Abstract
Kidney transplantation remains limited by toxicities of calcineurin inhibitors (CNIs) and steroids. Belatacept is a less toxic CNI alternative, but existing regimens rely on steroids and have higher rejection rates. Experimentally, donor bone marrow and sirolimus promote belatacept’s efficacy. To investigate a belatacept-based regimen without CNIs or steroids, we transplanted recipients of live donor kidneys using alemtuzumab induction, monthly belatacept and daily sirolimus. Patients were randomized 1:1 to receive unfractionated donor bone marrow. After 1 year, patients were allowed to wean from sirolimus. Patients were followed clinically and with surveillance biopsies. Twenty patients were transplanted, all successfully. Mean creatinine (eGFR) was 1.10±0.07mg/dl (89±3.56ml/min) and 1.13±0.07 mg/dl (and 88±3.48 ml/min) at 12- and 36-months, respectively. Excellent results were achieved irrespective of bone marrow infusion. Ten patients elected oral immunosuppressant weaning, seven of whom were maintained rejection-free on monotherapy belatacept. Those failing to wean were successfully maintained on belatacept-based regimens supplemented by oral immunosuppression. Seven patients declined immunosuppressant weaning and three patients were denied weaning for associated medical conditions; all remained rejection-free. Belatacept and sirolimus effectively prevent kidney allograft rejection without CNIs or steroids when used following alemtuzumab induction. Selected, immunologically low-risk patients can be maintained solely on once monthly intravenous belatacept.
Keywords: alemtuzumab, belatacept, costimulation, immunosuppressive regimens, minimization/withdrawal, sirolimus
Introduction
Transplantation effectively treats most causes of end stage renal disease; but its clear benefits are tempered by a continuous requirement for drug-induced immunosuppression [1–3]. Calcineurin inhibitors (CNIs) are the centerpiece maintenance immunosuppressant, used in over 94% of transplant recipients [1]. They effectively prevent graft rejection and are approved for use with glucocorticosteroids. However, both agents impact broad metabolic pathways causing significant chronic side effects. Nevertheless, transplant recipients typically remain dependent on the daily use of these drugs, often in combination with other immunosuppressive agents, for life [1].
Belatacept is a fusion protein administered by monthly infusion that has recently been approved for use as a CNI alternative [3–8]. It blocks the CD28-B7 costimulation pathway, a highly specific effect that avoids most chronic side effects [5–9]. Furthermore, extensive evidence suggests that costimulation blockade (CoB) fosters immune processes that reduce dependence on maintenance immunosuppression over time [10–12]. Unfortunately, belatacept is less effective than CNIs in preventing early [5,7], but not late [13, 14], acute rejection, and its approved use remains dependent on chronic steroids [15]. The mechanisms of early CoB resistant rejection (CoBRR) have been shown to relate to the action of short-lived, alloreactive memory T cells that have differentiated beyond the requirements for CD28-B7 costimulation [16–19]. While these cells are controlled by CNIs, CNIs are known to antagonize the mechanisms by which CoB facilitates long-term allograft acceptance [10–12, 20]. Conversely, mechanistic target of rapamycin inhibitors (mTORi) have been shown to promote the effects of CoB, particularly when donor antigen is abundant or even augmented through donor hematopoietic cell infusion [10–12]. Lymphocyte depletion has been shown to substantially reduce the risk of early acute rejection [21,22], and in particular to allow for patients to be transplanted with mTORi without CNIs or steroids [23–25]. We therefore reasoned that transient T cell depletion and treatment with mTORi would satisfy the requirements for control of CoBRR without inhibiting the progressive, salutary effects of CoB, and in doing so, promote a regimen that could lead to once monthly immune therapy devoid of the side effects of CNIs and steroids. Herein, we demonstrate that CoB can be used effectively to prevent kidney allograft rejection without CNIs or maintenance steroids, and that with time, selected patients can avoid rejection solely on a once monthly infusion of belatacept.
Methods
Patients and General Medical Care
Adult, Epstein-Barr Virus (EBV) seropositive recipients of a first, HLA non-identical, live donor kidney allograft were prospectively consented to an Institutional Review Board approved (IRB00005064) clinical trial (NCT00565773). Patients with a history of immunosuppression within one year prior to transplant, prior lymphodepletion, known immune deficiency, coagulopathy, malignancy or glomerulopathy with potential for recurrence were excluded from enrollment. Patients receiving grafts from CMV seropositive donors were required to be CMV seropositive, but CMV seronegative recipients were enrolled if their donor also was CMV seronegative. Transplantation was performed using standard surgical techniques. Peri-operative surgical and medical management, excepting the immunosuppressive strategy described below, were consistent with standard transplant care. Allograft function and other relevant parameters were assessed in keeping with standard clinical practice augmented by the studies detailed below.
Immune Management
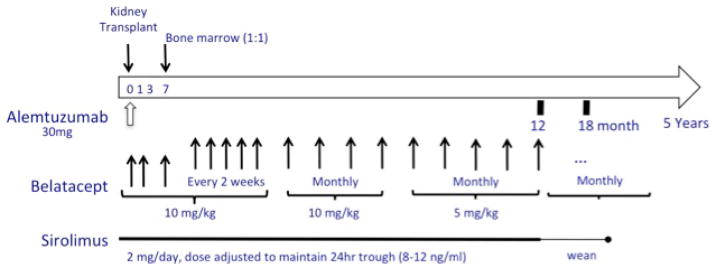
Immune therapy (Figure 1A) began intra-operatively with a single 500mg intravenous dose of methylprednisolone, 50mg of diphenhydramine intravenously and 650mg of acetaminophen rectally. One hour after premedication, a single 30mg dose of alemtuzumab was given intravenously over 3 hours.
Figure 1.
(A) Scheme of the immune therapy and monitoring for the trial. (B) Trial conduct with regard to weaning of oral immunosuppressive medication.
Intravenous belatacept (10mg/kg) was started on the first postoperative day, repeated on days 3, 7, 14, every 2 weeks for 4 additional doses, and monthly through month 6; thereafter belatacept was given at a dose of 5mg/kg monthly. Belatacept was intentionally given remote from alemtuzumab to avoid co-infusion of biologics and facilitate the ability to attribute any adverse events that occurred.
Oral sirolimus (2mg/day) was started on postoperative day 1 and dose adjusted to maintain 24-hour trough levels of 8–12ng/ml. If the drug was not weaned, a lower trough of 3–8ng/ml was targeted after 1 year. Sirolimus trough targets were dropped by up to 50% if side effects referable to this drug (e.g. mouth ulcers, arthralgias, anemia) occurred. If dose reduction was insufficient to control these effects, patients were converted to mycophenolate mofetil (MMF; 1000mg twice daily) covered by an oral steroid taper (20mg to off over 4 weeks).
