Summary
Neuropilin-1 (NRP1) is a receptor for vascular endothelial growth factor (VEGF). A soluble isoform of Nrp1 (sNrp1) has not been described in the mouse. Our goal was to examine the expression of mouse sNrp1 during liver development and regeneration. sNrp1 was cloned from mouse liver. The expression of sNrp1 and VEGF was examined in mouse liver during postnatal development and regeneration using northern blot, western blot, in situ hybridization, and immunohistochemical analyses. HGF/NRP1 binding was examined in vitro. A novel 588-amino acid sNrp1 isoform was found to contain the ligand binding regions of Nrp1. The adult liver expressed more sNrp1 than full-length Nrp1. In vivo, hepatocytes constitutively expressed VEGF and sNrp1 in the quiescent state. sNrp1 was highly upregulated at P20, a time point coinciding with a plateau in liver and body weights. Following hepatectomy, endogenous levels of sNrp1 decreased during the rapid growth phase; and VEGF levels were highest just prior to and during the angiogenic phase. sNrp1 levels again rose 5-10 days post-hepatectomy, presumably to control regeneration. HGF protein bound NRP1 and binding was competed with sNRP1. We cloned a novel mouse sNrp1 isoform from liver and provide evidence that this endogenous angiogenesis inhibitor may regulate VEGF or HGF bioavailability during normal physiological growth and development as well as during liver regeneration.
Keywords: liver, hepatectomy, hepatocyte, endothelial cell, angiogenesis, anti-angiogenesis, neuropilin, VEGF, HGF, development
Introduction
All physiological development and growth is dependent on angiogenesis 1. Organ expansion requires new capillary growth to nourish the proliferating tissues. VEGF is a potent angiogenic factor capable of stimulating new vessels. Under hypoxia, VEGF is upregulated and recruits new vessels toward the hypoxic area. VEGF stimulates endothelial cell (EC) proliferation, migration and permeability 2. VEGF is also an endothelial survival factor made constitutively in the adult liver 3.
VEGF-A (hereafter, VEGF) binds two high affinity tyrosine-kinase transmembrane receptors, VEGFR1 and VEGFR2, with most downstream effects attributed to VEGFR2 signaling 4. VEGF also binds to transmembrane receptors called neuropilins (NRPs). NRP1 and NRP2 bind VEGF and act as co-receptors for VEGFR1 or -2 5. NRPs, which can also bind members of the class 3 semaphorin (SEMA3) family of chemorepulsive proteins, have short cytoplasmic domains with no known kinase activity 6. Sinusoidal EC of the liver express VEGFR1 and VEGFR2; and genetic ablation of VEGFR2 or inhibition of VEGFR2 activity with tyrosine kinase inhibitors impairs liver regeneration 7,8. Human liver expresses NRP1 mRNA 5; and recently, NRP1 protein was detected on human and rat liver EC 9,10. In contrast, the liver does not express Nrp2 11.
More recently, it has been suggested that NRP1 may also bind another heparan sulfate binding protein, hepatocyte growth factor (HGF) 12,13. HGF is secreted as an inactive, single polypeptide and cleaved by serine proteases into a 69-kD alpha chain and a 34-kD beta chain to produce the active, disulfide-linked, heterodimeric HGF protein 14. HGF binds the c-met tyrosine kinase receptor. Besides its mitogenic activity for hepatocytes 15, HGF is also a potent angiogenesis factor 16. NRP1-Fc was shown to bind to immobilized HGF in microtiter plates (solid-phase binding) 13. NRP1 is suggested to act as a co-receptor for HGF/c-met, similar to its function with VEGF/VEGFR 13. Others suggest that VEGF may signal via NRP1/c-met in tumor cells 17.
Various human NRP1 isoforms have been identified including the full-length receptor (NRP1) that encompasses 17 exons and an alternative receptor that lacks exon 16 18. Several smaller soluble isoforms lacking the MAM/c, transmembrane, and cytoplasmic domains of NRP1 were identified. Our group previously isolated two human sNRP1 isoforms, designated s12NRP1 and s11NRP1, indicating they contain intron 12- or intron 11-derived sequences 19,20. Other human sNRP1 isoforms were subsequently identified: sIIINRP1, which lacks exons 10-11, reads into intron 12 and is 551 aa; and sIVNRP1, which lacks exon 11, reads into intron 12 and is 609 aa 21.
The function of the endogenous sNRP1 isoforms is presumably to bind and sequester ligand 19,21. Specifically, sNRP1 binds VEGF165, competes binding of I125-VEGF165 to the surface of EC, and inhibits VEGF-induced phosphorylation of VEGFR2 on EC 19. Transfection of tumor cells with sNRP1 results in necrotic and hemorrhagic tumors 19. Transgenic mice expressing K14-sNRP1 display changes in the vascular architecture and impaired cutaneous vascular permeability 22. Similarly, sNRP1-Fc monomers inhibit vascular development in a mouse embryo explant model 23.
Although many angiogenesis inhibitors have been used as exogenous anti-angiogenic agents in pre-clinical models, the distribution and regulation of the endogenous proteins are poorly understood. Our previous in situ hybridization (ISH) analysis revealed that hepatocytes are the major source of sNRP1 in the body 19. To further study the endogenous soluble isoform, we cloned mouse sNrp1. This novel protein is similar to the human sNRP1 in its inclusive a1a2/b1b2 domain structures but differs in its splicing pattern. Herein, we report that sNrp1 is upregulated in the liver at postnatal day 20 and downregulated during liver regeneration. We demonstrate, for the first time, NRP1 binding to HGF on the surface of cells. Our data support the concept that sNrp1 may alter the VEGF or HGF bioavailability in vivo.
