Abstract
A liver, heart, iliac vessel, and two kidneys were recovered from a 39-year-old man who died of traumatic head injury and were transplanted into five recipients. The liver recipient eighteen days post-transplantation presented with headache, ataxia and fever, followed by rapid neurologic decline and death. Diagnosis of granulomatous amebic encephalitis (GAE) was made on autopsy. Balamuthia mandrillaris infection was confirmed with immunohistochemical and PCR assays. Donor and recipients' sera were tested for B. mandrillaris antibodies. Donor brain was negative for Balamuthia by immunohistochemistry and PCR; donor serum Balamuthia antibody titer was positive (1:64). Antibody titers in all recipients were positive (range, 1:64 to 1:512). Recipients received a 4- to 5-drug combination of miltefosine or pentamidine, azithromycin, albendazole, sulfadiazine, and fluconazole. Nausea, vomiting, elevated liver transaminases and renal insufficiency was common. All other recipients survived and have remained asymptomatic 24-months post-transplant. This is the third donor-derived Balamuthia infection cluster described in solid organ transplant recipients in the U.S. As Balamuthia serologic testing is only available through a national reference laboratory, it is not feasible for donor screening, but may be useful to determine exposure status in recipients and to help guide chemotherapy.
Keywords: Balamuthia mandrillaris, amebic encephalitis, donor-derived infection, miltefosine
Introduction
In February 2012, the U.S. Centers for Disease Control and Prevention (CDC) was asked to evaluate brain sections from a liver transplant recipient who, eighteen days post-transplantation in November 2011, presented with headache, ataxia, fever, rapid neurologic decline and progressed to death. Diagnosis of granulomatous amebic encephalitis (GAE) was made on autopsy and Balamuthia mandrillaris infection was confirmed with immunohistochemical and PCR assays at CDC. Organs procured from the same donor included (in addition to the liver): heart, iliac vessel, and two kidneys transplanted into four recipients.
When the liver recipient became ill the host organ procurement organization (OPO) and transplant centers were contacted about a potential donor derived infection. All recipients were well and without evidence of disease. The CDC confirmed the diagnosis of Balamuthia in the liver recipient and notified the transplant center of the findings. The liver recipient's transplant center rapidly notified the organ procurement organization, who, in turn, notified the Organ Procurement and Transplantation Network (OPTN). A public health investigation was undertaken directed by the CDC to evaluate the other recipients for exposure to B. mandrillaris and to provide serologic testing to monitor response to anti-Balamuthia medications.
Balamuthia mandrillaris is a free-living ameba that is distributed in the natural environment. Infection is rare with fewer than 200 cases reported worldwide; however, it is likely that this entity is misdiagnosed as other types of encephalitis or neurologic disease (1-3). The exact ecological niche is unknown, but the organism has been isolated on several occasions from soil, dust and water (1, 4-6,7). Disease caused by Balamuthia appears to be associated with contact with soil or stagnant water. Balamuthia disease affects both immunocompetent and immunocompromised patients with underlying co-morbidities such as HIV infection, diabetes and drug abuse; it appears to occur more frequently in persons of Hispanic ethnicity (1-3, 8-11).
Balamuthia cysts and trophozoites can be introduced into the body through inhalation into the lower respiratory tract and through ulcerated or broken skin. Disease can occur weeks to months after exposure, and manifests typically as encephalitis, known as granulomatous amebic encephalitis (GAE). Symptoms of central nervous system (CNS) infection include headache, stiff neck, nausea, fever and changes in mental status. Skin involvement, if present, appears as papular, erythematous lesions which evolve to violaceous plaques. Optimal treatment is undefined but consists typically of three or more of the following agents administered for a prolonged period: macrolides, pentamidine, antifungal agents (amphotericin B, azoles, flucytosine), albendazole, sulfadiazine and miltefosine (12-14). Despite aggressive combination antimicrobial therapy the disease is associated with high mortality.
Two clusters of donor-derived Balamuthia infection have been described previously in the United States (10, 15). Of eight organ recipients exposed in these two clusters, four developed GAE and three of the four died. Four patients without proven infection were administered preemptive therapy of variable duration. Herein, we describe a third transmission of Balamuthia through organ transplantation among five solid organ transplant (SOT) recipients and use of serologic testing to monitor response to therapy in four patients.
Methods
Epidemiologic Investigation
We reviewed donor and recipient medical records and conducted patient interviews to characterize clinical history, potential risk factors for infection, diagnostic studies and outcomes.
