Abstract
Objective
Experimental evidence indicates that circulating insulin-like growth factor-1 (IGF-1) counteracts vascular aging and atherosclerosis, for which increased carotid artery intima-media thickness (IMT) is a marker. Yet, IGF-1 concentrations have been inconsistently associated with carotid IMT in epidemiological studies. Since vitamin D is also implicated in vascular protection and affects IGF-1 biology, we hypothesized that it would influence the effect of IGF-1 on IMT.
Methods
The relationship between carotid IMT and fasting serum IGF-1 was examined across strata of 25-hydroxyvitamin D [25(OH)D] in 472 participants in the Baltimore Longitudinal Study of Aging (BLSA) with well-controlled blood pressure and in 165 treatment-naive patients with essential hypertension from the Microalbuminuria: A Genoa Investigation on Complications (MAGIC) study. Moreover, the interplay between vitamin D and IGF-1 was preliminarily explored in EA.hy926 endothelial cells.
Results
After adjusting for age, sex, BMI, renal function, smoking, systolic blood pressure, LDL-cholesterol, glycemia, antihypertensive or lipid-lowering therapy, season, parathyroid hormone, and vitamin D supplementation, IGF-1 was significantly and negatively associated with carotid IMT only within the lowest 25(OH)D quartile (range 6.8–26 ng/mL) of the BLSA (β −0.095, p = 0.03). Similarly, a significant negative correlation between IGF-1 and carotid IMT was found after full adjustment only in MAGIC patients with 25(OH)D concentrations below either the deficiency cut-off of 20 ng/mL (β −0.214, p = 0.02) or 26 ng/mL (β −0.174, p = 0.03). Vitamin D dose-dependently decreased hydrogen peroxide-induced endothelial cell oxidative stress and apoptosis, which were further inhibited by IGF in the presence of low, but not high vitamin D concentration.
Conclusions
Circulating IGF-1 is vasoprotective primarily when vitamin D levels are low. Future studies should address the mechanisms of vitamin D/IGF-1 interaction.
Keywords: IGF-1, Vitamin D, Intima-media thickness, Aging, Atherosclerosis, Endothelial
1. Introduction
Insulin-like growth factor-1 (IGF-1) exerts effects on the vasculature that are important in physiology and disease [1]. IGF-1 coming from the bloodstream or released within the vessel wall stimulates endothelial and vascular smooth muscle cells in an endocrine and paracrine/autocrine way, respectively. Experimental data suggest that circulating IGF-1 is protective against aspects of arterial aging, such as oxidative stress, inflammation, and endothelial progenitor cell dysfunction, as well as against atherogenesis [2,3]. Mice with adult-onset deficiency in endocrine IGF-1 exhibit impaired vascular antioxidant responses, which result in the exacerbation of superoxide generation, endothelial dysfunction, and apoptosis following oxidative challenges [4]. Treatment with exogenous IGF-1 increases the number of endothelial progenitor cells, improves their colony forming and migratory capacity, and prevents their senescence in both mice and humans [5]. In the ApoE−/− strain, even a mild reduction in circulating IGF-1 leads to increased atherosclerotic burden [6], while IGF-1 infusion inhibits superoxide formation and inflammation in the aortic wall, stimulates endothelial nitric oxide synthase activity, and decreases the severity of atherosclerosis [7].
Thickening of the intimal-medial layer of the carotid artery is an established feature of the age-related vascular phenotype and is interrelated with atherogenesis [8]. In agreement with the evidence from mouse models [4–7], an inverse association between IGF-1 concentrations and carotid intima-media thickness (IMT) has been described in humans [9–11]. However, other authors reported a positive correlation between circulating IGF-1 levels and carotid IMT [12–14].
Pre-clinical and physiology studies have shown that vitamin D can affect the synthesis and/or activity of IGF-1 at the tissue level, as well as the amount of IGF-1 in the circulation [15]. Surprisingly, vitamin D modulation of IGF-1 in the cardiovascular system has been little investigated. Here, we tested the hypothesis that vitamin D influences the relationship between circulating IGF-1 and carotid IMT in a sample of relatively healthy elderly individuals, most of which had well-controlled blood pressure. Then, we tested the generalizability of our findings by replicating the analysis in an independent cohort of hypertensive patients. Furthermore, the combined effect of vitamin D and IGF-1 on endothelial cell oxidative stress and apoptosis, two distinguishing features of arterial aging [16,17] and atherosclerosis [18], was studied in vitro.
2. Methods
2.1. Study populations
Data analyzed for this report were from the Baltimore Longitudinal Study of Aging (BLSA) and the Microalbuminuria: A Genoa Investigation on Complications (MAGIC) cohorts, which have been described in details elsewhere [19,20].
Briefly, the BLSA is an ongoing study that enrolls community-dwelling adult volunteers mainly from the Baltimore–Washington area (Maryland and DC, USA; latitude 39°N) and follows them up with repeated thorough visits. Participants must be healthy at the time of recruitment, but are not excluded if a disease develops later on. For the present work, we selected 472 subjects who had undergone measurement of fasting IGF-1 and 25-hydroxyvitamin D [25(OH)D] concentrations and carotid artery IMT at the same visit between March 2007 and May 2010. In all cases parathyroid hormone (PTH) levels had also been determined.
The MAGIC investigation involved 18 year old or older outpatients with grade 1 or 2 primary hypertension from Genova and surroundings (Italy, latitude 44°N). Participants underwent drug washout if on antihypertensive therapy, clinical examination, ambulatory blood pressure monitoring, selected blood tests, and instrumental assessment of target organ damage including carotid artery ultrasonography. Serum and plasma samples were also prospectively collected after overnight fasting, frozen, and stored at −80 °C The present analysis comprised all the subjects enrolled in 1999–2001, whose sera were assayed for 25(OH)D, IGF-1, and PTH. In addition, levels of high sensitivity C-reactive protein (hsCRP) and aldosterone were available for this population.
Both the BLSA and the MAGIC study complied with the Declaration of Helsinki and were approved by the relevant Institutional Review Board and Ethics Committees. Written informed consent was given by every participant.
2.2. Measurement of IGF-1 and 25(OH)D concentrations
Serum total IGF-1 was measured by a validated [21] chemiluminescent immunoassay (Siemens Medical Solutions Diagnostics, New York, NY, USA) in both the BLSA and the MAGIC samples. The inter-assay coefficient of variation (CV) was 8.4% and the lower detection limit was 20 ng/mL Concentrations of 25(OH)D, the marker of vitamin D status, were assessed by liquid chromatography-mass spectrometry at Mayo Clinic laboratories (Rochester, MN, USA) in the BLSA and by chemiluminescent immunoassay on the DiaSorin Liaison® System (DiaSorin, Saluggia, Italy) in the MAGIC population. The inter-assay CV was 10% and 12.6%, respectively, while the lower detection limit was 4 ng/mL for both assays. Previous head-to-head comparisons demonstrated a good overall agreement between 25(OH)D immunoassays and the liquid chromatography-mass spectrometry method [22].
Serum levels of intact PTH were measured by chemiluminescent immunoassay in the BLSA (Siemens Medical Solutions Diagnostics, New York, NY, USA) and by a third generation sandwich-type immunoassay specific for human 1–84 PTH in the MAGIC cohort (DiaSorin, Saluggia, Italy). The inter-assay CV was 8% for both assays.
