Abstract
Background
Subtle, but clinically detectable, neurological abnormalities (SNAs) are associated with impaired physical performance in elderly persons without overt neurological diseases. We investigated whether SNAs were prospectively associated with cognitive and functional status, death, and cerebrovascular events (CVEs) in older community-dwelling individuals.
Methods
In participants without history of stroke, parkinsonism and dementia, or cognitive impairment, a score (NSNA) was obtained by summing SNAs detected with a simple neurological examination. Cognitive status and disability were reassessed 4 years later, and deaths and CVEs were documented over 8 years.
Results
Of 506 participants free of neurological diseases (mean [SEM] age, 71.9[0.3] years; 42% were men), 59% had an NSNA of 1 or more (mean [SEM], 1.1[0.06]; range, 0–8). At baseline, the NSNA increased with age and with declining cognitive and physical performance, depressive symptoms, and disability, after adjusting for several covariates, but did not increase with falls and urinary incontinence. The NSNA prospectively predicted worsening cognitive status and disability, adjusting for demographics and for baseline comorbidity and cognitive and physical performance. The mortality rates were 22.6, 23.3, 23.9, 58.6, and 91.9 per 1000 person-years in participants with an NSNA of 0, 1, 2, 3, and 4 or higher, respectively. Compared with an NSNA of less than 3, having an NSNA of 3 or higher was associated with an increased adjusted risk of death (hazard ratio, 1.77; 95% confidence interval [CI], 1.25–2.74) and of CVE (hazard ratio, 1.94; 95% CI, 1.07–3.54) over 8 years.
Conclusion
In this sample of older community-dwelling persons without overt neurological diseases, multiple SNAs were associated with cognitive and functional decline and independently predicted mortality and CVEs.
Studies of apparently healthy, older community-dwelling persons have shown that subclinical diseases of different organs are associated with poor physical performance1,2 and frailty.3 In the Cardiovascular Health Study (CHS),4 a lower extent of subclinical cardiovascular disease predicted successful aging, defined as the years lived without physical and cognitive impairment, overt cardiovascular disease, cancer, and chronic obstructive pulmonary disease.
In further CHS analyses, the severity of neurological disease at neuroimaging predicted accelerated functional decline and onset of physical dependency.5 Also subtle, but clinically detectable, neurological abnormalities (SNAs) seemed to be associated with poor physical performance and falls in a cross-sectional investigation of 818 older community-dwelling persons without major neurological diseases.6 However, to our knowledge, no study has ever investigated whether SNAs independently predict poor health outcomes in older persons.
We evaluated whether SNAs are associated, both cross-sectionally and longitudinally, with reduced cognitive and functional status and falls in older persons free of overt neurological diseases. We also tested the association between SNAs and urinary incontinence, which may be an early manifestation of subclinical neurological disease.7 Finally, we verified whether SNAs independently predict mortality and incident cerebrovascular events (CVEs) over an extended follow-up.
METHODS
STUDY PROTOCOL AND SAMPLE SELECTION
The “Insufficienza Cardiaca negli Anziani Residenti a Dicomano” (ICARe Dicomano) study enrolled all the older (≥65 years) community-dwelling individuals living in Dicomano, a small rural town near Florence, Italy. The methods of the study, which followed the principles of the Declaration of Helsinki, have been detailed elsewhere.8 Participants gave informed consent. Adherence rates ranged from 91.2% to 80.7% in different phases of the study. Participants and nonparticipants had similar sex and age distribution, although slightly fewer men (77.4%) than women (83.1%) underwent the final clinical assessment.8 From the 1995 baseline study sample, we excluded participants with previous stroke, parkinsonism, dementia, or cognitive impairment. Participants were reinterviewed in 1999. Vital status and hospitalizations were followed up until December 2003.
DATA COLLECTION
Diagnosis of Overt Neurological Disease
Neurological diseases were identified with standardized algorithms based on previous diagnoses, medical records, structured medical interviews, and clinical examinations.8 The diagnosis of stroke was adjudicated when a previous diagnosis or positive questionnaire items (deficit in strength in an upper and/or lower extremity, speech, or visual field) were confirmed by hospital discharge records, neuroimaging, or clinical examination. The diagnosis of Parkinson disease or parkinsonism was based on clinical examination or a previous physician’s diagnosis in current specific drug treatment. Participants who scored 26 or less on the Mini-Mental State Examination (MMSE)9 received an extensive neuropsychological evaluation by an expert geriatrician, who adjudicated the final diagnosis of dementia. Conservatively, in the present study we also excluded participants with cognitive impairment (MMSE score <24) who refused or missed the complete neuropsychological evaluation necessary to diagnose dementia.
