:9182–9185. doi: 10.1073/pnas.0911517107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garner EC, Bernard R, Wang W, Zhuang X, Rudner DZ, Mitchison T. Coupled, circumferential motions of the cell wall synthesis machinery and MreB filaments in B. subtilis. Science. 2011;333:222–225. doi: 10.1126/science.1203285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dominguez-Escobar J, Chastanet A, Crevenna AH, Fromion V, Wedlich-Soldner R, Carballido-Lopez R. Processive movement of MreB-associated cell wall biosynthetic complexes in bacteria. Science. 2011;333:225–228. doi: 10.1126/science.1203466. [DOI] [PubMed] [Google Scholar]
- 9.Hayhurst EJ, Kailas L, Hobbs JK, Foster SJ. Cell wall peptidoglycan architecture in Bacillus subtilis. Proc Natl Acad Sci USA. 2008;105:14603–14608. doi: 10.1073/pnas.0804138105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gan L, Chen S, Jensen GJ. Molecular organization of Gram-negative peptidoglycan. Proc Natl Acad Sci USA. 2008;105:18953–18957. doi: 10.1073/pnas.0808035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniel RA, Errington J. Control of cell morphogenesis in bacteria: two distinct ways to make a rod-shaped cell. Cell. 2003;113:767–776. doi: 10.1016/s0092-8674(03)00421-5. [DOI] [PubMed] [Google Scholar]
- 12.Swulius MT, Chen S, Jane Ding H, Li Z, Briegel A, Pilhofer M, Tocheva EI, Lybarger SR, Johnson TL, Sandkvist M, et al. Long helical filaments are not seen encircling cells in electron cryotomograms of rod-shaped bacteria. Biochem Biophys Res Commun. 2011;407:650–655. doi: 10.1016/j.bbrc.2011.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SY, Gitai Z, Kinkhabwala A, Shapiro L, Moerner WE. Single molecules of the bacterial actin MreB undergo directed treadmilling motion in Caulobacter crescentus. Proc Natl Acad Sci USA. 2006;103:10929–10934. doi: 10.1073/pnas.0604503103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayer JA, Amann KJ. Assembly properties of the Bacillus subtilis actin, MreB. Cell Motil Cytoskel. 2009;66:109–118. doi: 10.1002/cm.20332. [DOI] [PubMed] [Google Scholar]
- 15.Defeu Soufo HJ, Graumann PL. Dynamic movement of actin-like proteins within bacterial cells. EMBO Rep. 2004;5:789–794. doi: 10.1038/sj.embor.7400209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young KD. Bacterial shape: two-dimensional questions and possibilities. Annu Rev Microbiol. 2010;64:223–240. doi: 10.1146/annurev.micro.112408.134102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dye NA, Pincus Z, Fisher IC, Shapiro L, Theriot JA. Mutations in the nucleotide binding pocket of MreB can alter cell curvature and polar morphology in Caulobacter. Mol Microbiol. 2011;81:368–394. doi: 10.1111/j.1365-2958.2011.07698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takacs CN, Poggio S, Charbon G, Pucheault M, Vollmer W, Jacobs-Wagner C. MreB drives de novo rod morphogenesis in Caulobacter crescentus via remodeling of the cell wall. J Bacteriol. 2010;192:1671–1684. doi: 10.1128/JB.01311-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holtje JV. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol Mol Biol Rev. 1998;62:181–203. doi: 10.1128/mmbr.62.1.181-203.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cabeen MT, Charbon G, Vollmer W, Born P, Ausmees N, Weibel DB, Jacobs-Wagner C. Bacterial cell curvature through mechanical control of cell growth. EMBO J. 2009;28:1208–1219. doi: 10.1038/emboj.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]