Abstract
Conifer and broadleaf trees emit volatile organic compounds in the summer. The major components of these emissions are volatile monoterpenes. Using solid phase microextraction fiber as the adsorbant, monoterpenes were successfully detected and identified in forest air samples. Gas chromatography/mass chromatogram of monoterpenes in the atmosphere of a conifer forest and that of serum from subjects who were walking in a forest were found to be similar each other. The amounts of α-pinene in the subjects became several folds higher after forest walking. The results indicate that monoterpenes in the atmosphere of conifer forests are transferred to and accumulate in subjects by inhalation while they are exposed to this type of environment.
Keywords: conifer, monoterpenes, pinene, forest walking, forest therapy
INTRODUCTION
Most vegetation emits an array of volatile organic compounds (VOCs).1)
The major components of conifer-derived VOCs are monoterpenes (MTs) such as α-pinene, β-pinene, camphene, β-myrcene, 3-carene, limonene, β-phellandrene, and bornyl acetate. The proportions of MTs vary with season, species and locality. According to the documented physicochemical properties of MTs,2) they are sparingly soluble in water but are soluble in blood and lipophilic tissues. α- and β-Pinene dominate emissions from conifer trees and research investigations have raised concerns regarding the physiological effect of α-pinene on humans.3,4) Exposure of workers to high concentrations of α-pinene (10–450 mg/m3) causes an increase in its concentration in the blood4) and human metabolism of α-pinene has also been documented.5,6) For example, anti-inflammatory and anti-cancer effects of essential oils containing geraniol or limonene have been reported.7–9) More interestingly, it has been reported that exposure of rat brain tissues to citrus flavor components results in the release of dopamines.10)
The concentrations of MTs in air in a forest are below 1 ppm.3) Inhalation of MTs within such a concentration range causes no irritation or injury. The safety levels of α-pinene in humans are estimated to be below 40–70 ppm. It has been suggested that walking in a forest reduces the stress levels of walkers.11) However, no quantitative study is available regarding the transfer of MTs from conifer trees to subjects. In the present study, a solid-phase microextraction (SPME) method was successfully applied to adsorb aromatic compounds, which were then desorbed and identified by gas chromatography/mass spectrometry (GC/MS).12,13) The results indicate a direct relationship between the concentration of conifer-derived MTs in the atmosphere of a conifer forest and in serum from subjects who walk in conifer forests.
MATERIALS AND METHODS
Venous blood samples (10 mL) were obtained from 4 volunteers before and after forest walking. The subjects were assembled at Asahikawa in the morning before forest walking and blood samples were collected. All subjects were taken to the town of Tsubetsu by car. Forest walking was performed for 60 min in the afternoon at Tsubetsu forest on July 7, 2013. The blood samples were collected after walking and were kept cold and transferred to tight head space vials. For a comparison of monoterpene composition in the blood, forest walking was also performed in a different forest located in Nakatombetsu on July, 2013 and in Iinan on October, 2013. The subjects were 24 inhabitants. Information regarding the aim of the experiment was provided to all of the healthy subjects and informed consent was obtained. MTs in the forest atmosphere and in blood were adsorbed on solid phase micro extraction (SPME) fibers for 60 min and identified by GC-MS (JEOL JMS T100 GCV). We exposed the SPME fiber to the forest atmosphere or in the head space (HS) of a test tube containing subjects’ blood for 60 min. The concentrations of α-pinene in the blood were determined by comparison with standard curves prepared in the same concentration range after the addition of α-pinene to blood. The linearity of the calibration curve ranged from 500 pM to 500 nM. SPME fiber, the carboxen/polydimethylsiloxane (CAR/PDMS) fiber (80 μm) was obtained from SUPELCO.
GC/MS parameters were as follows: a DB-1MS fused silica capillary column was used for separation (30 m×0.25 μm, Agilent Technologies, USA). The carrier gas was helium with a flow rate of 1.5 mL/min, and the injector unit temperatures were set to 280°C. The oven temperature was initially 32°C for 3 min, then increased to 100°C at 5°C/min and increased to 300°C at 80°C/min, and held for 2 min. Transfer line temperature was 280°C. Mass detector conditions were: electron ionization (EI) mode at 70 eV; source temperature: 260°C; mass acquisition range: 10–500.
α-Pinene concentrations in the blood from subjects after walking in the Tsubetsu forest were statistically compared with those before walking using the paired Student’s t-test.
