Abstract
Background
The challenge of antibiotic resistance and the emergence of new infections have generated considerable interest in the exploration of natural products from plant origins as combination therapy. In this context, crude ethanolic extract (CEE), ethyl acetate fraction (EAF), and methanolic fraction (MF) from Anacardium microcarpum were tested alone or in combination with antibiotics (amikacin, gentamicin, ciprofloxacin, and imipenem) against Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus.
Methods
Antibiotic resistance-modifying activity was performed using the microdilution method by determining the minimal inhibitory concentration (MIC). In addition, phytochemical prospecting analyses of tested samples were carried out.
Results
Our results indicated that all the extracts showed low antibacterial activity against multidrug-resistant strains (MIC =512 μg/mL). However, addition of CEE, EAF, and MF to the growth medium at the subinhibitory concentration (MIC/8=64 μg/mL) significantly modulated amikacin- and gentamicin-resistant E. coli 06. CEE and EAF also demonstrated a significant (P<0.001) synergism with imipenem against S. aureus. In contrast, MF antagonized the antibacterial effect of ciprofloxacin and gentamicin against P. aeruginosa 03 and S. aureus 10, respectively. Qualitative phytochemical analysis of the extracts revealed the presence of secondary metabolites including phenols, flavonoids, xanthones, chalcones, and tannin pyrogallates.
Conclusion
Taken together, our results suggest that A. microcarpum is a natural resource with resistance-modifying antibacterial activity that needs to be further investigated to overcome the present resistant-infection problem.
Keywords: multidrug-resistant, A. microcarpum, antibacterial activity
Introduction
Infectious diseases caused by microorganisms including bacterial species remain a major therapeutic problem in hospitals, leading to significant morbidity and mortality.1–3 This is mainly due to the emergence of new infections (eg, mycoses and bacterial illnesses) and the widespread of antibiotic resistance.4
Among several pathogenic species, the most significant ones are Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus. E. coli belongs to the normal flora of humans and plays an important role in the human intestinal tract. However, some strains can cause chronic diarrhea, food poisoning,5 or illness outside the intestinal tract. P. aeruginosa, which is occasionally associated with opportunistic human diseases,6 can cause a wide range of acute and chronic infections including infections of the urinary tract, skin, and respiratory tract as well as cystic fibrosis.7 P. aeruginosa was reported by the Centers for Disease Control and Prevention (CDC) to be the second most common pathogen in health care-associated infections, behind E. coli.8 S. aureus is one of the five most common causes of nosocomial postsurgical wound infections.9,10 It is also an opportunistic pathogen in humans.11
Normally, the principal mechanism by which antibacterial agents act is by interference with cell wall synthesis, inhibition of protein synthesis, interference with nucleic acid synthesis, inhibition of a metabolic pathway, and disruption of bacterial membrane structure.12 However, these actions of antibacterial agents have failed due to multidrug resistance, which is related to the simple/plastic genomes and DNA exchanges with other bacterial strains.13 This situation represents a serious public health problem and greatly affects the economy, necessitating the urgent development of new antimicrobial agents.
Less than 2 decades ago, natural products from plant origins have generated considerable interest in the search for new antibacterial agents and/or new compounds able to potentiate the antibacterial activity of old antibiotics.14–20 Additionally, substantial evidence from the literature has shown the potential of crude extracts in controlling multidrug resistance in in vitro studies21–23 and the efficacy of plant-derived antimicrobial compounds in food applications.24–26
The Brazilian plant Anacardium microcarpum, popularly known as “cajui”, belongs to the Anacardiaceae family. It is found in the Northeast Region, Brazil. Its fruits are rich in vitamin C, proteins, lipids, carbohydrates, and phenolic compounds. Infusions of A. microcarpum bark are used in traditional Brazilian medicine as a tonic for the treatment of a variety of diseases, including infectious diseases, inflammation, rheumatism, and tumor. Recently, we reported for the first time that the popular use of A. microcarpum in the prevention and/or treatment of the above-listed diseases (which are associated with oxidative stress) has a scientific basis due to its antioxidative activity,27 but no study has been carried out to investigate its potential use as an antibacterial agent. Therefore, the purpose of this study was to evaluate the antibacterial activity of crude ethanolic extract (CEE) and fractions (ethyl acetate and methanolic) from A. microcarpum bark against different bacterial strains and their potential to modulate antibiotic drugs used in clinical infections. Furthermore, the phytochemical prospecting of A. microcarpum was investigated for the presence or absence of secondary metabolites.