Laboratory Investigation
Recipient cerebrospinal fluid (CSF) and heart-recipient lung biopsy samples were processed for culture as follows. The CSF was divided into two aliquots; one aliquot was inoculated into human lung fibroblast (HLF) cell monolayer and the other aliquot was used to extract DNA for PCR analysis. Lung biopsy tissue was minced and inoculated into a different HLF monolayer. The HLF flasks inoculated with the CSF and lung samples were incubated at 37°C and examined daily under an inverted microscope equipped with phase contrast optics (16).
Donor and recipient serum samples were tested by an immunofluorescence assay (IFA as described previously) (17). Serum samples were considered to be positive for exposure in transplant patients if ≥1:64. Additionally, IFA was performed on tissue sections using anti-Acanthamoeba castellanii and anti-Balamuthia mandrillaris sera as described previously (16).
Immunohistochemical assays (IHC) were performed on tissue samples using a polymer-based indirect immunoalkaline phosphatase detection system with colorimetric detection of antibody/polymer complex with Fast Red Chromogen (Thermo Fisher Scientific or Biocare Medical). Tissues were evaluated with a pool of anti-amoebic antibodies which include a rabbit polyclonal against Naegleria fowleri, rabbit polyclonal against Acanthamoeba culbertsoni, and a rabbit polyclonal against Balamuthia mandrillaris.
DNA was extracted directly from formalin fixed clinical samples using the DNeasy blood and tissue DNA extraction kit (QIAGEN, Valencia, CA). Samples were tested by PCR using a TaqMan assay that detects Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri as described elsewhere (18).
Results
An autopsy of the liver transplant recipient was performed. Results were suggestive of GAE and the specimens were sent to the CDC for evaluation. Laboratory testing confirmed the diagnosis as GAE caused by Balamuthia mandrillaris. The donor's organ procurement agency was notified as described above, and an investigation was initiated.
Case Reports
Donor
The donor was a 39-year-old white man who presented to an emergency room with head trauma secondary to an assault in October, 2011. He was lethargic, confused and potentially had an unwitnessed seizure per his mother's report. He had been involved in an altercation and sustained a contusion on his posterior scalp. Past medical history included seizure disorder, for which he received carbamazapine, substance abuse and previous homelessness. He was incarcerated briefly in 1997 and 2011. On presentation he was afebrile. His white blood cell count was elevated (18,600 cells/μL). A toxicological screen was positive for cocaine. Head CT imaging showed bilateral frontal and temporal contusions and a left parietal contusion. Two days post-admission cerebral perfusion studies showed no blood flow. He died the next day with cause of death documented as blunt head trauma/homicide. Permission from the family was granted for organ donation. The heart, liver, iliac vessel, and kidneys were recovered; no other tissues were procured.
After the liver recipient was diagnosed with GAE post-mortem, immunohistochemical (IHC) testing was performed by the CDC on donor brain, lung, and kidney tissue which was negative for Balamuthia and other free-living amebae. Formalin-fixed brain and lung tissue were also PCR-negative for Balamuthia and other free-living amebae. Archived serum and plasma from the donor, drawn at time of organ procurement, were positive for anti-Balamuthia antibodies with titers of 1:64 obtained on both samples when tested with IFA.
Liver Recipient
The liver recipient was a 58-year-old white male with a history of cirrhosis and end-stage liver disease secondary to hepatitis C virus infection (Table 1). His immediate post-operative course was uneventful and he was discharged 10-days post-transplant. He presented eight days after discharge complaining of headaches for one week, neck stiffness, ataxia and fever. Shortly thereafter, he developed altered mental status and neuroimaging revealed multiple brain infarcts and moderate edema. CSF studies showed neutrophilic pleocytosis (975cells/μL; 63% polymorphs, 20% macrophages, 16% lymphocytes and 1% eosinophils). CSF protein was elevated (221mg/dL) and CSF glucose was normal. Blood, CSF, and urine cultures were negative. CSF was negative for cryptococcal antigen, but positive for Toxoplasma IgG. He had a rapid neurologic decline and care was withdrawn by the family 12 days after admission. Samples of brain tissue from autopsy were consistent with ameba on histopathologic examination (Figure 1) and were positive for Balamuthia by IHC and PCR.