Neurological Clinical Evaluation
The neurological assessment, conducted by expert geriatricians and summarized in Table 1, required up to 15 minutes and included a traditional neurological examination plus 2 simple tests (ie, dynamometry and the Purdue Pegboard Test).8 Handgrip and hip flexion strength were assessed on both the right and left sides with handheld dynamometers, according to a standard protocol.10 Consistent with published criteria for lower extremity strength testing,10 clinically relevant asymmetry for handgrip strength was defined as a difference of more than 20% between the 2 sides. In the Purdue Pegboard Test, participants are timed while putting 10 small sticks into an equal number of holes in a tablet. Originally introduced to evaluate manual dexterity,11 this test also explores frontal lobe cognitive function because it requires integrity of attention, sequencing, planning, and motor coordination. We used the score obtained with the dominant hand. Failure to complete the task, or a time to complete above the 90th percentile of the study sample, indicated an altered performance. The reliability of the neurological examination had been previously evaluated in a small sample of older outpatients with characteristics similar to participants in the present survey. For the standard neurological examination, interrater agreement in the distinction between normal vs abnormal findings was very high (κ > 0.95). Other items, based on continuous measures (eg, muscle strength) initially reported greater variability, which, after training and standardization, was contained to an interrater difference of 10% to 15%.
Table 1.
Prevalence of SNAs and Their Bivariate Associations With Physical Performance (SPPB Score) and Cognitive Function (MMSE) in 506 Older Community-Dwelling Persons
| Items of the Neurological Evaluation | Prevalence, % | P Valuea | |
|---|---|---|---|
| SPPB | MMSE | ||
| Muscle strength–physical examination | |||
| Reduced shoulder elevation, detectable difference between the 2 sides while participant is shrugging against resistance | 0.4 | .02 | .21 |
| Reduced or absent foot extension, detectable difference between the 2 sides while participant is pushing against resistance (each foot is tested separately) | 3.0 | <.001 | .11 |
| Pronator drift or shift of 1 arm while both are extended frontally | 0.6 | .01 | .42 |
| Shift of 1 leg while both hips and knees are flexed at 90° in the supine position | 0.0 | NA | NA |
| Muscle strength–dynamometry (difference of >20% between the 2 sides) | |||
| Handgrip | 4.9 | .11 | .16 |
| Hip flexion | 11.3 | .66 | .49 |
| Sensitivity–physical examination, reduced foot plant sensitivity (light touch) in at least 2 of 3 tests | 4.3 | <.001 | .95 |
| Deep tendon reflexes, absent or diminished (present but slightly detectable) muscle contraction in response to tendon stimulation with a reflex hammer using standard techniques | |||
| Absent or reduced patellar reflexes | 3.0 | .03 | .64 |
| Absent or reduced Achilles reflexes | 29.9 | .002 | .01 |
| Plantar (Babinski) reflex, extension of the big toe or fanning of the other toes or contraction of the fascia lata while rubbing the lateral margin of the sole from heel to toe | 3.6 | .09 | .83 |
| Extrapyramidal signs | |||
| Bradykinesia, slowness, reduction in amplitude or arrest during thumb-finger tapping, hand movements (opening-closing, pronosupination), heel tapping, or arising from a chair | 0.2 | .14 | .01 |
| Resting tremor while the participant is relaxed, with both hands resting in the lap | 3.6 | .02 | .01 |
| Muscle rigidity, steady resistance throughout the entire range of movement at passive rotation of the neck and flexion and extension of elbows and knees | 2.4 | <.001 | .001 |
| Postural instability–retropulsion at the pull test | 20.0 | .02 | .01 |
| Superior functions | |||
| Frontal lobe function (Purdue Pegboard Test)–dominant hand | 9.1 | <.001 | <.001 |
Abbreviations: MMSE, Mini-Mental State Examination; NA, not applicable; SNAs, subtle neurological abnormalities; SPPB, Short Physical Performance Battery.
P values indicate significant differences in mean SPPB and mean MMSE between participants with and without each specific subtle neurological abnormality.
To obtain a final score of neurological damage, we calculated, for each participant, the number of SNAs (NSNA) detected with the neurological examination (defined as “subtle” because they neither related to definite neurological diseases nor were reported by the participants).
Covariates
Functional Status
Functional status was measured as self-reported disability in the activities of daily living (ADLs) and, objectively, using the Short Physical Performance Battery (SPPB) for lower extremities12 and the 6-minute walking test (6MWT).13 According to a modified version of the World Health Organization questionnaire,8,14 severity of disability was expressed as the number of basic and instrumental ADLs (BADLs and IADLs, respectively) in which the participant was dependent. The BADLs included 6 tasks: walking in the house, washing (hands and face), dressing oneself, toileting, transferring from bed to chair, and eating. The IADLs included 9 tasks: walking outside, walking 400mwithout rest, climbing stairs, shopping, bathing or showering, cooking, housekeeping (both light and heavy tasks), and trimming nails.
