Abstract
The association between parity and endometrial cancer risk is inconsistent from observational studies. We aimed to quantitatively assess the relationship by summarizing all relevant epidemiological studies. PubMed (MEDLINE), Embase and Scopus were searched up to February 2015 for eligible case–control studies and prospective studies. Random-effects model was used to pool risk estimations. Ten prospective studies, 35 case-control studies and 1 pooled analysis of 10 cohort and 14 case-control studies including 69681 patients were identified. Pooled analysis revealed that there was a significant inverse association between parity and risk of endometrial cancer (relative risk (RR) for parous versus nulliparous: 0.69, 95% confidence interval (CI) 0.65–0.74; I2 = 76.9%). By evaluating the number of parity, we identified that parity number of 1, 2 or 3 versus nulliparous demonstrated significant negative association (RR = 0.73, 95% CI 0.64–0.84, I2 = 88.3%; RR = 0.62, 95% CI 0.53–0.74, I2 = 92.1%; and RR = 0.68, 95% CI 0.65–0.70, I2 = 20.0% respectively). The dose-response analysis suggested a nonlinear relationship between the number of parity and endometrial cancer risk. The RR decreased when the number of parity increased. This meta-analysis suggests that parity may be associated with a decreased risk of endometrial cancer. Further studies are warranted to replicate our findings.
As the most common tumor of the female reproductive tract, endometrial cancer remains the fourth most common malignancy in females1. Parity, a representative reproductive factor, is demonstrated to potentially modulate risk of endometrial cancer through affecting estrogen and progesterone levels2. A lot of observational studies also suggest such an association. For example, in comparison to nulliparous, parous was detected to be associated with decreased risk of developing endometrial cancer in several prospective studies3,4, case-control studies5,6,7,8,9,10,11,12, as well as pooled analysis13. However, such an inverse association was not detected in several other epidemiological studies14,15,16,17.
Considering that results from individual epidemiological studies can be strongly affected by available sample sizes, a better way to clarify the association between parity and risk of endometrial cancer is to summarize all available evidence from relevant observational studies. In the current study, we aimed to conduct a comprehensive meta-analysis to evaluate this research question. We also conducted analysis to clarify the dose-response relationship between number of parity and risk of endometrial cancer.
Results
Literature Search and Study Characteristics
The detailed procedures of the literature search and article screening were demonstrated in Fig. 1. The database search yielded 7906 publications, among which 7852 were excluded based on the screening of titles and/or abstracts. Combined with 35 studies identified through manual search of references of relevant review articles, the whole contents of a total of 89 publications were assessed. Among them, 43 articles were further excluded due to various reasons: 5 did not meet the eligibility criteria; 20 involved duplicated study individuals with other articles; and 18 did not report sufficient data or information (the complete list of the 43 excluded articles is available upon request). Finally, a total of 46 studies were included in the current meta-analysis (references are within the supplementary material). The detailed characteristics of the involved studies were demonstrated in Table 1. In total, 10 prospective cohort studies, 35 case-control studies and 1 pooled analysis of 10 cohort and 14 case-control studies were involved. Overall, 18 studies were conducted in Europe, 18 in America, 9 in Asia, and 1 was an international report. The studies enrolled 69681 patients. The quality assessments of these studies were demonstrated in Tables 2 and 3. Overall, 9 of the 10 cohort studies (90%) and 26 of the 35 case-control studies (74%) were categorized as high-quality studies. Others were categorized as low-quality studies.
Figure 1. Flow chart for selection of eligible studies.
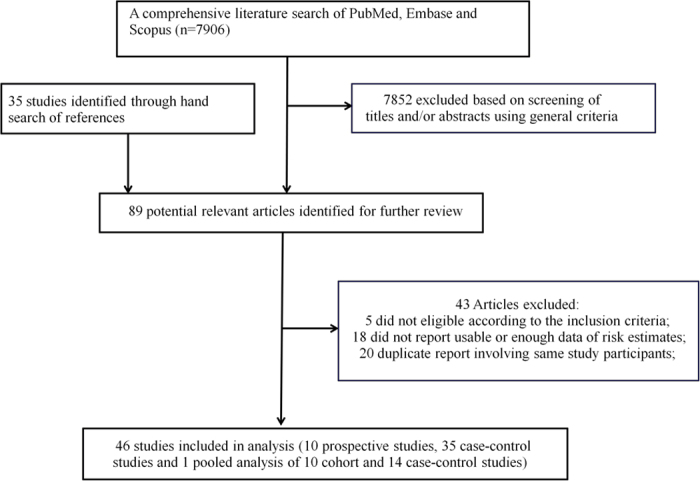
Table 1. Characteristics of studies evaluating parity with endometrial cancer risk.
| First author’s last name, publication year, Country, Study design | Cases/subject (age), duration of follow up | Parity categories (exposure/case assessment) | RR (95% CI) | Matched/Adjusted factors | |||||
|---|---|---|---|---|---|---|---|---|---|
| Prospective studies | |||||||||
| Setiawan, 2013,International, 10 cohort and 14 case-control studies | 14,069/35,312 (mean from 54.6–71.6y) | Nulliparous | 1.0 (ref.) | unadjusted | |||||
| parous | 0.73 (0.71–0.76) | ||||||||
| 1 | 0.88 (0.84–0.92) | ||||||||
| 2 | 0.78 (0.75–0.81) | ||||||||
| 3 | 0.68 (0.65–0.70) | ||||||||
| ≥4 | 0.60 (0.57–0.64) | ||||||||
| (questionnaire or interview/cancer registry, pathology report, medical chart or slide review) | |||||||||
| Dossus, 2010, Europe, CS | 1,017/302,618 (mean 50.5y), mean 8.7y | Nulliparous | 1.0 (ref.) | Age, study center, body mass index (BMI), physical activity, alcohol, diabetes, smoking status and education | |||||
| Parous | 0.65 (0.54–0.77) | ||||||||
| Parity = 1 | 1.0 (ref.) | ||||||||
| 2 | 0.92 (0.76–1.11) | ||||||||
| 3 | 0.80 (0.64–0.99) | ||||||||
| ≥4 | 0.58 (0.44–0.78) | ||||||||
| (Self-questionnaire/Cancer registry, histology confirmation) | |||||||||
| Wernli, 2006, China, CS | 206/267,400 (N/A), mean 7.6y | Nulliparous | 3.95 (1.43–10.86) | Age at baseline | |||||
| 1 | 1.00 (ref.) | ||||||||
| 2 | 0.77 (0.42–1.42) | ||||||||
| 3 | 1.07 (0.57–2.04) | ||||||||
| 4 | 0.93 (0.46–1.86) | ||||||||
| ≥5 | 0.75 (0.36–1.56) | ||||||||
| (Trained interviewer/Cancer registry and medical record ) | |||||||||
| Hinkula, 2002, Finland, CS | 419/86,978 (N/A), mean 19.3y | Parity number | Age at first birth, birth intensity | ||||||
| 5 | 1.0 (ref.) | ||||||||
| 6 | 0.72 (0.57–0.92) | ||||||||
| 7 | 0.87 (0.62–1.22) | ||||||||
| ≥8 | 0.71 (0.57–1.02) | ||||||||
| (Registry/Cancer registry) | |||||||||
| physical activity, fruit and vegetable consumption, diabetes, social-economic status, cigarette smoking, alcohol consumption | |||||||||
| Terry, 1999, Sweden, CS | 133/11,659 (median56.2y), mean 20.4y | Nulliparous | 1.0 (ref.) | ||||||
| parous | 0.83 (0.55–1.25) | ||||||||