Table 1. Organ recipient characteristics.
| Organ transplanted | Liver | Heart | Iliac Vessel | Left kidney | Right kidney |
|---|---|---|---|---|---|
| Demographics | 58-year-old white male | 62-year-old white male | 61-year-old white male | 69-year-old black male | 60-year-old white female |
| Underlying Disease | Hepatitis C, cirrhosis | Ischemic cardiomyopathy | Alcoholic cirrhosis, hepatocellular carcinoma | HTN, DM, ESRD | HTN, DM, CAD, ESRD, |
| Induction | basiliximab | basiliximab methylprednisolone | basiliximab | basiliximab followed by ATG for delayed graft function | alemtuzumab methylprednisolone |
| Immunosuppression | Tacrolimus mycophenolate | cyclosporine mycophenolate prednisone | tacrolimus mycophenolate prednisone | tacrolimus mycophenolate prednisone | mycophenolate tacrolimus |
| Clinical course | Fever, malaise, ataxia, headache, neck stiffness that began on post-operative day 12. Had rapid neurologic decline and death | Complicated by leukocytosis and fever. No source was clearly identified, but patient recovered fully | Asymptomatic | Asymptomatic | Asymptomatic |
| Laboratory data | CSF: 975 WBC/μL; 63% PMNs, 20% macrophages, 16% lymphocytes and 1% eosinophils; protein elevated at 221mg/dL and glucose normal. CSF cultures were negative | CSF : 2 WBC/μL, protein elevated at 196mg/dL and normal glucose CSF samples were PCR-negative for Balamuthia and other free-living ameba. One sample revealed unusual cells similar to that of Balamuthia (Figure 2) |
CSF: WBC 0/μL, normal glucose and protein; samples were PCR-negative for Balamuthia and other free-living ameba | CSF: WBC 0/μL, protein elevated at 82 mg/dL, glucose 94 CSF PCR negative for Balamuthia and other free living ameba |
CSF : WBC 24/μL; (15% PMNs, 9% lymphocytes, 14% monocytes, 2% eosinophils). RBC 6000/μL; protein mildly elevated at 93 mg/dl, glucose of 50mg/dl CSF PCR negative for Balamuthia and other free living ameba |
| Baseline Balamuthia serology | Not applicable | 1:512 | 1:128 | 1:256 | 1:128 |
| Neuroradiology (MRI) | MRI brain: multiple brain infarcts and moderate edema | No acute intracranial process | No acute intracranial process | No acute intracranial process | No acute intracranial process; signs of chronic white matter changes |
| Initial Balamuthia medications | No specific therapy (diagnosis made post-mortem) | azithromycin 20 mg/kg daily; albendazole 400 mg bid; fluconazole 400 mg daily; sulfadiazine 1.5 grams every 6 hrs; pentamidine 4mg/kg IV daily replaced with miltefosine 50 mg tid two days later | azithromycin 20 mg/kg daily; albendazole 400mg bid; fluconazole 400mg daily; pentamidine 4mg/kg IV daily replaced with miltefosine 50 mg tid five days later | azithromycin 20 mg/kg daily; albendazole 400 mg PO bid; fluconazole 400 mg PO daily; sulfadiazine 1.5 grams PO every 6 hours; pentamidine 4mg/kg IV daily replaced with miltefosine 7 days later | Initially clarithromycin and fluconazole; albendazole added 10 days later; miltefosine was started a few days later; azithromycin replaced clarithromycin |
| Duration of Balamuthia medications | 143 days (not including additional 180 days of fluconazole for cryptococosis) | 175days | 162 days | 68 days | |
| Adverse effects | Nausea, vomiting, weight loss, renal failure, elevated transaminases requiring two admissions and temporary discontinuation of some medications | Nausea, anorexia, weight loss, elevated transaminases requiring discontinuation of some medications | Mildly elevated transaminases, intermittent diarrhea. Did not require discontinuation of therapy | Nausea, dizziness, vomiting, elevated creatinine, tacrolimus toxicity. Required two admissions and discontinuation of some medications | |
| Outcome | Died Diagnosis at autopsy; Balamuthia mandrillaris confirmed by PCR and immunohistochemical staining of brain tissue |
Survived Currently asymptomatic |
Survived Currently asymptomatic |
Survived Currently asymptomatic |
Survived Currently asymptomatic |
HTN=hypertension; DM=diabetes mellitus; ESRD=end-stage renal disease; CAD=coronary artery disease; ATG=anty-thymocyte globulin; CSF=cerebrospinal fluid; WBC=white blood cell; PMN=polymorphonuclear cell; RBC=red blood cell; MRI=magnetic resonance imaging; PCR=polymerase chain reaction.
Figure 1. Liver recipient autopsy findings.