One year after transplantation, patients without evidence of rejection on biopsy or donor-specific antibody (DSA) were offered the choice of continuing sirolimus (or MMF), or weaning from oral immunosuppression completely (Figure 1B). Weaning from oral immunosuppression required additional informed consent.
Bone Marrow Infusion
Bone marrow infusion was performed based on formal randomization controlled by the Emory investigational pharmacy service. Donors were not mandated to donate marrow and randomization was performed regardless of their desires in this regard. In the single incident that a donor refused marrow procurement, the recipient proceeded in the study without receiving marrow.
Donor bone marrow was procured from the iliac crest at the time of kidney donation. A dose of 1×108 nucleated cells/kg of recipient weight was targeted; the volume needed to achieve this result was estimated from a cell count on the product performed after approximately 200 ml had been collected. The marrow was collected and filtered using a sealed system, and anticoagulated in heparinized media. Following the procurement, the product was cryopreserved in a solution containing albumin and 10% dimethyl sulfoxide, and stored at ≤140°C. On postoperative day 7, the product was thawed and infused at a dose of 1×108 nucleated cells/kg using standard techniques [26] in the outpatient clinic after the scheduled belatacept infusion. Recipients were pre-medicated before marrow infusion with diphenhydramine, hydrocortisone and ondansetron. Patients were monitored for complications for 4 hours after the infusion. Based on the absence of myelospecific conditioning, chimerism was not anticipated, but was assessed using a PCR-based assay for short-tandem repeat polymorphisms weekly for 1 month and monthly for 3 months.
Surveillance Biopsy and Treatment of Rejection
Patients underwent surveillance renal biopsies at 6 months, and annually for 3 years. Biopsies were required as a condition of sirolimus weaning. Biopsies were scored by the clinical pathology service as described [27]. For the purposes of determining eligibility for weaning, any rejection, including borderline changes, contraindicated weaning. Suspected rejection was verified by biopsy and treated based on the standard of care.
Viral Monitoring
Patients were monitored monthly for BK virus viremia for one year, then every 3 months or as clinically indicated using a polymerase chain reaction (PCR)-based assay specific for the BK Virus VP1 gene performed by the clinical lab. Any level detected was considered positive (levels < 1070 copies/ml were reported as “low positive”). Patients were monitored every 3 months, or more frequently if clinically indicated, for EBV and cytomegalovirus (CMV) viremia using PCR-based assays specific for the EBNA-1 gene or the Major Immediate Early gene, respectively. For both targets, positive results with values <300 copies/ml were reported as “low positive.”
Alloantibody Monitoring
Recipients were required to be DSA-free and have a calculated panel reactive antibody (PRA) ≤20% as a condition of enrollment. All samples were screened for the presence of alloantibody using a flow cytometric, microparticle-based screening assay (FlowPRA®; One Lambda, Inc, Canoga Park, CA) as described [28] every 3 months. Positive samples were studied to define individual specificities to HLA-A, B, C, DRB1, DQA, DQB1, DRB3, 4, 5 and/or DPB1 antigens using a Luminex™-based assay. Antibody positivity was defined as a baseline normalized median fluorescence intensity (MFI) >1000 [29].
Flow Cytometry
Absolute lymphocyte count (ALC) and lymphocyte phenotype were evaluated weekly for 4 weeks, monthly for 12 months, and every 6 months thereafter, as previously described [30]. The fluorochrome labeled monoclonal antibodies anti-CD3-Alexa 700, anti-CD3-PerCP, anti-CD4-V450, anti-CD4-PE, anti-CD8-APC Fluor780, anti-CD8-PacBlue, anti-CD16-FITC, anti-CD20 PECy7, anti-CD45-PerCP, anti-CD45RA-APC, anti-CD56-APC, anti-Ki67-FITC and anti-CD197 PECy7 (BD Biosciences, Franklin Lakes, NJ), and anti-CD45RA-QDOT655 (Invitrogen, Carlsbad, CA) were used. ALC was determined using Trucount beads (BD Biosciences). Cells were interrogated using an LRSII Flow Cytometer (BD Biosciences), and data were analyzed using FlowJo software (Tree Star, San Carlos, CA). Memory and regulatory phenotypes were defined as previously described [31–34].
Results
Administration and tolerance of the therapy
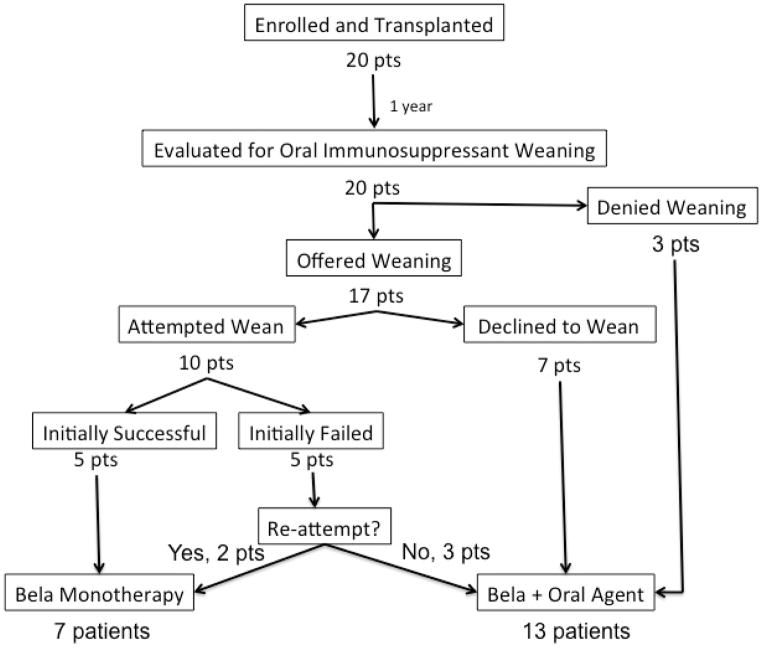
Twenty patients received a transplant. Their median age was 45 years (range 20–69). Twelve were male; sixteen were Caucasian and four were African American. The donor-recipient pairs were mostly unrelated (13 unrelated, 7 related); HLA identical pairs were specifically excluded. The mean HLA mismatch was 3.6/6 with mean mismatches of 1.2, 1.5 and 1.1 for HLA A, B and DR loci, respectively. The clinical status of all patients is depicted in Figure 2. Graft function was immediate in all patients. All tolerated the transplant, induction therapy, and belatacept infusions well. Seventeen patients tolerated sirolimus therapy. Three patients (pts. 2, 11 and 12) were switched to MMF at months 2, 1 and 5 for arthalgias, mouth ulcers and lymphocele, respectively. No result segregated with age, diagnosis or mismatch status. Nine patients received donor bone marrow, all without adverse event. Chimerism was not anticipated and was not detected in any patient.