| 1–2 | 0.9 (0.6–1.5) | ||||||||
| ≥3 | 0.4 (0.2–0.8) | ||||||||
| (Self-questionnaire/Cancer registry) | |||||||||
| Albrektsen, 1995, Norway, CS | 554/765,756 (30–56y), mean 12.2y | Nulliparous | 1.94 (1.46–2.59) | Age, birth cohort | |||||
| 1 | 1.00 | ||||||||
| 2 | 0.84 (0.64–1.09) | ||||||||
| 3 | 0.61 (0.46–0.82) | ||||||||
| ≥4 | 0.48 (0.34–0.69) | ||||||||
| (Registry/cancer registry) | |||||||||
| Kvale, 1988, Norway, CS | 420/62,079 (27–69y), 19y | Nulliparous | 1.0 (ref.) | Age, urban/rural place of residence | |||||
| parous | 0.66 (0.53–0.84) | ||||||||
| 1 | 0.80 (0.59–1.10) | ||||||||
| 2 | 0.72 (0.55–0.96) | ||||||||
| 3 | 0.55 (0.39–0.77) | ||||||||
| 4 | 0.72 (0.50–1.06) | ||||||||
| ≥5 | 0.41 (0.26–0.66) | ||||||||
| (Trained interviewer/Cancer registry) | |||||||||
| PLCO, US, CS | 417/40562 (mean 62.8y), ~13y | Nulliparous | 1.0 (ref.) | birth year and entry year, age at last menstrual period, age at menarche, BMI, oral contraceptive use, menopausal hormone therapy use, diabetes, and smoking status | |||||
| parous | 0.76 (0.57–1.01) | ||||||||
| (questionnaire/cancer registry and questionnaire) | |||||||||
| USRT, US, CS | 125/10050 (mean ~57y), ~15y | Nulliparous | 1.0 (ref.) | birth year and entry year, age at last menstrual period, age at menarche, BMI, oral contraceptive use, menopausal hormone therapy use, diabetes, and smoking status | |||||
| parous | 0.60 (0.40–0.88) | ||||||||
| (questionnaire/database link and questionnaire) | |||||||||
| de Warrd, 1996, Netherlands, CS | 147/1047 (40–65y), up to 18 y | Nulliparous | 1.0 (ref.) | unadjusted | |||||
| Parous | 0.61 (0.45–0.84) | ||||||||
| 1–2 | 0.74 (0.52–1.04) | ||||||||
| ≥3 | 0.49 (0.33–0.72) | ||||||||
| (questionnaire/database link) | |||||||||
| Bevier, 2011, Sweden, CS | 31118/5759120 (NA), up to 45 y | Nulliparous | 1.0 (ref.) | age, period, region, socioeconomic status | |||||
| 1 | 0.47 (0.42–0.52) | ||||||||
| 2 | 0.41 (0.37–0.46) | ||||||||
| 3–4 | 0.36 (0.32–0.40) | ||||||||
| 5–9 | 0.29 (0.25–0.34) | ||||||||
| 10+ | 0.25 (0.10–0.58) | ||||||||
| (database/database link) | |||||||||
| First author, publication year, Country, Study design | Cases/control (age) | Parity categories (exposure/case assessment) | RR (95% CI) | Matched/Adjusted factors | |||||
| Case-control studies | |||||||||
| Parslov, 2000, Denmark, PC-CS | Nulliparous | 1.0 (ref.) | Age, residence, family history of endometrial cancer, BMI, diabetes mellitus, hypertension, menarche, pregnancy, number of pregnancy, number of induced abortions, age of first birth, hyperandrogenism, amenorrhea, oral contraceptive use, hormone replacement therapy, cigarette smoking, and years of schooling | ||||||
| parous | 0.62 (0.50–0.77) | ||||||||
| 1 | 0.6 (0.3–1.1) | ||||||||
| 2 | 0.3 (0.2–0.6) | ||||||||
| ≥3 | 0.2 (0.1–0.4) | ||||||||
| (Self-questionnaire/histology confirmation) | |||||||||
| Salazar-Martinez, 1999, Mexico, HC-CS | 85/668 (54.9y) | Nulliparous | 1.0 (ref.) | Age, hormonal use, breastfeeding, smoking, diabetes mellitus, hypertension, physical activity, menopausal status, BMI | |||||
| parous | 0.25 (0.12–0.49) | ||||||||
| 1–2 | 0.41 (0.19–0.86) | ||||||||
| 3–4 | 0.15 (0.06–0.36) | ||||||||
| ≥5 | 0.16 (0.06–0.40) | ||||||||
| (Trained interviewer/biopsy confirmation) | |||||||||
| Parazzini, 1998 Italy, HC-CS | 752/2,606 (25–74y) | Nulliparous | 1.0 (ref.) | Age, calendar year at interview, education, BMI, menopausal status, use of hormonal replacement therapy, smoking, history of diabetes, hypertension, abortions, age at first birth, time since last birth | |||||
| parous | 0.91 (0.78–1.06) | ||||||||
| 1 | 0.9 (0.7–1.1) | ||||||||
| 2 | 0.8 (0.6–1.0) | ||||||||
| ≥3 | 0.7 (0.5–0.8) | ||||||||
| (Trained interviewer/histology confirmation) | |||||||||
| Kalandidi, 1996, Greece, HC-CS | 145/298 (NA) | Nulliparous | 1.0 (ref.) | Age, schooling, occupation, age at menopause, age at menarche, oral contraceptive, menopausal estrogen, smoking, alcohol intake, coffee intake, BMI, energy intake | |||||
| parous | 0.71 (0.53–0.96) | ||||||||
| 1 | 0.75 (0.27–2.11) | ||||||||
| 2 | 0.66 (0.26–1.67) | ||||||||
| 3 | 0.36 (0.13–1.03) | ||||||||
| ≥4 | 0.34 (0.11–1.05) | ||||||||
| (Trained interviewer/histologic confirmation) | |||||||||
| Shu, 1993, China, PC-CS | 268/268 (18–74y) | Nulliparous | 1.0 (ref.) | Age | |||||
| parous | 0.58 (0.48–0.69) | ||||||||
| 1 | 0.3 (0.1–0.8) | ||||||||
| 2–3 | 0.2 (0.1–0.7) | ||||||||
| ≥4 | 0.1 (0.1–0.4) | ||||||||
| (Trained interviewer/Cancer registry) | |||||||||
| Koumantaki, 1989, Greece, HC-CS | 83/164 (40–79y) | Nulliparous | 1.0 (ref.) | unadjusted | |||||
| parous | 1.04 (0.65–1.66) | ||||||||
| 1–2 | 1.19 (0.73–1.94) | ||||||||
| ≥3 | 0.81 (0.47–1.43) | ||||||||
| (Trained interviewer/Biopsy-confirmation) | |||||||||
| Kelsey, 1982, US, HC-CS | 167/903 (45–74y) | Nulliparous | 1.0 (ref.) | Race, education, age at menopause, weight, history of diabetes, oral contraceptive use, age, menopausal status, estrogen replacement therapy use | |||||
| 1 | 0.8 (0.7–0.9) | ||||||||
| (Trained interviewer/pathology confirmation) | |||||||||
| Baron, 1986, US, HC-CS | 476/2128 (40–89y) | Nulliparous | 1.0 (ref.) | unadjusted | |||||
| parous | 0.75 (0.63–0.91) | ||||||||
| 1–2 | 0.85 (0.69–1.05) | ||||||||
| 3–4 | 0.68 (0.54–0.86) | ||||||||
| ≥5 | 0.70 (0.55–0.90) | ||||||||
| (interview/clinic diagnosis) | |||||||||
| Castellsague, 1993, US, PC-CS | 437/3200 (20–54y) | Nulliparous | 1.0 (ref.) | Location, age, time interval | |||||
| parous | 0.54 (0.45–0.66) | ||||||||
| 1–2 | 0.59 (0.48–0.74) | ||||||||
| 3–4 | 0.54 (0.43–0.68) | ||||||||
| ≥5 | 0.41 (0.29–0.59) | ||||||||
| (interview/histological confirmation) | |||||||||
| Dahlgren, 1991, Sweden, PC-CS | 147/1409 (31–65y) | Nulliparous | 1.0 (ref.) | unadjusted | |||||
| parous | 0.43 (0.31–0.60) | ||||||||
| (interview and/or questioinnaire/hospital records) | |||||||||
| Damon, 1960, US, HC-CS | 197/233 (NA) | Nulliparous | 1.0 (ref.) | unadjusted | |||||
| parous | 0.81 (0.66–0.995) | ||||||||
| (hospital records/pathology diagnosis) | |||||||||
| Elwood, 1977, US, PC-CS | 212/1198 (40–89y) | Nulliparous | 1.0 (ref.) | age | |||||
| parous | 0.57 (0.45–0.73) | ||||||||
| 1 | 0.74 (0.49–1.13) | ||||||||
| 2 | 0.61 (0.44–0.86) | ||||||||
| 3 | 0.51 (0.33–0.76) | ||||||||
| 4+ | 0.48 (0.33–0.70) | ||||||||
| (Questionnaire/histological confirmation) | |||||||||