The modified version of the SPPB used in this study15 included 3 subtests, for balance (standing in5tasks of increasing difficulty), walking (4-m gait speed), and muscle strength (time to stand up 5 times from a chair). In the ICARe Dicomano study population, the SPPB summary score (range, 0–12, where 0 is worst performance and 12 is the best) predicted disability and death.16
Sustainable walking capacity was assessed with the 6MWT,13 which measures the distance walked in 6 minutes at the preferred velocity and represents a valid and reliable indicator of physical performance in elderly persons.17,18
Falls and Urinary Incontinence
Specific questionnaires were used to evaluate the number of falls in the preceding 12 months (0, 1–2, and ≥3 falls) and urge urinary incontinence (never, rare, or persistent).8
Cognitive and Emotional Status
Cognitive status was assessed with the MMSE9; depressive symptoms, with the Geriatric Depression Scale.19
Comorbidity
Comorbidity was measured with the Index of Coexistent Diseases, calculated as the sum of individual severity scores assigned to 14 chronic conditions, which were identified with structured diagnostic algorithms.16,20
Follow-up
In 1999, cognitive status, ADL disability, and falls were assessed using the same instruments as in 1995. Vital status and hospital admissions forCVEthrough2003wereascertained using administrative databases of the Tuscany region of Italy.
STATISTICAL ANALYSIS
Continuous variables are expressed as mean (SEM), and t tests were used to examine differences in MMSE and SPPB between participants with and those without each SNA. Differences in baseline characteristics across increasing NSNA were assessed with the χ2 test for trend for categorical variables and with analysis of variance (ANOVA) (or analysis of covariance [ANCOVA], to adjust for age and sex) for continuous variables. Multivariable logistic regression was used to evaluate the association between NSNA, taken as the independent variable in a cross-sectional design, and the prevalence of falls (0 vs ≥1) and urinary incontinence (never vs rare or persistent), adjusting for age and sex.
The ANOVA (or ANCOVA, when continuous variables were entered as covariates) was used to model the longitudinal associations between NSNA and declines in functional status (difference in the number of limitations in BADL and IADL from 1995 to 1999), cognitive status (change in MMSE from 1995 to 1999), and falls in the 12 months preceding the follow-up evaluation. Age, sex, comorbidity, SPPB, MMSE, and disability were entered in the models as covariates. Polynomial contrasts were applied to test for linear trends between contiguous categories of the NSNA (0, 1, 2, 3, and ≥4).
Cox proportional hazards regression models were used to evaluate NSNA as a predictor of death and hospitalization for CVE, adjusting for age, sex, comorbidity, SPPB, MMSE, BADL, IADL, and, limited to the outcome of CVE, for previous transient ischemic attacks. Because mortality rates seemed to sharply increase for NSNA values of 3 or higher, this was used as a cutoff point in survival analyses. Age and sex were forced in the model, whereas NSNA (≥3 vs <3) and the other baseline variables were entered at the first step and backward deleted thereafter (P = .05). Additional Cox models were constructed to examine the effect of adding NSNA to age, sex, comorbidity, and functional and cognitive status in the prediction of either death or CVE. For each outcome, the statistical significance of the difference between the −2 log likelihood of 2 models, 1 without and the other with NSNA, was tested against a distribution (P < .05) to determine the improvement of the models after the inclusion of NSNA.
A 2-tailed P <.05 was considered statistically significant. Analyses were performed using SPSS statistical software (version 12.0; SPSS Inc, Chicago, Illinois).
RESULTS
BASELINE EVALUATION
Of 864 eligible participants, 697 underwent baseline clinical evaluation.7 Exclusion of 118 participants with history of stroke, parkinsonism, dementia, or cognitive impairment, and of another 73 with incomplete data led to a final sample of 506 participants (mean [SD] age, 72.5[0.3] years; 57% were women). Participants excluded owing to incomplete data were older (77.5 [0.9] years; P < .001) than those included but did not differ in sex, BADL disability, severity of comorbidity, or level of physical performance (data not shown).
Of the 15 neurological signs considered, 5 were associated only with the SPPB, 1 only with the MMSE, and 5 with both tests (Table 1). The 2 most prevalent SNAs were reduced Achilles reflexes and postural instability. The greatest observed NSNA was 8 (mean [SD], 1.1[0.06]), in a possible range of 0 to 19 (considering that 4 signs could be bilateral). The number of participants with an NSNA of 0, 1, 2, 3, and 4 or more was 208, 131, 96, 38, and 33, respectively. The mean age increased across NSNA levels, whereas the proportion of women was similar (Table 2). Comorbidity increased with higher NSNA in unadjusted comparisons (P < .001; data not shown), although after adjusting for age and sex the linear trend was no longer significant (P = .08) (Table 2). After controlling for age and sex, an increasing NSNA was associated with a greater severity of IADL disability, more depressive symptoms, and worse cognitive and functional status (Table 2).