2.3. Carotid artery ultrasonography
The right common carotid artery was studied by high-resolution B-mode ultrasonography with a linear-array 5- to 10-MHz transducer (Ultramark 9 HDI, Advanced Technology Laboratories, USA in the BLSA and Diasonic Spectra System, Diasonic, CA, USA in the MAGIC study). A segment about 1 cm proximal to the carotid bifurcation was imaged in the longitudinal plane and IMT was measured as the distance between the luminal–intimal interface and the medial–adventitial interface on the far wall. Frozen frames of suitable images were magnified to improve accuracy of the measurements. IMT was assessed in three contiguous sites at 1 -mm intervals and the average of the three values was used for analyses [23].
2.4. Other variables
Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Smoking was ascertained by a questionnaire and study subjects were classified as active smokers vs. past or never smokers.
In the BLSA, blood pressure was measured at the right brachial artery before carotid ultrasonography. Three consecutive readings were performed and the average was recorded. In the MAGIC investigation, blood pressure was monitored over the 24-h of a routine working day with a validated oscillometric device (Space-labs 90207; SpaceLabs Inc, Redmond, WA, USA). Measurements were done at the non-dominant arm every 15 min from 07.00 to 23.00 pm and every 30 min in the remaining hours. Patients were instructed to live their normal life but to avoid over-exertion and to hold the arm still and relaxed during the readings.
By study design, participants in the MAGIC study were not diabetic, nor were they taking antihypertensive or lipid-lowering drugs or vitamin D supplements at the time of evaluation [20]. In the BLSA diabetes mellitus was diagnosed if the American Diabetes Association criteria were met or if subjects were on hypoglycemic medications. Use of drugs with Anatomical Therapeutic Chemical codes related to the cardiovascular system (C codes) was reviewed to identify patients on antihypertensive or lipid-lowering therapy. The first included diuretics, beta blockers, calcium channel antagonists, ACE inhibitors, angiotensin receptor blockers, and peripheral vasodilators. Subjects using vitamin D and analogs (A11CC), vitamin D and A in combination (A11CB), and vitamin D with other vitamins (AHA, A11B, A11H, A11JC) were considered as taking vitamin D supplements.
Serum creatinine, low-density lipoprotein (LDL)-cholesterol, and glucose were measured by automated assay. Estimated glomerular filtration rate (eGFR) was calculated by means of the CKD-EPI formula. Concentrations of hsCRP and aldosterone in the MAGIC study were assessed by immunonephelometry (Beckman Coulter Inc., Fullerton, CA) and radioimmunoassay (Sorin Bio-medica, Saluggia, Italy), respectively.
2.5. In vitro experiments
EA.hy926 human umbilical vein cells (ATCC, VA, USA) were cultured in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum at 37 °C in a 5% CO2 humidified incubator [24]. For experiments, cells grown to 50–60% confluence were treated with 100 mM hydrogen peroxide (H2O2) alone (Sigma–Aldrich, St. Louis, MO, USA), 100 rrM H2O2 + 0.1,10, or 100 nM 1,25-dihydroxyvitamin D [l,25(OH)2D] (Sigma–Aldrich), or 100 mM H2O2 + 0.1,10, or 100 nM l,25(OH)2D + 100 ng/mL IGF-1 (Pepro-Tech, Rocky Hill, NJ, USA). Vitamin D and IGF-1 were added to the culture medium 30 min before H2O2. The 0.1 nM and 10 nM l,25(OH)2D concentrations were used because they were 10 times lower and higher, respectively, than the one that had already been shown to counteract endothelial cell oxidative stress and apoptosis caused by H2O2 [25]. In the experiments evaluating oxidative stress, l,25(OH)2D was also used at 100 nM, since this concentration had also been reported to antagonize H2O2 oxidant damage in endothelial cells [26]. To quantify oxidative stress, 20 mM 2′,7′-dichlor-odihydrofluorescein (DCFH) was added 20 min after H2O2 and incubated for 30 min at 37 °C. After washing cells with PBS, dichlorofluorescein production by oxidation of DCFH was assessed by flow cytometry (Becton Dickinson and Company, Franklin Lakes, NJ, USA). To evaluate apoptosis, cells were exposed to H2O2 for 18 h, washed with PBS, resuspended in binding buffer at a concentration of 106/mL, and incubated with 5 rrL of annexin V-Alexa Fluor 488 and 1 mL of propidium iodide (Life Technologies, Carlsbad, CA, USA) for 15 min in the dark. Annexin V-positive cells were counted by flow cytometry.
2.6. Statistical analysis
Continuous variables are presented as mean ± SD and categorical variables as absolute and/or relative frequencies. Comparisons were drawn by chi-square test, t-test, and one-way analysis of variance, as appropriate. Adjusted means of IGF-1 concentrations were compared by analyses of co-variance.
For both the BLSA and the MAGIC investigation, the relationship between serum IGF-1 and carotid IMT was initially evaluated in the entire population by univariate regression analysis and after adjustment for age and gender, which strongly modify circulating IGF-1 levels [21]. Next, the relation of IGF-1 with IMT was assessed as function of vitamin D status. Only 42 (8.9%) BLSA participants had 25(OH)D values below the 20 ng/mL cut-off defining vitamin D deficiency [27] (Supplementary Fig. 1). Conversely, 25(OH)D levels below 20 ng/mL were found in around half of MAGIC patients. Therefore, to perform stratified analyses the BLSA cohort was divided into quartiles of 25(OH)D, and the MAGIC sample in subjects with < or ≥20 ng/mL 25(OH)D. In order to improve the comparability of the results obtained with the two study populations, MAGIC patients were also divided in those who had 25(OH)D concentrations below or above the maximum value of the first 25(OH)D quartile in the BLSA (i.e. 26 ng/mL). Although it has also been proposed that 30 ng/mL 25(OH)D identifies vitamin D sufficiency [28], we did not use this cut-off as only few MAGIC patients had 25(OH)D levels above it.
Within each vitamin D subgroup, the association of IGF-1 with IMT was tested by linear regression accounting for age, gender, and variables that might impact IMT, namely BMI and eGFR; the established cardiovascular risk factors smoking, systolic blood pressure (SBP), LDL-cholesterol, and fasting glucose; and antihypertensive or lipid-lowering drugs. Moreover, concentrations of hsCRP and aldosterone were included in the analysis of the MAGIC dataset as markers of inflammation and activity of the renin–angiotensin–aldosterone system (RAAS), respectively. The regression models were further adjusted for the season in which subjects were evaluated, categorized as November–April vs. May–October, and PTH levels, as they both can lie behind the association of vitamin D with health outcomes [29,30]. The analysis of the BLSA sample was also corrected for the use of vitamin D supplements, since it may result in increased circulating IGF-1 [31]. To achieve a normal distribution, IGF-1 concentrations were naturally log-transformed. The variance inflation factor was calculated for each model to assess collinearity and was considered acceptable when ≤2.
Analyses were performed using the SAS package (version 9.2, SAS Institute Inc., Cary, NC). Statistical significance was set at p < 0.05.