| Fox, 1970, US, PC-CS | 300/300 (NA) | Nulliparous | 1.0 (ref.) | age | |||||
| parous | 0.74 (0.63–0.86) | ||||||||
| (records/histological confirmation) | |||||||||
| Garnet, 1958, US, HC-CS | 50/50 (30–80y) | Nulliparous | 1.0 (ref.) | unadjusted | |||||
| Parous | 0.63 (0.44–0.92) | ||||||||
| 1–3 | 0.56 (0.37–0.85) | ||||||||
| 4+ | 0.95 (0.59–1.51) | ||||||||
| (unclear/clinic diagnosis) | |||||||||
| Henderson, 1983, US, PC-CS | 110/110 (45y−) | Nulliparous | 1.0 (ref.) | age | |||||
| Parous | 0.61 (0.48–0.78) | ||||||||
| 1 | 0.91 (0.66–1.24) | ||||||||
| 2 | 0.70 (0.52–0.95) | ||||||||
| 3 | 0.51 (0.34–0.79) | ||||||||
| 4+ | 0.33 (0.18–0.60) | ||||||||
| (trained interviewer/microscopical confirmation) | |||||||||
| Hirose, 1996, Japan, HC-CS | 145/26751 (20y+) | Nulliparous | 1.0 (ref.) | Age, first-visit year | |||||
| Parous | 0.83 (0.56–1.25) | ||||||||
| 1 | 0.63 (0.35–1.14) | ||||||||
| 2 | 0.62 (0.40–0.96) | ||||||||
| 3+ | 0.41 (0.25–0.69) | ||||||||
| (questionnaire/histology diagnosis) | |||||||||
| Hosono, 2011, Japan, HC-CS | 222/2162 (mean 56y) | Nulliparous | 1.0 (ref.) | Age, menstrual-status | |||||
| Parous | 0.51 (0.39–0.68) | ||||||||
| 1–2 | 0.56 (0.42–0.74) | ||||||||
| ≥3 | 0.40 (0.27–0.60) | ||||||||
| (questionnaire/histological confirmation) | |||||||||
| Jaakkola, 2011, Finland, PC-CS | 7261/19490 (50–80y) | Nulliparous | 1.0 (ref.) | age | |||||
| Parous | 0.84 (0.80–0.88) | ||||||||
| 1–2 | 0.90 (0.85–0.94) | ||||||||
| ≥3 | 0.76 (0.72–0.80) | ||||||||
| (registry/cancer registry) | |||||||||
| Kakuta, 2009, Japan, HC-CS | 152/285 (mean ~54y) | Nulliparous | 1.0 (ref.) | Age, area of residence | |||||
| Parous | 0.63 (0.44–0.89) | ||||||||
| 1–3 | 0.94 (0.65–1.36) | ||||||||
| ≥4 | 0.89 (0.55–1.44) | ||||||||
| (questionnaire/histopathological confirmation) | |||||||||
| Lawrence, 1989, US, PC-CS | 84/168 (40–69y) | Nulliparous | 1.0 (ref.) | Age, county of residence, weight, time since last medical visit, education, diabetes, estrogen pill use | |||||
| Parous | 0.80 (0.68–0.95) | ||||||||
| (Trained interviewer/medical record review) | |||||||||
| Lesko, 1991, US, HC-CS | 483/693 (30–69y) | Nulliparous | 1.0 (ref.) | Age, race, religion, BMI, diabetes history, hypertension history, alcohol use, tobacco use, durations of oral contraceptive and non-contraceptive estrogen use, menopausal status, age at menopause, age at first pregnancy, years of education, date of interview, geographic region | |||||
| Parous | 0.98 (0.84–1.15) | ||||||||
| 1–2 | 1.3 (0.9–1.9) | ||||||||
| 3–4 | 1.0 (0.7–1.5) | ||||||||
| ≥5 | 0.5 (0.3–0.9) | ||||||||
| (Trained interviewer/clinic diagnosis) | |||||||||
| Levi, 1991, Switzerland, HC-CS | 122/309 (75y−) | Nulliparous | 1.0 (ref.) | unadjusted | |||||
| Parous | 0.84 (0.61–1.16) | ||||||||
| (Trained interviewer/histological confirmation) | |||||||||
| Littman, 2001, US, PC-CS | 679/944 (45–74y) | Nulliparous | 1.0 (ref.) | Age, location | |||||
| Parous | 0.74 (0.64–0.85) | ||||||||
| 1 | 0.91 (0.75–1.11) | ||||||||
| >1 | 0.71 (0.62–0.82) | ||||||||
| (Trained interviewer//histological confirmation) | |||||||||
| Macdonald, 1977, US, PC-CS | 145/580 (unknown) | Nulliparous | 1.0 (ref.) | age | |||||
| Parous | 0.56 (0.38–0.83) | ||||||||
| (Medical record linkage/pathology confirmation) | |||||||||
| Newcomer, 2001, US, PC-CS | 740/2372 (40–79y) | Nulliparous | 1.0 (ref.) | age | |||||
| Parous | 0.68 (0.58–0.80) | ||||||||
| 1–2 | 0.8 (0.6–1.0) | ||||||||
| 3–4 | 0.6 (0.5–0.8) | ||||||||
| ≥5 | 0.4 (0.3–0.6) | ||||||||
| (Trained interviewer/registry link and histologic confirmation) | |||||||||
| Pettersson, 1986, Sweden, PC-CS | 254/254 (30–94y) | Nulliparous | 1.0 (ref.) | Age, county of residence | |||||
| Parous | 0.6 (0.4–0.9) | ||||||||
| 1 | 0.7 (0.4–1.2) | ||||||||
| 2 | 0.7 (0.4–1.1) | ||||||||
| 3 | 0.6 (0.3–1.1) | ||||||||
| 4 | 0.4 (0.2–0.8) | ||||||||
| ≥5 | 0.3 (0.1–0.6) | ||||||||
| (Trained interviewer/histologic confirmation) | |||||||||
| Spengler, 1981, Canada, PC-CS | 88/177 (40–74y) | Nulliparous | 1.0 (ref.) | age | |||||
| Parous | 1.10 (0.65–1.86) | ||||||||
| (Trained interviewer/pathology confirmation) | |||||||||
| Wynder, 1966, US, HC-CS | 112/200 (unknown) | Nulliparous | 1.0 (ref.) | unadjusted | |||||
| Parous | 0.85 (0.63–1.16) | ||||||||
| 1 | 1.09 (0.73–1.64) | ||||||||
| 2 | 0.65 (0.42–1.01) | ||||||||
| 3 | 0.86 (0.53–1.38) | ||||||||
| 4 | 0.96 (0.53–1.73) | ||||||||
| 5 | 1.51 (0.71–3.20) | ||||||||
| 6 | 1.88 (1.02–3.48) | ||||||||
| 7 | 0.31 (0.05–1.99) | ||||||||
| (Trained interviewer/histologic diagnosis) | |||||||||
| Wang, 1990, China, HC-CS | 102/102 (mean 58y) | Nulliparous | 1.0 (ref.) | Same hospital, time at diagnosis, age, marriage status | |||||
| Parous | 0.65 (0.45–0.92) | ||||||||
| 1–2 | 0.81 (0.55–1.20) | ||||||||
| 3–4 | 0.59 (0.39–0.88) | ||||||||
| ≥5 | 0.58 (0.38–0.91) | ||||||||
| (Trained interviewer/pathology confirmation) | |||||||||
| Hachisuga, 1998, Japan, HC-CS | 242/1021 (20–79y) | Nulliparous | 1.0 (ref.) | Age, BMI, hypertension, diabetes | |||||
| Parous | 0.43 (0.34–0.54) | ||||||||
| 1–3 | 0.23 ((0.16–0.34) | ||||||||
| ≥4 | 0.33 (0.23–0.48) | ||||||||
| (Medical record/histology comfirmation) | |||||||||
| Brons, 2015, Denmark, PC-CS | 5382/72127 (30–84y) | Nulliparous | 1.0 (ref.) | Age | |||||
| Parous | 0.81 (0.76–0.86) | ||||||||
| 1 | 0.92 (0.85–0.99) | ||||||||
| 2 | 0.83 (0.77–0.88) | ||||||||
| ≥3 | 0.71 (0.66–0.77) | ||||||||
| (Database/Cancer Registry) | |||||||||
| La Vecchia, 1984, Italy, HC-CS | 283/566 (33–74y) | Nulliparous | 1.0 (ref.) | age | |||||
| Parous | 0.85 (0.69–1.05) | ||||||||
| 1 | 0.77 (0.58–1.01) | ||||||||
| ≥2 | 0.89 (0.72–1.11) | ||||||||
| (Trained interviewer/histology confirmation) | |||||||||
| Salmi, 1979, Finland, PC-CS | 282/282 (31–82y) | Nulliparous | 1.0 (ref.) | Age, weight, social class | |||||
| Parous | 0.95 (0.79–1.15) | ||||||||
| 1–2 | 0.89 (0.72–1.10) | ||||||||
| 3–4 | 1.06 (0.84–1.32) | ||||||||
| ≥5 | 1.02 (0.72–1.44) | ||||||||
| (Trained interviewer/histology confirmation) | |||||||||
| Asakura, 2009, Japan, PC-CS | 191/419 (NA) | Nulliparous | 1.0 (ref.) | Age, area, BMI | |||||
| Parous | 0.40 (0.26–0.61) | ||||||||
| 1 | 0.40 (0.22–0.74) | ||||||||
| 2 | 0.39 (0.25–0.61) | ||||||||
| ≥3 | 0.44 (0.24–0.79) | ||||||||
| (questionnaire/histology confirmation) | |||||||||