Materials and methods
Ethics Approval/Mice
C57Bl/6 mice were purchased from Charles River Laboratories. Partial hepatectomies (67%) were performed surgically as described 24. VEGFLacZ mice 3 were obtained from Dr. Andras Nagy (Toronto, Canada). Mice were maintained under pathogen-free conditions and procedures were performed in accordance with regulations of the National Institutes of Health (NIH), the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), and the Institutional Animal Care & Use Committee (IACUC) at Boston Children’s Hospital, Boston, MA (annual renewal approval on July 2, 2013).
sNrp1 cloning
Endogenous mouse sNrp1 cDNA was cloned by 3’ RACE 25. Briefly, a 580-bp PCR product was cloned using the TOPO-TA cloning kit (Invitrogen) from mouse liver. The 3’ cDNA clone sequence was identified as a truncated Nrp1 (sNrp1) cDNA. The full-length mouse sNrp1 was amplified from mouse liver cDNA by PCR using primers 5’ Nrp1a (ATGGAGAGGGGGCTGCCGTT) and 3’ sNrp1 (CAGATAAGTATGTGAGCCCAAGTGC).
IHC
Paraffin sections were de-waxed and rehydrated. Antigens were retrieved by HIER (pH 8). Endogenous peroxidase was quenched with 3% H2O2 in methanol. Sections were blocked in blocking reagent (PerkinElmer) and incubated (4°C) in anti-VEGF Ab1 (Lab Vision). Sections were incubated in biotin-conjugated anti-rabbit (Vector) and HRP-conjugated avidin (Vector). Staining was visualized with DAB (Vector) and counterstained with hematoxylin (Sigma).
X-gal staining
For detection of β-galactosidase activity, cryosections from VEGFLacZ mice were fixed in methanol and incubated (37ºC) in X-gal [1 mg/ml X-gal (5-bromo-4-chloro-3-indolyl-b-D-galactopyranoside) in DMSO; 5 mM K3Fe(CN)6; 5mM K4Fe(CN)6; 2 mM MgCl2 in PBS; pH 6.5]. Sections were counterstained with Eosin (Sigma).
ISH – oligonucleotide probes
ISH was used to detect human sNRP1 using an oligonucleotide probe and was performed as described 19. Sections were dewaxed, rehydrated, and digested with pepsin. Biotinylated probes included: anti-sense sNRP1-specific (TTCTGTCACATTTCGTATTTTATTTGATAC), sense, and poly-dT oligonucleotide. Following hybridization, samples were washed with SSC, incubated with alkaline phosphatase-labeled avidin (Dako), and developed in Fast Red (Research Genetics).
ISH – RNA probes
Sections were de-waxed, rehydrated, and digested with proteinase k; then, post-fixed, dehydrated, and air-dried. Anti-sense human NRP1-specific riboprobe and sense probe were synthesized from 750-bp 3’UTR-derived cDNA using a digoxigenin RNA-labeling kit (Roche). Anti-sense mouse sNrp1 riboprobe and sense probe were created similarly from 300-bp cDNA. Probes were hybridized and washed in SSC. Alkaline phosphatase-labeled anti-digoxigenin and BM Purple (Roche) were used to visualize the reaction.
Northern blot
Polyadenylated mRNA preparation from mouse organs, electrophoresis, blotting, and hybridization were performed as described 5,19. Probes were labeled with P32-dCTP (New England Nuclear) using RediPrime DNA labeling Kit (GE Healthcare). Probes were generated to mouse Nrp1 a/CUB, b/factorV/VIII, 3'UTR, and intron 10-derived (soluble specific 300 bp) domains.
Western blot
Lysates of mouse organs were used neat or precipitated with Con A Sepharose (GE Healthcare). Proteins were reduced, resolved with SDS-PAGE, transferred, and blocked with 5% milk. Blots were incubated with anti-mouse Nrp1 (N-terminal ) #AF566 (R&D Systems) or rabbit monoclonal anti-mouse Nrp1 (C-terminal) #2621-1 (Epitomics). Membranes were incubated in HRP-linked secondary antibody, washed, and exposed to ECL (PerkinElmer).
HGF binding
Porcine aortic endothelial cells (PAE) and PAE NRP1 cells were grown in Ham’s F12 media with 10% FBS as previously described 26. MDA-MB-231 breast cancer cells (positive control) were obtained from ATCC (HTB26) and cultured in DMEM with 10% FBS. Cells were grown on glass slides and incubated for 30 min on ice in 400 ng biotinylated human HGF (R&D Systems) followed by 30 min in avidin-FITC. Cells were washed twice in RDF1 buffer (R&D Systems) and fixed in 4% PFA. Alternately, cells were incubated for 30 min on ice in 200 ng biotinylated human HGF or 10:1 (2μg:200ng) mixture of human sNRP1:biotinylated human HGF (both in same volume), incubated in avidin-FITC, washed and fixed as above. Slides were mounted with fluorescent mount and coverslips.