Materials and methods
Plant material and extraction
Stem bark of A. microcarpum was collected from Barrero Grande, Crato, CE (7°22′S; 39°28′W; 892 m above sea level), Brazil, in November 2011. The plant material was identified by Dr Maria Arlene Pessoa da Silva of the herbarium Caririense Dárdano de Andrade-Lima (HCDAL) of the Regional University of Cariri (URCA) (Crato, CE, Brazil), and a voucher specimen was deposited (number 6702). The fresh bark of A. microcarpum was macerated with 99.9% ethanol and water (1:1, v/v) for 3 days. The suspension was filtered and the solvent evaporated under reduced pressure and lyophilized to obtain 490 g of CEE. One hundred and fifty grams of this was partitioned with ethyl acetate and methanol to obtain 12.5 g of ethyl acetate fraction (EAF) and 105.23 g of methanolic fraction (MF). Prior to use, the CEE, EAF, and MF were prepared by dissolving 10 mg of each in 1 mL of DMSO (10%), thus starting with an initial concentration of 10 mg/mL. The resulting solutions were then diluted to 1,024 μg/mL in sterile water and used in the experiments.
Preliminary phytochemical analysis
The CEE, EAF, and MF were screened according to the method described by Matos,28 with slight modifications, for detecting the presence of different classes of secondary metabolites such as phenols, flavones, flavonoids, chalcones, xanthones, alkaloids, flavonones, aurones, and tannin pyrogallates.
Drugs and microorganisms
The antibiotics used were amikacin, gentamicin, ciprofloxacin, and imipenem. These were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). The stock solutions of the antibiotics (5 mg/mL) were prepared in sterile water and then diluted to 2,500 μg/mL, which was used for the experiments. Resazurin sodium (Sigma-Aldrich Co.) was used as an indicator of the growth of bacteria.
The Gram-negative bacteria used in this study were E. coli (25922) and P. aeruginosa (9027). The Gram-positive bacterium that was used in this study was S. aureus (25923). These were used for the determination of minimal inhibitory concentration (MIC). However, for the modulation of the antibiotic activity P. aeruginosa 03, E. coli 06, and S. aureus 10 were used. Their origins and resistance profiles to antibiotics are shown in Table 1. All the strains were obtained from the Laboratory of Clinical Microbiology of the Federal University of Paraíba (João Pessoa, PB, Brazil) and maintained on nutrient agar slants at 4°C.
Table 1.
Origins of bacterial strains and their resistance to antibiotics
| Bacteria | Origin | Resistance profile |
|---|---|---|
| Escherichia | Surgical | Aztreonam, amoxicillin, ampicillin, amicilin, |
| coli 06 | wound | amoxicillin, cefadroxil, cefaclor, cephalothin, Ceftazidime, ciprofloxacin, chloramphenicol, imipenem, kanamycin, sulphametrim, tetracycline, tobramycin |
| Pseudomonas | Urine | Ceftazidime, imipenem, ciprofloxacin, |
| aeruginosa 03 | culture | piperacillin–tazobactam, levofloxacin, meropenem, amicilin |
| Staphylococcus | Surgical | Oxacillin, gentamicin, tobramycin, amicilin, |
| aureus 10 | wound | kanamycin, neomycin, paromomycin, butirosin, sisomicin, netilmicin |
Growth media and culture conditions
Heart infusion agar (Difco Laboratory, Ltd., MI, USA) and the Caldo brain heart infusion (BHI) broth (10%; Acumedia Manufacturers Inc., NJ, USA) were used for bacteria media culture. The culture media were prepared according to the guidelines of the suppliers. The cultures of bacteria were maintained at 4°C in heart infusion agar. Briefly, the microbial suspensions were prepared by making a sterile saline suspension of isolated colonies selected from BHI (for bacteria) and the agar plates were grown for 24 hours at 37°C. The suspension was adjusted to 0.5 of McFarland turbidity scale, which corresponds to 105 colony-forming units (CFUs)/mL.29 Resazurin sodium at a concentration of 0.01% was added to the culture media as a growth indicator, after incubation at 37°C.
Determination of antibacterial activity
A preliminary evaluation of the antibacterial activity of the CEE, EAF, and MF from the bark of A. microcarpum was determined by the microdilution method,30 by determining the smallest amount of plant extract required to inhibit the visible growth of the tested pathogens. This was carried out using twofold dilutions. Briefly, 100 μL of culture medium containing different bacterial strains (105 CFUs/mL) were distributed in 96-well plates and then subjected to serial dilution using 100 μL of each extract, with final concentrations varying from 512 to 8 μg/mL. The negative control contained bacterial suspension and the solvent (DMSO, 10%), while the positive control was made of the culture medium plus different bacterial strains. Antibacterial activity was detected by adding 20 μL of a 0.01% resazurin stain aqueous solution in each well at the end of the incubation period. The bacterial growth was observed by the irreversible reduction of resazurin to resorufin, characterized by a change from blue color to pink color. The MIC of the extracts was determined as the lowest concentration at which they were able to inhibit bacterial growth.29 All experiments were performed in triplicate, and the procedure was repeated at least three times.