| Hao, 2009, China, PC-CS | 421/1263 (22–84y) | Nulliparous | 1.0 (ref.) | Age, area | |||||
| Parous | 0.223 (0.115–0.435) | ||||||||
| (questionnaire/cancer registry) | |||||||||
BMI: body mass index; CI: confidence interval; CS: cohort study; HC-CS: hospital-based case-control study; NA: not available; NC-CS: nested case-control study; OR: odds ratio; PC-CS: population-based case-control study; ref.: reference; RR: relative risk.
Table 2. Quality Assessment of Reviewed Case-Control Studies.
| Study | Case defined with independent validation | Representativeness of the cases | Selection of controls from community | Statement that controls have no history of outcome | Cases and controls matched and/or adjusted by factors | Ascertain exposure by blinded structured interview | Same method of ascertainment for cases and controls | Same response rate for both groups | Overall Score |
|---|---|---|---|---|---|---|---|---|---|
| Parslov, 2000 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 8 |
| Salazar-Martinez, 1999 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 1 | 7 |
| Parazzini, 1998 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 1 | 7 |
| Kalandidi, 1996 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 1 | 7 |
| Shu, 1993 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| Koumantaki, 1989 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 5 |
| Kelsey, 1982 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 8 |
| Baron, 1986 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 6 |
| Castellsague, 1993 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 |
| Dahlgren, 1991 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
| Damon, 1960 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 6 |
| Elwood, 1977 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 6 |
| Fox, 1970 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| Garnet, 1958 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 5 |
| Henderson, 1983 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| Hirose, 1996 | 1 | 1 | 0 | 1 | 2 | 0 | 1 | 1 | 7 |
| Hosono, 2011 | 1 | 1 | 0 | 1 | 2 | 0 | 1 | 1 | 7 |
| Jaakkola, 2011 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Kakuta, 2009 | 1 | 1 | 0 | 1 | 2 | 0 | 1 | 1 | 7 |
| Lawrence, 1989 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 |
| Lesko, 1991 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 8 |
| Levi, 1991 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 6 |
| Littman, 2001 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Macdonald, 1977 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Newcomer, 2001 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Pettersson, 1986 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Spengler, 1981 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Wynder, 1966 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 6 |
| Wang, 1990 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 8 |
| Hachisuga, 1998 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 8 |
| Brons, 2015 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 6 |
| La Vecchia, 1984 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Salmi, 1979 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 |
| Asakura, 2009 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 7 |
| Hao, 2009 | 0 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 7 |
1 means study adequately fulfilled a quality criterion (2 for case-control fully matched and adjusted), 0 means it did not. Quality scale does not imply that items are of equal relevant importance.
Table 3. Quality Assessment of Reviewed Cohort Studies.
| Study | Exposed cohort represents average in community | Selection of the non-exposed cohort from same community | Ascertain exposure through records or structured interviews | Demonstrate that outcome not present at study start | Exposed and non-exposed matched and/or adjusted by factors | Ascertain outcome via independent blind assessment or record linkage | Follow-up long enough for outcome to occur | Loss to follow-up<20% | Overall Score |
|---|---|---|---|---|---|---|---|---|---|
| Dossus, 2010 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 8 |
| Wernli, 2006 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| Hinkula, 2002 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 |
| Terry, 1999 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 8 |
| Albrektsen, 1995 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 |
| Kvale, 1988 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 |
| PLCO, US | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 8 |
| USRT, US | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 7 |
| de Warrd, 1996 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 5 |
| Bevier, 2011 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 |
1 means study adequately fulfilled a quality criterion, 0 means it did not. Quality scale does not imply that items are of equal relevant importance.
Parous vs. Nulliparous
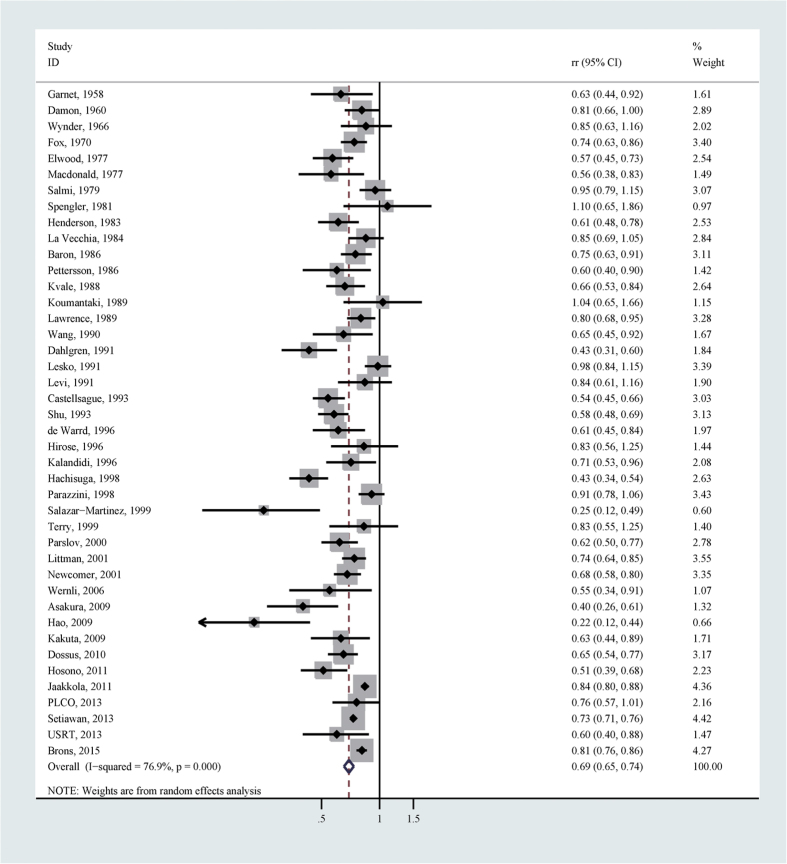
A total of 42 studies reported the association between risk of endometrial cancer and parity for parous versus nulliparous. After summarizing all available estimates, there was a significant inverse association between parity and endometrial cancer risk (relative risk (RR) = 0.69, 95% confidence interval (CI) 0.65–0.74), with considerable heterogeneity (I2 = 76.9%; Table 4 and Fig. 2). There was no significant publication bias as suggested by Begg’s test (p for bias: 0.104). Sensitivity analysis revealed that the 42 study-specific RRs of parous versus nulliparous ranged from as low as 0.69 (95% CI 0.64–0.73; I2 = 76.9%) after omitting the study by Setiawan et al.13 to as high as 0.70 (95% CI 0.67–0.75; I2 = 74.2%) after omitting the study by Hachisuga et al.11. The subgroup analyses revealed that the significant negative association was detected in all strata according to study design, location, number of cases, study publication time, estimate adjustment, control resources and study quality (Table 4), although in a lot of subgroups the high heterogeneity persisted. According to the Galbraith plot (Supplementary Figure 1), 14 studies contributed to the heterogeneity7,9,10,11,15,18,19,20,21,22,23,24,25,26. After excluding these studies from the pooled analysis, the overall effect size remained similar (RR = 0.73, 95% CI 0.71–0.75), with no heterogeneity (I2 = 0.0%).