Results
Human sNRP1 versus mouse sNrp1
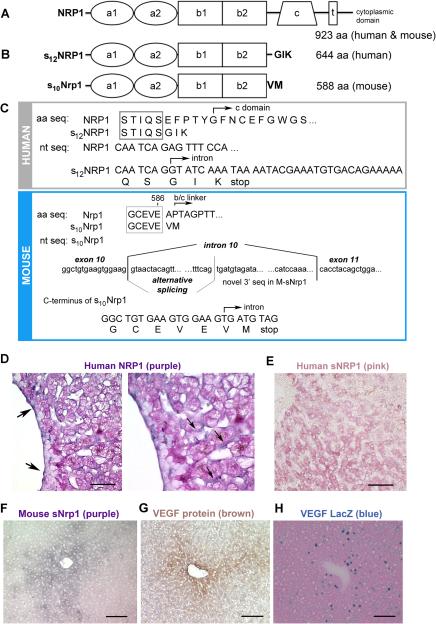
NRP1 is a transmembrane receptor consisting of 923 aa and an overall aa sequence identity of 93% between human and mouse NRP1. Human and mouse NRP1 have similar domain structures (Figure 1A). The largest and most abundant endogenous human soluble NRP1 isoform found in tissues and cells is s12NRP1, hereafter called sNRP1 (Figure 1B) 19. This 644 aa soluble receptor is created via alternative splicing and is identical to human NRP1 through the first 12 exons. Its sequence reads into intron 12 and results in an mRNA with a unique 28 bp sequence and a protein ending in 3 novel amino acids (GIK), not found in NRP1 (Figure 1C, upper panel). This endogenous sNRP1 protein contains all of the ligand binding domains of NRP1, yet lacks the c domain; therefore, it is likely a monomer.
Figure 1. Comparison of human and mouse Neuropilin 1 isoforms.
(A) Diagram of human and mouse NRP1 structure. (B) Upper panel: diagram of human s12NRP1. Lower panel: diagram of mouse s10Nrp1. (C) Upper panel: Human sNRP1 ends in 3 novel aa (GIK). Lower panel: The novel mouse sNrp1 reads through into intron 10, has an alternatively spliced region, and 300 unique nt encoding 2 novel aa (VM) and a stop codon. (D) ISH demonstrates human NRP1 (purple color) in EC (arrows) of sinusoids and veins. Eosin counterstain (pink color). Scale bar = 30 μm. Right panel is magnification of left panel. (E) ISH demonstrates human sNRP1 (pink color) in hepatocytes. Scale bar = 50 μm. (F) ISH demonstrates mouse sNrp1 (purple color) in hepatocytes surrounding central veins. (G) IHC for VEGF protein (brown color) in hepatocytes surrounding central veins. Hematoxylin counterstain (blue nuclei). (H) X-gal staining demonstrates LacZ signal (blue color) in hepatocytes. Eosin counterstain (pink color). Scale bars F-H = 0.1 mm.
In order to study the regulation and function of this soluble receptor in greater detail, we turned to a mouse model. We cloned mouse sNrp1 from mouse liver tissue using 3' RACE. The resulting product had a predicted size of 588 aa and contained the a1a2 and b1b2 ligand binding domains, but lacked all domains 3’ of b2 including the b/c-linker, c, transmembrane and cytoplasmic domains (Figure 1 B-C, lower panels).
sNrp1 expression in the liver
The distribution of NRP1 and sNRP1 was investigated in human liver sections by ISH. Although NRP1 and sNRP1 are alternatively spliced isoforms of the same gene, their patterns of distribution and localization within the liver were non-overlapping. The full-length NRP1 mRNA was expressed in EC of veins and sinusoids (Figure 1D, purple color) whereas, sNRP1 mRNA was highly expressed by hepatocytes (Figure 1E, pink color). The sense probe for both NRP1 and sNRP1 did not show any specific staining (data not shown).
ISH was used to localize mouse sNrp1 mRNA (Figure 1F, purple color). In both human liver and mouse liver, hepatocytes were shown to highly express the soluble isoform (compare Figure 1E and Figure 1F). Mouse sNrp1 was abundantly localized in hepatocytes surrounding central veins, and its expression was diminished in hepatocytes more distant from the veins (Figure 1F). All hepatocytes had intact mRNA as shown by positive staining using a polydT probe (data not shown).
VEGF expression in the liver
Since the sNrp1 protein contains domains capable of binding VEGF, we also examined the tissue distribution of VEGF protein in sections of adult mouse liver (Figure 1G). Using IHC, we determined that the VEGF protein was expressed by mouse hepatocytes in a pattern similar to that of sNrp1 (compare Figure 1F and G). VEGF is a secreted protein that can bind heparan sulfate proteoglycans and may be sequestered in the extracellular matrix. Therefore, to confirm that the hepatocytes were indeed the source of the VEGF protein, adult mouse liver sections from transgenic VEGFLacZ mice 3 were stained with x-gal. Blue nuclei (due to the nuclear translocation signal of the β-gal gene) were detected only in hepatocytes and not in EC (Figure 1H).
Comparing the expression of Nrp1 and sNrp1 in mouse organs
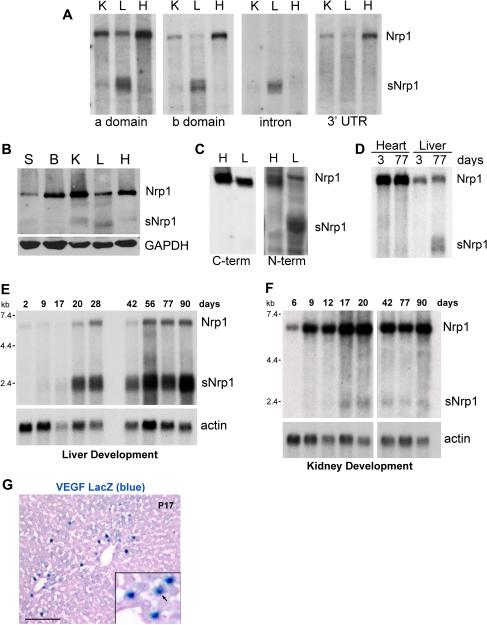
We previously compared the expression of full-length Nrp1 mRNA to sNrp1 mRNA in various adult mouse organs by northern blot analysis and found that kidney, lung, and brain primarily expressed Nrp1 mRNA, whereas the liver expressed a smaller mRNA band (presumed to be sNrp1) in addition to Nrp1 22. To determine whether the 2 kb band was indeed the same soluble isoform that we had cloned, a northern blot containing mRNA from adult mouse kidney, liver, and heart was serially incubated with probes to the a domain, b domain, intron 10-derived sequence, and Nrp1 3’ untranslated region (UTR). As expected, the probes to the a and b domains detected both the 7 kb Nrp1 mRNA and the 2 kb sNrp1 mRNA (Figure 2A, left panels). The intronic probe only hybridized to the 2 kb sNrp1 band whereas the 3’UTR probe identified only the receptor isoform (Figure 2A, right panels). In fact, the liver was the only organ that expressed more soluble than full-length receptor.