3. Results
3.1. Lack of association of circulating IGF-1 with carotid artery IMT not accounting for vitamin D status
The characteristics of the BLSA and MAGIC samples are shown in Supplementary Table 1. Two-hundred ninety four participants in the BLSA were excluded because they did not have measurements of IMT, 25(OH)D, and IGF-1 at the same visit over the 2007–2010 period; compared to the study cohort, they were significantly younger and had significantly higher IGF-1 concentrations and lower carotid IMT (Supplementary Table 2). All the patients recruited in the MAGIC investigation between 1999 and 2001 were considered in the present analysis.
In the entire BLSA cohort, logIGF-1 values were negatively correlated with carotid IMT in univariate regression analysis (β −0.076, p < 0.001), but the correlation became no longer significant after correcting for age and gender (β −0.009, p = 0.64). In the MAGIC sample, the relationship between IGF-1 and carotid IMT was not significant even without adjustments (β −0.060, p = 0.17). Concentrations of 25(OH)D were associated with carotid IMT in MAGIC patients (unadjusted β −0.201, p = 0.01), but not in the BLSA (unadjusted β 0.0001, p = 0.68).
To verify the assumption that vitamin D status might modulate the effect of IGF-1 on IMT, we then performed separate analyses across strata of 25(OH)D.
3.2. Circulating IGF-1 is inversely related to carotid artery IMT in subjects with low vitamin D levels
The variables of interest across the quartiles of 25(OH)D in the BLSA population are presented in Table 1. Unexpectedly, age progressively increased from the lowest to the highest vitamin D quartile, which is counterintuitive as aging predisposes to vitamin D deficiency [32]. On the other hand, the older was the age, the more frequent was the use of vitamin D supplements and, according to the trend of BMI, the lower was the amount of adipose tissue, which is also a well-known cause of low vitamin D levels [33].
Table 1.
Characteristics of the BLSA cohort by quartiles of 25-hydroxyvitamin D (25(OH)D).
| First quartile | Second quartile | Third quartile | Fourth quartile | p for Trend | |
|---|---|---|---|---|---|
| No. | 120 | 111 | 125 | 116 | |
| 25(OH)D (ng/mL) | 20.0 ± 5.1 | 29.4 ± 1.7 | 36.3 ± 2.4 | 49.0 ± 8.0 | |
| Range | 6.8–26 | 27–32 | 33–40 | 41–83 | |
| Males | 64 (53.3%) | 67 (60.4%) | 58 (46.4%) | 41 (35.3%) | 0.001 |
| Age (years) | 65.6 ± 10.9 | 70.6 ± 12.6 | 70.6 ± 12.3 | 71.5 ± 12.5 | <0.001 |
| BMI (kg/m2) | 29.3 ± 5.2 | 26.6 ± 4.1 | 26.8 ± 4.6 | 25.0 ± 3.5 | <0.0001 |
| eGFR (mL/min/m2) | 92.3 ± 13.6 | 91.9 ± 11.2 | 91.9 ± 12.3 | 90.8 ± 13.6 | 0.39 |
| Active smokers | 3 (2.5%) | 4 (3.6%) | 4 (3.2%) | 2 (1.7%) | 0.83 |
| SBP (mm/Hg) | 118.2 ± 15.8 | 117.4 ± 14.9 | 114.1 ± 15.9 | 112.2 ± 13.7 | <0.001 |
| LDL (mg/dL) | 119.5 ± 37.0 | 109.6 ± 36.0 | 109.7 ± 32.5 | 110.5 ± 32.2 | 0.05 |
| Fasting glucose (mg/dL) | 97.0 ± 35.7 | 89.9 ± 14.6 | 88.3 ± 15.1 | 87.3 ± 14.9 | <0.001 |
| Diabetes mellitus | 32 (26.7%) | 26 (23.4%) | 30 (24%) | 26 (22.4%) | 0.89 |
| Antihypertensive drugs | 57 (47.5%) | 60 (54.1%) | 58 (46.4%) | 62 (53.3%) | 0.53 |
| Lipid-lowering drugs | 47 (39.2%) | 58 (52.3%) | 65 (52.0%) | 65 (56.0%) | 0.05 |
| Vitamin D supplements | 48 (40.0%) | 76 (68.5%) | 88 (70.4%) | 86 (74.1%) | <0.0001 |
| Season | |||||
| November–April | 64 (53.3%) | 55 (49.6%) | 67 (53.6%) | 58 (50%) | |
| May–October | 56 (46.7%) | 56 (50.4%) | 58 (46.4%) | 58 (50%) | 0.89 |
| PTH (pg/mL) | 45.4 ± 18.9 | 36.9 ± 15.8 | 36.0 ± 16.6 | 32.8 ± 16.6 | <0.0001 |
| IGF-1 (ng/mL) | 123.5 ± 45.5 | 120.6 ± 43.8 | 119.7 ± 48.5 | 117.4 ± 43.2 | 0.30 |
| Adjusted for age and sex | 117.6 ± 3.9 | 120.8 ± 4.1 | 121.4 ± 3.8 | 121.5 ± 4.0 | 0.89 |
| Carotid IMT (mm) | 0.75 ± 0.19 | 0.72 ± 0.18 | 0.75 ± 0.18 | 0.73 ± 0.16 | 0.74 |
BMI, body mass index; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; LDL, low-density lipoprotein cholesterol; PTH, parathyroid hormone; IGF-1, insulin-like growth factor-1; IMT, intima-media thickness.
In the lowest 25(OH)D quartile, logIGF-1 was inversely correlated with carotid IMT both in univariate regression analysis (β −0.111, p = 0.01) and after accounting for age and gender (Table 2). Further adjustment for factors that might affect IMT, season, PTH, and use of vitamin D supplements did not substantially modify the result (Table 2 and Supplementary Fig. 2). In contrast, relationships between IGF-1 and IMT in the second to fourth vitamin D quartiles were not significant (Supplementary Fig. 2).
Table 2.
Adjusted regression coefficients of the relationship between log-transformed insulin-like growth factor-1 (IGF-1) concentrations and carotid artery intima-media thickness (IMT) within the lowest 25-hydroxyvitamin D quartile of the BLSA cohort.