RESULTS AND DISCUSSION
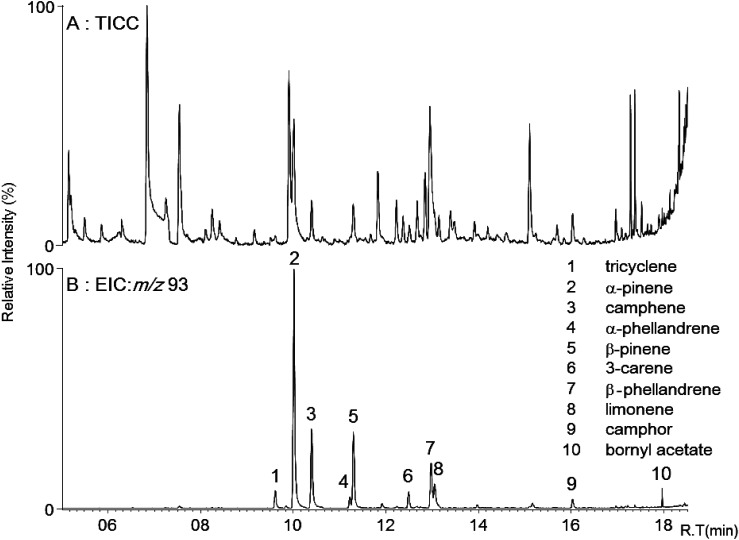
The concentrations of MTs in the atmosphere of forest air range from 0.001 to 1 ppm.3) The major constituents of conifer-derived MTs are α- and β-pinene in the summer.1) These compounds with exo- or endocyclic double bonds react with active oxygen to protect plants from oxidative stress. We initially attempted to detect the MTs in the air in the woods using a SPME fiber as the adsorbent. MTs were effectively detected in the atmosphere of the Tsubetsu forest using SPME GC/MS (Fig. 1). The mass chromatogram indicates the presence of tricyclene (peak 1), α-pinene (peak 2), camphene (peak 3), α-phellandrene (peak 4), β-pinene (peak 5), 3-carene (peak 6), β-phellandrene (peak 7), limonene (peak 8), camphor (peak 9), and bornyl acetate (peak 10) (Fig. 1b). Oxygenated MTs such as camphor and bornyl acetate are much less abundant and the concentration of 3-carene is lower relative to other MTs in the summer.1) The Tsubetsu forest mainly consists of conifer trees. We separately identified the conifer-derived and broadleaf-derived MTs and were able to differentiate compounds collected from conifer trees and from broadleaf trees by GC/MS (Figs. 2a and 2b). The extracted ion chromatograms of samples from the atmosphere of conifer trees (Picea jezoensis, Abies sachalinensis Masters) and broadleaf trees (Ulmusdavidiana var. japonica, Cerasussargenti H. Ohta), indicate that conifer trees emit many MTs, with α-pinene being the major component, while a higher abundance of α-phellandrene (peak 4) relative to β-pinene (peak 5) is characteristic of broadleaf trees (Fig. 2b). These results are compatible with the report that pinenes are a major component of conifer-derived MTs.1)
Fig. 1. GC mass chromatogram of monoterpenes in the atmosphere of Tsubetsu forest.
A: Total ion current chromatogram (TICC). B: Extracted ion chromatogram (EIC). Arabic numerals on peaks indicate identified monoterpenes.
Fig. 2. GC/MS extracted ion chromatogram of monoterpenes in the atmosphere of conifer trees (Picea jezoensis, Abies sachalinensis Masters) (a) and broadleaf trees (Ulmus davidiana var. japonica, Cerasus sargenti H. Ohba) (b).