Figure 2. sNrp1 mRNA and protein is expressed in the adult liver.
(A) Northern blot. sNrp1 is detected with a, b, and intron probes in the liver (L); while Nrp1 is detected with a, b, and 3'UTR probes in kidney (K), liver, and heart (H). (B) Western blot of mouse organs. GAPDH = loading control. Skin (S), Brain (B). (C) Western blot. N-terminal anti-Nrp1 detects Nrp1 in adult liver and heart and sNrp1 in liver. C-terminal anti-Nrp1 detects Nrp1 in heart and liver. (D) Northern blot. Nrp1 mRNA in adult and neonatal (P3) heart and liver. sNrp1 in the adult liver. (E) sNrp1 is upregulated in P20 livers and remains elevated. (F) sNrp1 is slightly upregulated in P17-20 kidneys. Nrp1 is more abundant than sNrp1 in kidney. Actin = loading control. (G) VEGF (blue nuclei) in P17 VEGFLacZ mouse liver. Eosin counterstain. Scale bar = 0.1 mm.
To determine whether sNrp1 protein was expressed in vivo, western blot analysis was performed. Whole cell protein lysates were isolated from various adult mouse organs and all organs were found to express the full-length Nrp1 receptor protein (130 kD) (Figure 2B). Kidney, liver and heart also expressed a smaller protein (sNrp1) detected with neuropilin 1 antibody. To more clearly identify this smaller isoform, protein lysates were concentrated with ConA-sepharose beads (which bind glycoproteins), run on SDS-PAGE, and the western blot was serially incubated with anti-Nrp1 antibodies specific to either the N terminus or the C terminus. Full-length Nrp1 protein (130 kD) was detected in both liver and heart tissue with both antibodies; the soluble isoform was only detected with N-terminal-specific antibodies (Figure 2C).
Developmental regulation of sNrp1
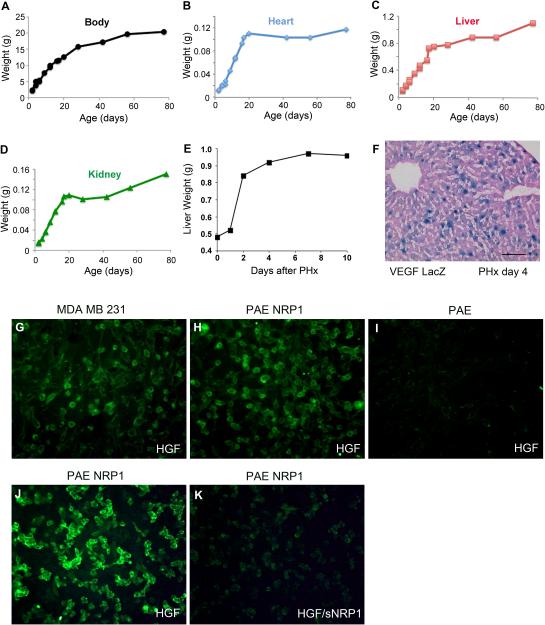
Comparing neonatal mouse liver and heart to their mother’s (11 week old) liver and heart revealed a dramatic difference in the expression of sNrp1 mRNA. sNrp1 was not detected in P3 livers by northern blot analysis (Figure 2D). To further examine this apparent age-dependent regulation of sNrp1, livers from mice ranging from 2 to 90 days postnatal were examined. Levels of mouse sNrp1 were dramatically elevated at postnatal day 20 (Figure 2E). sNrp1 expression in kidney was upregulated at similar time points (day 17-20) but to a lesser extent (Figure 2F). To determine whether VEGF was expressed in the liver at P17, VEGFLacZ mice were examined by x-gal staining. β-gal expressing hepatocytes confirmed that VEGF was produced at this time point (Figure 2G). P20 also coincided with a time point at which mouse body weight and organ weight (heart, liver, and kidney) began to slow or plateau in the mice used for our study (Figure 3A-D).
Figure 3. NRP1 binds HGF.
The weight of the overall mouse (A), heart (B), liver (C), and kidney (D) were measured at various time points after birth. All weights plateaued around 20 days. (E) Graph of liver weight following PHx. (F) VEGF (blue nuclei) is expressed in many hepatocytes 4 days following PHx. Eosin counterstain. Scale bar = 0.1 mm. (G-K) HGF binding assay using biotinylated human HGF and avidin-FITC. HGF binds MDAMB231 cells (G) and PAE NRP1 cells (H, J), but not PAE cells (I). sNRP1 competes the binding of HGF to NRP1 (K). Note green color is on the cell membrane.