Modulation of the antibiotic activity
In order to evaluate the potential modulatory effect of CEE, EAF, and MF on the tested antibiotics, the extracts were tested at the subinhibitory concentration (ie, MIC/8) as described by Coutinho et al31 using different bacterial strains. For this set of experiments, we used P. aeruginosa 03, E. coli 06, and S. aureus 10. These bacteria are known to be resistant to a wide range of antibiotics (Table 1). Briefly, 100 μL of a solution mixture containing BHI (10%) and the bacterial inocula with or without different extracts (64 μg/mL) was placed in each well plate. Then, 100 μL of the antibiotic drugs at the concentration of 1,250 μg/mL was added in the first well, which was two-fold diluted to obtain concentrations ranging from 1.22 to 1,250 μg/mL.
Statistical Analysis
The results are expressed as mean ± standard error of mean (SEM). Statistical analysis was performed using two-way ANOVA followed by Bonferroni posttests and P<0.05 was considered statistically significant.
Results
Phytochemical analysis of CEEand fractions from the bark of A. microcarpum
The phytochemical prospecting of the CEE and fractions (EAF and MF) revealed the presence of a variety of classes of secondary metabolites (Table 2). On all products, the presence of phenols, flavones, flavonoids, chalcones, xanthones, alkaloids, flavanones, aurones, and tannin pyrogallates was detected (Table 2). It should be noted that the chemical composition of the CEE, EAF, and MF used in the present study was previously determined by Barbosa Filho et al27 using high-performance liquid chromatography coupled with a diode-array detector.
Table 2.
Qualitative phytoconstituent analysis of crude ethanolic extract (CEE), ethyl acetate fraction (EAF) and methanolic fraction (MF) from the stem bark of Anacardium microcarpum
| Secondary metabolites | CEE | EAF | MF |
|---|---|---|---|
| Phenols | + | + | + |
| Flavones | + | + | + |
| Flavonoids | + | + | + |
| Chalcones | + | + | + |
| Xanthones | + | + | + |
| Alkaloids | + | + | + |
| Flavanones | + | + | + |
| Aurones | + | + | + |
| Tannin pyrogallates | + | + | + |
Note: “+” indicates the presence of the metabolite.
Antibacterial activity of CEE, EAF, and MF from A. microcarpum bark extract
All the extracts showed growth inhibition of E. coli (25922), P. aeruginosa (9027), and S. aureus (25923) only at the highest concentration tested (512 μg/mL), suggesting that they had an MIC value of 512 μg/mL (data not shown). Taking into account that the extracts presented weak inhibitory activity since their MIC value was more than 500 μg/mL, we tested their possible potential to modulate the action of antibiotics at the subinhibitory concentration (MIC/8=64 μg/mL).
Modulation of antibiotic activity by CEE, EAF, and MF from A. microcarpum bark extract
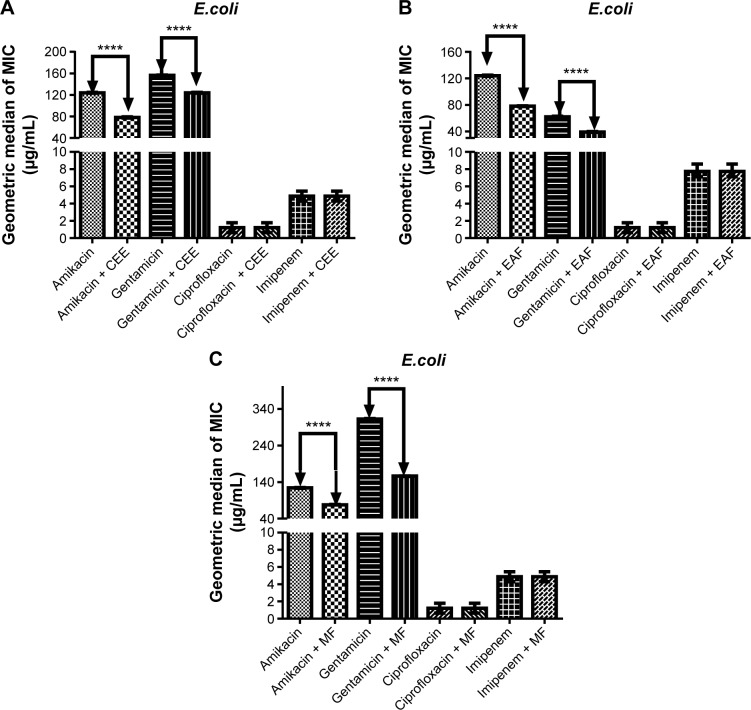
The ability of CEE, EAF, and MF to modulate the effects of the antibiotics amikacin, gentamicin, ciprofloxacin, and imipenem against E. coli 06 is depicted in Figure 1. The results indicate that the addition of CEE, EAF, and MF to the growth medium at the subinhibitory concentration caused a significant reduction of the MIC when compared to amikacin and gentamicin alone (P<0.001) (Figure 1). However, the addition of CEE, EAF, and MF to the growth medium in the presence of ciprofloxacin and imipenem did not have any effect (Figure 1). The synergistic action of the CEE, EAF, and MF with amikacin caused, respectively, a 30%, 40%, and 40% reduction in the MIC when compared to amikacin alone (Figure 1), while the combination of gentamicin with MF resulted in the highest reduction of the MIC (from 364.58 to 156.25 μg/mL), with a 57.14% reduction in comparison to gentamicin alone (Figure 1A–C).