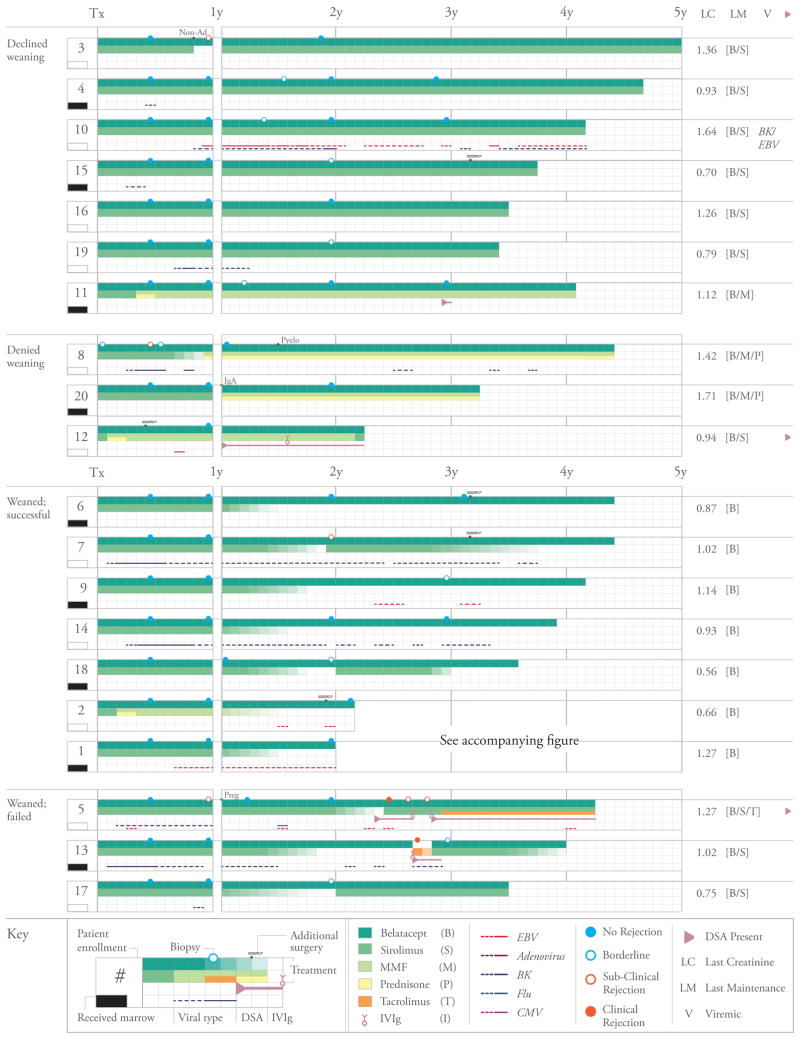
Figure 2.
The clinical course of all patients group with relation to their participation and success of oral immunosuppressant weaning. Patient numbers refer to their order of enrollment into the trial (not necessarily the order in which they were transplanted). Shown for each patient is the immunosuppressive therapy received by month, receipt of bone marrow, deviation from the base regimen, duration and type of all episodes of viremia, results of all biopsies, any occurrence of DSA, and creatinine, maintenance therapy and presence of DSA or viremia at last follow-up. Dotted lines refer to viremia defined as low positive (present at the limit of the clinical assay) whereas solid lines refer to viremia occurring in the quantifiable range. The scalpel icon indicates surgical procedures performed after transplantation (e.g. native nephrectomy or hernia repair). See pictorial key for details
Efficacy of the initial therapy in controlling alloimmunity
The base regimen was successful in preventing clinical allograft rejection (Figure 2). No patient experienced clinical, biopsy proven, acute rejection, nor did any patient develop DSA within the first year. Nineteen patients had no functional concern for rejection within the first year. One patient (pt. 8) experienced a rise in serum creatinine (1.0 to 1.3mg/dl) in the second post-operative week that prompted a biopsy revealing an interstitial macrophage infiltrate [24] that did not reach Banff criteria for rejection. He was treated with a 3-day course of methylprednisolone and returned to belatacept and sirolimus therapy.
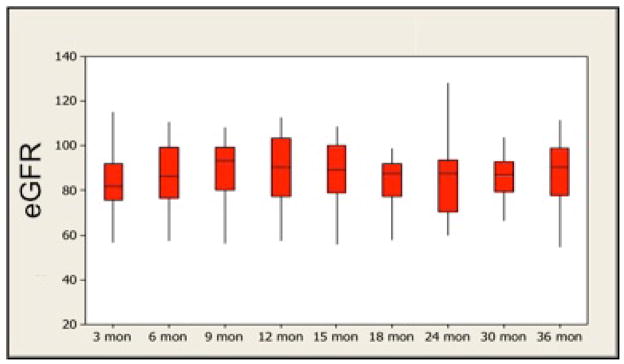
Subclinical rejection was detected on surveillance biopsy in the first year in 3 patients: one (also pt. 8) was found to have subclinical Banff grade 1B rejection at 6 months for which he received a 5-day oral prednisone taper. Two additional patients (pts. 3 and 5) were found to have subclinical Banff grade 1A and 1B rejections, respectively, 12 months post transplant; patient 3 admitted to non-adherence with oral sirolimus in the prior 2 months. For these two patients treatment was limited to having their sirolimus reinstituted (patient 3) and their sirolimus trough reduction delayed (both patients). All patients found to have subclinical rejection maintained stable function, remained on protocol therapy, and resolved their histological findings on subsequent surveillance biopsies. All three episodes of sub-clinical rejection found on protocol biopsy were detected in patients who did not receive donor bone marrow; however, this secondary endpoint difference was not statistically significant (P=0.22, Fisher’s exact test). When considering all patients regardless of subsequent weaning, allograft function remained excellent. Mean serum creatinine was 1.10±0.07mg/dl and 1.13±0.07mg/dl, and eGFR (Nankivell formula)[35] was 89±3.56ml/min and 88±3.48 ml/min at 12 and 36 months, respectively (Figure 3).
Figure 3.
Estimated glomerular filtration (eGFR; calculated using the Nankivell formula)[35] rate for all patients through 36 months of follow-up.