HGF binding to NRP1
The slowing of liver growth around postnatal day 20 (Figure 3C) may be do to the direct inhibition of hepatocyte proliferation (via the inhibition of HGF) or the inhibition of angiogenesis (via the inhibition of VEGF or HGF). HGF has been shown to bind to immobilized NRP1-Fc on plastic but not on cells 13. PAE cells transfected with human NRP1 bound HGF protein in binding assays (Figure 3H), while untransfected PAE cells (negative control) did not (Figure 3I). PAE cells may express porcine c-met but cannot bind human HGF due to its species-specific nature 27. Human breast cancer cells (MDAMB231), which express c-met and NRP1, served as a positive control (Figure 3G). Additionally, human sNRP1 (purified a1a2b1b2 domain) 28 inhibited HGF binding to cells when pre-incubated with HGF (Figure 3J-K).
sNrp1 and VEGF expression during liver regeneration
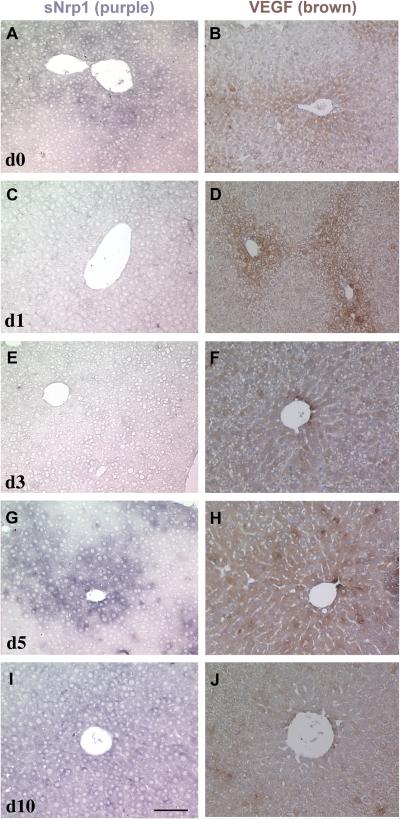
The expression of sNrp1 and VEGF was next examined in a model of liver angiogenesis and regeneration. ISH for sNrp1 and IHC for VEGF was conducted in sections of adult mouse liver at day 0,1, 3, 5, and 10 following 67% PHx. VEGF protein (brown color) was detected in hepatocytes surrounding veins in normal liver (day 0), and this pattern did not change until day 3 following PHx when VEGF was found in virtually all hepatocytes (Figure 4B, D, F). VEGF protein expression remained high at day 4-5 but was decreased dramatically by day 10 (Figure 4H, J; Figure 3F). Peri-venous hepatocytes also expressed sNrp1 (purple) in normal livers (day 0) (Figure 4A). sNrp1 was dramatically downregulated or nearly undetectable on day 1 after PHx (Figure 4C) and remained low until day 5 following PHx (Figure 4G). The pattern of sNrp1 on day 5 was similar to normal liver although the signal intensity was slightly stronger. At 10 days after PHx, sNrp1 was increased above normal levels and its expression was detected in nearly all hepatocytes (Figure 4I). The increase in levels of sNrp1 at day 5 coincided with a plateau in liver weight (Figure 3E).
Figure 4. Expression and regulation of sNrp1 and VEGF following PHx (days 0-10).
ISH for sNrp1 (A, C, E, G, I) and IHC for VEGF (B, D, F, H, J) after 67% PHx. Day 0 (A, B), day 1 (C, D), day 3 (E, F), day 5 (G, H), day 10 (I, J) after PHx. By day 3, sNrp1 is downregulated and VEGF is upregulated.
Discussion
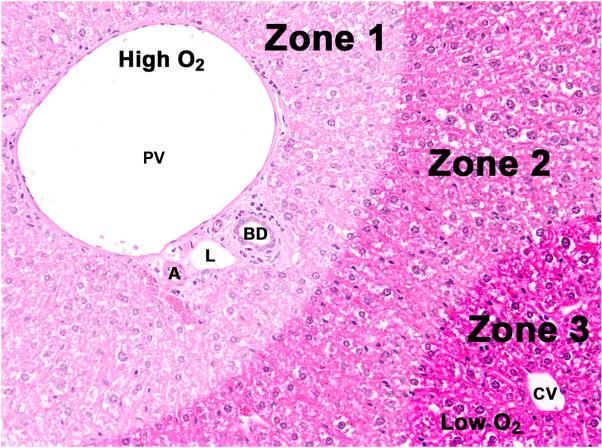
The liver is a unique organ with specialized epithelial cells (hepatocytes) and sinusoidal capillaries. Most hepatocytes are in direct contact with a sinusoid. Blood flow in the liver originates from both the hepatic artery and the portal vein and travels through sinusoids to collect in the central veins, which coalesce into the hepatic vein. Oxygenation is highest in zone 1 of the lobule near the triad and lowest in zone 3 near the central vein (Figure 5). This oxygen distribution pattern may explain why VEGF is highest in zone 3, which is relatively hypoxic. Although VEGF is a potent inducer of angiogenesis, its constitutive expression by hepatocytes in the normal adult liver, in the absence of angiogenesis, may point to a role as a survival factor, an association that has been documented for fenestrated EC in other tissues 3.
Figure 5. Diagram of liver.
Oxygenation (O2) is highest in zone 1 near the portal vein (PV), artery (A), and bile duct (BD) (triad) and lowest near the central vein (CV). L, lymphatic. Image was false-colored to depict the relative zones.
Homeostasis is based upon a balance between stimulatory and inhibitory molecules. Among the VEGF family of receptors, VEGFR1 has a soluble isoform called sFlt-1 29. sFlt-1 is associated with pre-eclampsia, a pathology of pregnancy characterized by hypertension and proteinuria 30. Endogenous sVEGFR2 was cloned from mouse cornea where it functions as a monomer to bind VEGF-A and -C and inhibit lymphangiogenesis 31.
A dichotomy exists in the liver such that NRP1 is found only in EC and sNRP1 only in hepatocytes. Our EC-specific results of NRP1 are in agreement with IHC previously reported 9,10. NRP1 is typically found in arteries, not veins, during development 32; yet in the adult liver, NRP1 is expressed on arteries, veins, and capillaries 9. There is currently no antibody that can specifically identify only sNRP1 (and not NRP1) as it includes only 3 novel amino acids at its C-terminus.