Figure 1.
MIC of antibiotics in the absence and presence of CEE (A), EAF (B), and MF (C) from Anacardium microcarpum at the subinhibitory concentration (64 μg/mL) for Escherichia coli strain 06.
Note: ****P<0.001 indicates a significant difference when the CEE, EAF, or MF was added to the medium.
Abbreviations: CEE, crude ethanolic extract; EAF, ethyl acetate fraction; MF, methanolic fraction; MIC, minimal inhibitory concentration.
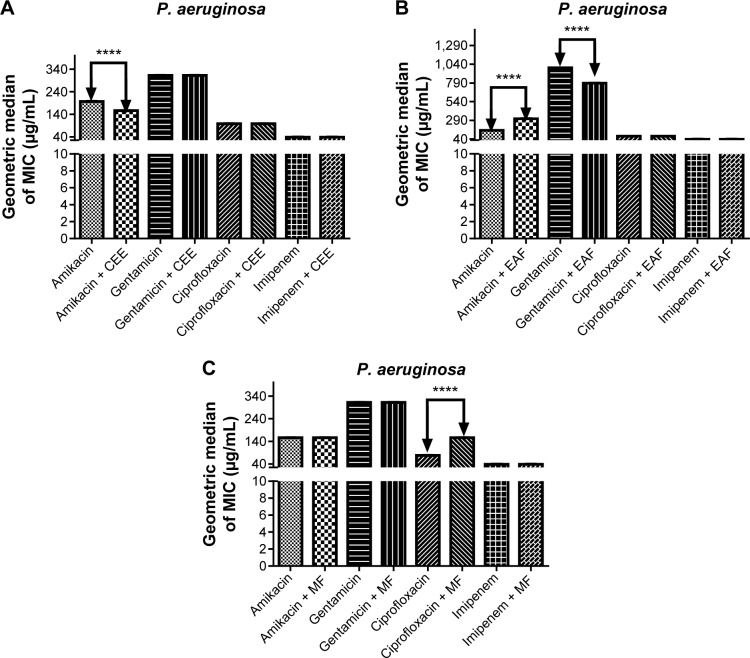
In contrast to what was observed in E. coli, CEE only modulated the action of amikacin against P. aeruginosa, with a percent reduction of 25% in the MIC (from 208.33 to 156.25 μg/mL) when compared to amikacin alone (P<0.001, Figure 2A). Similarly, the synergistic action of gentamicin with EAF caused a significant reduction in the MIC when compared to gentamicin alone (P<0.001) (Figure 2B). Surprisingly, the addition of EAF to the growth medium showed a significant antagonistic effect on the activity of the antibiotic amikacin (Figure 2B). CEE was not able to modulate the antibiotics gentamicin, ciprofloxacin, and imipenem (Figure 2A). No modulatory effect on the activity of the antibiotics ciprofloxacin and imipenem against P. aeruginosa was observed when EAF was added to the growth medium (Figure 2B). Similarly, MF did not have any effect on amikacin, gentamicin, and imipenem, but significantly decreased the MIC of ciprofloxacin (Figure 2C).
Figure 2.
MIC of antibiotics in the absence and presence of CEE (A), EAF (B), and MF (C) from Anacardium microcarpum at the subinhibitory concentration (64 μg/mL) for Pseudomonas aeruginosa strain 03.
Note: ****P<0.001 indicates a significant difference when the CEE, EAF, or MF was added to the medium.