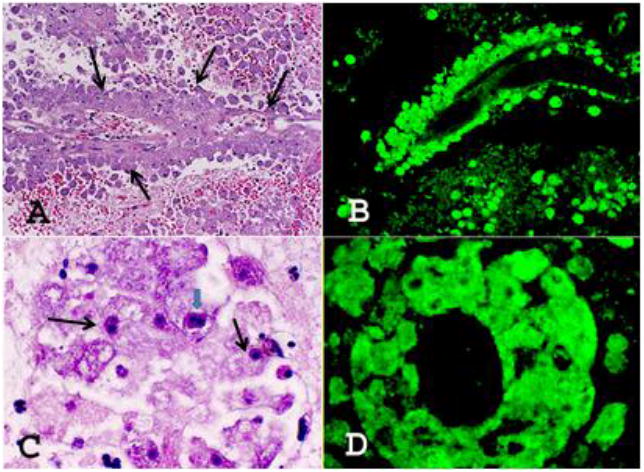
CNS sections of the liver recipient stained with H&E (A & C) and reacted with the anti-Balamuthia antibodies (B & D). A. Large numbers of Balamuthia amebae (at arrows) are seen around a blood vessel. H&E, ×200. B. A similar section reacted with the anti-Balamuthia antibodies in the immunofluorescence test. Balamuthia amebae around the blood vessel are intensely fluorescing, staining bright apple green. ×200. C. Large numbers of Balamuthia (at arrows) around a blood vessel. Note the darkly staining nucleus. A CNS section stained with H&E. One ameba is seen with double nucleolus (big arrow), × 1,000. D. A section as in B with large numbers of intensely staining apple green amebae are seen around a blood vessel, × 1,000.
Heart Recipient
The heart recipient was a 62-year-old white male with a history of ischemic cardiomyopathy. Approximately four months after transplantation he underwent evaluation for Balamuthia exposure with serologic testing, brain imaging and lumbar puncture (Table 1). Microscopic examination of the Human Lung Fibroblast culture of one CSF sample revealed a few unusual cells approximately 30 μm in size, with pseudopodial movement and nuclear morphology similar to that of Balamuthia and other free-living amebas (Figure 2). These cells failed to grow in culture and decayed. Initial serology for Balamuthia antibodies was positive at 1:512 (Figure 3).
Figure 2. Heart recipient CSF.
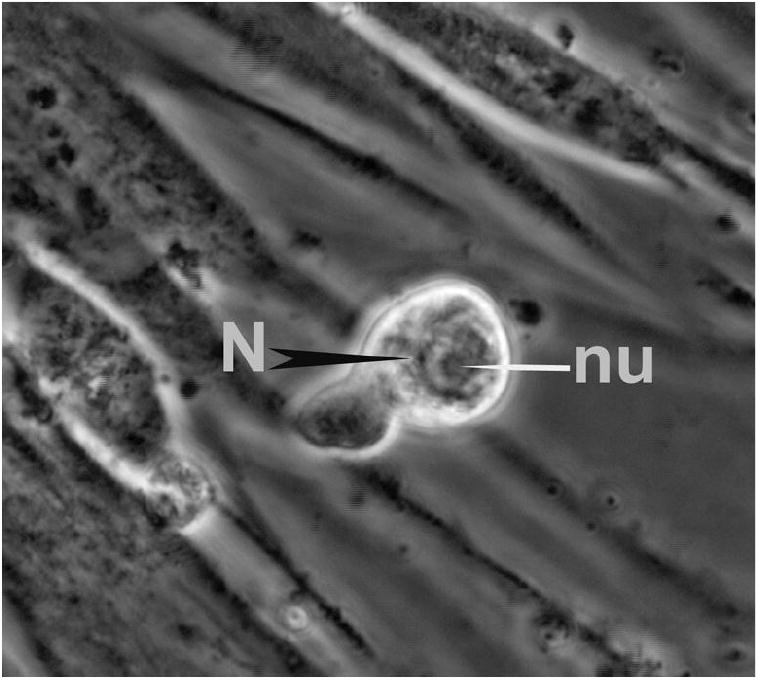
A cell with the characteristic morphology of an ameba in the HLF CSF culture of the heart transplant recipient. Note the nucleus (N) and nucleolus (nu); phase contrast, ×600.
Figure 3.
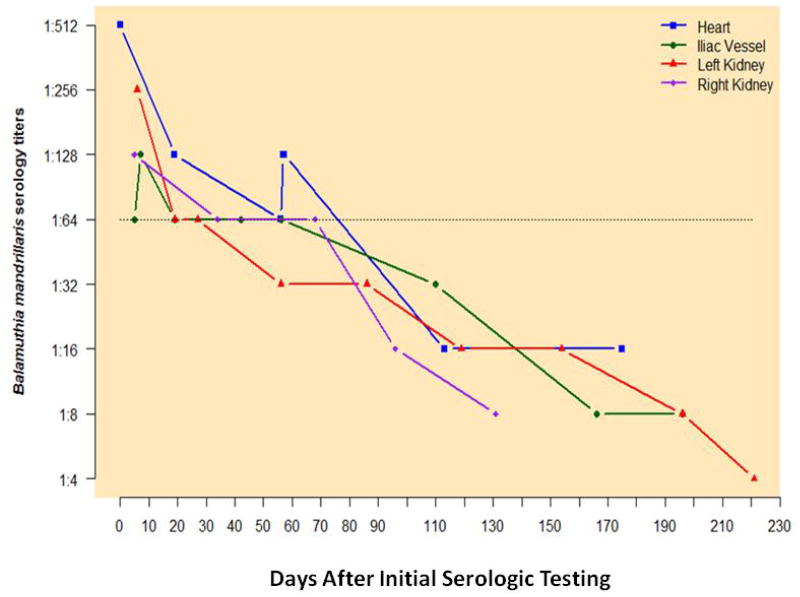
Recipient Balamuthia serologies. Days on x-axis represent days from initial serologic testing. Initial serologic testing in all patients occurred approximately 4 months post-transplant.