Table 2.
Demographic and Clinical Characteristics of 506 Older Participants, by NSNA
| Characteristic | NSNA, No. of Participantsa | P Value | ||||
|---|---|---|---|---|---|---|
| 0 (n = 208) |
1 (n = 131) |
2 (n = 96) |
3 (n = 38) |
<4 (n = 33) |
||
| Age, yb | 71.2 ± 0.4 | 71.2 ± 0.4 | 73.7 ± 0.5 | 73.9 ± 1.1 | 78.1 ± 1.2 | <.001 |
| Women, No. (%) | 113 (54.3) | 77 (58.8) | 54 (56.3) | 26 (68.9) | 17 (51.5) | .52 |
| ICEDc | 6.5 ± 0.2 | 7.1 ± 0.3 | 7.5 ± 0.3 | 7.5 ± 0.5 | 7.6 ± 0.5 | .08 |
| MMSE scorec | 26.9 ± 0.1 | 26.8 ± 0.2 | 26.7 ± 0.2 | 26.4 ± 0.3 | 25.6 ± 0.4 | .003 |
| GDS scorec | 6.6 ± 0.4 | 8.6 ± 0.6 | 8.0 ± 0.6 | 8.9 ± 1.0 | 8.9 ± 1.0 | .047 |
| SPPB scorec | 9.8 ± 0.1 | 9.5 ± 0.1 | 9.5 ± 0.1 | 8.9 ± 0.1 | 7.4 ± 0.1 | <.001 |
| 6-MWTc | 344 ± 91 | 326 ± 79 | 311 ± 91 | 306 ± 90 | 253 ± 93 | <.001 |
| BADLs, No. with dependencyb | 0.02 ± 0.02 | 0.05 ± 0.03 | 0.07 ± 0.04 | 0.06 ± 0.05 | 0.06 ± 0.06 | .50 |
| IADLs, No. with dependencyb | 0.3 ± 0.08 | 0.4 ± 0.10 | 0.6 ± 0.11 | 0.6 ± 0.16 | 1.4 ± 0.21 | <.001 |
Abbreviations: 6-MWT, walking distance during a 6-minute walking test; BADL, basic activity of daily living; IADL, instrumental activity of daily living; GDS, Geriatric Depression Scale; ICED, Index of Coexistent Diseases; MMSE, Mini-Mental State Examination; NSNA, number of subtle neurological abnormalities; SPPB, Short Physical Performance Battery score.
Data are given as mean ± SEM except where noted.
Unadjusted means from analysis of variance models.
Means adjusted for age and sex from analysis of covariance models.
In bivariate comparisons, increasing NSNA was also associated with more falls in the previous year (χ2 = 4.46; P value for trend = .04) and with more frequent urge urinary incontinence (χ2 = 5.88; P value for trend = .02). After adjusting for age and sex, these associations were no longer statistically significant (P value for trend = .12 for falls; P value for trend = .47 for urinary incontinence).
LONGITUDINAL ANALYSIS
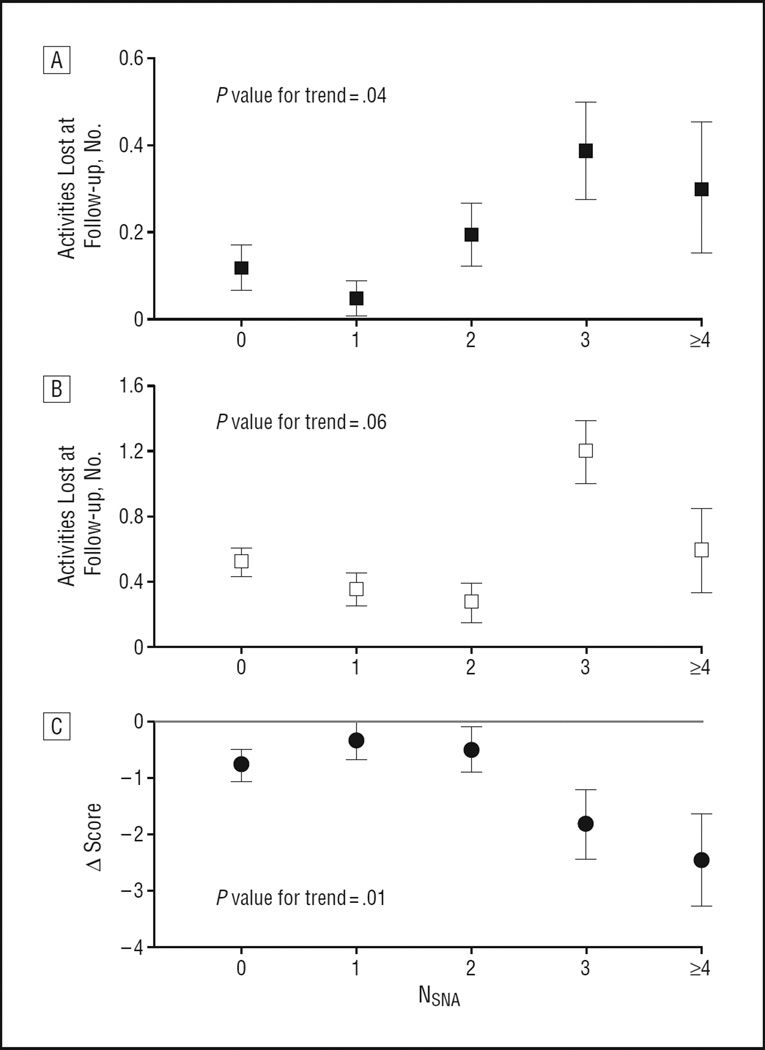