Abbreviations: CEE, crude ethanolic extract; EAF, ethyl acetate fraction; MF, methanolic fraction; MIC, minimal inhibitory concentration.
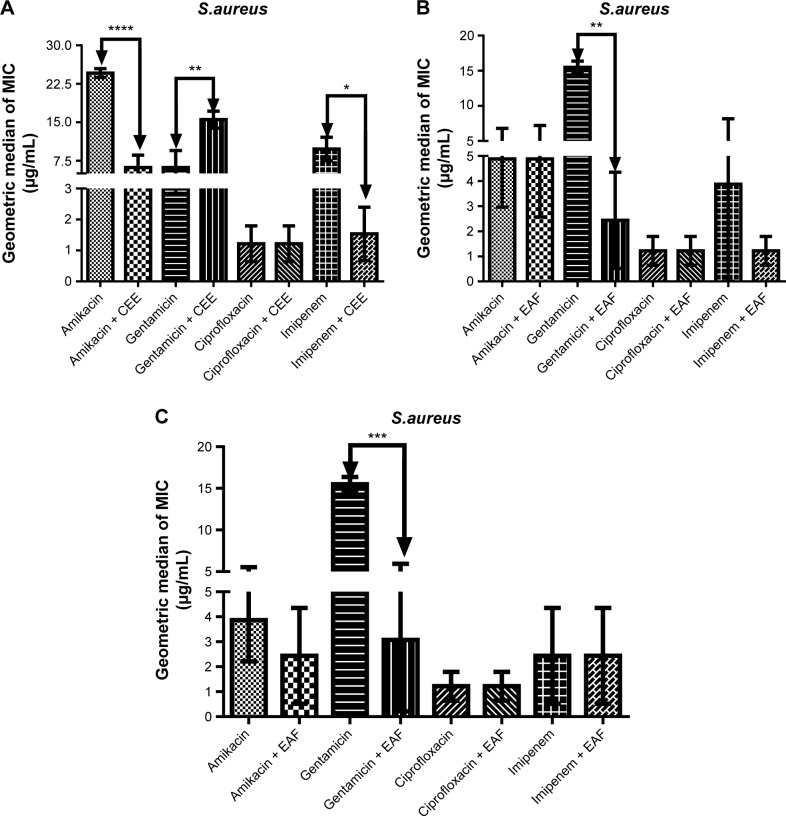
Against multidrug-resistant S. aureus, CEE demonstrated synergism with the antibiotics amikacin and imipenem (Figure 3A), showing a reduction of MIC of 60.94% and 90.46%, respectively. Similarly, EAF (Figure 3B) and MF (Figure 3C), when combined with gentamicin, significantly modulated the effect of the antibiotic. However, CEE exhibited a significant antagonistic effect on the activity of gentamicin in comparison to gentamicin alone (P<0.001) (Figure 3A). The combination of EAF with imipenem caused a dramatic decrease in the MIC of the antibiotic (P<0.001) (Figure 3B). Ciprofloxacin, when combined with CEE (Figure 3A), EAF (Figure 3B), or MF (Figure 3C), did not promote any reduction of the MIC. Similarly, MF did not show any synergism with amikacin or imipenem (Figure 3C).
Figure 3.
MIC of antibiotics in the absence and presence of CEE (A), EAF (B), and MF (C) from Anacardium microcarpum at the subinhibitory concentration (64 μg/mL) for Staphylococcus aureus strain 10.
Note: *P<0.5; **P<0.1; ***P<0.01; ****P<0.001 indicates a significant difference when the CEE, EAF, or MF was added to the medium.
Abbreviations: CEE, crude ethanolic extract; EAF, ethyl acetate fraction; MF, methanolic fraction; MIC, minimal inhibitory concentration.
Discussion
The challenge of antibiotic resistance has recently generated considerable interest in the exploration of natural products from plant origins as a combination therapy in order to modulate the action of antibiotics.19,20,31 In this context, the aim of this study was to evaluate the potential modulatory action of four antibiotics (amikacin, gentamicin, ciprofloxacin, and imipenem) by CEE and fractions (ethyl acetate and methanolic) from A. microcarpum against different bacterial strains (E. coli 06, P. aeruginosa 03, and S. aureus 10). The results presented herein indicate that A. microcarpum exhibited significant synergistic action with the tested antibiotics, especially those from the class of aminoglycosides (amikacin and gentamicin), against the tested bacterial strains.