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| β | SE | P | β | SE | P | β | SE | P | |
| logIGF-1 | −0.078 | 0.039 | 0.048 | −0.094 | 0.042 | 0.030 | −0.095 | 0.044 | 0.035 |
| Age | 0.008 | 0.001 | <0.0001 | 0.007 | 0.002 | 0.001 | 0.006 | 0.002 | 0.002 |
| Male gender | 0.068 | 0.030 | 0.027 | 0.062 | 0.033 | 0.065 | 0.054 | 0.034 | 0.110 |
| BMI | – | – | – | −0.002 | 0.003 | 0.478 | −0.003 | 0.003 | 0.369 |
| eGFR | – | – | – | −0.001 | 0.001 | 0.283 | −0.001 | 0.001 | 0.303 |
| Smoking | – | – | – | −0.055 | 0.103 | 0.591 | −0.039 | 0.105 | 0.710 |
| SBP | – | – | – | 0.002 | 0.001 | 0.012 | 0.003 | 0.001 | 0.018 |
| LDL | – | – | – | 0.0003 | 0.0005 | 0.467 | 0.0003 | 0.0004 | 0.529 |
| Fasting glucose | – | – | – | 0.0002 | 0.0004 | 0.644 | 0.0003 | 0.0004 | 0.545 |
| Antihypertensive tp. | – | – | – | 0.028 | 0.038 | 0.469 | 0.028 | 0.039 | 0.471 |
| Lipid-lowering tp. | – | – | – | 0.004 | 0.037 | 0.907 | 0.005 | 0.039 | 0.888 |
| Season (Nov–April) | – | – | – | – | – | – | −0.027 | 0.033 | 0.404 |
| PTH | – | – | – | – | – | – | 0.0005 | 0.0009 | 0.572 |
| Vitamin D supplements | – | – | – | – | – | – | 0.024 | 0.033 | 0.465 |
Model 1: adjusted for age and sex; model 2: further adjusted for variables affecting IMT; model 3: further adjusted for season, PTH, and vitamin D supplements. BMI, body mass index; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; LDL, low-density lipoprotein cholesterol; tp., therapy; PTH, parathyroid hormone.
In the final regression model investigating the correlates of IMT within the lowest 25(OH)D quartile of the BLSA sample, the magnitude of the effect of IGF-1 and SBP was similar, but of opposite direction (standardized estimates −0.19 and 0.21, respectively), and only second to the magnitude of the effect of age (standardized estimate 0.36).
As SBP was well controlled in BLSA participants (Supplementary Table 1), we sought to verify whether vitamin D status would also modify the relation of circulating IGF-1 with carotid IMT in subjects with untreated hypertension. For this reason, we replicated the analysis with the MAGIC population. This also allowed us to use more accurate mean 24-h SBP values instead of office measurements, as well as to take into account the concentrations of hsCRP and aldosterone. MAGIC patients were divided according to the 25(OH)D cut-offs of 20 and 26 ng/mL, corresponding to the definition of vitamin D deficiency and to the upper limit of the first vitamin D quartile in the BLSA, respectively (Table 3 and Supplementary Table 3). A significant negative correlation between logIGF-1 and IMT was found in subjects with <20 ng/mL 25(OH)D (unadjusted β −0.159, p < 0.01) and persisted after adjusting for multiple potential confounders (Table 4), while the relationship between IGF-1 and IMT was not significant in participants with ≥20 ng/mL 25(OH)D (data not shown). The standardized estimate of the association between logIGF-1 and IMT within the vitamin D-deficient MAGIC subgroup was −0.35, even higher than the one of age (0.26). Similar results were obtained with the 25(OH)D threshold of 26 ng/mL (Supplementary Table 4).
Table 3.
Characteristics of vitamin D-deficient and vitamin D-sufficient hypertensive patients from the MAGIC study.
| <20 ng/mL 25(OH)D | ≥20 ng/mL 25(OH)D | P for Comparison | |
|---|---|---|---|
| No. | 86 | 79 | |
| 25(OH)D (ng/mL) | 13.4 ± 4.3 | 28.7 ± 8.1 | |
| Males | 60 (69.8%) | 53 (67.1%) | 0.65 |
| Age (years) | 47.1 ± 9.5 | 46.3 ± 9.5 | 0.59 |
| BMI (kg/m2) | 26.5 ± 3.7 | 25.8 ± 2.9 | 0.18 |
| eGFR (mL/min/m2) | 92.7 ± 14.5 | 89.4 ± 15.9 | 0.17 |
| Active smokers | 18 (20.9)% | 19 (24.1%) | 0.58 |
| SBP | 154.9 ± 13.2 | 152.7 ± 10.2 | 0.22 |
| 24-h SBP (mm/Hg) | 141.3 ± 15.6 | 140.2 ± 12.2 | 0.62 |
| LDL (mg/dL) | 145.6 ± 35.8 | 126.9 ± 50.7 | 0.01 |
| Fasting glucose (mg/dL) | 92.9 ±11.0 | 89.8 ± 11.7 | 0.09 |
| hsCRP (mg/L) | 0.24 ± 0.31 | 0.21 ± 0.27 | 0.49 |
| Aldosterone (pg/mL) | 104.9 ± 58.0 | 121.1 ± 70.1 | 0.14 |
| Season | |||
| November–April | 63 (73.3%) | 32 (40.5%) | |
| May–October | 23 (26.7%) | 47 (59.5%) | <0.0001 |
| PTH (pg/mL) | 8.7 ± 6.4 | 8.8 ± 6.1 | 0.87 |
| IGF-1 (ng/mL) | 100.1 ± 54.3 | 1133 ± 57.3 | 0.13 |
| Age- and sex-adjusted | 100.9 ± 5.7 | 1123 ± 5.9 | 0.17 |
| Carotid IMT (mm) | 0.72 ± 0.19 | 0.66 ± 0.16 | 0.01 |
25(OH)D, 25-hydroxyvitamin D; BMI, body mass index; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; LDL, low-density lipoprotein cholesterol; hsCRP, high sensitivity C-reactive protein; PTH, parathyroid hormone; IGF-1, insulin-like growth factor-1; IMT, intima-media thickness.
Table 4.
Adjusted regression coefficients of the relationship between log-transformed insulin-like growth factor-1 (IGF-1) concentrations and carotid artery intima-media thickness (IMT) in vitamin D-deficient hypertensive patients from the MAGIC study.
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| β | SE | P | β | SE | P | β | SE | P | |
| logIGF-1 | −0.144 | 0.060 | 0.018 | −0.249 | 0.082 | 0.004 | −0.214 | 0.088 | 0.020 |
| Age | 0.003 | 0.002 | 0.101 | 0.006 | 0.004 | 0.130 | 0.006 | 0.004 | 0.114 |
| Male gender | −0.040 | 0.041 | 0.329 | −0.013 | 0.057 | 0.814 | −0.021 | 0.058 | 0.717 |
| BMI | – | – | – | 0.010 | 0.008 | 0.192 | 0.008 | 0.008 | 0.297 |
| eGFR | – | – | – | 0.003 | 0.002 | 0.209 | 0.003 | 0.002 | 0.218 |
| Smoking | – | – | – | 0.095 | 0.067 | 0.162 | 0.093 | 0.069 | 0.189 |
| 24-h SBP | – | – | – | −0.001 | 0.002 | 0.415 | −0.001 | 0.002 | 0.481 |
| LDL | – | – | – | 0.001 | 0.001 | 0.081 | 0.001 | 0.001 | 0.152 |
| Fasting glucose | – | – | – | −0.001 | 0.003 | 0.586 | −0.001 | 0.003 | 0.835 |
| hsCRP | – | – | – | 0.024 | 0.082 | 0.773 | 0.038 | 0.087 | 0.662 |
| Aldosterone | – | – | – | 0.001 | 0.0005 | 0.768 | 0.001 | 0.0005 | 0.795 |
| Season | – | – | – | – | – | – | 0.026 | 0.068 | 0.706 |
| PTH | – | – | – | – | – | – | −0.006 | 0.005 | 0.290 |
Model 1: adjusted for age and sex; model 2: further adjusted for variables affecting IMT; model 3: further adjusted for season and PTH.