In 1999, 420 of the original 506 participants were reinterviewed. Increasing NSNA predicted 4-year functional and cognitive decline, expressed as the number of BADLs and IADLs lost, and the reduction in MMSE score between baseline and follow-up, respectively. The analyses were adjusted for age, sex, and baseline comorbidity, SPPB, MMSE, and BADL and IADL (Figure 1). In bivariate analysis, NSNA predicted the number of falls in the 12 months preceding the 1999 evaluation (P = .03). This result was no longer significant after adjustment for demographics and comorbidity (P = .13).
Figure 1.
Prediction of functional and cognitive decline from 1995 to 1999 in 420 older community-dwelling persons free of overt neurological diseases at baseline. All analyses adjusted for age, sex, baseline comorbidity, physical performance, cognitive functioning, and basic and instrumental activities of daily living (BADLs and IADLs, respectively). NSNA indicates the number of subtle neurological abnormalities; the error bars indicate the mean ± SE. A, BADL analysis; B, IADL analysis; C, Mini-Mental State Examination (MMSE) analysis.
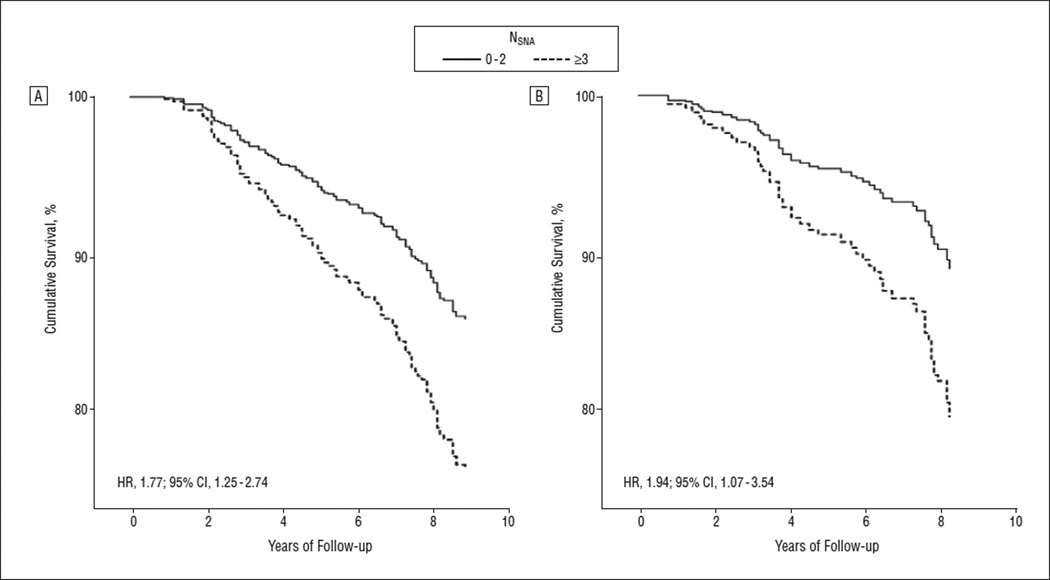
From 1995 through 2003, 113 participants died and 62 were hospitalized for CVE. The mortality rates were 22.6, 23.3, 23.9, 58.6, and 91.9 per 1000 person-years in participants with an NSNA of 0, 1, 2, 3, and 4 or greater, respectively. Compared with a value of less than 3, having an NSNA of 3 or greater was associated with a 2-fold greater risk of death (hazard ratio [HR], 1.77; 95% confidence interval [CI], 1.25–2.74, adjusted for age, sex, baseline comorbidity, SPPB, MMSE, and BADL and IADL) and CVE (HR, 1.94; 95% CI, 1.07–3.54, adjusted also for history of transient ischemic attacks) (Figure 2). When added to age, sex, comorbidity, cognitive and physical functions, and functional status in Cox models, NSNA improved the prediction of mortality (P = .006 for the likelihood ratio test), whereas the improvement of the model predicting CVE did not reach statistical significance (P = .08 for the likelihood ratio test).