Numerous studies32,33 have reported the antibacterial activity of plant extracts by measuring their capacity to inhibit bacterial growth. In the current study, we investigated the antibacterial activity of CEE, EAF, and MF using the microdilution method. The results indicate that all the extracts showed weak antibacterial activity against the bacterial strains tested, since their MIC value was greater than 500 μg/mL. According to Dall’Agnol et al32 and Tanaka et al,33 plant extracts with 500≤ MIC ≤1,000 μg/mL present weak inhibitory activity.
Aminoglycosides are a class of bactericidal antibiotics characterized by the presence of a six-carbon aminocyclitol ring covalently bonded to multiple amino sugar groups. They can act by impairing bacterial protein synthesis through irreversible binding to the 30S subunit of the bacterial ribosome.34 The resistance of bacteria (Gram-positive and -negative) to aminoglycosides mainly includes enzyme inactivation and the presence of efflux proteins that are able to pump the antibiotic to the extracellular space. The effects of the aminoglycosides, amikacin and gentamicin, used in this study were significantly modulated by CEE, EAF, and MF from A. microcarpum against E. coli. Interestingly, CEE and EAF also demonstrated significant synergism with imipenem against S. aureus. Similarly, MF modulated the action of ciprofloxacin-resistant P. aeruginosa and gentamicin-resistant S. aureus. The inhibition of bacterial growth by plant extracts and chemicals occurs through diverse mechanisms which may be related to the hydrophobic nature of the compound(s). Although we did not investigate the mechanism by which these extracts modulate aminoglycoside activity, we hypothesize that it might be attributed to the chemical constituent of these extracts. It is possible that compounds from A. microcarpum are interacting with the lipid bilayer of the cell membrane or with proteins present on the bacterial outer plasma membrane,35 thereby facilitating the permeability to antibiotics, leading to the interruption of vital cellular activity.36 Phytochemical prospection of A. microcarpum revealed the presence of a variety of secondary metabolites, including phenols, flavonoids, tannins, and alkaloids, which are known to be responsible for the antimicrobial activity of most plants. Of particular interest, a recent study has shown that compounds such as polyphenols can interact with signal transduction pathways and cell receptors, leading to interruption of the microbial growth, and induce cell death.37
Natural compounds from plant origin can cause an alteration in the effect of antibiotics, either by increasing or antagonizing the antibiotic activity.31 In the present study, CEE antagonized the activity of gentamicin against S. aureus, while EAF antagonized the activity of amikacin-resistant P. aeruginosa, by significantly increasing the MIC of the antibiotics. Our results corroborate those of Veras et al38 who observed a significant increase in the MIC value when they combined natural products and aminoglycosides. This antagonism may be partly due to mutual interaction/chelation of the antibiotic with the chemical component of the plant extracts.39,40
Conclusion
This study reports for the first time the potential of CEE and fractions (ethyl acetate and methanolic) from A. microcarpum to enhance, in a synergic way, different antibiotics’ resistance against different bacterial strains (E. coli 06, P. aeruginosa 03, and S. aureus 10) in vitro. Of note is the fact that all the extracts exhibited significant synergistic action with aminoglycosides (amikacin and gentamicin) against E. coli 06. The findings of the present investigation represent an important step in the search for and development of new antibacterial agents, which could be useful in overcoming the present resistant-infection problem.
Acknowledgments
Valter M Barbosa-Filho would like to specially thank CAPES for financial support. Valter M Barbosa-Filho is a beneficiary of the CAPES postgraduate (doctoral) fellowship. Jean P Kamdem especially thanks The World Academy of Sciences for the developing countries (TWAS), TWAS-CNPq, and the Alexander von Humboldt Foundation for the 2013 AGNES Grant for Junior Researchers. This work was also supported by FAPERGS and FAPERGS-PRONEX-CNPq.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rapp RP. Changing strategies for the management of invasive fungal infections. Pharmacotherapy. 2004;24:4S–28S. [PubMed] [Google Scholar]