Table 4. Summary risk estimates of the association between parity and endometrial cancer risk (parous versus nulliparous).
| No of reports | RR (95% CI) | I2 (%) | P for heterogeneity | |
|---|---|---|---|---|
| Overall | 42 | 0.69 (0.65–0.74) | 76.9 | <0.001 |
| Subgroup analysis | ||||
| Study design | ||||
| Prospective | 7 | 0.66 (0.60–0.74) | 0.0 | 0.790 |
| Case–control | 34 | 0.69 (0.64–0.74) | 79.7 | <0.001 |
| Location | ||||
| Europe | 15 | 0.76 (0.70–0.82) | 67.9 | <0.001 |
| America | 17 | 0.71 (0.64–0.78) | 66.5 | <0.001 |
| Asia | 9 | 0.53 (0.44–0.63) | 58.6 | 0.013 |
| International | 1 | 0.73 (0.71–0.76) | — | — |
| Number of cases | ||||
| <200 | 19 | 0.68 (0.60–0.76) | 57.1 | 0.001 |
| ≥200 | 23 | 0.70 (0.65–0.75) | 83.3 | <0.001 |
| Study publication time | ||||
| Earlier than 1992 | 19 | 0.74 (0.68–0.82) | 63.0 | <0.001 |
| 1992– | 23 | 0.66 (0.61–0.71) | 82.8 | <0.001 |
| Estimate adjustment | ||||
| Yes | 33 | 0.68 (0.63–0.73) | 79.4 | <0.001 |
| No | 9 | 0.72 (0.65–0.81) | 52.1 | 0.033 |
| Estimate adjusted for age | ||||
| Yes | 32 | 0.68 (0.63–0.73) | 80.1 | <0.001 |
| No | 10 | 0.73 (0.66–0.81) | 47.3 | 0.048 |
| Estimate adjusted for BMI | ||||
| Yes | 10 | 0.63 (0.51–0.77) | 85.7 | <0.001 |
| No | 32 | 0.71 (0.67–0.75) | 72.7 | <0.001 |
| Estimate adjusted for smoking | ||||
| Yes | 9 | 0.72 (0.61–0.85) | 75.5 | <0.001 |
| No | 33 | 0.68 (0.64–0.73) | 77.8 | <0.001 |
| Estimate adjusted for age, BMI and smoking | ||||
| Yes | 8 | 0.71 (0.59–0.85) | 78.5 | <0.001 |
| No | 34 | 0.69 (0.64–0.73) | 77.1 | <0.001 |
| Sources of controls | ||||
| Population based | 18 | 0.66 (0.60–0.73) | 82.9 | <0.001 |
| Hospital based | 16 | 0.72 (0.63–0.83) | 76.3 | <0.001 |
| Study quality | ||||
| high | 31 | 0.67 (0.62–0.73) | 79.5 | <0.001 |
| low | 10 | 0.72 (0.63–0.81) | 65.7 | 0.002 |
Figure 2.
Forest plot (random effects model) of parity (parous vs. nulliparous) and endometrial cancer risk.
Different number of parity
The associations between different number of parity (1, 2 or 3) and endometrial cancer risk were evaluated respectively. Parity number of 1 versus nulliparous was inversely associated with risk of endometrial cancer (RR = 0.73, 95% CI 0.64–0.84; I2 = 88.3%), after summarizing estimates from 19 studies (Table 5). The significant inverse association was detected in almost all strata of subgroup analyses (Table 5). According to the Galbraith plot (Supplementary Figure 2), 6 studies contributed to the heterogeneity10,13,26,27,28,29. The heterogeneity disappeared after excluding these studies in the pooled analysis (I2 = 0.0%). Similarly, after summarizing 13 studies, parity number of 2 versus nulliparous demonstrated a significant inverse association with risk of endometrial cancer (RR = 0.62, 95% CI 0.53–0.74; I2 = 92.1%), which was also identified in different strata of subgroup analyses (Table 6). Five studies contributed to the heterogeneity according to the Galbraith plot (Supplementary Figure 3)6,10,13,26,29. The heterogeneity disappeared after excluding these studies in the pooled analysis (I2 = 0.0%). Additionally, parity number of 3 versus nulliparous showed a significant inverse association with endometrial cancer risk (RR = 0.68, 95% CI 0.65–0.70; I2 = 20.0%), after pooling 7 studies.
Table 5. Summary risk estimates of the association between parity and endometrial cancer risk (parity number of 1 versus nulliparous).
| No of reports | RR (95% CI) | I2 (%) | P for heterogeneity | |
|---|---|---|---|---|
| Parity number of 1 vs. nulliparous | 19 | 0.73 (0.64–0.84) | 88.3 | <0.001 |
| Subgroup analysis | ||||
| Study design | ||||
| Prospective | 4 | 0.54 (0.40–0.72) | 74.7 | 0.008 |
| Case-control | 14 | 0.83 (0.76–0.91) | 35.2 | 0.093 |
| Location | ||||
| Europe | 9 | 0.70 (0.53–0.91) | 92.8 | <0.001 |
| America | 5 | 0.85 (0.77–0.93) | 0.0 | 0.494 |
| Asia | 4 | 0.43 (0.29–0.63) | 6.1 | 0.362 |
| International | 1 | 0.88 (0.84–0.92) | — | — |
| Number of cases | ||||
| <200 | 6 | 0.79 (0.64–0.97) | 41.4 | 0.129 |
| ≥200 | 13 | 0.71 (0.60–0.84) | 91.7 | <0.001 |
| Study publication time | ||||
| Earlier than 1992 | 7 | 0.81 (0.74–0.89) | 0.0 | 0.782 |
| 1992– | 12 | 0.66 (0.54–0.81) | 92.7 | <0.001 |
| Estimate adjustment | ||||
| Yes | 17 | 0.69 (0.58–0.82) | 87.6 | <0.001 |
| No | 2 | 0.89 (0.82–0.96) | 5.7 | 0.303 |
| Estimate adjusted for age | ||||
| Yes | 17 | 0.69 (0.58–0.82) | 87.6 | <0.001 |
| No | 2 | 0.89 (0.82–0.96) | 5.7 | 0.303 |
| Estimate adjusted for BMI | ||||
| Yes | 4 | 0.66 (0.43–1.01) | 56.0 | 0.078 |
| No | 15 | 0.74 (0.63–0.86) | 90.4 | <0.001 |
| Estimate adjusted for smoking | ||||
| Yes | 3 | 0.86 (0.69–1.06) | 0.0 | 0.496 |
| No | 16 | 0.72 (0.62–0.84) | 90.1 | <0.001 |
| Estimate adjusted for age, BMI and smoking | ||||
| Yes | 3 | 0.86 (0.69–1.06) | 0.0 | 0.496 |
| No | 16 | 0.72 (0.62–0.84) | 90.1 | <0.001 |
| Study quality | ||||
| high | 15 | 0.67 (0.55–0.80) | 83.2 | <0.001 |
| low | 3 | 0.92 (0.85–0.99) | 0.0 | 0.424 |
Table 6. Summary risk estimates of the association between parity and endometrial cancer risk (parity number of 2 versus nulliparous).