He was administered initial Balamuthia treatment using the following medications: azithromycin 20 mg/kg daily; albendazole 400 mg bid; fluconazole 400 mg daily; sulfadiazine 1.5grams every 6 hrs; pentamidine 4mg/kg IV daily (replaced with miltefosine 50 mg tid two days later). During his course of therapy her developed weight loss, anorexia and nausea and was admitted on several occasions for acute kidney injury and elevated liver function tests (Table 1). Several medications (miltefosine, albendazole) were discontinued and then restarted when he improved. Balamuthia titers decreased to negative (1:16) over a 110-day period (Figure 3).
Iliac Vessel Recipient
The vessel recipient was a 61-year-old white male with a history of cirrhosis secondary to alcohol use, complicated by hepatocellular carcinoma (Table 1). In October 2012 he received an iliac vessel graft from the donor for use as a vascular conduit. He received a liver transplant from a different donor. Four months after transplantation he underwent evaluation for Balamuthia exposure with serologic testing, brain imaging and lumbar puncture. CSF samples were PCR-negative for Balamuthia and other free-living ameba. Initial serology for Balamuthia antibodies was positive (1:128; Figure 3).
Balamuthia treatment was begun with the following: azithromycin 20 mg/kg daily; albendazole 400mg bid; fluconazole 400mg daily; pentamidine 4mg/kg IV daily (replaced with miltefosine 50 mg tid five days later). During his treatment course he developed intermittent nausea, anorexia, malaise and elevated liver function tests that necessitated discontinuing of some medications and re-starting medications when laboratory abnormalities improved. Balamuthia titers decreased to negative (1:8) over a 160-day period (Figure 3)
Left Kidney Recipient
The left kidney recipient was a 69-year-old black male with a history of hypertension and diabetes (Table 1). Approximately four months after transplantation he underwent serologic testing, brain imaging and lumbar puncture. Initial serology for Balamuthia antibodies was positive at 1:256 (Figure 3). CSF PCR was negative for Balamuthia and other free-living amebae.
Balamuthia treatment was initiated with the following: azithromycin 20 mg/kg daily (1,000 mg PO daily); albendazole 400 mg PO bid; fluconazole 400 mg PO daily; pentamidine 4mg/kg IV daily (300 mg IV daily replaced with miltefosine 50 mg tid 7 days later); sulfadizine 1.5 grams PO Q6 hours. The patient remained this regimen throughout the duration of treatment (162 days). Adverse drug effects included diarrhea and mild AST and ALT elevation. Balamuthia antibody titers decreased to negative (1:16) by approximately 100 days after initiation of treatment (Figure 3).
Right Kidney Recipient
The right kidney recipient was a 60-year-old female with a history of diabetes and hypertension (Table 1). As part of her evaluation she had serum testing which revealed an initial antibody titer for Balamuthia of 1:128. Her initial regimen included clarithromycin 1000 mg daily and fluconazole 400mg daily, drugs she could obtain while she was travelling. Several days later miltefosine was added and azithromycin was substituted for clarithromycin Adverse effects included unsteadiness, inability to walk, nausea, postprandial vomiting and intermittent hallucinations. She developed dehydration, acute kidney injury (Cr 9.7 mg/dL), and tacrolimus toxicity (level> than 60 ng/ml), requiring hospitalization and temporary discontinuations of several medications. The Balamuthia serology was negative (1:16) approximately 90 days after initiation of medications.
Discussion
We describe donor-derived Balamuthia exposure among four SOT recipients and one recipient of an iliac vessel, one of whom developed GAE and died. B. mandrillaris is an uncommon pathogen but is emerging as a cause of donor-derived infection (2, 11, 19). Transplant professionals should consider Balamuthia as a potential cause of unexplained encephalitis in organ donors and recipients; however, it important to note that our donor did not have any signs of encephalitis or skin lesions consistent with Balamuthia disease.