- 2.Tenover FC. Mechanisms of antimicrobial resistance in bacteria. Am J Med. 2006;119:S3–S10. doi: 10.1016/j.amjmed.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Gullo A. Invasive fungal infections: the challenge continues. Drugs. 2009;69(Suppl 1):65–73. doi: 10.2165/11315530-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . Traditional Medicine – Growing Needs and Potential. Geneva: World Health Organization; 2002. (WHO Policy Perspectives on Medicine No. 2). Available from: http://apps.who.int/medicinedocs/en/d/Js2293e/ [Google Scholar]
- 5.Fleckenstein JM, Hardwidge PR, Munson GP, Rasko DA, Sommerfelt H, Steinsland H. Molecular mechanisms of enterotoxigenic Escherichia coli infection. Microbes Infect. 2010;12:89–98. doi: 10.1016/j.micinf.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin SJ, Yost RJ. Infectious diseases in the critically ill patients. J Pharm Pract. 2011;24:35–43. doi: 10.1177/0897190010388906. [DOI] [PubMed] [Google Scholar]
- 7.Lavoie EG, Wangdi T, Kazmierczak BI. Innate immune responses to Pseudomonas aeruginosa infection. Microbes Infect. 2011;13:1133–1145. doi: 10.1016/j.micinf.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hidron AI, Edwards JR, Patel J, et al. National Healthcare Safety Network Team. Participating National Healthcare Safety Network Facilities NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 9.Bibi Y, Nisa S, Chaudhary F, Zia M. Antibacterial activity of some selected medicinal plants of Pakistan. BMC Complement Altern Med. 2011;11:52. doi: 10.1186/1472-6882-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lima AF, Costa LB, Silva JL, Maia MB, Ximenes EC. Interventions for wound healing among diabetic patients infected with Staphylococcus aureus: a systematic review. Sao Paulo Med J. 2011;129:165–170. doi: 10.1590/S1516-31802011000300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colonna C, Dorati R, Conti B, Caliceti P, Genta I. Sub-unit vaccine against S. aureus-mediated infections: set-up of nano-sized polymeric adjuvant. Int J Pharm. 2013;452:390–401. doi: 10.1016/j.ijpharm.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 12.Tripathi KD. Essentials of Medicinal Pharmacology. Jaypee Brothers Medical Publishers; New Delhi: 2008. Antimicrobial drugs; pp. 667–818. [Google Scholar]
- 13.Sahu MC, Dubey D, Rath S, Debata NK, Padhy RN. Multidrug resistance of Pseudomonas aeruginosa as known from surveillance of nosocomial and community infections in an Indian teaching hospital. Journal of Public Health. 2012;20:413–423. [Google Scholar]
- 14.Kumar K, Chopra S. New drugs for methicillin-resistant Staphylococcus aureus: an update. J Antimicrob Chemother. 2013;68:1465–1470. doi: 10.1093/jac/dkt045. [DOI] [PubMed] [Google Scholar]
- 15.Tran TD, Do TH, Tran NC, et al. Synthesis and anti methicillin resistant Staphylococcus aureus activity of substituted chalcones alone and in combination with non-beta-lactam antibiotics. Bioorg Med Chem Lett. 2012;22:4555–4560. doi: 10.1016/j.bmcl.2012.05.112. [DOI] [PubMed] [Google Scholar]
- 16.Matias EF, Santos KA, Almeida TS, Costa JG, Coutinho HD. Phytochemical prospection and modulation of aminoglycoside antibiotic activity by Croton campestris A. Chemotherapy. 2011;57:305–309. doi: 10.1159/000328975. [DOI] [PubMed] [Google Scholar]
- 17.Sánchez-Medina A, García-Sosa K, May-Pat F, Peña-Rodríguez LM. Evaluation of biological activity of crude extracts from plants used in Yucatecan traditional medicine part I. Antioxidant, antimicrobial and beta-glucosidase inhibition activities. Phytomedicine. 2001;8:144–151. doi: 10.1078/0944-7113-00020. [DOI] [PubMed] [Google Scholar]
- 18.Weckesser S, Engel K, Simon-Haarhaus B, Wittmer A, Pelz K, Schempp CM. Screening of plant extracts for antimicrobial activity against bacteria and yeasts with dermatological relevance. Phytomedicine. 2007;14:508–516. doi: 10.1016/j.phymed.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 19.de Almeida TS, Rocha JB, Rodrigues FF, Campos AR, da Costa JG. Chemical composition, antibacterial and antibiotic modulatory effect of Croton campestris essential oils. Ind Crops Prod. 2013;44:630–633. [Google Scholar]
- 20.Barreto HM, Silva Filho EC, Lima EO, et al. Chemical composition and possible use as adjuvant of the antibiotic therapy of the essential oil of Rosmarinus officinalis L. Ind Crops Prod. 2014;59:290–294. [Google Scholar]