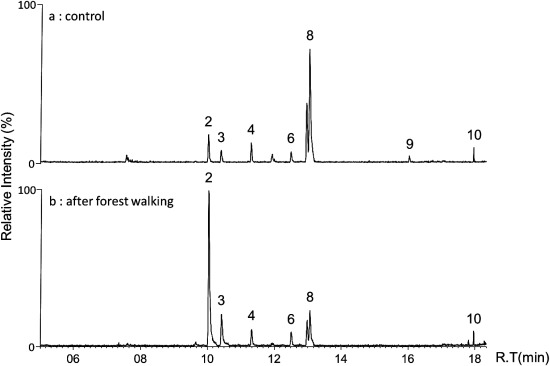
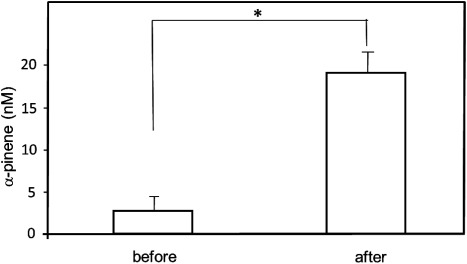
Exposure of subjects to a forest environment has been associated with an improvement in their physical condition. Walking in a broadleaf forest reduces the stress levels of subjects compared with walking in a city area.11) Bornyl acetate, which is a component of forest air induces the autonomic nervous system to relax.14) Four healthy volunteers walked for 60 min in a forest located at Tsubetsu. Figure 3a shows the GC-mass chromatogram of blood from one subject in the morning before the forest walk. Peaks corresponding to, β-phellandrene and limonene were abundant, and weak signals assigned with other MTs were also observed. We found a high abundance of allyl isothiocyanate, menthol and limonene in blood from other subjects (data not shown). These findings suggest that the abundance of β-phellandrene and limonene are derived from foods or fragrance-containing products that are ingested by the subjects. On the contrary, a small level of MTs (peaks 1 to 10, except for 7 and 8) originate from street trees (Fig. 3a). The height of peak 2 was six fold greater after walking in the forest (Fig. 3b). Quantification was obtained by a calculation based on the percentage of the peak area for α-pinene (peak 2) to a calibration curve. The concentration of α-pinene in the blood from volunteers (n=4) before and after walking was 2.6±1.7 and 19.4±2.1 (nM) (mean±S.E.), respectively (Fig. 4). Walking in the Tsubetsu forest for 60 min resulted in an increase in the concentration of α-pinene in the blood from subjects. It is important to note that both the GC mass chromatogram of a forest atmosphere (Fig. 1b) and blood from forest walkers (Fig. 3b) were similar to each other. It has been reported that α-pinene and limonene are rapidly distributed in the tissues, and both unchanged MTs are excreted into the urine and in exhaled air.2,15) α-Pinene is metabolized and converted to verbenone through the formation of verbenol.4–6) Verbenol has been reported to have anti-ischemic and anti-inflammatory activities.9) The conversion of limonene to oxygenated metabolites by humans has also been reported.15) However no such metabolites were found in the blood from forest walkers (Fig. 3b).
Fig. 3. GC/MS extracted ion chromatogram of monoterpenes in blood from subject in the morning (a) and after Tsubetsu forest walking (b).
Fig. 4. Concentrations of α-pinene in the blood from subjects before and after Tsubetsu forest walking.
Values are presented as mean±S.E., n=4. α-Pinene concentration in the blood was significantly increased after walking (*p<0.05).
HS-SPME is a very sensitive for the detection of lipophilic MTs and less lipophilic MTs. We performed forest walking in different areas, and the blood samples from forest walkers were found to consistently contain α-pinene, irrespective of the area that they were walking in.
MTs were found to accumulate in blood by the inhalation of the conifer forest atmosphere. The results suggest the possibility that extensive forest walking could result in an increase in the concentration of MTs in blood up to the μM levels.4) Volatile organic compounds even at the low mM level are known to inhibit acetylcholinesterase activity by 50%.16) The uncompetitive and reversible inhibition of acetylcholinesterase activity by MTs indicate that α-pinene interacts with the enzyme–substrate complex. The reversible inhibition of acetylcholinesterase activity is known to delay the progression of Alzheimer’s disease. MTs accumulate in lipophilic tissues such as brain and are present for as long as several days.2,4) Determining the distribution of MTs and their local concentration in the tissues will provide more evidence regarding the potential benefits of forest walking.
CONCLUSION
MTs in the atmosphere of a conifer forest and in blood from conifer forest walkers were effectively detected using an HS-SPME method. α-Pinene was a major component and oxygenated MTs including camphor and bornyl acetate were minor components. The atmospheres of conifer and broad leaf forests in the summer can be distinguished from each other by GC/MS. A marked increase of α-pinene in the blood after forest walking was observed. Conifer and broad-leaf derived MTs are easily transferred to and accumulate in subjects who are walking in a forest.
Acknowledgments
We thank Dr. Richard S. Magliozzo (Brooklyn College, CUNY) for proofreading the manuscript.