BMI, body mass index; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; LDL, low-density lipoprotein cholesterol; hsCRP, high sensitivity C-reactive protein; PTH, parathyroid hormone.
Consistent with the findings above, there was a significant interaction on carotid IMT between logIGF-1 and 25(OH)D quartiles in the BLSA (p = 0.01), as well as between logIGF-1 and vitamin D deficiency (p = 0.01) or having 25(OH)D values below 26 ng/mL (p = 0.03) in the MAGIC sample.
We also tested the interaction between IGF-1 and markers of dyslipidemia, diabetes, and inflammation, all of which have been put in relation with vitamin D levels and might affect the association of circulating IGF-1 with carotid IMT. There was no significant interaction with total and LDL-cholesterol (p = 0.71 and p = 0.80 in the BLSA sample, respectively; and p = 0.56 and p = 0.22 in the MAGIC one), glycated hemoglobin (p = 0.14 in the BLSA sample, which included diabetic subjects), and hsCRP (p = 0.65 in the MAGIC sample, in which CRP values were available). The IGF-1 system is also influenced by the RAAS [34,35]. Consistent with this, a significant interaction was found between IGF-1 and aldosterone in the MAGIC population (p = 0.003). Conversely, no interaction was observed between IGF-1 and use of RAAS-modifying drugs in the BLSA cohort (p = 0.11).
3.3. IGF-1 enhances the antioxidant and anti-apoptotic effect of low-dose vitamin D in cultured endothelial cells
In the attempt to explain the modulation exerted by vitamin D status on the relationship between circulating IGF-1 and carotid IMT, we speculated that the activity of endocrine IGF-1 on the vasculature could be affected by vitamin D levels. By contrast, a biologically relevant effect of vitamin D on the amount of IGF-1 in the bloodstream seemed unlikely, since age- and sex-adjusted serum IGF-1 was not significantly different across vitamin D groups in both study samples (Tables 1 and 3, Supplementary Table 4). No significant difference was found either when IGF-1 concentrations in the BLSA cohort were additionally adjusted for vitamin D supplementation (118.0 ± 4.0,120.7 ± 4.1,121.3 ± 3.8, and 121.3 ± 4.0 in the first to the fourth quartile, p for trend = 0.93). To gain preliminary confirmation of our hypothesis, we challenged EA.hy926 cells with H2O2 and different concentrations of l,25(OH)2D alone or together with a fixed dose of IGF-1. As shown in Fig. 1A, l,25(OH)2D blunted oxidative stress triggered by H2O2, the 100 nM concentration being the most effective. However, while IGF-1 enhanced the protection conferred by 0.1 and 10 nM l,25(OH)2D, it did not potentiate the inhibition of oxidative stress attained by 100 nM l,25(OH)2D. Similarly, vitamin D dose-dependently ameliorated the survival of EA.hy926 exposed to H2O2 and IGF-1 further reduced the percentage of apoptotic cells when added to 0.1 nM, but not to 10 nM l,25(OH)2D (Fig. 1B).
Fig. 1.
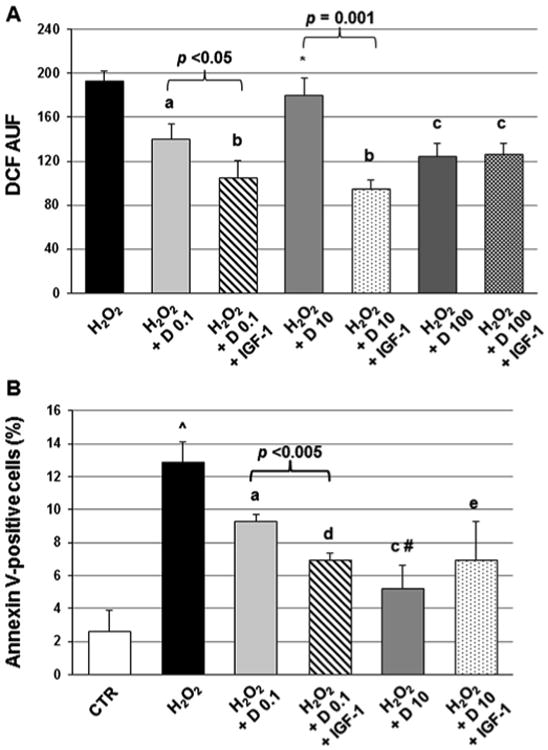
Mean dichlorofluorescein (DCF) fluorescence, expressed as arbitrary units of fluorescence (AUF), (A) and mean percentage of apoptotic EA.hy926 endothelial cells (B) after treatment with 100 mM H2O2 alone or 100 mM H2O2 together with 0.1,10, or 100 nM 1,25-dihydroxyvitamin D (D) ± 100 ng/mL insulin-like growth factor-1 (IGF-1). Vertical bars represent standard deviations for three independent experiments, a, p < 0.01; b, p < 0.001; c, p < 0.005; and d, p = 0.001 vs. H2O2. *, p < 0.05; and #, p < 0.01 vs. 0.1 nM 1,25-dihydroxyvitamin D. ˆ, p < 0.01 vs. control.
4. Discussion
This study found a significant association between circulating IGF-1 and carotid artery IMT, a hallmark of vascular aging strongly linked to atherogenesis, limited to subjects with low vitamin D levels. The influence of vitamin D status on the relationship of IGF-1 with IMT was demonstrated in two unrelated cohorts and was independent of major cardiovascular risk factors, especially hypertension. In the setting of low vitamin D concentrations, serum IGF-1 was inversely correlated with carotid IMT, pointing to a protective action. In agreement with these findings, in preliminary experiments in vitro IGF-1 improved the decrease in H2O2-induced endothelial cell oxidative stress and apoptosis achieved by low-, but not high-dose vitamin D.
Experimental evidence indicates that endocrine IGF-1 inhibits endothelial dysfunction and vascular inflammation that occur with aging and drive atherosclerosis development and progression [2,3]. Mice with reduced blood levels, but normal tissue expression of IGF-1 display higher vascular production of superoxide and apoptosis in response to oxidative stressors than controls [4]. In the ApoE−/− model, a 20% decline in circulating IGF-1 results in increased atherosclerotic burden with more intense macrophage infiltration and higher TNF-a levels in the aorta [6]. Consistently, infusion of exogenous IGF-1 to ApoE−/− mice causes a decrease in aortic oxidative stress, inflammation, and atherosclerosis [7].
Vitamin D, too, has been reported to be beneficial for the vasculature by virtue of a number of mechanisms, such as preservation of endothelial function and integrity, and inhibition of arterial inflammation and immune activation [36]. Vascular cells express the vitamin D receptor and the 1a-hydroxylase enzyme that converts 25(OH)D into the hormonally active metabolite l,25(OH)2D [37]. Although l,25(OH)2D is also present in the circulation and might theoretically act on arteries in an endocrine way, locally produced l,25(OH)2D is thought to mediate most vitamin D effects on the vasculature [36].
Vitamin D has also been shown to modulate the IGF-1 system at multiple levels [15]. In particular, Wu-Wong and colleagues found that vitamin D up-regulates IGF-1 mRNA in human coronary artery smooth muscle cells [38], providing evidence that vitamin D may affect IGF-1 in the vascular system. Here we show, for the first time, that vitamin D may moderate the relation of IGF-1 with vascular outcomes.