- 21.Dubey D, Sahu MC, Rath S, Paty DP, Debata NK, Padhy RN. Antibacterial activity of medicinal plants used by aborigines of Kalahandi, Orissa, India against multidrug resistant bacteria. Asian Pac J Trop Biomed. 2012;2:S846–S854. [Google Scholar]
- 22.Dubey D, Padhy RN. Surveillance of multidrug resistance of two Gram-positive pathogenic bacteria in a teaching hospital and in vitro efficacy of 30 ethnomedicinal plants used by an aborigine of India. Asian Pac J Trop Dis. 2012;2:273–281. [Google Scholar]
- 23.Arokiyaraj S, Sripriya N, Bhagya R, Radhika B, Prameela L, Udayaprakash NK. Phytochemical screening, antibacterial and free radical scavenging effects of Artemisia nilagirica, Mimosa pudica and Clerodendrum siphonanthus – an in–vitro study. Asian Pac J Trop Biomed. 2012;2:S601–S604. [Google Scholar]
- 24.Tajkarimi MM, Ibrahim SA, Cliver DO. Antimicrobial herb and spice compounds in food. Food Control. 2010;21:1199–1218. [Google Scholar]
- 25.Gyawali R, Ibrahim SA. Impact of plant derivatives on the growth of foodborne pathogens and the functionality of probiotics. Appl Microbiol Biotechnol. 2012;95:29–45. doi: 10.1007/s00253-012-4117-x. [DOI] [PubMed] [Google Scholar]
- 26.Gyawali R, Adkins A, Minor RC, Ibrahim SA. Behavior and changes in cell morphology of Escherichia coli O157:H7 in liquid medium and skim milk in the presence of caffeine. CyTA: Journal of Food. 2014;12:235–241. [Google Scholar]
- 27.Barbosa Filho VM, Waczuk EP, Kamdem JP, et al. Phytochemical constituents, antioxidant activity, cytotoxicity and osmotic fragility effects of caju (Anacardium microcarpum) Ind Crops Prod. 2014;55:280–288. [Google Scholar]
- 28.Matos FJ. Introdução à Fitoquímica Experimental. Fortaleza: Edições UFC; 1997. Portuguese. [Google Scholar]
- 29.National Committee for Clinical Laboratory Standards (NCCLS) Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. 6th ed. Villanova: NCCLS; 2003. (NCCLS approved standard M7-A5, v 20, n 2). [Google Scholar]
- 30.Javadpour MM, Juban MM, Lo WC, et al. De novo antimicrobial peptides with low mammalian cell toxicity. J Med Chem. 1996;39:3107–3113. doi: 10.1021/jm9509410. [DOI] [PubMed] [Google Scholar]
- 31.Coutinho HD, Costa JG, Lima EO, Falcão-Silva VS, Siqueira-Júnior JP. Enhancement of the antibiotic activity against a multiresistant Escherichia coli by Mentha arvensis L. and chlorpromazine. Chemotherapy. 2008;54:328–330. doi: 10.1159/000151267. [DOI] [PubMed] [Google Scholar]
- 32.Dall’Agnol R, Ferraz A, Bernardi AP, et al. Antimicrobial activity of some Hypericum species. Phytomedicine. 2003;10:511–516. doi: 10.1078/094471103322331476. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka JC, da Silva CC, Filho BP, Nakamura CV, de Carvalho JE, Foglio MA. Chemical constituents of Luehea divaricata Mart. (Tiliaceae) Quim Nova. 2005;28:834–837. Portuguese. [Google Scholar]
- 34.Chen LF, Kaye D. Current use for old antibacterial agents: polymyxins, rifamycins, and aminoglycosides. Med Clin North Am. 2011;95:819–842. doi: 10.1016/j.mcna.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Tsuchiya H, Sato M, Miyazaki T, et al. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J Ethnopharmacol. 1996;50:27–34. doi: 10.1016/0378-8741(96)85514-0. [DOI] [PubMed] [Google Scholar]
- 36.Juven BJ, Kanner J, Schved F, Weisslowicz H. Factors that interact with the antibacterial action of thyme essential oil and its active constituents. J Appl Bacteriol. 1994;76:626–631. doi: 10.1111/j.1365-2672.1994.tb01661.x. [DOI] [PubMed] [Google Scholar]
- 37.Rodrigues FF, Costa JG, Coutinho HD. Synergy effects of the antibiotics gentamicin and the essential oil of Croton zehntneri. Phytomedicine. 2009;16:1052–1055. doi: 10.1016/j.phymed.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Veras HN, dos Santos IJ, dos Santos AC, et al. Comparative evaluation of antibiotic and antibiotic modifying activity of quercetin and isoquercetin in vitro. Curr Top Nutraceutical Res. 2011;9:25–30. [Google Scholar]
- 39.Behling EB, Sendão MC, Francescato HD, Antunes LM, Bianchi ML. Flavonoid quercetin: general aspects and biological actions. Alimentos e Nutricao. 2004;15:285–292. [Google Scholar]
- 40.Granowitz EV, Brown RB. Antibiotic adverse reactions and drug interactions. Crit Care Clin. 2008;24:421–442. doi: 10.1016/j.ccc.2007.12.011. [DOI] [PubMed] [Google Scholar]