Figure 2.
Cumulative survival and cumulative event-free survival during the follow-up period. Mortality (A) and incident hospitalization for cerebrovascular events (B) in 506 older community-dwelling persons free of overt neurological diseases, by number of subtle neurological abnormalities (NSNA) (<3 vs ≥3). Cox proportional hazard models were adjusted for age, sex, baseline comorbidity, cognitive and physical performance, and functional status at baseline (A and B) and history of transient ischemic attacks (B). CI indicates confidence interval; HR, hazard ratio.
COMMENT
In our sample of older community-dwelling persons free of overt neurological diseases, a standardized examination disclosed at least 1 SNA in more than half of the participants, with a prevalence increasing with age. A strong and independent association was demonstrated cross-sectionally among the NSNA and IADL disability, worse cognitive and physical performances, and more depressive symptoms. In longitudinal analyses, the NSNA predicted functional and cognitive decline over 4 years and mortality and CVE over 8 years.
Despite some differences in the neurological examination, our baseline results reinforce those obtained in a similar population-based, cross-sectional study of older persons.6 In both investigations, a mean number of approximately 1.1 SNAs was detected, with similar associations among the NSNA and physical performance and falls. Compared with the previous report, the present analysis has a more accurate control for covariates. Moreover, the longitudinal design provides support to the hypothesis of a causal relationship among the NSNA and functional and cognitive decline, death, and CVE. To our knowledge, this is the first study showing this association prospectively in a relatively large sample of unselected elderly persons. A previous report21 was limited in terms of number of participants (only 59), setting (clinical selection), and signs tested (tendon reflexes and vibration sense).
Performance-based tests for cognitive and physical functions and depressive symptoms are relevant clinical markers of cardiorespiratory, skeletal, muscular, and nervous functions and predict subsequent disability, CVE, and mortality in apparently healthy older adults.12,22–25 In the present study, SNA predicted cognitive and functional decline, CVE, and mortality independent of all these other measures of cognitive function, physical performance, and mood. Thus, a simple neurological examination seems to be an additional prognosticator of hard outcomes, particularly death, above and beyond other measures used in clinical practice. It is likely that the neurological examination might capture additional information about the integrity of the nervous system in apparently healthy older adults.
A previous study26 documented the relationship between specific SNA and diffuse brain white matter lesions using computed tomographic scans in older adults without dementia. Similarly, in CHS participants without a history of stroke, the number of neurological findings was independently associated with silent infarctions at brain magnetic resonance imaging.27 In turn, neuroimaging evidence of subclinical brain disease is associated with cognitive decline, executive dysfunction, depression, gait disorders, falls, urinary incontinence,7 poor physical performance,5,28 and incident ADL dependency.29 Subclinical brain disease also predicts CVE30 and mortality31 in elderly individuals. Thus, we speculate that our findings reflect the presence of diffuse white matter lesions or brain infarcts. However, although most of the neurological signs included in the NSNA could be an expression of damage in the central nervous system, some of them may be caused by peripheral neuropathy, a condition that can indeed contribute to physical performance decline in elderly persons.32
Our results suggest a nonlinear relationship between NSNA and the long-term hard outcomes of death and CVE, the risk of which dramatically increased in the presence of 3 or more SNAs. The absence of a dosage-response relationship does not exclude per se causation. Rather, a given level of neurological damage, up to a critical threshold, might be required before overt consequences become apparent. This threshold effect might reflect, for example, diffuse or confluent white matter hyperintensities rather than isolated lesions. Alternatively, it is possible that the associations we found were driven only by a limited number of specific neurological abnormalities, which more likely occurred when more SNAs were detected. Given the limited sample size, we could not discriminate between these alternatives; further studies are needed to confirm these findings and to highlight their pathogenesis and consequences.
Additional study limitations must be considered. First, our clinical summary score of neurological damage is probably less sensitive and specific than neuroimaging, and, therefore, it is of limited value in etiologic studies. Nevertheless, because of its low cost and ease of use, it might prove useful in clinical practice and in epidemiologic studies. Second, we were unable to prospectively investigate the association between SNAs and changes in physical performance because the SPPB and the 6MWT were not reassessed during the follow-up.
With these limitations in mind, our findings indicate that SNAs are frequent and must be systematically investigated in elderly persons. Subtle neurological abnormalities can be used to reveal otherwise undetected brain damage with relevant functional correlates and a clear prognostic value on survival and the development of CVE. Moreover, should the hypothesized association with cerebrovascular disease be demonstrated in future studies, the detection of multiple SNAs might prompt further cardiovascular investigation and eventually lead to a more intensive management of cardiovascular risk factors.