Effects of the initial therapy on protective immunity
There were no readmissions for opportunistic infection and no malignancies. However, protective immunity, which was intensively monitored, was measurably impaired. Ten patients were found to have transient BK viremia within the first post transplant year (Figure 2). SV40-staining was detected on protocol biopsy in 2 patients, one each at 6 and 12 months, without detectable changes in renal function. All BK viremia resolved with expectant management or transient reduction in oral immunosuppression. Low-level, transient EBV viremia was detected in 5 patients (Figure 2) and resolved spontaneously. CMV viremia was detected in one patient (pt. 12) and resolved following an increase in prophylactic valganciclovir from 450mg daily to 900mg twice daily for 1 month. One patient (pt. 5) contracted and resolved influenza post transplant.
One patient (pt. 8) developed pyelonephritis. This patient had been discontinued from sirolimus and converted to infliximab, MMF and prednisone to treat a flair of ulcerative colitis. His pyelonephritis resolved, his allograft function remains excellent (creatinine 1.37mg/dl), and his colitis has been in remission for 3 years.
Results of Oral Immunosuppressant Weaning
At one year, 7 patients were offered the opportunity to wean their oral immunosuppression, but declined to do so (Declined Weaning, Figure 2). These patients were satisfied with their immune management consisting of a single oral drug plus monthly belatacept and/or did not want to be encumbered by the increased surveillance required during the weaning period. All remained rejection-free without DSA (follow-up 38–58 months). Six of these patients were on sirolimus and one had converted to MMF. Their serum creatinine was 1.15±0.11mg/dl at 36 months post transplant.
Three patients were denied weaning for associated medical conditions (Denied Weaning, Figure 2) including ulcerative colitis (pt. 8), histological evidence of IgA nephropathy on surveillance biopsy (pt. 20), and low level DSA (DQ specific antibodies with MFIs of 2062–3092; pt. 12), respectively. The trial was written so that immunosuppression reduction would not be offered if the attending transplant physicians or the principal investigator felt that the risk/benefit ratio of immunosuppressive reduction was potentially adverse, and in these cases there was consensus that the medical conditions did not favor reduced immunosuppression. All remained rejection-free, two on belatacept, MMF and prednisone (IgA and colitis), and one on belatacept and MMF (DSA) with excellent function.
Five patients (pts. 1, 2, 6, 9, 14) were successfully weaned to belatacept monotherapy without incident. They maintained stable, excellent allograft function for over 1 year without DSA on monotherapy belatacept. Follow-up biopsies showed no rejection and their clinical courses on belatacept were unremarkable.
Five patients initially failed sirolimus weaning. Two failures were due to subclinical, borderline changes on surveillance biopsy prompting reinstitution of oral sirolimus, 1mg/day. One of these patients (pt. 18) discontinued their sirolimus after 1 year of observation and moved to monotherapy belatacept. One (pt. 17) elected to stay on sirolimus. Three weaning failures experienced biopsy-proven rejection. One clinical rejection (Banff 2A with DSA) occurred 10 months after weaning and was treated with a methylprednisolone taper and IVIg. The rejection and DSA resolved, the patient (pt. 13) has remained clinically stable for 11 months (creatinine 0.95mg/dl), and is again weaning sirolimus. One, (pt. 7), was found to have a sub clinical Banff 1A rejection without DSA post weaning and was treated with methylprednisolone. One year afterwards this patient elected again to wean, and achieved belatacept monotherapy. The remaining wean failure (pt. 5), a clinical Banff 2A rejection with DSA, was treated with methylprednisolone and IVIg. The renal function improved, but the DSA persisted despite IVIg and rituximab. This patient had tacrolimus added to her regimen and maintained stable graft function therafter (creatinine 1.22mg/dl); a low level (MFI 2264) DSA to Bw6 persists. Of note, this patient became pregnant 12 months post transplant and underwent an elective abortion, both potentially sensitizing events. Thus, of the 5 weaning failures, all achieved stable allograft function and two subsequently re-weaned to belatacept monotherapy. In all, 7 patients weaned to belatacept monotherapy (Weaned; Successful, Figure 2) and 3 patients failed weaning (Weaned; Failed, Figure 2).
Two of the patients who were successfully weaned to monotherapy belatacept were followed for 1 year on belatacept, confirmed by biopsy to be rejection free and by HLA testing to be DSA free, and then reconsented to discontinue belatacept (Figure 4). The first patient (pt. 1), a bone marrow recipient, had an unremarkable clinical course off all immunosuppression for 7 months, at which time, coincident with an upper respiratory infection, he developed a Banff 2A cellular rejection with DSA prompting treatment with rabbit antithymocyte globulin, IVIg and plasmapheresis. He was converted to a tacrolimus regimen as dictated by the protocol. His rejection and DSA resolved, and he has maintained stable allograft function at a baseline creatinine of 1.40mg/dl for over 2 years. The second patient (pt. 2) remained off all immunosuppression for 4 months, at which time she developed low-level DSA without biopsy findings. She was treated with IVIg and returned to belatacept and MMF (sirolimus intolerant). Her DSA resolved. After 1 year she was weaned back to monotherapy belatacept and has remained clinically stable (creatinine 0.68mg/dl) without DSA for over 1 year.
Figure 4.
The clinical course of two patients who were weaned off of all immunosuppressive therapy. Shown for both patients is the immunosuppressive therapy received by month, receipt of bone marrow, deviation from the base regimen, duration and type of all episodes of viremia, results of all biopsies, any occurrence of DSA, and creatinine, maintenance therapy and presence of DSA or viremia at last follow-up beginning from the 1 year time point post transplantation (initiation of initial weaning). Dotted lines refer to viremia defined as low positive (present at the limit of the clinical assay) whereas solid lines refer to viremia occurring in the quantifiable range. The scalpel icon indicates a surgical procedure (in this case total knee replacement) performed after transplantation. See pictorial key for details.
