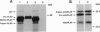Abstract
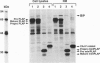
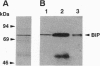
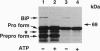
Nascent proteins destined to be processed to a glycosylphosphatidylinositol (GPI)-anchored membrane form contain NH2-terminal and COOH-terminal signal peptides. The first directs a nascent protein into the endoplasmic reticulum; the second peptide targets the protein to a putative COOH-terminal signal transamidase where cleavage of the peptide and addition of the GPI anchor occur. We recently showed that ATP hydrolysis is required for maturation of GPI proteins at a stage prior to transamidation. Here we show that one of the ATP-requiring proteins involved in processing of GPI-anchored proteins in the endoplasmic reticulum is the immunoglobulin heavy chain binding protein (BiP; GRP 78). This and related findings indicate that GPI transamidase is localized in the endoplasmic reticulum.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amthauer R., Kodukula K., Brink L., Udenfriend S. Phosphatidylinositol-glycan (PI-G)-anchored membrane proteins: requirement of ATP and GTP for translation-independent COOH-terminal processing. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6124–6128. doi: 10.1073/pnas.89.13.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross G. A. Glycolipid anchoring of plasma membrane proteins. Annu Rev Cell Biol. 1990;6:1–39. doi: 10.1146/annurev.cb.06.110190.000245. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Donaldson J. G., Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992 Mar;116(5):1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodukula K., Cines D., Amthauer R., Gerber L., Udenfriend S. Biosynthesis of phosphatidylinositol-glycan (PI-G)-anchored membrane proteins in cell-free systems: cleavage of the nascent protein and addition of the PI-G moiety depend on the size of the COOH-terminal signal peptide. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1350–1353. doi: 10.1073/pnas.89.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodukula K., Gerber L. D., Amthauer R., Brink L., Udenfriend S. Biosynthesis of glycosylphosphatidylinositol (GPI)-anchored membrane proteins in intact cells: specific amino acid requirements adjacent to the site of cleavage and GPI attachment. J Cell Biol. 1993 Feb;120(3):657–664. doi: 10.1083/jcb.120.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodukula K., Micanovic R., Gerber L., Tamburrini M., Brink L., Udenfriend S. Biosynthesis of phosphatidylinositol glycan-anchored membrane proteins. Design of a simple protein substrate to characterize the enzyme that cleaves the COOH-terminal signal peptide. J Biol Chem. 1991 Mar 5;266(7):4464–4470. [PubMed] [Google Scholar]
- Low M. G. The glycosyl-phosphatidylinositol anchor of membrane proteins. Biochim Biophys Acta. 1989 Dec 6;988(3):427–454. doi: 10.1016/0304-4157(89)90014-2. [DOI] [PubMed] [Google Scholar]
- Micanovic R., Gerber L. D., Berger J., Kodukula K., Udenfriend S. Selectivity of the cleavage/attachment site of phosphatidylinositol-glycan-anchored membrane proteins determined by site-specific mutagenesis at Asp-484 of placental alkaline phosphatase. Proc Natl Acad Sci U S A. 1990 Jan;87(1):157–161. doi: 10.1073/pnas.87.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micanovic R., Kodukula K., Gerber L. D., Udenfriend S. Selectivity at the cleavage/attachment site of phosphatidylinositol-glycan anchored membrane proteins is enzymatically determined. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7939–7943. doi: 10.1073/pnas.87.20.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell. 1989 Nov 17;59(4):591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Taguchi R., Asahi Y., Ikezawa H. Purification and properties of phosphatidylinositol-specific phospholipase C of Bacillus thuringiensis. Biochim Biophys Acta. 1980 Jul 14;619(1):48–57. [PubMed] [Google Scholar]
- Takami N., Oda K., Fujiwara T., Ikehara Y. Intracellular accumulation and oligosaccharide processing of alkaline phosphatase under disassembly of the Golgi complex caused by brefeldin A. Eur J Biochem. 1990 Dec 27;194(3):805–810. doi: 10.1111/j.1432-1033.1990.tb19473.x. [DOI] [PubMed] [Google Scholar]
- Tsang V. C., Peralta J. M., Simons A. R. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 1983;92:377–391. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]
- Udenfriend S., Micanovic R., Kodukula K. Structural requirements of a nascent protein for processing to a PI-G anchored form: studies in intact cells and cell-free systems. Cell Biol Int Rep. 1991 Sep;15(9):739–759. doi: 10.1016/0309-1651(91)90030-m. [DOI] [PubMed] [Google Scholar]
- Waheed A., Zhu X. L., Sly W. S. Membrane-associated carbonic anhydrase from rat lung. Purification, characterization, tissue distribution, and comparison with carbonic anhydrase IVs of other mammals. J Biol Chem. 1992 Feb 15;267(5):3308–3311. [PubMed] [Google Scholar]