There have been two previous recent reports of donor-derived Balamuthia infection in the US (10, 15). In the first, the donor was a four-year-old boy who developed a febrile illness, seizures and headache (10). Donor brain tissue from autopsy showed amebae histopathologically consistent with Balamuthia and PCR was positive for Balamuthia. Two of the four organ recipients (right and left kidney) developed Balamuthia encephalitis and were treated with a combination of pentamidine, sulfadiazine, flucytosine, fluconazole, azithromycin, and later miltefosine; one progressed to death three months post-transplant. The heart recipient did not develop Balamuthia antibodies but was nonetheless treated empirically (pentamidine, azithromycin and fluconazole) and remained asymptomatic. The seropositive liver recipient was treated with pentamidine, azithromycin, fluconazole and sulfadiazine.
The second cluster occurred in 2010 (15). The organ donor was a Hispanic male with a chronic skin lesion who died of presumed stroke. The liver and kidney-pancreas recipients developed fatal encephalitis 18 days and 24 days post-transplant. Two other recipients (heart, second kidney) remained asymptomatic and were given prophylactic therapy with multi-drug combinations.
The donor associated with this third cluster had no skin lesions, no fever at time of death, and a negative brain biopsy for Balamuthia; nevertheless, transplant-associated transmission of Balamuthia from his donor due to amebae in his blood and/or organ tissues likely occurred. The donor was seropositive (1:64) and all of the recipients of his tissues were seropositive (>1:64), suggesting potentially efficient (100%) transmission. Further, GAE occurred in the liver recipient. The identification of an ameba-like organism in the CSF of the asymptomatic heart recipient who had the highest serum antibody titer (1:512) among all the recipients is also suggestive, although the cells failed to grow in culture.
In this cluster described herein, a gradual reduction in titers to negative levels (1:16) was achieved in all asymptomatic recipients over a period of 306 days post transplantation; maximum duration of Balamuthia treatment was 175 days. Previously, in non-transplant clusters, serum samples were considered positive for titers ≥1:128 (17). Because of immunosuppression in transplant patients, lower titers to Balamuthia might be found with infection, so exposure in transplant patients is considered to be a titer of ≥1:64 and a two-fold reduction in titers is considered to be seronegative under these circumstances (17, 24).
Organ donor screening has reduced the risk of transmission of many important pathogens such as HIV and hepatitis C virus. If a recipient is thought to potentially have a transplant-transmitted illness, Organ Procurement and Transplantation Network (OPTN) policy requires reporting of potential donor-derived disease from both OPOs and transplant centers. The transplant center should notify the OPO and evaluation of other recipients should occur. OPTN should be notified by the OPO and transplant center. Any illness of uncertain cause found to be present in more than one recipient should be immediately reported to the OPO, OPTN, and public health authorities upon discovery by treating healthcare providers (25).
Because of the rarity of Balamuthia infection among SOT recipients and lack of an FDA approved screening test, laboratory donor screening is not feasible or justified, highlighting the importance of communication between the OPO and transplant centers. We used a serologic screening test developed at the CDC to identify infected but asymptomatic patients and to monitor patients monthly while receiving therapy. In all cases, negative titers (≤1:16) were achieved before medicines were discontinued. All patients at risk also received lumbar punctures with CSF studies sent for culture and PCR. Tissue biopsies for imunohistochemistry from brain or skin lesions may also be helpful to determine Balamuthia infection, if present.
Although combination Balamuthia therapy has led to successful in cases of GAE (12, 20-23), effective treatment for B. mandrillaris disease has not been established. Mortality is high and optimal medications combinations are undefined; GAE has been treated in the past with various combinations of macrolides, pentamidine, antifungal agents (amphotericin B, azoles, flucytosine), sulfadiazine, albendazole, miltefosine, and additional drugs. The advantages of medications for patients exposed to Balamuthia are unclear. In the three transplant-associated clusters to date, none of the asymptomatic patients exposed to Balamuthia through solid-organ transplantation who received empiric therapy developed GAE; however, side effects were problematic. In our cluster, prolonged (range, 68-175 days) empiric therapy was given to our asymptomatic recipients, which proved challenging. We added miltefosine to the regimen, as it has been used with some success with other therapies for Balamuthia GAE and was used similarly in a previous cluster (10, 12). Most patients in our 2012 cluster developed nausea, vomiting, malaise, dehydration, renal insufficiency, and abnormal liver studies. Three patients required admission because of adverse drug effects and only one patient tolerated a five-drug regimen without interruption for the treatment duration. Additionally, abnormal drug levels of immunosuppressive agents caused by interactions with the anti-Balamuthia regimen were noted. Administrative issues also arose as emergency Food and Drug Administration (FDA) investigational new drug protocols and Institutional Review Board (IRB) approvals were required to obtain miltefosine.