| No of reports | RR (95% CI) | I2 (%) | P for heterogeneity | |
|---|---|---|---|---|
| Parity number of 2 vs. nulliparous | 13 | 0.62 (0.53–0.74) | 92.1 | <0.001 |
| Subgroup analysis | ||||
| Study design | ||||
| Prospective | 2 | 0.54 (0.31–0.93) | 92.7 | <0.001 |
| Case-control | 10 | 0.63 (0.53–0.76) | 68.8 | 0.001 |
| Location | ||||
| Europe | 7 | 0.61 (0.43–0.86) | 95.3 | <0.001 |
| America | 3 | 0.66 (0.54–0.80) | 0.0 | 0.835 |
| Asia | 2 | 0.49 (0.31–0.78) | 52.7 | 0.146 |
| International | 1 | 0.78 (0.75–0.81) | — | — |
| Number of cases | ||||
| <200 | 5 | 0.60 (0. 48–0.74) | 16.2 | 0.312 |
| ≥200 | 8 | 0.64 (0.52–0.78) | 95.1 | <0.001 |
| Study publication time | ||||
| Earlier than 1992 | 5 | 0.68 (0.58–0.79) | 0.0 | 0.957 |
| 1992- | 8 | 0.59 (0.47–0.74) | 95.4 | <0.001 |
| Estimate adjustment | ||||
| Yes | 11 | 0.59 (0.46–0.77) | 92.5 | <0.001 |
| No | 2 | 0.78 (0.75–0.81) | 0.0 | 0.417 |
| Estimate adjusted for age | ||||
| Yes | 11 | 0.59 (0.46–0.77) | 92.5 | <0.001 |
| No | 2 | 0.78 (0.75–0.81) | 0.0 | 0.417 |
| Estimate adjusted for BMI | ||||
| Yes | 4 | 0.50 (0.29–0.85) | 79.5 | 0.002 |
| No | 9 | 0.66 (0.54–0.79) | 94.0 | <0.001 |
| Estimate adjusted for smoking | ||||
| Yes | 3 | 0.55 (0.27–1.09) | 80.1 | 0.007 |
| No | 10 | 0.63 (0.53–0.76) | 93.7 | <0.001 |
| Estimate adjusted for age, BMI and smoking | ||||
| Yes | 3 | 0.55 (0.27–1.09) | 80.1 | 0.007 |
| No | 10 | 0.63 (0.53–0.76) | 93.7 | <0.001 |
| Study quality | ||||
| high | 9 | 0.56 (0.44–0.73) | 82.1 | <0.001 |
| low | 3 | 0.74 (0.59–0.92) | 52.1 | 0.124 |
Dose-response analysis
Assuming a linear relationship, we detected that the combined RR per an additional live birth was 0.86 (95% CI 0.84–0.89), with considerable heterogeneity (P for heterogeneity < 0.0001). After testing a potential non-linear relationship, the test for nonlinearity suggested that a non-linear relationship might exist (p for nonlinearity: 0.0058). Under this model the RR also decreased when the number of parity increased. The nonlinear relationship between the number of parity and endometrial cancer risk in females was demonstrated in Fig. 3.
Figure 3. Nonlinear dose-response relationship between number of parity and endometrial cancer risk.
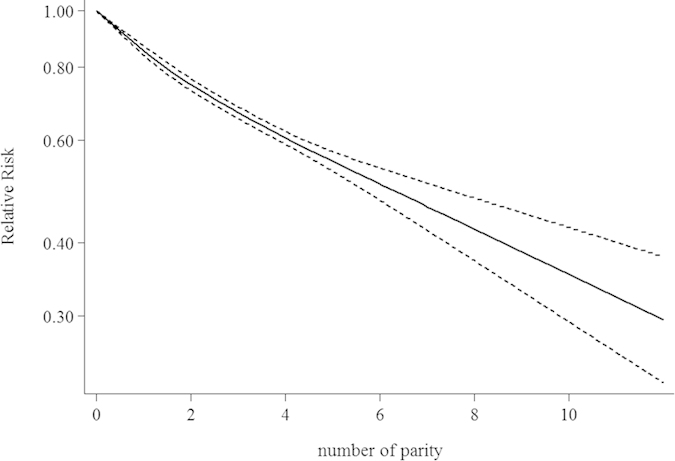
The solid line represents the estimated relationship. The dashed line represents the 95% confidence interval of the estimated relationship.
Discussion
We performed a comprehensive quantitative meta-analysis to evaluate the relationship between parity and endometrial cancer risk. After summarizing all available evidence, ever giving birth to children was associated with an inverse risk of developing endometrial cancer. The sensitivity analysis demonstrated that the result was not significantly affected by any individual study; also subgroup analyses revealed that the inverse association was detected in all strata. Additionally, analyses assessing each number of parity (1, 2 and 3) demonstrated that the inverse association persisted for all 3 scenarios. Furthermore, we identified a dose-response relationship between the number of parity and risk of endometrial cancer. Overall, our findings support that parity may be associated with risk of endometrial cancer.
Our findings are plausible based on understandings from basic research. Estrogens are known to stimulate proliferation of cells in the endometrium and increase mitotic activity, which can induce cancer development30,31. On the other hand, progestins can decrease risk of developing endometrial cancer through reducing cell proliferation and stimulating differentiation31. During live birth, there is a hormonal balance shift toward less estrogen and more progesterone, which may further affect risk of developing endometrial cancer32. Our finding of the dose-response relationship between the number of parity and endometrial cancer risk may be attributable to repeatedly long-term progesterone actions for the antiestrogenic endometrial effects33,34. Another potential explanation is that at each birth delivery there is mechanical shedding of malignant/premalignant endometrial cells28,35.
Our study has several strengths. To the best of our knowledge, this is the first comprehensive meta-analysis evaluating the association between parity and endometrial cancer. Besides conducting subgroup analyses and sensitivity analysis to further evaluate the association, we assessed associations of different numbers of parity and conducted dose-response analysis to fully understand the relationship. Our analyses suggested that the finding of the inverse association between parity and endometrial cancer risk might be robust.
Several potential limitations need be acknowledged for the appropriate interpretation of our findings. First, we do not have access to the individualized primary data from each of the included studies, which induces the possibility that the risk estimates used in our pooled analysis may not be fully adjusted for. For example, obesity and use of oral contraceptive are among the known factors affecting risk of developing endometrial cancer32,36. However, in some of the included studies, they were not adjusted for the association between parity and endometrial cancer risk. Residual confounding may thus be an issue. Second, for the dose-response analysis, the highest categories of number of live birth have wide range of values in different studies. The exposure values may not be accurately assigned based on our assumptions in the methods section. However, this limitation is difficult to eliminate and the method we used is in concordance with the general approach in this area. Third, our study mainly summarizes evidence from observational studies, which are known to confer several relevant biases due to the observational nature. Further large scale multi-center prospective studies are warranted to replicate our findings. Forth, we notice considerable heterogeneities across studies in our pooled analyses. We conducted numerous subgroup analyses with the hope of detecting potential factors for such heterogeneities; however, it appears that in many subgroups the heterogeneity remains relatively high. According to the Galbraith plots, a proportion of the included studies contribute to the high heterogeneities. The heterogeneities disappear after excluding these studies in the pooled analyses. These need to be considered when interpreting our findings. Last, we would like to acknowledge that I2 value should be interpreted with caution because it has certain uncertainty. The value has relatively low statistical power especially in scenarios of small numbers of available studies37. However, in the current meta-analysis there are a relatively large number of eligible studies. Thus the possibility of this limitation is low.