Mass Spectrom (Tokyo) 2015; 4(1): A0042
- VOCs
volatile organic compounds
- MTs
monoterpenes
- GC/MS
gas chromatography/mass spectrometry
- HS
head space
- SPME
solid phase microextraction
References
- 1) C. Geron, R. Rasmussen, R. R. Arnts, A. Guenther. A review and synthesis of monoterpene speciation from forests in the United States. Atmos. Environ. 34: 1761–1781, 2000. [Google Scholar]
- 2) A. Falk, E. Gullstrand, A. Lof, E. Wigaeus-Hjelm. Liquid/air partition coefficients of four terpenes. Br. J. Ind. Med. 47: 62–64, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3) G. D. Nielsen, S. T. Larsen, K. S. Hougaard, M. Hammer, P. Wolkoff, P. A. Clausen, C. K. Wilkins, Y. Alarie. Mechanisms of acute inhalation effects of (+) and (−)-α-pinene in BALB/c mice. Basic Clin. Pharmacol. Toxicol. 96: 420–428, 2005. [DOI] [PubMed] [Google Scholar]
- 4) A. A. Falk, M. T. Hagberg, A. E. Lof, E. M. Wigaeus-Hjelm, W. Zhiping. Uptake, distribution and elimination of α-pinene in man after exposure by inhalation. Scand. J. Work Environ. Health 16: 372–378, 1990. [DOI] [PubMed] [Google Scholar]
- 5) K. Eriksson, J.-O. Levin. Gas chromatographic-mass spectrometric identification of metabolites from α-pinene in human urine after occupational exposure to sawing fumes. J. Chromatogr. B Biomed. Appl. 677: 85–98, 1996. [DOI] [PubMed] [Google Scholar]
- 6) T. Vanek, J. Halik, R. Vankova, I. Valterova. Formation of trans-verbenol and verbenone from α-pinene catalysed by immobilised Picea abies cells. Biosci. Biotechnol. Biochem. 69: 321–325, 2005. [DOI] [PubMed] [Google Scholar]
- 7) R. Hirota, N. N. Roger, H. Nakamura, H.-S. Song, M. Sawamura, N. Suganuma. Anti-inflammatory effects of limonene from yuzu (Citrus junos Tanaka) essential oil on eosinophils. J. Food Sci. 75: 87–92, 2010. [DOI] [PubMed] [Google Scholar]
- 8) M. Kusuhara, K. Urakami, Y. Masuda, V. Zangiacomi, H. Ishii, S. Tai, K. Maruyama, K. Yamaguchi. Fragrant environment with α-pinene decreases tumor growth in mice. Biomed. Res. 33: 57–61, 2012. [DOI] [PubMed] [Google Scholar]
- 9) I.-Y. Choi, J. Lim, S. Hwang, J.-C. Lee, G.-S. Cho, W.-K. Kim. Anti-ischemic and anti-inflammatory activity of (S)-cis-verbenol. Free Radic. Res. 44: 541–551, 2010. [DOI] [PubMed] [Google Scholar]
- 10) S. Fukumoto, E. Sawasaki, S. Okuyama, Y. Miyake, H. Yokogoshi. Flavor components of monoterpenes in citrus essential oils enhance the release of monoamines from rat brain slices. Nutr. Neurosci. 9: 73–80, 2006. [DOI] [PubMed] [Google Scholar]
- 11) Y. Tsunetsugu, B.-J. Park, H. Ishii, H. Hirano, T. Kagawa, Y. Miyazaki. Physiological effects of shinrin-yoku (taking in the atmosphere of the forest) in an old-growth broad leaf forest in Yamagata prefecture, Japan. J. Physiol. Anthropol. 26: 135–142, 2007. [DOI] [PubMed] [Google Scholar]
- 12) J. Richter, I. Schellenberg. Comparison of different extraction methods for the determination of essential oils and related compounds from aromatic plants and optimization of solid-phase microextraction/gas chromatography. Anal. Bioanal. Chem. 387: 2207–2217, 2007. [DOI] [PubMed] [Google Scholar]
- 13) S. Matysik, F.-M. Matysik. Microextraction by packed sorbent coupled with gas chromatography-mass spectrometry: Application to the determination of metabolites of monoterpenes in small volumes of human urine. Mikrochim. Acta 166: 109–114, 2009. [Google Scholar]
- 14) E. Matsubara, M. Fukagawa, T. Okamoto, K. Ohnuki, K. Shimizu, R. Kondo. (−)-Bornyl acetate induces autonomic relaxation and reduces arousal level after visual display terminal work without any influences of task performance in low-dose condition. Biomed. Res. 32: 151–157, 2011. [DOI] [PubMed] [Google Scholar]
- 15) J. Sun. D-Limonene: Safety and clinical applications. Altern. Med. Rev. 12: 259–264, 2007. [PubMed] [Google Scholar]
- 16) M. Miyazawa, C. Yamafuji. Inhibition of acetylcholinesterase activity by bicyclic monoterpenoids. J. Agric. Food Chem. 53: 1765–1768, 2005. [DOI] [PubMed] [Google Scholar]