Lymphocyte Repopulation
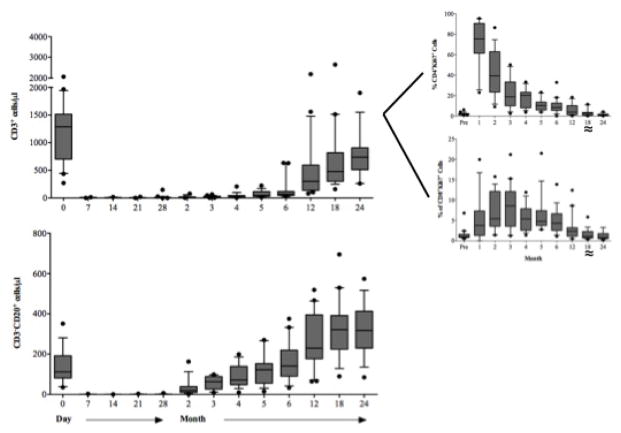
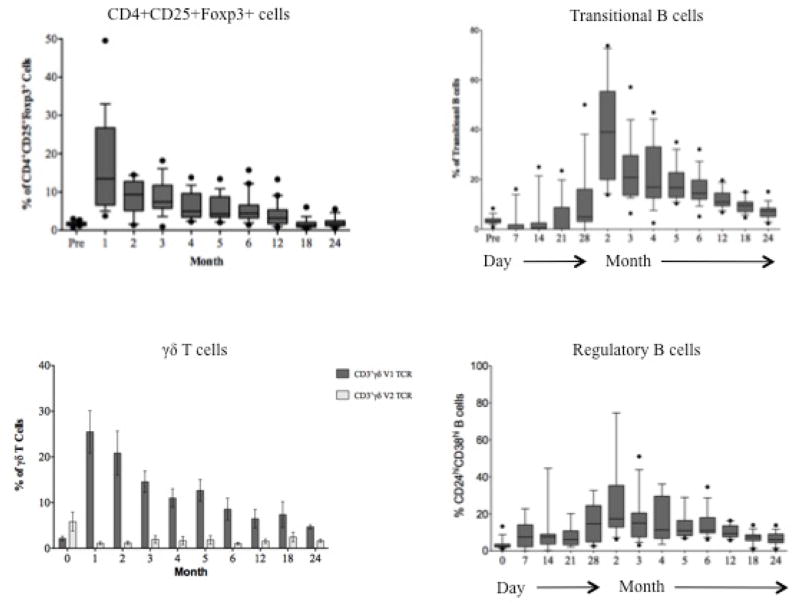
T and B cells were depleted peripherally following alemtuzumab induction (Figure 5A). Depletion and repopulation kinetics varied as a function of cell surface phenotype. As has been described [30], T cells expressing a terminally differentiated (CCR7−, CD45RA+) [31] effector phenotype enriched for CD28−CD8+ [34] T cells predominated for approximately 3 months. Homeostatic activation and repopulation occurred with a time course similar to that described [24], with ALCs returning to pre-transplant levels in 12 months. Homeostatic T cell activation (CD69+Ki67+) persisted 6 months beyond ALC normalization (Figure 5A). Several cell populations with potential regulatory function were disproportionately increased commensurate with the period of homeostatic activation (Figure 5B) including CD4+CD25+Foxp3+ T regulatory cells, CD3+Vδ1+ T cells (with an increased Vδ1+/Vδ2+ ratio), transitional (CD27−CD38+IgDhi) B cells, and B regulatory cells (CD20 hiCD24hiCD38hiIgM hi) [32,33].
Figure 5.
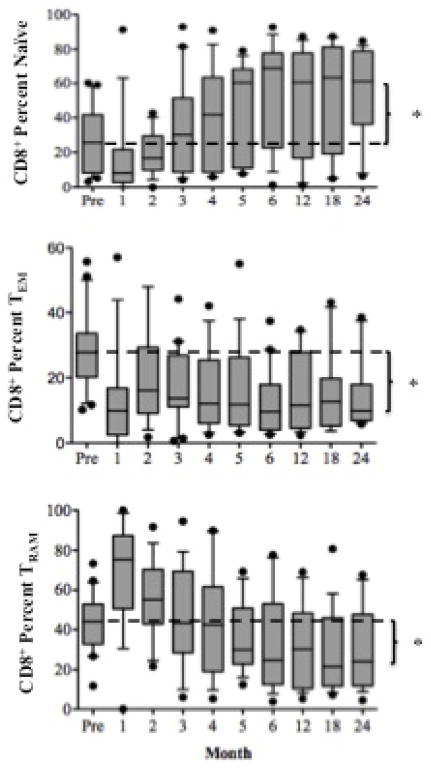
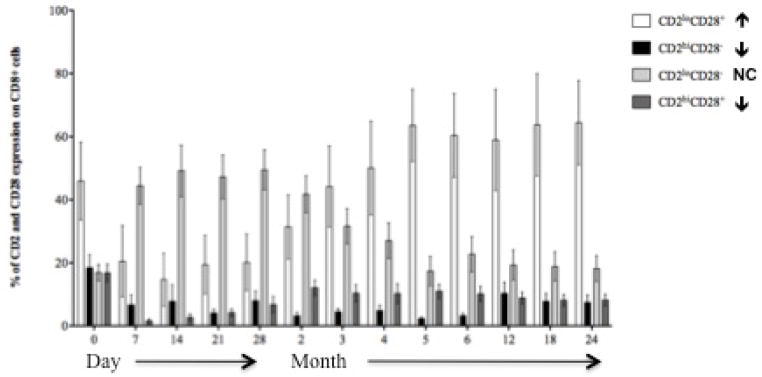
Lymphocyte repopulation and changes in lymphocyte phenotype over the course of the trial. (A) T cell (CD3+ cells) and B cell (CD3−, CD20+) absolute numbers prior to and following transplantation showing a return of B cells prior to T cells. T cells are further segregated by CD4 and CD8 (graphs, upper right) by Ki67 expression showing the duration and magnitude of homeostatic proliferation post depletion. Homeostatic activation reaches baseline at month 18 (≈). (B) Cells with potential regulatory function occurring during the period of homeostatic population following transplantation. Shown are surges of Regulatory T cells (CD4+CD25+FoxP3+), gamma-delta T cells (showing a reconstitution with an inverted Vδ1/Vδ2 ratio), Transitional B cells and B regulator cells corresponding to the period of homeostatic activation shown in 5a. (C) CD8+ T cell memory phenotypes showing reconstitution of the CD8+ T cell repertoire with significantly higher percentages of naïve and significantly lower percentages of effector memory and terminal effectors following the end of homeostatic repopulation. * p ≤0.05. (D) Reconstitution of the T cell repertoire with regard to CD28 expression, showing an increase in nonactivated (CD2lo) CD28 expressing cells (p=0.0394), and a decrease in activated CD2hi) CD28− and activated CD28+ cells (p=0.034 and 0.018, respectively). There is no change (NC) seen in nonactivated CD28− cells.
CD4+ T cells reconstituted a repertoire phenotypically indistinguishable from that seen pre-transplant (not shown). However, CD8+ T cells repopulated enriched for naïve phenotype T cells expressing CD28 (Figures 5C and D). There was a proportionate decrease in CD28− effector or memory T cells. B cells repopulated more rapidly than T cells, reaching, and then exceeding, baseline levels by 6 and 18 months, respectively. Repopulated B cells were predominantly naïve, with significantly reduced numbers of memory B cells. Thus, the final lymphocyte repertoire was characterized by more naïve T and B cells, fewer differentiated T and B effectors, and increased expression of CD28, the receptor pathway targeted by belatacept.