This investigation was limited by the inability to identify Balamuthia in culture or by PCR in the organ donor and surviving organ recipients. Formalin fixed tissue available from the organ donor, is not the optimal specimen for PCR as formalin may interfere with test performance. The serologic assay is experimental and has not been validated in the post-transplant population; however, given the specificity of the assay, it is unlikely that the positive serologic results in this cluster are secondary to cross-reactivity (16,17). This, coupled with positive serologic findings in all recipients and the donor, suggests donor-derived exposure.
In summary, we describe a cluster of donor-derived Balamuthia transmission in solid organ transplant recipients. Only one of five exposed patients developed encephalitis. To date among three transplant clusters, symptomatic organ recipients have received chemotherapy for treatment of transplant-transmitted GAE and asymptomatic organ recipients have received prophylactic chemotherapy, guided by serology, for presumed B. mandrillaris exposure. Asymptomatic organ recipients in the 2012 cluster were considered exposed and received chemotherapy if serologies reached ≥1:64 and continued therapy, adjusted for side effects, until their serologies fell to 1:16 or lower. Although the serological treatment points were experimental and require further confirmation, and optimal preemptive therapy regimens are undefined, asymptomatic organ recipients in these three clusters who had initial titers ≥1:64 and who were managed in this way did not develop GAE.
Acknowledgments
We would like to thank the many members of the outbreak investigation team: Alabama Department of Health: Mary McIntyre, Tina Pippin, Joanna Roberson, Michael Davis, Sharon Massingale, Tom Miller Alabama Organ Procurement Agency: Virginia Guindon CDC: Rama Sriram; Infectious Diseases Pathology Branch: Dianna M. Blau, Clifton Drew, Lindy Liu, Christopher Paddock, Jana Ritter, Wun-Ju Shieh, Christopher Taylor, Sherif R. Zaki
This work was presented in part at the American Society of Transplant Surgeon's 13th Annual State of the Art Winter Symposium, January 31-Februrary 3, 2013, Miami, FL.
Footnotes
Publisher's Disclaimer: Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of CDC.
Disclosures: All authors have no disclosures or conflicts to report.
References
- 1.Matin A, Siddiqui R, Jayasekera S, Khan NA. Increasing importance of Balamuthia mandrillaris. Clinical microbiology reviews. 2008;21(3):435–448. doi: 10.1128/CMR.00056-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuster FL, Yagi S, Gavali S, Michelson D, Raghavan R, Blomquist I, et al. Under the radar: balamuthia amebic encephalitis. Clin Infect Dis. 2009;48(7):879–887. doi: 10.1086/597260. [DOI] [PubMed] [Google Scholar]
- 3.Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS immunology and medical microbiology. 2007;50(1):1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 4.Dunnebacke TH, Schuster FL, Yagi S, Booton GC. Balamuthia mandrillaris from soil samples. Microbiology (Reading, England) 2004;150(Pt 9):2837–2842. doi: 10.1099/mic.0.27218-0. [DOI] [PubMed] [Google Scholar]
- 5.Niyyati M, Lorenzo-Morales J, Rezaeian M, Martin-Navarro CM, Haghi AM, Maciver SK, et al. Isolation of Balamuthia mandrillaris from urban dust, free of known infectious involvement. Parasitology research. 2009;106(1):279–281. doi: 10.1007/s00436-009-1592-9. [DOI] [PubMed] [Google Scholar]
- 6.Schuster FL, Dunnebacke TH, Booton GC, Yagi S, Kohlmeier CK, Glaser C, et al. Environmental isolation of Balamuthia mandrillaris associated with a case of amebic encephalitis. Journal of clinical microbiology. 2003;41(7):3175–3180. doi: 10.1128/JCM.41.7.3175-3180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmad AF, Andrew PW, Kilvington S. Development of a nested PCR for environmental detection of the pathogenic free-living amoeba Balamuthia mandrillaris. The Journal of eukaryotic microbiology. 58(3):269–271. doi: 10.1111/j.1550-7408.2011.00541.x. [DOI] [PubMed] [Google Scholar]