In conclusion, based on a summarization of all available evidence from epidemiological studies, parous versus nulliparous was inversely associated with risk of endometrial cancer. There was a nonlinear dose-response relationship between the number of live births and risk of endometrial cancer. Our findings suggested that parity might be a risk factor for endometrial cancer, suggesting roles of reproductive factors in the etiology of endometrial cancer.
Materials and Methods
Data Sources and Search Strategies
A literature search of PubMed (MEDLINE), Embase and Scopus databases was conducted from the inception to February 2015. We used the following search keywords: (((((((((parity) OR pregnancy) OR livebirth) OR reproductive) OR reproduction) OR reproductive factors) OR reproductive factor)) AND ((endometrium) OR endometrial)) AND ((((((((((malignancies) OR malignancy) OR neoplasm) OR neoplasms) OR cancer) OR cancers) OR adenoma) OR adenomas) OR carcinoma) OR carcinomas). We also screened references of included articles and relevant review papers to identify other potential studies.
Study Selection
Studies were eligible if they (i) were prospective studies or case–control studies or pooled analysis of epidemiological studies; (ii) evaluated the association between parity and risk of endometrial cancer; (iii) presented RR, odds ratio (OR), or hazard ratio (HR) values with 95% CI or necessary data for determination. Cross-sectional studies were excluded. Epidemiological studies comparing endometrial cancer cases with controls with gynecology conditions were excluded as well. If we identified multiple articles involving same participants, the study with the largest number of patients and most relevant information was included.
Data Extraction and Quality Assessment
Two investigators independently carried out the abstract screening, full text screening, and data extraction. Disagreements were resolved by discussion, with input from other investigators. Data extracted from each study included: the first author’s name, publication year, study country, study design, characteristics of study population (sample size, age, length of follow-up, measures and numbers of parity, and association effect sizes). If more than 1 estimate were reported, we used the estimate that was adjusted for the most appropriate covariates, like the previous studies38,39,40,41,42. In situations where only unadjusted estimates were provided, we used the crude estimate in the analysis.
The qualities of included studies were assessed with the Newcastle-Ottawa Quality Assessment Scale43. Specifically, aspects of population and sample methods, exposure and outcome descriptions, and statistical matching/adjustments of the data were assessed. With this scale each study was assigned a score (maximum score is 9 points). Studies with an overall score of higher than or equal to 7 points were categorized as high-quality studies; others were categorized as low-quality studies.
Statistical Methods
The RR and 95% CI from included studies were used as the measure of association. Due to the rarity of endometrial cancer, ORs and HRs were deemed equivalent to RRs and RRs were used to represent measures. I2 was used to assess the heterogeneity across studies, where a I2>50% suggests considerable heterogeneity44. We pooled the log transformed RR using the fixed-effects model45 when there was no considerable heterogeneity. We used the random-effects model46 when there was high heterogeneity. Besides pooling results for parous vs. nulliparous, we summarized effect sizes according to different numbers of parity. We evaluated parity number of 1 vs. nulliparous, parity number of 2 vs. nulliparous, and parity number of 3 vs. nulliparous respectively, according to the characteristics of the included studies. Subgroup analyses were conducted according to design of study (case-control vs. prospective studies), study location (America, Europe, Asia or International), number of cases (<200 vs. ≥200), study publication time (earlier than 1992 vs. 1992-), estimate adjustment, control source, and study quality (high-quality vs. low-quality) . We also conducted sensitivity analyses excluding one study at a time to explore whether any specific study strongly affected the results.
With regards to the dose-response analysis, we explored potential linear relationship between the number of parity and risk of endometrial cancer47. If studies reported the parity number by ranges, we used the midpoint of each category in the analysis. For studies in which the highest category did not have an upper end, the width of the highest category was assumed to be the same as the adjacent category, like previous studies48,49. Furthermore, we assessed potential non-linear relationship for the association. For this analysis, fractional polynomial models with restricted cubic splines and 3 knots at fixed percentiles (10%, 50%, and 90%) of the distribution were used50,51. We then performed a likelihood ratio test to determine whether nonlinear or linear relationship was suggested.
Publication bias was evaluated via Begg’s test52. A P-value of 0.05 was used as the threshold to determine significant publication bias. All statistical analyses were performed with Stata (version 13; StataCorp, College Station, TX).
Additional Information
How to cite this article: Wu, Q.-J. et al. Parity and endometrial cancer risk: a meta-analysis of epidemiological studies. Sci. Rep. 5, 14243; doi: 10.1038/srep14243 (2015).
Supplementary Material
Acknowledgments
This work was supported by The National Natural Science Foundation of China (81472438 and 81272874 for Xiao-Xin Ma), and the Younger research fund of Shengjing Hospital (Grant 2014sj09 for Qi-Jun Wu). This publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. The funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. We would also like to thank Mr. Larry J. Prokop of the Mayo Clinic Libraries for the help in the database search, as well as staffs at Mayo Clinic Libraries for their help in obtaining the full text of relevant articles.
Footnotes
Author Contributions Conception and design of the experiments: C.T. and L.W. Execution of the experiments: Q.J.W., Y.Y.L., C.T., K.Q.Q., T.B.F., C.L., L.W., X.X.M., J.Z. Analysis of the data: Q.J.W., L.W., J.Z. Contribution of reagents/materials/analytical tools: Q.J.W., Y.Y.L., C.T., K.Q.Q., T.B.F., C.L., L.W., X.X.M. Composition of the manuscript: Q.J.W., L.W., X.X.M.
References
- Hill H. A. & Austin H. Nutrition and endometrial cancer. Cancer Causes Control 7, 19–32 (1996). [DOI] [PubMed] [Google Scholar]
- Key T. J. & Pike M. C. The dose-effect relationship between ‘unopposed’ oestrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk. Br J Cancer 57, 205–212 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossus L. et al. Reproductive risk factors and endometrial cancer: the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 127, 442–451, doi: 10.1002/ijc.25050 (2010). [DOI] [PubMed] [Google Scholar]
- Kvale G., Heuch I. & Ursin G. Reproductive factors and risk of cancer of the uterine corpus: a prospective study. Cancer Res 48, 6217–6221 (1988). [PubMed] [Google Scholar]
- Fujita M. et al. Smoking, earlier menarche and low parity as independent risk factors for gynecologic cancers in Japanese: a case-control study. Tohoku J Exp Med 216, 297–307 (2008). [DOI] [PubMed] [Google Scholar]
- Parslov M. et al. Risk factors among young women with endometrial cancer: a Danish case-control study. Am J Obstet Gynecol 182, 23–29 (2000). [DOI] [PubMed] [Google Scholar]
- Salazar-Martinez E. et al. Reproductive factors of ovarian and endometrial cancer risk in a high fertility population in Mexico. Cancer Res 59, 3658–3662 (1999). [PubMed] [Google Scholar]
- Kalandidi A. et al. A case-control study of endometrial cancer in relation to reproductive, somatometric, and life-style variables. Oncology 53, 354–359 (1996). [DOI] [PubMed] [Google Scholar]
- Shu X. O. et al. A population-based case-control study of dietary factors and endometrial cancer in Shanghai, People’s Republic of China. Am J Epidemiol 137, 155–165 (1993). [DOI] [PubMed] [Google Scholar]