Discussion
We have shown that kidney transplantation can be performed using a belatacept-based regimen without reliance on maintenance CNIs or steroids. Additionally, we have shown that when adjuvant agents are chosen to exploit the mechanisms known to facilitate experimental CoB-based therapies, immunosuppressive requirements decrease with time, allowing selected, immunologically low-risk patients to remain rejection-free solely on monthly low dose [7] belatacept. Several aspects of this experience deserve comment.
First, the base regimen, one that can be deployed without pre-conditioning using only agents approved for clinical use, clearly controls rejection in live donor recipients. This is demonstrated by the success of all patients in the first year, and emphasized by the prolonged clinical stability of those patients who, thereafter, did not wean from their oral immunosuppression. Thus, we believe the base regimen to be a logistically feasible immunosuppressive approach worthy of multicenter validation and expansion into the setting of deceased donor kidney transplantation.
Numerous pre-clinical studies have advanced the promise of CoB-based therapy as a means of preventing rejection without the burden of chronic immunosuppression, a goal regularly achieved in experimental animals. However, CoB’s clinical translation has been hampered by early, high-grade acute rejections [5,7], prompting intense investigation into the mechanisms differentiating the experimental from the clinic settings. Substantial data have implicated terminally differentiated, effector T cells, arising in part through differences in environmental antigen exposure between humans and experimental animals, as the prime mediators of CoBRR [18, 36]. These cells have significant cytolytic potential and typically have lost CD28 expression during the course of late phase differentiation [34]; as such, they are indifferent to the direct effects of CD28-B7 pathway blockade. However, their terminally differentiated state renders them largely non-proliferative and susceptible to gradual elimination by activation induced cell death or apoptosis; mTORi and donor antigen exposure are both thought to foster this elimination [10–12, 37]. This is the first clinical study to prospectively address these mechanisms and further to prospectively randomize the use of donor bone marrow. We have shown that, although bone marrow infusion is feasible and well tolerated, it may not be necessary for primary efficacy–a logistical advantage. While, no measured parameter was significantly influenced by marrow infusion, we cannot comment definitively on trends associated with this maneuver in this proof-of-concept study. We thus view this as a matter worthy of further controlled investigation.
We find that the lymphocyte depletion achieved with this regimen promotes the ultimate emergence of a lymphocyte repertoire that is perhaps more accommodating to belatacept’s intended effect, being characterized by more naïve T and B cells, and fewer differentiated CD28− T cells and memory B cells than seen prior to transplantation. While this does not eliminate the possibility of residual donor-alloreactive belatacept-resistant T cells, it is reasonable to consider that these repertoire changes contribute to the eventual capacity to maintain the patients on belatacept alone. Indeed, we have recently demonstrated that belatacept’s immunosuppressive effect is greatest when considering naïve CD28+ T cells, and diminishes with progressive T cell differentiation and loss of CD28 expression [38] In addition, the regimen has allowed for the emergence of numerous cell types with potential regulatory function, and given the temporal, perhaps compensatory, association with homeostatic repopulation, we hypothesize that this is one reason we have not seen homeostatic activation-dependent CoBRR [39]. Other factors that may foster late, as opposed to early, immunosuppression withdrawal include the absence of surgical trauma, peritransplant reperfusion or other similarly activating events at the time of withdrawal. Several patients (pts. 2, 6, 7, 12 and 15) have subsequently undergone additional surgical procedures, to include bilateral native nephrectomy or partial hepatectomy for massive polycystic disease, without acute consequences. Thus, in this setting, surgical trauma alone appears insufficient to provoke allograft rejection late after transplantation.
It is clear that this regimen, like every efficacious anti-rejection regimen, impairs protective immunity. No major infectious events were seen, but the relative degree of impairment of this regimen compared to the myriad regimens used in kidney transplantation remains speculative. As reactivation of CMV and EBV was not notable, it is unlikely that a marked impairment of immunity to latent herpesviruses exists. However, the presence of transient BK viremia in 10 patients suggests that the agents used in this combination could disproportionately influence immunity toward this particular virus. Additional experience will be required to understand this further, but it is worth noting for future trials. All episodes of BK virus were subclinical, detected only due to aggressive surveillance, so a sampling bias may be in effect. Also, all episodes resolved with reduction of the sirolimus dose, suggesting that sirolimus was contributing to the burden of immunosuppression and not providing an anti-BK effect as has been suggested [40]. It is important to point out that the vast majority of the episodes of viremia occurred at the limit of detection of the PCR assays employed (dotted lines in Figures 2 and 4), and reflect aggressive surveillance with highly sensitive methods rather than clinical viral illness. In addition, at the end of follow-up, only 1 patient had any detectable viremia (patient 10, a patient with BK and EBV detected below the level of quantification and who was eligible to wean sirolimus had they wished to), indicating a resolution of viral protective immune capacity without a resurgence of rejection or alloantibody formation.
Several aspects of this study prevent its immediate generalizability. This is an uncontrolled, single center, proof-of-concept experience, albeit one that included Caucasian and African American patients of both genders across a broad age range (18–69). Nevertheless, numerous aspects of the decision-making have been left to the attending physicians (specifically with regard to preventing 3 patients from weaning in pursuit of beneficence) or the patients themselves (particularly the ability for patients to opt-out of weaning at one year even if they were eligible, preserving patient autonomy). Also, while the general care that these patients received was consistent with the prevailing clinical standard with regard to infectious prophylaxis and clinical follow-up, monitoring was more intensively in several ways, including serial monitoring for DSA and viremia, and regular surveillance biopsies. It is not clear whether the subclinical findings of these techniques helped prevent progression to clinical disease and are important to this approach, or were indicative of self-limiting conditions that prompted unnecessary alterations in the therapeutic course. Our bias would be to maintain a similarly intensive monitoring scheme until multicenter validation can be secured. Our two patients who were withdrawn from all immunosuppression indicate that continued immune therapy is required. Nevertheless, the rigor of that immune therapy appears to be substantially reduced compared to the clinical standard. We cannot comment on the potential clinical course of the 7 patients who chose not to wean or the 3 who were prohibited from weaning except to say that they have excellent graft function and tolerable immunosuppressive regimens. Whether they would have tolerated elimination of oral immunosuppression is a matter of speculation.