Similar to human liver, the adult mouse liver expressed Nrp1 mRNA and another band at approximately 2 kb (Figure 2A). Using 3’ RACE with mouse liver cDNA, a novel isoform of mouse Nrp1 was identified. The sNrp1 transcript differed in sequence from any of the previously reported soluble isoforms. sNrp1 (also called s10Nrp1 according to the previous convention) is generated from a read-through into intron 10 and contained 300 bp at the 3’ end not found in Nrp1. An intronic probe confirmed that the 2 kb band found in adult mouse liver corresponded to the cloned sequence. Kidney and heart tissue weakly express sNrp1. The sNrp1 protein contains only two novel amino acids. Importantly, sNrp1 contains both VEGF and SEMA3 ligand-binding domains as confirmed by northern blotting with probes specific to each domain. A second soluble Nrp1 isoform is likely as probes to the b domain clearly showed two bands in the region near 2 kb (Figure 2A).
Both membrane-bound and soluble Nrp1 proteins were detected in mouse liver using antibodies specific to the N-terminus. As expected, antibodies to Nrp1 C-terminus detected only 130-kD receptor protein. NRP1 protein expression in various tissues and tumors has been examined using IHC 33,34. In light of our identification of the soluble Nrp isoform, such results must be interpreted with caution when antibodies specific to extracellular epitopes have been used, as this antibody would detect both NRP1 isoforms. Thus, the interpretation of such results may be the opposite when dealing with soluble (antiangiogenic) versus receptor (angiogenic) isoforms.
The expression of sNrp1 during postnatal mouse development was evaluated. A dramatic upregulation in sNrp1 was observed in the liver at P20. The mechanism for this upregulation is unclear, but it did correlate with a plateau in the weight of the developing liver. HGF is a potent mitogen and survival factor in the liver 35. HGF mRNA levels increased in the rat postnatally at day 5 and peaked at day 14 36. We examined HGF ligand binding to NRP1 on the surface of cells in vitro. HGF binding assays on cells are complicated by the fact that most cells also express the c-met tyrosine kinase receptor. However, HGF is species-specific, therefore human HGF binds only to human cells or human receptors 27. We exploited this fact and used PAE cells as our background or negative control. Even though PAE cells may express porcine c-met, they did not bind human biotinylated HGF. Human NRP1, on the cell surface, did bind HGF (Figure 3H). Importantly, sNRP1 could sequester HGF and compete HGF from binding to the cell surface. Thus, we speculate that as the liver reaches its full size, sNrp1 increases to curb growth and angiogenesis by neutralizing HGF and/or VEGF and halting organ growth.
Liver regeneration can be a model to study angiogenesis 35,37. The process following 67% partial hepatectomy involves massive hepatocyte proliferation (days 1-3), followed by EC proliferation and sprouting (days 3-5), with the total liver mass returning to approximately normal size within 7-10 days 24,38,39. In our experiments, liver weight nearly doubled in the first 2 days. VEGF protein was elevated in hepatocytes 3-5 days after PHx. These data are consistent with other studies of VEGF expression during regeneration 38,39. IHC localization of VEGF suggested alterations in its localization during regeneration with VEGF initially found in peri-venous hepatocytes (day 0-1), then in nearly all hepatocytes (days 3-5), and finally widely distributed at low levels (day 10).
Like VEGF, sNrp1 was constitutively expressed around central veins in normal liver. Unlike its ligand, sNrp1 was dramatically downregulated immediately following PHx, remained low until day five and elevated above normal levels by 10 days (Figure 4). Taken together, our studies suggest that sNrp1 levels in the liver are low during times of active angiogenesis such as during the growth phase of the neonate (P1-20) or during the proliferative stage following hepatectomy (d1-5). sNrp1 may act as an endogenous angiogenesis inhibitor as it is expressed at higher levels at times when growth is slowed, which would be expected to be associated with reduced VEGF signaling.
EGF dramatically upregulates NRP1 and sNRP1 in epithelial cells and tumor cells 40. Therefore, it is surprising that mouse sNrp1, which shares the same promoter as Nrp1, would be downregulated in the liver one day after hepatectomy when TGFα/EGF levels are known to be elevated 41. This suggests that another factor may be responsible for inhibiting sNrp1 or that EGFR ligands may not regulate Nrp1 in hepatocytes.
We have shown that human sNRP1 binds VEGF, inhibits VEGF binding to EC, and inhibits the activation of VEGFR2 by VEGF on EC 19. Since mouse sNrp1 contains the same ligand binding domains as human sNRP1, it is reasonable to assume that sNrp1 in the mouse liver also sequesters VEGF, thereby acting to inhibit VEGF-induced neovascularization. sNrp1 is expected to bind and sequester not only VEGF but also other Nrp1 ligands including PlGF, VEGF-B, and SEMA3A. PlGF, an angiogenic ligand, is upregulated after PHx 42; whereas SEMA3A, an anti-angiogenic ligand, is significantly downregulated during the first 6 days following PHx 10.
Although knocking-out sNRP1 in the liver would be useful to confirm our strong correlative results shown here, such efforts are likely to lead to ambiguous outcomes. As mentioned, Nrp1 and sNrp1 both arise from the same gene—therefore, knocking-out sNrp1 would also deplete the Nrp1 receptor in the liver. Additionally, Nrp1 KO mice die at E12.5-13.5 with severe vascular defects 43.