- Brøns N., Baandrup L., Dehlendorff C. & Kjaer S. K. Use of nonsteroidal anti-inflammatory drugs and risk of endometrial cancer: a nationwide case–control study. Cancer Causes and Control 26, 973–981, doi: 10.1007/s10552-015-0578-4 (2015). [DOI] [PubMed] [Google Scholar]
- Hachisuga T. et al. Risk factors for endometrial cancer in Japanese women. International Journal of Gynecological Cancer 8(4), 292–297, doi: 10.1046/j.1525-1438.1998.09786.x (1998). [DOI] [Google Scholar]
- Wang P. [A case-control study on endometrial carcinoma]. Zhonghua Liu Xing Bing Xue Za Zhi 11, 356–359 (1990). [PubMed] [Google Scholar]
- Setiawan V. W. et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol 31, 2607–2618, doi: 10.1200/JCO.2012.48.2596 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry P. et al. Lifestyle and endometrial cancer risk: a cohort study from the Swedish Twin Registry. Int J Cancer 82, 38–42 (1999). [DOI] [PubMed] [Google Scholar]
- Parazzini F. et al. Role of reproductive factors on the risk of endometrial cancer. Int J Cancer 76, 784–786 (1998). [DOI] [PubMed] [Google Scholar]
- Koumantaki Y. et al. A case-control study of cancer of endometrium in Athens. Int J Cancer 43, 795–799 (1989). [DOI] [PubMed] [Google Scholar]
- La Vecchia C., Franceschi S., Decarli A., Gallus G. & Tognoni G. Risk factors for endometrial cancer at different ages. J Natl Cancer Inst 73, 667–671 (1984). [PubMed] [Google Scholar]
- Castellsague X., Thompson W. D. & Dubrow R. Intra-uterine contraception and the risk of endometrial cancer. Int J Cancer 54, 911–916 (1993). [DOI] [PubMed] [Google Scholar]
- Dahlgren E. et al. Endometrial carcinoma; ovarian dysfunction–a risk factor in young women. Eur J Obstet Gynecol Reprod Biol 41, 143–150 (1991). [DOI] [PubMed] [Google Scholar]
- Elwood J. M., Cole P., Rothman K. J. & Kaplan S. D. Epidemiology of endometrial cancer. J Natl Cancer Inst 59, 1055–1060 (1977). [DOI] [PubMed] [Google Scholar]
- Hosono S. et al. Weight gain during adulthood and body weight at age 20 are associated with the risk of endometrial cancer in Japanese women. J Epidemiol 21, 466–473 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakkola S., Lyytinen H. K., Dyba T., Ylikorkala O. & Pukkala E. Endometrial cancer associated with various forms of postmenopausal hormone therapy: a case control study. Int J Cancer 128, 1644–1651, doi: 10.1002/ijc.25762 (2011). [DOI] [PubMed] [Google Scholar]
- Lesko S. M. et al. Endometrial cancer and age at last delivery: evidence for an association. Am J Epidemiol 133, 554–559 (1991). [DOI] [PubMed] [Google Scholar]
- Salmi T. Risk factors in endometrial carcinoma with special reference to the use of estrogens. Acta Obstetricia et Gynecologica Scandinavica 58, 1–119 (1979). [PubMed] [Google Scholar]
- Hao J. Z., Zhang S. W., Wang T. & Deng X. H. Epidemiological study of endometrial cancer in Beijing. [Chinese]. Chinese Journal of Cancer Prevention and Treatment 16(11), 805–809 (2009). [Google Scholar]
- Asakura S., Mori M., Suzuki T. & Saito T. A case-control study of endometrial cancer especially with reference to lifestyle and other factors of Japanese women. Sapporo Medical journal 78(1-6), 19–30 (2009). [Google Scholar]
- Wernli K. J. et al. Menstrual and reproductive factors in relation to risk of endometrial cancer in Chinese women. Cancer Causes Control 17, 949–955, doi: 10.1007/s10552-006-0034-6 (2006). [DOI] [PubMed] [Google Scholar]
- Albrektsen G., Heuch I., Tretli S. & Kvale G. Is the risk of cancer of the corpus uteri reduced by a recent pregnancy? A prospective study of 765,756 Norwegian women. Int J Cancer 61, 485–490 (1995). [DOI] [PubMed] [Google Scholar]
- Bevier M., Sundquist J. & Hemminki K. Does the time interval between first and last birth influence the risk of endometrial and ovarian cancer? Eur J Cancer 47, 586–591, doi: 10.1016/j.ejca.2010.10.004 (2011). [DOI] [PubMed] [Google Scholar]
- Henderson B. E. & Feigelson H. S. Hormonal carcinogenesis. Carcinogenesis 21, 427–433 (2000). [DOI] [PubMed] [Google Scholar]
- Akhmedkhanov A., Zeleniuch-Jacquotte A. & Toniolo P. Role of exogenous and endogenous hormones in endometrial cancer: review of the evidence and research perspectives. Ann N Y Acad Sci 943, 296–315 (2001). [DOI] [PubMed] [Google Scholar]
- Kaaks R., Lukanova A. & Kurzer M. S. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 11, 1531–1543 (2002). [PubMed] [Google Scholar]
- Brinton L. A. et al. Reproductive, menstrual, and medical risk factors for endometrial cancer: results from a case-control study. Am J Obstet Gynecol 167, 1317–1325 (1992). [DOI] [PubMed] [Google Scholar]
- Preston-Martin S., Pike M. C., Ross R. K., Jones P. A. & Henderson B. E. Increased cell division as a cause of human cancer. Cancer Res 50, 7415–7421 (1990). [PubMed] [Google Scholar]
- Lambe M., Wuu J., Weiderpass E. & Hsieh C. C. Childbearing at older age and endometrial cancer risk (Sweden). Cancer Causes Control 10, 43–49 (1999). [DOI] [PubMed] [Google Scholar]
- Gierisch J. M. et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: a systematic review. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 22, 1931–1943, doi: 10.1158/1055-9965.EPI-13-0298 (2013). [DOI] [PubMed] [Google Scholar]
- Ioannidis J. P., Patsopoulos N. A. & Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. Bmj 335, 914–916, doi: 10.1136/bmj.39343.408449.80 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q. J. et al. Consumption of fruit and vegetables reduces risk of pancreatic cancer: evidence from epidemiological studies. Eur J Cancer Prev, doi: 10.1097/CEJ.0000000000000171 (2015). [DOI] [PubMed] [Google Scholar]
- Wu L., Zhu J., Prokop L. J. & Hassan Murad M. Pharmacologic Therapy of Diabetes and Overall Cancer Risk and Mortality: A Meta-Analysis of 265 Studies. Sci Rep 5, 10147, doi: 10.1038/srep10147 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. et al. Nut consumption and risk of cancer and type 2 diabetes: a systematic review and meta-analysis. Nutr Rev 73, 409–425, doi: 10.1093/nutrit/nuv006 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Z., Wu Q. J., Zhu J. & Wu L. Fish consumption and risk of myeloma: a meta-analysis of epidemiological studies. Cancer Causes Control, doi: 10.1007/s10552-015-0625-1 (2015). [DOI] [PubMed] [Google Scholar]
- Wu L. & Zhu J. Linear reduction in thyroid cancer risk by oral contraceptive use: a dose-response meta-analysis of prospective cohort studies. Hum Reprod, doi: 10.1093/humrep/dev160 (2015). [DOI] [PubMed] [Google Scholar]
- Wells G. A., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. (Date of access: 07/04/2015)
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses. Bmj 327, 557–560, doi: 10.1136/bmj.327.7414.557 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P. & Thompson S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558, doi: 10.1002/sim.1186 (2002). [DOI] [PubMed] [Google Scholar]
- DerSimonian R. & Laird N. Meta-analysis in clinical trials. Controlled clinical trials 7, 177–188 (1986). [DOI] [PubMed] [Google Scholar]
- Greenland S. & Longnecker M. P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135, 1301–1309 (1992). [DOI] [PubMed] [Google Scholar]
- Guan H. B., Wu L., Wu Q. J., Zhu J. & Gong T. Parity and pancreatic cancer risk: a dose-response meta-analysis of epidemiologic studies. PloS one 9, e92738, doi: 10.1371/journal.pone.0092738 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan N. N. et al. Nonlinear reduction in risk for colorectal cancer by oral contraceptive use: a meta-analysis of epidemiological studies. Cancer Causes Control, doi: 10.1007/s10552-014-0483-2 (2014). [DOI] [PubMed] [Google Scholar]
- Orsini N., Li R., Wolk A., Khudyakov P. & Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 175, 66–73, doi: 10.1093/aje/kwr265 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Cook N. R., Bergstrom A. & Hsieh C. C. A two-stage hierarchical regression model for meta-analysis of epidemiologic nonlinear dose–response data. Computational Statistics and Data Analysis 53, 4157–4167 (2009). [Google Scholar]
- Begg C. B. & Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.