These data are consistent with the favorable short-term results of Ferguson, et al (41) in suggesting that depletional induction is an effective means of reducing early belatacept resistant rejection, and that mTORi are appropriate maintenance agents to pair with belatacept. They are distinct from the relatively poor results of Ciancio, et al (42), whose trial used alemtuzumab, donor stem cell infusion and tacrolimus conversion to sirolimus in the absence of belatacept. In this latter trial, failure due to recurrent glomerulopathy (a contraindication to inclusion in our trial) was seen, pointing out that immunosuppressive agents are at times best not withdrawn, and rejection was seen, suggesting that belatacept is an important aspect of this regimen. Our results extend these concepts well beyond the acute setting of both trials, clearly demonstrating a durable effect beyond the completion of homeostatic lymphocyte repopulation. Most importantly, they suggest that prolonged avoidance of CNIs and steroids in the presence of belatacept does more than limit morbidity; rather it allows for the requirements for immunosuppression to relax with time, facilitating a once monthly immune therapy for human transplantation. We believe that this approach is worthy of additional consideration.
Acknowledgments
Funding for this work was provided by United States Food and Drug Administration Office of Orphan Products Development grant 1RO1FD003539 (ADK). Belatacept was provided by Bristol-Myers Squibb. This trial is registered as NCT00565773 on ClinicalTrials.gov. The authors acknowledge the assistance of Satyen S. Tripathi, MA, CMI in creation of the figures.
Abbreviations
- ALC
absolute lymphocyte count
- CNI
calcineurin inhibitor
- CoB
costimulation blockade
- CoBRR
costimulation blockade resistant rejection
- CMV
cytomegalovirus
- DSA
donor-specific antibody
- EBV
Epstein-Barr virus
- eGFR
estimated glomerular filtration rate
- HLA
human leukocyte antigen
- IVIg
intravenous immunoglobulin
- MFI
median fluorescence intensity
- mTORi
mechanistic target of rapamycin inhibitor
- MMF
mycophenolate mofetil
- PRA
panel reactive antibody
- PCR
polymerase chain reaction
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry or Transplant Recipients (SRTR) OPTN/SRTR 2010 Annual Data Report. Department of Health and Human Services, Health and Services Administration, Healthcare Systems Bureau, Division of Transplantation. Am J Transplant. 2012;12(Suppl1):S1–154. [Google Scholar]
- 2.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 3.Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant. 2011;11:450–462. doi: 10.1111/j.1600-6143.2010.03283.x. [DOI] [PubMed] [Google Scholar]
- 4.Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobert E, Anderson D, Cowan S, Price K, Naemura J, Emswiler J, Greene J, Turk LA, Bajorath J, Townsend R, Hagerty D, Linsley PS, Peach RJ. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5:443–453. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 5.Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, Lang P, Grinyo J, Halloran PF, Solez K, Hagerty D, Levy E, Zhou W, Natarajan K, Charpentier B Belatacept Study Group. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353:770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 6.Larsen CP, Knechtle SJ, Adams A, Pearson T, Kirk AD. A new look at blockade of T-cell co-stimulation: a therapeutic strategy for long-term maintenance immunosuppression. Am J Transplant. 2006;6:876–883. doi: 10.1111/j.1600-6143.2006.01259.x. [DOI] [PubMed] [Google Scholar]
- 7.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, Massari P, Mondragon-Ramirez GA, Agarwal M, Di Russo G, Lin CS, Garg P, Larsen CP. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) Am J Transplant. 2010;10:535–546. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 8.Durrbach A, Pestana JM, Pearson T, Vincenti F, Garcia VD, Campistol J, Rial Mdel C, Florman S, Block A, Di Russo G, Xing J, Garg P, Grinyó J. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study) Am J Transplant. 2010;10:547–557. doi: 10.1111/j.1600-6143.2010.03016.x. [DOI] [PubMed] [Google Scholar]
- 9.Vanrenterghem Y, Bresnahan B, Campistol J, Durrbach A, Grinyó J, Neumayer HH, Lang P, Larsen CP, Mancilla-Urrea E, Pestana JM, Block A, Duan T, Glicklich A, Gujrathi S, Vincenti F. Belatacept-based regimens are associated with improved cardiovascular and metabolic risk factors compared with cyclosporine in kidney transplant recipients (BENEFIT and BENEFIT-EXT studies) Transplantation. 2011;91:976–983. doi: 10.1097/TP.0b013e31820c10eb. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999;5:1298–1302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 11.Wells AD, Li XC, Li Y, Walsh MC, Zheng XX, Wu Z, Nuñez G, Tang A, Sayegh M, Hancock WW, Strom TB, Turka LA. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5:1303–1307. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 12.Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant. 2007;7:1722–1732. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincenti F, Larsen CP, Alberu J, Bresnahan B, Garcia VD, Kothari J, Lang P, Urrea EM, Massari P, Mondragon-Ramirez G, Reyes-Acevedo R, Rice K, Rostaing L, Steinberg S, Xing J, Agarwal M, Harler MB, Charpentier B. Three-year outcomes from BENEFIT, a randomized, active-controlled, parallel-group study in adult kidney transplant recipients. Am J Transplant. 2012;12:210–217. doi: 10.1111/j.1600-6143.2011.03785.x. [DOI] [PubMed] [Google Scholar]
- 14.Pestana JO, Grinyo JM, Vanrenterghem Y, Becker T, Campistol JM, Florman S, Garcia VD, Kamar N, Lang P, Manfro RC, Massari P, Rial MD, Schnitzler MA, Vitko S, Duan T, Block A, Harler MB, Durrbach A. Three-year outcomes from BENEFIT-EXT: a phase III study of belatacept versus cyclosporine in recipients of extended criteria donor kidneys. Am J Transplant. 2012;12:630–639. doi: 10.1111/j.1600-6143.2011.03914.x. [DOI] [PubMed] [Google Scholar]
- 15. [accessed September 19, 2013];Belatacept package insert. at: http://packageinserts.bms.com/pi/pi_nulojix.pdf.