Thickening of the intima-media of the common carotid artery is a typical sign of arterial aging, to which a chronic elevation in local blood pressure contributes primarily [39]. Indeed, hypertensive patients from the MAGIC study had mean IMT values only 0.05-mm thinner than the mainly normotensive participants in the BLSA, despite being much younger (Supplementary Table 1). On the other hand, the same cellular and molecular alterations that underlie carotid intimal-medial thickening are also involved in atherogenesis [40] and, in fact, increased carotid IMT is associated with overt atherosclerosis at other sites and atherosclerosis-related cardiovascular events, even if to a lesser extent compared to carotid plaques [8]. Although this study was not conceived to determine the precise mechanism by which vitamin D modulates the relationship between IGF-1 and carotid IMT, we preliminary investigated the interplay between the two hormones on endothelial cell oxidative stress and apoptosis, which occur in both vascular aging and atherogenesis [16–18]. In EA.hy926 cells, IGF-1 added to the reduction in H2O2-induced oxidative stress and apoptosis attained by low-, but not high-dose l,25(OH)2D, suggesting a ceiling effect whereby the antioxidant and survival pathways activated by higher concentrations of l,25(OH)2D prevail over those initiated by IGF-1. Based on these findings, it can be speculated that increasing l,25(OH)2D levels within the vascular wall (which are function of the substrate 25(OH)D carried with the blood) may also overcome the inhibition attained by circulating IGF-1 on other biological events involved in vascular aging and atherogenesis, such as leukocyte activation and inflammation. Alternatively, vitamin D may modify the pattern of microRNAs that control the expression of the receptor and, thereby, the effects of IGF-1. In particular, a target of vitamin D might be miR-133a, which has been reported to prolong the half-life of the IGF-1 receptor mRNA and to sustain the proliferation of vascular smooth muscle cells in response to IGF-1 [41].
Other factors that are related to vitamin D status may interact with IGF-1 in affecting carotid IMT and, in general, the vasculature. For instance, indirect evidence from mouse experiments links the decrease in IGF-1 concentrations to the development of cardiovascular disease secondary to the combination of hyperglycemia and dyslipidemia [42]. Moreover, an inverse correlation exists between low vitamin D levels and markers of immune activation and inflammation [43], which are central to arterial aging and atheroma formation [40] and can also decrease systemic [44] and intravascular [45] IGF-1. Although we did not find any significant interaction between IGF-1 and cholesterol, HbAlc, or hsCRP concentrations, it remains possible that dyslipidemia, diabetes, and inflammation at least in part mediate the impact of low vitamin D levels on the relation of circulating IGF-1 with carotid IMT.
Besides being interrelated to one another, the vitamin D and IGF-1 systems are also interconnected to the RAAS [46–48]. With regards to this, the finding of a significant interaction between IGF-1 and aldosterone is extremely interesting and deserves further investigation.
It is becoming evident that vitamin D effects may be U-shaped, high levels of the hormone being as detrimental as deficiency [49]. In the cardiovascular system, this may be due to direct activation of cardiac and arterial VDR, but also to vitamin D-mediated non-cardiovascular responses that have deleterious consequences on the heart and vessels, such as hypercalcemia or hyperphosphoremia [50]. Moreover, under oxidative conditions vitamin D may be broken into reactive species that can be damaging. Hence, and regardless of the modulation of IGF-1, vitamin D may be associated or not with carotid IMT depending on the population examined, as we found in the MAGIC and BLSA cohort, respectively. In fact, previous investigations of the relationship between vitamin D status and carotid IMT yielded conflicting results [51,52]. Remarkably, a 25(OH)D-decreasing single nucleotide polymorphism in the DHCR7 gene, encoding for a 7-dehydrocholesterol reductase/NAD synthetase-1 responsible for vitamin D synthesis, has recently been associated to faster carotid IMT progression independent of 25(OH)D levels, suggesting that enzymes implicated in vitamin D metabolism affect IMT through still unknown pathways [53]. Prior studies also failed to consistently show an association between IGF-1 concentrations and carotid IMT [9–14]. In this case, the discrepancies might have stemmed from differences in vitamin D status, which was not taken into account.
We acknowledge that the present work has limitations. First, the independent variable tested by all analyses was circulating total IGF-1, which includes both the hormone bound to the IGF binding protein-3/acid labile subunit complex and the free one [54]. The latter is thought to principally, if not exclusively, interact with the IGF-1 receptor [55]. Therefore, adjustment for IGF binding protein-3 levels or direct measurement of free IGF-1 might have yielded a better estimate of IGF-1 endocrine activity. Second, we focused on carotid IMT because it reflects vascular aging and is also strictly correlated with atherosclerosis, both processes being antagonized by circulating IGF-1 in animal models. It would be worth investigating the interaction between vitamin D and IGF-1 on other clinical manifestations of vascular aging, such as aortic stiffness, and on plaque formation. Third, although epidemiological results are substantiated by the reproducibility in two cohorts, it must be stressed that the populations analyzed here were heterogenous, with differences in 25(OH)D and IMT determination, in the cova-riates available, and likely also in sun exposure and other life habits contributing to vitamin D status. Finally, the experiments with EAhy926 cells addressed only endothelial oxidative stress and apoptosis, and did not consider the other vascular cell types affected by aging and atherosclerosis.
In conclusion, our results indicate that, in humans, circulating IGF-1 is protective against arterial aging and, possibly, atherosclerosis only when vitamin D levels are low. Further studies are needed to fully characterize the interaction between vitamin D and IGF-1 within the vasculature and pinpoint the mechanisms behind it. More in general, the present work highlights the importance of trying to integrate the information about the cardiovascular effects of different hormones into complex models.
Supplementary Material
Acknowledgments
Sources of funding: This study was supported by the Intramural Research Program of the National Institute on Aging (BLSA) and by the Progetto di Ricerca d'Ateneo 2011 research grant funded by the University of Genova, Italy (MAGIC).
Footnotes
Conflict of interest: The authors do not have any financial or other conflicts of interest to declare.
Appendix A. Supplementary data: Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.atherosclerosis.2014.08.022.