Our data support the hypothesis that SNAs in elderly individuals are a manifestation of early brain damage, a finding that may have important implications in research studies on the prevention of age-related cognitive and functional decline. Understanding the nature of dysfunctions underlying the decline in physical performance and disability contributes to planning specific preventive interventions. If our findings are confirmed by future research, NSNA might be also considered as a surrogate outcome for such intervention studies.
Acknowledgments
Funding/Support: This work was sponsored by the Italian Ministry of Scientific and Technological Research (National Special Project on Heart Failure), by the government of Tuscany, and by the Azienda Ospedaliero-Universitaria Careggi-Firenze, Italy.
Role of the Sponsor: The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr Inzitari had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the analysis. Study concept and design: Inzitari, Ferrucci, and Di Bari. Acquisition of data: Ferrucci, Pini, Masotti, Marchionni, and Di Bari. Analysis and interpretation of data: Inzitari, Pozzi, Ferrucci, Chiarantini, Rinaldi, Baccini, Pini, Masotti, Marchionni, and Di Bari. Drafting of the manuscript: Inzitari, Pozzi, Chiarantini, Rinaldi, Baccini, and Di Bari. Critical revision of the manuscript for important intellectual content: Inzitari, Ferrucci, Pini, Masotti, Marchionni, and Di Bari. Statistical analysis: Inzitari, Ferrucci, and Di Bari. Obtained funding: Masotti, Marchionni, and Di Bari. Administrative, technical, and material support: Inzitari, Pozzi, Chiarantini, and Di Bari. Study supervision: Di Bari.
Financial Disclosure: None reported.
Additional Contributions: Subashan Perera, PhD, and Mindi Spencer, PhD, from the University of Pittsburgh, Pittsburgh, Pennsylvania, provided advice in revising the manuscript.
REFERENCES
- 1.Enright PL, McBurnie MA, Bittner V, et al. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003;123(2):387–398. doi: 10.1378/chest.123.2.387. [DOI] [PubMed] [Google Scholar]
- 2.Newman AB, Haggerty CL, Kritchevsky SB, Nevitt MC, Simonsick EM. Walking performance and cardiovascular response: associations with age and morbidity: the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2003;58(8):715–720. doi: 10.1093/gerona/58.8.m715. [DOI] [PubMed] [Google Scholar]
- 3.Newman AB, Gottdiener JS, Mcburnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56(3):M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 4.Newman AB, Arnold AM, Naydeck BL, et al. “Successful aging”: effect of subclinical cardiovascular disease. Arch Intern Med. 2003;163(19):2315–2322. doi: 10.1001/archinte.163.19.2315. [DOI] [PubMed] [Google Scholar]
- 5.Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT, Jr, Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc. 2005;53(4):649–654. doi: 10.1111/j.1532-5415.2005.53214.x. [DOI] [PubMed] [Google Scholar]
- 6.Ferrucci L, Bandinelli S, Cavazzini C, et al. Neurological examination findings to predict limitations in mobility and falls in older persons without a history of neurological disease. Am J Med. 2004;116(12):807–815. doi: 10.1016/j.amjmed.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Kuo HK, Lipsitz LA. Cerebral white matter changes and geriatric syndromes: is there a link? J Gerontol A Biol Sci Med Sci. 2004;59(8):818–826. doi: 10.1093/gerona/59.8.m818. [DOI] [PubMed] [Google Scholar]
- 8.Di Bari M, Marchionni N, Ferrucci L, et al. Insufficienza Cardiaca negli Anziani Residenti a Dicomano. Heart failure in community-dwelling older persons: aims, design and adherence rate of the ICARe Dicomano project: an epidemiologic study. J Am Geriatr Soc. 1999;47(6):664–671. doi: 10.1111/j.1532-5415.1999.tb01587.x. [DOI] [PubMed] [Google Scholar]
- 9.Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry. 1983;40(7):812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- 10.Bandinelli S, Benvenuti E, Del L, et al. Measuring muscular strength of the lower limbs by hand-held dynamometer: a standard protocol. Aging (Milano) 1999;11(5):287–293. doi: 10.1007/BF03339802. [DOI] [PubMed] [Google Scholar]
- 11.Costa LD, Vaughan HG, Jr, Levita E, Farber N. Purdue Pegboard as a predictor of the presence and laterality of cerebral lesions. J Consult Psychol. 1963;27:133–137. doi: 10.1037/h0040737. [DOI] [PubMed] [Google Scholar]