Endogenous angiogenesis inhibitors have been shown to be expressed by specific tissues. For example, both sFlt1 and sVEGFR2 are expressed in the cornea where they functions to maintain the tissue in an avascular state 31,44. By comparison, we conclude that sNrp1 is an endogenous angiogenesis inhibitor secreted by hepatocytes to sequester its ligands and control the balance of angiogenesis. sNrp1 protein (or specifically peptides including the b domain region) may be useful for the management of a variety of angiogenesis-related diseases including arthritis, various proliferative retinopathies, hemangiomas, and cancer.
Acknowledgements
Michael Gagnon cloned human sNRP1 19. The authors would like to thank Ricardo Sanchez, H.T.L., for histological sections and consultations, and Kristin Johnson for graphic design and figures.
Sources of funding
This study was supported by the National Institutes of Health (USA) CA118732 and CA155728 to DRB; CA148633 to DP, CA037392 to MK, EY015435 to PAD.
Footnotes
Conflict of interest
The authors state that there are no conflicts of interest to disclose.
References
- 1.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 2.Brown LF, Detmar M, Claffey K, et al. Vascular permeability factor/vascular endothelial growth factor: a multifunctional angiogenic cytokine. Exs. 1997;79:233–269. doi: 10.1007/978-3-0348-9006-9_10. [DOI] [PubMed] [Google Scholar]
- 3.Maharaj AS, Saint-Geniez M, Maldonado AE, et al. Vascular endothelial growth factor localization in the adult. Am J Pathol. 2006;168:639–648. doi: 10.2353/ajpath.2006.050834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Soker S, Takashima S, Miao HQ, et al. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 6.Bielenberg DR, Klagsbrun M. Targeting endothelial and tumor cells with semaphorins. Cancer Metastasis Rev. 2007;26:421–431. doi: 10.1007/s10555-007-9097-4. [DOI] [PubMed] [Google Scholar]
- 7.Ding BS, Nolan DJ, Butler JM, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hora C, Romanque P, Dufour JF. Effect of sorafenib on murine liver regeneration. Hepatology. 2011;53:577–586. doi: 10.1002/hep.24037. [DOI] [PubMed] [Google Scholar]
- 9.Berge M, Allanic D, Bonnin P, et al. Neuropilin-1 is upregulated in hepatocellular carcinoma and contributes to tumour growth and vascular remodelling. J Hepatol. 2011;55:866–875. doi: 10.1016/j.jhep.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 10.Fu LKT, Iwabuchi K, Ichinose S, Yanagida M, Ogawa H, Watanabe S, Maruyama T, Suyama M, Takamori K. Interplay of neuropilin-1 and semaphorin 3A after partial hepatectomy in rats. World J Gastroenterol. 2012;18:5034–5041. doi: 10.3748/wjg.v18.i36.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bielenberg DR, Seth A, Shimizu A, et al. Increased Smooth Muscle Contractility in Mice Deficient for Neuropilin 2. Am J Pathol. 2012;181:548–559. doi: 10.1016/j.ajpath.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.West DC, Rees CG, Duchesne L, et al. Interactions of multiple heparin binding growth factors with neuropilin-1 and potentiation of the activity of fibroblast growth factor-2. The Journal of biological chemistry. 2005;280:13457–13464. doi: 10.1074/jbc.M410924200. [DOI] [PubMed] [Google Scholar]
- 13.Sulpice E, Plouet J, Berge M, et al. Neuropilin-1 and neuropilin-2 act as coreceptors, potentiating proangiogenic activity. Blood. 2008;111:2036–2045. doi: 10.1182/blood-2007-04-084269. [DOI] [PubMed] [Google Scholar]
- 14.Mizuno K, Nakamura T. Molecular characteristics of HGF and the gene, and its biochemical aspects. Exs. 1993;65:1–29. [PubMed] [Google Scholar]
- 15.Matsumoto K, Nakamura T. Hepatocyte growth factor: molecular structure and implications for a central role in liver regeneration. Journal of gastroenterology and hepatology. 1991;6:509–519. doi: 10.1111/j.1440-1746.1991.tb00897.x. [DOI] [PubMed] [Google Scholar]
- 16.Aoki M, Morishita R, Taniyama Y, Kaneda Y, Ogihara T. Therapeutic angiogenesis induced by hepatocyte growth factor: potential gene therapy for ischemic diseases. Journal of atherosclerosis and thrombosis. 2000;7:71–76. doi: 10.5551/jat1994.7.71. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S, et al. Vascular endothelial growth factor regulates myeloid cell leukemia-1 expression through neuropilin-1-dependent activation of c-MET signaling in human prostate cancer cells. Molecular cancer. 2010;9:9. doi: 10.1186/1476-4598-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tao Q, Spring SC, Terman BI. Characterization of a new alternatively spliced neuropilin-1 isoform. Angiogenesis. 2003;6:39–45. doi: 10.1023/a:1025884628155. [DOI] [PubMed] [Google Scholar]
- 19.Gagnon ML, Bielenberg DR, Gechtman Z, et al. Identification of a natural soluble neuropilin-1 that binds vascular endothelial growth factor: In vivo expression and antitumor activity. Proc Natl Acad Sci U S A. 2000;97:2573–2578. doi: 10.1073/pnas.040337597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossignol M, Gagnon ML, Klagsbrun M. Genomic organization of human neuropilin-1 and neuropilin-2 genes: identification and distribution of splice variants and soluble isoforms. Genomics. 2000;70:211–222. doi: 10.1006/geno.2000.6381. [DOI] [PubMed] [Google Scholar]