References
- 1.Delafontaine P, Song YH, Li Y. Expression, regulation, and function of IGF-1, IGF-1 R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vase Biol. 2004;24:435–44. doi: 10.1161/01.ATV.0000105902.89459.09. [DOI] [PubMed] [Google Scholar]
- 2.Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P. Aging, atherosclerosis, and IGF-1. J Gerontol A Biol Sci Med Sci. 2012;67:626–39. doi: 10.1093/gerona/gls102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ungvari Z, Csiszar A. The emerging role of IGF-1 deficiency in cardiovascular aging: recent advances. J Gerontol A Biol Sci Med Sci. 2012;67:599–610. doi: 10.1093/gerona/gls072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:313–29. doi: 10.1093/gerona/glr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thum T, Hoeber S, Froese S, Mink I, Stichtenoth DO, Galuppo P, et al. Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1. Ore Res. 2007;100:434–43. doi: 10.1161/01.RES.0000257912.78915.af. [DOI] [PubMed] [Google Scholar]
- 6.Shai SY, Sukhanov S, Higashi Y, Vaughn C, Rosen CJ, Delafontaine P. Low circulating insulin-like growth factor I increases atherosclerosis in ApoE-deficient mice. Am J Physiol Heart Circ Physiol. 2011;300:H1898–906. doi: 10.1152/ajpheart.01081.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sukhanov S, Higashi Y, Shai SY, Vaughn C, Mohler J, Li Y, et al. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler Thromb Vase Biol. 2007;27:2684–90. doi: 10.1161/ATVBAHA.107.156257. [DOI] [PubMed] [Google Scholar]
- 8.Johnsen SH, Mathiesen EB. Carotid plaque compared with intima-media thickness as a predictor of coronary and cerebrovascular disease. Curr Cardiol Rep. 2009;11:21–7. doi: 10.1007/s11886-009-0004-1. [DOI] [PubMed] [Google Scholar]
- 9.Janssen JA, Stolk RP, Pols HA, Grobbee DE, Lamberts SW. Serum total IGF-I, free IGF-I, and IGFB-1 levels in an elderly population: relation to cardiovascular risk factors and disease. Arterioscler Thromb Vase Biol. 1998;18:277–82. doi: 10.1161/01.atv.18.2.277. [DOI] [PubMed] [Google Scholar]
- 10.van den Beld AW, Bots ML, Janssen JA, Pols HA, Lamberts SW, Grobbee DE. Endogenous hormones and carotid atherosclerosis in elderly men. Am J Epidemiol. 2003;157:25–31. doi: 10.1093/aje/kwf160. [DOI] [PubMed] [Google Scholar]
- 11.Boquist S, Ruotolo G, Skoglund-Andersson C, Tang R, Björkegren J, Bond MG, et al. Correlation of serum IGF-I and IGFBP-1 and -3 to cardiovascular risk indicators and early carotid atherosclerosis in healthy middle-aged men. Clin Endocrinol (Oxf) 2008;68:51–8. doi: 10.1111/j.1365-2265.2007.02998.x. [DOI] [PubMed] [Google Scholar]
- 12.Kawachi S, Takeda N, Sasaki A, Kokubo Y, Takami K, Sarui H, et al. Circulating insulin-like growth factor-1 and insulin-like growth factor binding protein-3 are associated with early carotid atherosclerosis. Arterioscler Thromb Vase Biol. 2005;25:617–21. doi: 10.1161/01.ATV.0000154486.03017.35. [DOI] [PubMed] [Google Scholar]
- 13.Spilcke-Liss E, Friedrich N, Dörr M, Schminke U, Völzke H, Brabant G, et al. Serum insulin-like growth factor-I and its binding protein 3 in their relation to intima media thickness: results of the study of health in Pomerania (SHIP) Clin Endocrinol (Oxf) 2011 Feb 9; doi: 10.1111/j.1365-2265.2011.04010.x. http://dx.doi.org/10.1111/j.l365-2265.2011.04010.x [Epub ahead of print] [DOI] [PubMed]
- 14.Hietaniemi M, Pöykkö SM, Ukkola O, Päivänsalo M, Antero Kesäniemi Y. IGF-I concentrations are positively associated with carotid artery atherosclerosis in women. Ann Med. 2005;37:373–82. doi: 10.1080/07853890510011967. [DOI] [PubMed] [Google Scholar]
- 15.Ameri P, Giusti A, Boschetti M, Murialdo G, Minuto F, Ferone D. Interactions between vitamin D and IGF-I: from physiology to clinical practice. Clin Endocrinol (Oxf) 2013;79:457–63. doi: 10.1111/cen.12268. [DOI] [PubMed] [Google Scholar]
- 16.Al Csiszar, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-tumor necrosis factor-alpha treatment in aging. Am J Pathol. 2007;170:388–98. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–44. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stacker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 19.Shock NW, Greulich RC, Costa PT, Andres R, Lakatta EG, Arenberg D, et al. NIH publication no 84-2450. Washington, DC: U.S. Government Printing Office; 1984. Normal human aging: the Baltimore longitudinal study of aging; p. 45. [Google Scholar]
- 20.Pontremoli R, Sofia A, Ravera M, Nicolella C, Viazzi F, Tirotta A, et al. Prevalence and clinical correlates of microalbuminuria in essential hypertension: the MAGIC study. Microalbuminuria: a Genoa investigation on complications Hypertension. 1997;30:1135–43. doi: 10.1161/01.hyp.30.5.1135. [DOI] [PubMed] [Google Scholar]
- 21.Frystyk J, Freda P, Clemmons DR. The current status of IGF-I assays – a 2009 update. Growth Horm IGF Res. 2010;20:8–18. doi: 10.1016/j.ghir.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koivula MK, Turpeinen U, Laitinen P, Risteli J. Comparison of automated 25-OH vitamin D immunoassays with liquid chromatography isotope dilution tandem mass spectrometry. Clin Lab. 2012;58:1253–61. [PubMed] [Google Scholar]
- 23.Nagai Y, Metter EJ, Earley CJ, Kemper MK, Becker LC, Lakatta EG, et al. Increased carotid artery intimal-medial thickness in asymptomatic older subjects with exercise-induced myocardial ischemia. Circulation. 1998;98:1504–9. doi: 10.1161/01.cir.98.15.1504. [DOI] [PubMed] [Google Scholar]
- 24.Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIH-related antigen established by hybridization. Proc Natl Acad Sci U S A. 1983;80:3734–7. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uberti F, Lattuada D, Morsanuto V, Nava U, Bolis G, Vacca G, et al. Vitamin D protects human endothelial cells from oxidative stress through the autophagic and survival pathways. J Clin Endocrinol Metab. 2014;99:1367–74. doi: 10.1210/jc.2013-2103. [DOI] [PubMed] [Google Scholar]
- 26.Polidoro L, Properzi G, Marampon F, Gravina GL, Festuccia C, Di Cesare E, et al. Vitamin D protects human endothelial cells from H2O2 oxidant injury through the Mek/Erk-Sirtl axis activation. J Cardiovasc Transl Res. 2013;6:221–31. doi: 10.1007/s12265-012-9436-x. [DOI] [PubMed] [Google Scholar]
- 27.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 29.Wood AD, Secombes KR, Thies F, Aucott L, Black AJ, Mavroeidi A, et al. Vitamin D3 supplementation has no effect on conventional cardiovascular risk factors: a parallel-group, double-blind, placebo-controlled RCT. J Clin Endocrinol Metab. 2012;97:3557–68. doi: 10.1210/jc.2012-2126. [DOI] [PubMed] [Google Scholar]
- 30.Richart T, Thijs L, Nawrot T, Yu J, Kuznetsova T, Balkestein EJ, et al. The metabolic syndrome and carotid intima-media thickness in relation to the parathyroid hormone to 25-OH-D(3) ratio in a general population. Am J Hypertens. 2011;24:102–9. doi: 10.1038/ajh.2010.124. [DOI] [PubMed] [Google Scholar]