- 12.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 14.Heikkinen E, Waters WE, Brzezinski ZJ. The Elderly in Eleven Countries: A Socio-medical Survey. Copenhagen, Denmark: World Health Organization Regional Office for Europe; 1983. Public Health in Europe, No. 21. [Google Scholar]
- 15.Di Bari M, Pozzi C, Cavallini MC, et al. The diagnosis of heart failure in the community: comparative validation of four sets of criteria in unselected older adults: the ICARe Dicomano Study. J Am Coll Cardiol. 2004;44(8):1601–1608. doi: 10.1016/j.jacc.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 16.Di Bari M, Virgillo A, Matteuzzi D, et al. Predictive validity of measures of comorbidity in older community dwellers: the Insufficienza Cardiaca negli Anziani Residenti a Dicomano Study. J Am Geriatr Soc. 2006;54(2):210–216. doi: 10.1111/j.1532-5415.2005.00572.x. [DOI] [PubMed] [Google Scholar]
- 17.Bean JF, Kiely DK, Leveille SG, et al. The 6-minute walk test in mobility-limited elders: what is being measured? J Gerontol A Biol Sci Med Sci. 2002;57(11):M751–M756. doi: 10.1093/gerona/57.11.m751. [DOI] [PubMed] [Google Scholar]
- 18.Harada ND, Chiu V, Stewart AL. Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil. 1999;80(7):837–841. doi: 10.1016/s0003-9993(99)90236-8. [DOI] [PubMed] [Google Scholar]
- 19.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 20.Greenfield S, Apolone G, McNeil BJ, Cleary PD. The importance of co-existent disease in the occurrence of postoperative complications and one-year recovery in patients undergoing total hip replacement: comorbidity and outcomes after hip replacement. Med Care. 1993;31(2):141–154. doi: 10.1097/00005650-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Baloh RW, Ying SH, Jacobson KM. A longitudinal study of gait and balance dysfunction in normal older people. Arch Neurol. 2003;60(6):835–839. doi: 10.1001/archneur.60.6.835. [DOI] [PubMed] [Google Scholar]
- 22.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 23.Inouye SK, Peduzzi PN, Robison JT, Hughes JS, Horwitz RI, Concato J. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279(15):1187–1193. doi: 10.1001/jama.279.15.1187. [DOI] [PubMed] [Google Scholar]
- 24.Penninx BW, Guralnik JM, Ferrucci L, Simonsick EM, Deeg DJ, Wallace RB. Depressive symptoms and physical decline in community-dwelling older persons. JAMA. 1998;279(21):1720–1726. doi: 10.1001/jama.279.21.1720. [DOI] [PubMed] [Google Scholar]
- 25.Whooley MA, Browner WS. Association between depressive symptoms and mortality in older women: study of Osteoporotic Fractures Research Group. Arch Intern Med. 1998;158(19):2129–2135. doi: 10.1001/archinte.158.19.2129. [DOI] [PubMed] [Google Scholar]
- 26.Steingart A, Hachinski VC, Lau C, et al. Cognitive and neurologic findings in demented patients with diffuse white matter lucencies on computed tomographic scan (leuko-araiosis) Arch Neurol. 1987;44(1):36–39. doi: 10.1001/archneur.1987.00520130028013. [DOI] [PubMed] [Google Scholar]
- 27.Price TR, Manolio TA, Kronmal RA, et al. Cardiovascular Health Study; CHS Collaborative Research Group. Silent brain infarction on magnetic resonance imaging and neurological abnormalities in community-dwelling older adults. Stroke. 1997;28(6):1158–1164. doi: 10.1161/01.str.28.6.1158. [DOI] [PubMed] [Google Scholar]
- 28.Wolfson L, Wei X, Hall CB, et al. Accrual of MRI white matter abnormalities in elderly with normal and impaired mobility. J Neurol Sci. 2005;232(1–2):23–27. doi: 10.1016/j.jns.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 29.Inzitari D, Simoni M, Pracucci G, et al. Risk of rapid global functional decline in elderly patients with severe cerebral age-related white matter changes: the LADIS study. Arch Intern Med. 2007;167(1):81–88. doi: 10.1001/archinte.167.1.81. [DOI] [PubMed] [Google Scholar]
- 30.Inzitari D. Leukoaraiosis: an independent risk factor for stroke? Stroke. 2003;34(8):2067–2071. doi: 10.1161/01.STR.0000080934.68280.82. [DOI] [PubMed] [Google Scholar]
- 31.Kuller LH, Arnold AM, Longstreth WT, Jr, et al. White matter grade and ventricular volume on brain MRI as markers of longevity in the cardiovascular health study. Neurobiol Aging. 2007;28(9):1307–1315. doi: 10.1016/j.neurobiolaging.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Inzitari M, Carlo A, Baldereschi M, et al. Risk and predictors of motor-performance decline in a normally functioning population-based sample of elderly subjects: the Italian Longitudinal Study on Aging. J Am Geriatr Soc. 2006;54(2):318–324. doi: 10.1111/j.1532-5415.2005.00584.x. [DOI] [PubMed] [Google Scholar]