- 21.Cackowski FC, Xu L, Hu B, et al. Identification of two novel alternatively spliced Neuropilin-1 isoforms. Genomics. 2004;84:82–94. doi: 10.1016/j.ygeno.2004.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mamluk R, Klagsbrun M, Detmar M, et al. Soluble neuropilin targeted to the skin inhibits vascular permeability. Angiogenesis. 2005;8:217–227. doi: 10.1007/s10456-005-9009-6. [DOI] [PubMed] [Google Scholar]
- 23.Yamada Y, Takakura N, Yasue H, et al. Exogenous clustered neuropilin 1 enhances vasculogenesis and angiogenesis. Blood. 2001;97:1671–1678. doi: 10.1182/blood.v97.6.1671. [DOI] [PubMed] [Google Scholar]
- 24.Greene AK, Puder M. Partial hepatectomy in the mouse: technique and perioperative management. J Invest Surg. 2003;16:99–102. [PubMed] [Google Scholar]
- 25.Bates D, Taylor GI, Minichiello J, et al. Neurovascular congruence results from a shared patterning mechanism that utilizes Semaphorin3A and Neuropilin-1. Dev Biol. 2003;255:77–98. doi: 10.1016/s0012-1606(02)00045-3. [DOI] [PubMed] [Google Scholar]
- 26.Bielenberg DR, Hida Y, Shimizu A, et al. Semaphorin 3F, a chemorepulsant for endothelial cells, induces a poorly vascularized, encapsulated, nonmetastatic tumor phenotype. The Journal of clinical investigation. 2004;114:1260–1271. doi: 10.1172/JCI21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francone TD, Landmann RG, Chen CT, et al. Novel xenograft model expressing human hepatocyte growth factor shows ligand-dependent growth of c-Met-expressing tumors. Molecular cancer therapeutics. 2007;6:1460–1466. doi: 10.1158/1535-7163.MCT-06-0466. [DOI] [PubMed] [Google Scholar]
- 28.Mamluk R, Gechtman Z, Kutcher ME, et al. Neuropilin-1 binds vascular endothelial growth factor 165, placenta growth factor-2, and heparin via its b1b2 domain. The Journal of biological chemistry. 2002;277:24818–24825. doi: 10.1074/jbc.M200730200. [DOI] [PubMed] [Google Scholar]
- 29.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baumwell S, Karumanchi SA. Pre-eclampsia: clinical manifestations and molecular mechanisms. Nephron Clin Pract. 2007;106:c72–81. doi: 10.1159/000101801. [DOI] [PubMed] [Google Scholar]
- 31.Albuquerque RJ, Hayashi T, Cho WG, et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat Med. 2009;15:1023–1030. doi: 10.1038/nm.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herzog Y, Kalcheim C, Kahane N, et al. Differential expression of neuropilin-1 and neuropilin-2 in arteries and veins. Mech Dev. 2001;109:115–119. doi: 10.1016/s0925-4773(01)00518-4. [DOI] [PubMed] [Google Scholar]
- 33.Jubb AM, Strickland LA, Liu SD, et al. Neuropilin-1 expression in cancer and development. J Pathol. 2012;226:50–60. doi: 10.1002/path.2989. [DOI] [PubMed] [Google Scholar]
- 34.Hansel DE, Wilentz RE, Yeo CJ, et al. Expression of neuropilin-1 in high-grade dysplasia, invasive cancer, and metastases of the human gastrointestinal tract. Am J Surg Pathol. 2004;28:347–356. doi: 10.1097/00000478-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Jia C. Advances in the regulation of liver regeneration. Expert review of gastroenterology & hepatology. 2011;5:105–121. doi: 10.1586/egh.10.87. [DOI] [PubMed] [Google Scholar]
- 36.Kagoshima M, Kinoshita T, Matsumoto K, Nakamura T. Developmental changes in hepatocyte growth factor mRNA and its receptor in rat liver, kidney and lung. European journal of biochemistry / FEBS. 1992;210:375–380. doi: 10.1111/j.1432-1033.1992.tb17431.x. [DOI] [PubMed] [Google Scholar]
- 37.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida D, Akahoshi T, Kawanaka H, et al. Roles of vascular endothelial growth factor and endothelial nitric oxide synthase during revascularization and regeneration after partial hepatectomy in a rat model. Surg Today. 2011;41:1622–1629. doi: 10.1007/s00595-010-4484-9. [DOI] [PubMed] [Google Scholar]
- 39.Taniguchi E, Sakisaka S, Matsuo K, et al. Expression and role of vascular endothelial growth factor in liver regeneration after partial hepatectomy in rats. J Histochem Cytochem. 2001;49:121–130. doi: 10.1177/002215540104900112. [DOI] [PubMed] [Google Scholar]
- 40.Parikh AA, Fan F, Liu WB, et al. Neuropilin-1 in human colon cancer: expression, regulation, and role in induction of angiogenesis. Am J Pathol. 2004;164:2139–2151. doi: 10.1016/S0002-9440(10)63772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stromblad S, Andersson G. The coupling between transforming growth factor-alpha and the epidermal growth factor receptor during rat liver regeneration. Exp Cell Res. 1993;204:321–328. doi: 10.1006/excr.1993.1039. [DOI] [PubMed] [Google Scholar]
- 42.Vanheule E, Fan YD, Van Huysse J, et al. Expression of placental growth factor in regenerating livers after partial hepatectomy in the rat. Eur J Gastroenterol Hepatol. 2011;23:66–75. doi: 10.1097/MEG.0b013e328341ef35. [DOI] [PubMed] [Google Scholar]
- 43.Kawasaki TKT, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999;126:4895–4902. doi: 10.1242/dev.126.21.4895. [DOI] [PubMed] [Google Scholar]
- 44.Sela S, Itin A, Natanson-Yaron S, et al. A novel human-specific soluble vascular endothelial growth factor receptor 1: cell-type-specific splicing and implications to vascular endothelial growth factor homeostasis and preeclampsia. Circ Res. 2008;102:1566–1574. doi: 10.1161/CIRCRESAHA.108.171504. [DOI] [PubMed] [Google Scholar]