- 31.Ameri P, Giusti A, Boschetti M, Bovio M, Teti C, Leoncini G, et al. Vitamin D increases circulating IGF1 in adults: potential implication for treatment of growth hormone deficiency. Eur J Endocrinol. 2013;169:767–72. doi: 10.1530/EJE-13-0510. [DOI] [PubMed] [Google Scholar]
- 32.Oudshoorn C, van der Cammen TJ, McMurdo ME, van Leeuwen JP, Colin EM. Ageing and vitamin D deficiency: effects on calcium homeostasis and considerations for vitamin D supplementation. Br J Nutr. 2009;101:1597–606. doi: 10.1017/S0007114509338842. [DOI] [PubMed] [Google Scholar]
- 33.Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013;10:el001383. doi: 10.1371/journal.pmed.1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen ZZ, Cai MY, Mai Z, Jin DM, Chen YX, Huang H, et al. Angiotensin II receptor blocker attenuates intrarenal renin-angiotensin-system and podocyte injury in rats with myocardial infarction. PLoS One. 2013;8:e67242. doi: 10.1371/journal.pone.0067242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis LK, Rodgers BD, Kelley KM. Angiotensin II- and glucose-stimulated extracellular matrix production: mediation by the insulin-like growth factor (IGF) axis in a murine mesangial cell line. Endocrine. 2008;33:32–9. doi: 10.1007/s12020-008-9055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kassi E, Adamopoulos C, Basdra EK, Papavassiliou AG. Role of vitamin D in atherosclerosis. Circulation. 2013;128:2517–31. doi: 10.1161/CIRCULATIONAHA.113.002654. [DOI] [PubMed] [Google Scholar]
- 37.Merke J, Milde P, Lewicka S, Hugel U, Klaus G, Mangelsdorf DJ, et al. Identification and regulation of 1,25-dihydroxyvitamin D3 receptor activity and biosynthesis of 1,25-dihydroxyvitamin D3. Studies in cultured bovine aortic endothelial cells and human dermal capillaries. J Clin Invest. 1989;83:1903–15. doi: 10.1172/JCI114097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu-Wong JR, Nakane M, Ma J, Ruan X, Kroeger PE. Effects of vitamin D analogs on gene expression profiling in human coronary artery smooth muscle cells. Atherosclerosis. 2006;186:20–8. doi: 10.1016/j.atherosclerosis.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka H, Dinenno FA, Monahan KD, DeSouza CA, Seals DR. Carotid artery wall hypertrophy with age is related to local systolic blood pressure in healthy men. Arterioscler Thromb Vase Biol. 2001;21:82–7. doi: 10.1161/01.atv.21.1.82. [DOI] [PubMed] [Google Scholar]
- 40.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–7. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 41.Gao S, Wassler M, Zhang L, Li Y, Wang J, Zhang Y, et al. MicroRNA-133a regulates insulin-like growth factor-1 receptor expression and vascular smooth muscle cell proliferation in murine atherosclerosis. Atherosclerosis. 2014;232:171–9. doi: 10.1016/j.atherosclerosis.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Shelat H, Wu H, Zhu M, Xu J, Geng YJ. Low circulating level of IGF-1 is a distinct indicator for the development of cardiovascular disease caused by combined hyperglycemia and dyslipidemia. Int J Cardiol. 2014;171:272–3. doi: 10.1016/j.ijcard.2013.11.091. [DOI] [PubMed] [Google Scholar]
- 43.Murr C, Pilz S, Grammer TB, Kleber ME, Meinitzer A, Boehm BO, et al. Vitamin D deficiency parallels inflammation and immune activation, the Ludwigshafen risk and cardiovascular health (LUR1C) study. Clin Chem Lab Med. 2012;50:2205–12. doi: 10.1515/cclm-2012-0157. [DOI] [PubMed] [Google Scholar]
- 44.Wolf M, Böhm S, Brand M, Kreymann G. Proinflammatory cytokines inter-leukin 1 beta and tumor necrosis factor alpha inhibit growth hormone stimulation of insulin-like growth factor I synthesis and growth hormone receptor mRNA levels in cultured rat liver cells. Eur J Endocrinol. 1996;135:729–37. doi: 10.1530/eje.0.1350729. [DOI] [PubMed] [Google Scholar]
- 45.Anwar A, Zahid AA, Scheidegger KJ, Brink M, Delafontaine P. Tumor necrosis factor-alpha regulates insulin-like growth factor-1 and insulin-like growth factor binding protein-3 expression in vascular smooth muscle. Circulation. 2002;105:1220–5. doi: 10.1161/hc1002.105187. [DOI] [PubMed] [Google Scholar]
- 46.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin–angiotensin system. J Clin Invest. 2002;110:229–38. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jia G, Aggarwal A, Yohannes A, Gangahar DM, Agrawal DK. Cross-talk between angiotensin II and IGF-1-induced connexin 43 expression in human saphenous vein smooth muscle cells. J Cell Mol Med. 2011;15:1695–702. doi: 10.1111/j.1582-4934.2010.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hannemann A, Wallaschofski H, Rettig R, Völzke H, Samietz S, Nauck M, et al. Association of IGF-I and the IGF-I/IGFBP-3 ratio with plasma aldosterone levels in the general population. Horm Metab Res. 2012;44:228–33. doi: 10.1055/s-0031-1301300. [DOI] [PubMed] [Google Scholar]
- 49.Ameri P, Canepa M, Milaneschi Y, Spallarossa P, Leoncini G, Giallauria F, et al. Relationship between vitamin D status and left ventricular geometry in a healthy population: results from the Baltimore longitudinal study of aging. J Intern Med. 2013;273:253–62. doi: 10.1111/joim.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lomashvili KA, Wang X, O'Neill WC. Role of local versus systemic vitamin D receptors in vascular calcification. Arterioscler Thromb Vase Biol. 2014;34:146–51. doi: 10.1161/ATVBAHA.113.302525. [DOI] [PubMed] [Google Scholar]
- 51.Deleskog A, Piksasova O, Silveira A, Gertow K, Baldassarre D, Veglia F, et al. Serum 25-hydroxyvitamin D concentration in subclinical carotid atherosclerosis. Arterioscler Thromb Vase Biol. 2013;33:2633–8. doi: 10.1161/ATVBAHA.113.301593. [DOI] [PubMed] [Google Scholar]
- 52.Blondon M, Sachs M, Hoofnagle AN, Ix JH, Michos ED, Korcarz C, et al. 25-Hydroxyvitamin D and parathyroid hormone are not associated with carotid intima-media thickness or plaque in the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vase Biol. 2013;33:2639–45. doi: 10.1161/ATVBAHA.113.301781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strawbridge RJ, Deleskog A, McLeod O, Folkersen L, Kavousi M, Gertow K, et al. A serum 25-hydroxyvitamin D concentration-associated genetic variant in DHCR7 interacts with type 2 diabetes status to influence subclinical atherosclerosis (measured by carotid intima-media thickness) Diabetologia. 2014;57:1159–72. doi: 10.1007/s00125-014-3215-y. [DOI] [PubMed] [Google Scholar]
- 54.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 55.Frystyk J. Free insulin-like growth factors – measurements and relationships to growth hormone secretion and glucose homeostasis. Growth IGF Res. 2004;14:337–75. doi: 10.1016/j.ghir.2004.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