- 16.Trambley J, Bingaman AW, Lin A, Elwood ET, Waitze SY, Ha J, Durham MM, Corbascio M, Cowan SR, Pearson TC, Larsen CP. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. 1999;104:1715–1722. doi: 10.1172/JCI8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford ML, Koehn BH, Wagener ME, Jiang W, Gangappa S, Pearson TC, Larsen CP. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. J Exp Med. 2007;204:299–309. doi: 10.1084/jem.20062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ford ML, Larsen CP. Overcoming the memory barrier in tolerance induction: molecular mimicry and functional heterogeneity among pathogen-specific T-cell populations. Curr Opin Organ Transplant. 2010;15:405–410. doi: 10.1097/MOT.0b013e32833b7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ford ML, Wagener ME, Hanna SS, Pearson TC, Kirk AD, Larsen CP. A critical precursor frequency of donor-reactive CD4+ T cell help is required for CD8+ T cell-mediated CD28/CD154-independent rejection. J Immunol. 2008;180:7203–7211. doi: 10.4049/jimmunol.180.11.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, Cho HR, Aruffo A, Hollenbaugh D, Linsley PS, Winn KJ, Pearson TC. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 21.Szczech LA, Berlin JA, Aradhye S, Grossman RA, Feldman HI. Effect of anti-lymphocyte induction therapy on renal allograft survival: a meta-analysis. J Am Soc Nephrol. 1997;8:1771–1777. doi: 10.1681/ASN.V8111771. [DOI] [PubMed] [Google Scholar]
- 22.Szczech LA, Berlin JA, Feldman HI. The effect of antilymphocyte induction therapy on renal allograft survival. A meta-analysis of individual patient-level data. Anti-Lymphocyte Antibody Induction Therapy Study Group. Ann Intern Med. 1998;128:817–826. doi: 10.7326/0003-4819-128-10-199805150-00004. [DOI] [PubMed] [Google Scholar]
- 23.Swanson SJ, Hale DA, Mannon RB, Kleiner DE, Chamberlain CE, Polly S, Harlan DM, Kirk AD. Kidney transplantation with rabbit antithymocyte globulin induction and sirolimus monotherapy. Lancet. 2002;360:1662–1664. doi: 10.1016/S0140-6736(02)11606-0. [DOI] [PubMed] [Google Scholar]
- 24.Kirk AD, Hale DA, Mannon RB, Kleiner DE, Hoffmann SC, Kampen RL, Cendales LK, Tadaki DK, Harlan DM, Swanson SJ. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody Campath-1H. Transplantation. 2003;76:120–129. doi: 10.1097/01.TP.0000071362.99021.D9. [DOI] [PubMed] [Google Scholar]
- 25.Knechtle SJ, Pirsch JD, Fechner JH, Jr, Becker BN, Friedl A, Colvin RB, Lebeck LK, Chin LT, Becker YT, Odorico JS, D’Alessandro AM, Kalayoglu M, Hamawy MM, Hu H, Bloom DD, Sollinger HW. Campath-1H induction plus rapamycin monotherapy for renal transplantation: results of a pilot study. Am J Transplant. 2003;3:722–730. doi: 10.1034/j.1600-6143.2003.00120.x. [DOI] [PubMed] [Google Scholar]
- 26.Standards for Cellular Product Services. 4. American Association of Blood Banks; 2009. [Google Scholar]
- 27.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M. Banff 07 Classification of Renal Allograft Pathology: Updates and Future Directions. Am Journal Transplant. 2008;8:753–760. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 28.Pei R, Wang G, Tarsitani C, Rojo S, Chen T, Takemura S, Liu A, Lee J. Simultaneous HLA Class I and Class II antibodies screening with flow cytometry. Hum Immunol. 1998;59:313–22. doi: 10.1016/s0198-8859(98)00020-2. [DOI] [PubMed] [Google Scholar]
- 29.Bray RA, Tarsitani C, Gebel HM, Lee J-H. Clinical cytometry and progress in HLA antibody detection. 2011. Meth Cell Biol. 103:285–310. doi: 10.1016/B978-0-12-385493-3.00012-7. Chapter. 12. [DOI] [PubMed] [Google Scholar]
- 30.Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, Swanson SJ, Mannon RB, Roederer M, Kirk AD. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5:465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 31.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 32.Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M, Burlingham WJ, Marks WH, Sanz I, Lechler RI, Hernandez-Fuentes MP, Turka LA, Seyfert-Margolis VL Immune Tolerance Network ST507 Study Group. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120:1836–1847. doi: 10.1172/JCI39933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li XC, Turka LA. An update on regulatory T cells in transplant tolerance and rejection. Nat Rev Nephrol. 2010;6:577–583. doi: 10.1038/nrneph.2010.101. [DOI] [PubMed] [Google Scholar]
- 34.Lo DJ, Weaver TA, Stempora L, Mehta AK, Ford ML, Larsen CP, Kirk AD. Selective targeting of human alloresponsive CD8+ effector memory T cells based on CD2 expression. Am J Transplant. 2011;11:22–33. doi: 10.1111/j.1600-6143.2010.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nankivell BJ, Gruenewald SM, Allen RD, Chapman JR. Predicting glomerular filtration rate after kidney transplantation. Transplantation. 1995;59:1683–1689. doi: 10.1097/00007890-199506270-00007. [DOI] [PubMed] [Google Scholar]
- 36.Adams AB, Pearson TC, Larsen CP. Heterologous immunity: an overlooked barrier to tolerance. Immunol Rev. 2003;196:147–160. doi: 10.1046/j.1600-065x.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 37.Lin H, Bolling SF, Linsley PS, Wei RQ, Gordon D, Thompson CB, Turka LA. Long-term acceptance of major histocompatibility complex mismatched cardiac allografts induced by CTLA4Ig plus donor-specific transfusion. J Exp Med. 1993;178:1801–1806. doi: 10.1084/jem.178.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu H, Perez SD, Cheeseman J, Mehta AK, Kirk AD. The allo- and viral-specific immunosuppressive effect of belatacept, but not tacrolimus, attenuates with progressive T cell maturation. Am J Transplant. 2014 doi: 10.1111/ajt.12574. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, Markmann JF, Kassaee A, Rosengard BR, Hancock WW, Sayegh MH, Turka LA. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suwelack B, Malyar V, Koch M, Sester M, Sommerer C. The influence of immunosuppressive agents on BK virus risk following kidney transplantation, and implications for choice of regimen. Transplant Rev. 2012;26:201–11. doi: 10.1016/j.trre.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Ferguson R, Grinyó J, Vincenti F, Kaufman DB, Woodle ES, Marder BA, Citterio F, Marks WH, Agarwal M, Wu D, Dong Y, Garg P. Immunosuppression with belatacept-based, corticosteroid-avoiding regimens in de novo kidney transplant recipients. Am J Transplant. 2011;11:66–76. doi: 10.1111/j.1600-6143.2010.03338.x. [DOI] [PubMed] [Google Scholar]
- 42.Ciancio G, Sageshima J, Akpinar E, Gaynor JJ, Chen L, Zarak A, Hanson L, Tueros L, Guerra G, Mattiazzi A, Kupin W, Roth D, Ricordi C, Burke GW., 3rd A randomized pilot study of donor stem cell infusion in living-related kidney transplant recipients receiving alemtuzumab. Transplantation. 2013;96:800–6. doi: 10.1097/TP.0b013e3182a0f68c. [DOI] [PMC free article] [PubMed] [Google Scholar]