- 8.Schuster FL, Glaser C, Honarmand S, Maguire JH, Visvesvara GS. Balamuthia amebic encephalitis risk, Hispanic Americans. Emerging infectious diseases. 2004;10(8):1510–1512. doi: 10.3201/eid1008.040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva-Vergara ML, Da Cunha Colombo ER, De Figueiredo Vissotto E, Silva AC, Chica JE, Etchebehere RM, et al. Disseminated Balamuthia mandrillaris amoeba infection in an AIDS patient from Brazil. The American journal of tropical medicine and hygiene. 2007;77(6):1096–1098. [PubMed] [Google Scholar]
- 10.Balamuthia mandrillaris transmitted through organ transplantation --- Mississippi. Mmwr. 2009;59(36):1165–1170. [PubMed] [Google Scholar]
- 11.Bravo FG, Alvarez PJ, Gotuzzo E. Balamuthia mandrillaris infection of the skin and central nervous system: an emerging disease of concern to many specialties in medicine. Current opinion in infectious diseases. 24(2):112–117. doi: 10.1097/QCO.0b013e3283428d1e. [DOI] [PubMed] [Google Scholar]
- 12.Martinez DY, Seas C, Bravo F, Legua P, Ramos C, Cabello AM, et al. Successful treatment of Balamuthia mandrillaris amoebic infection with extensive neurological and cutaneous involvement. Clin Infect Dis. 51(2):e7–11. doi: 10.1086/653609. [DOI] [PubMed] [Google Scholar]
- 13.Schuster FL, Guglielmo BJ, Visvesvara GS. In-vitro activity of miltefosine and voriconazole on clinical isolates of free-living amebas: Balamuthia mandrillaris, Acanthamoeba spp., and Naegleria fowleri. The Journal of eukaryotic microbiology. 2006;53(2):121–126. doi: 10.1111/j.1550-7408.2005.00082.x. [DOI] [PubMed] [Google Scholar]
- 14.Schuster FL, Visvesvara GS. Opportunistic amoebae: challenges in prophylaxis and treatment. Drug Resist Updat. 2004;7(1):41–51. doi: 10.1016/j.drup.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Notes from the field: transplant-transmitted Balamuthia mandrillaris --- Arizona. Mmwr. 2010;59(36):1182. [PubMed] [Google Scholar]
- 16.Visvesvara GS, Martinez AJ, Schuster FL, Leitch GJ, Wallace SV, Sawyer TK, et al. Leptomyxid ameba, a new agent of amebic meningoencephalitis in humans and animals. Journal of clinical microbiology. 1990;28(12):2750–2756. doi: 10.1128/jcm.28.12.2750-2756.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuster FL, Glaser C, Gilliam S, Visvesvara GS. Survey of sera from encephalitis patients for Balamuthia mandrillaris antibody. The Journal of eukaryotic microbiology. 2001;(1):10S–12S. doi: 10.1111/j.1550-7408.2001.tb00435.x. [DOI] [PubMed] [Google Scholar]
- 18.Qvarnstrom Y, Visvesvara GS, Sriram R, da Silva AJ. Multiplex real-time PCR assay for simultaneous detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri. Journal of clinical microbiology. 2006;44(10):3589–3595. doi: 10.1128/JCM.00875-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bravo FG, Seas C. Balamuthia mandrillaris amoebic encephalitis: an emerging parasitic infection. Current infectious disease reports. 14(4):391–396. doi: 10.1007/s11908-012-0266-4. [DOI] [PubMed] [Google Scholar]
- 20.Deetz TR, Sawyer MH, Billman G, Schuster FL, Visvesvara GS. Successful treatment of Balamuthia amoebic encephalitis: presentation of 2 cases. Clin Infect Dis. 2003;37(10):1304–1312. doi: 10.1086/379020. [DOI] [PubMed] [Google Scholar]
- 21.Doyle JS, Campbell E, Fuller A, Spelman DW, Cameron R, Malham G, et al. Balamuthia mandrillaris brain abscess successfully treated with complete surgical excision and prolonged combination antimicrobial therapy. Journal of neurosurgery. 114(2):458–462. doi: 10.3171/2010.10.JNS10677. [DOI] [PubMed] [Google Scholar]
- 22.Jung S, Schelper RL, Visvesvara GS, Chang HT. Balamuthia mandrillaris meningoencephalitis in an immunocompetent patient: an unusual clinical course and a favorable outcome. Archives of pathology & laboratory medicine. 2004;128(4):466–468. doi: 10.5858/2004-128-466-BMMIAI. [DOI] [PubMed] [Google Scholar]
- 23.Cary LC, Maul E, Potter C, Wong P, Nelson PT, Given C, 2nd, et al. Balamuthia mandrillaris meningoencephalitis: survival of a pediatric patient. Pediatrics. 125(3):e699–703. doi: 10.1542/peds.2009-1797. [DOI] [PubMed] [Google Scholar]
- 24.Schuster FL, Yagi S, Wilkins PP, Gavali S, Visvesvara GS, Glaser CA. Balamuthia mandrillaris, agent of amebic encephalitis: detection of serum antibodies and antigenic similarity of isolates by enzyme immunoassay. The Journal of eukaryotic microbiology. 2008;55(4):313–320. doi: 10.1111/j.1550-7408.2008.00333.x. [DOI] [PubMed] [Google Scholar]
- 25.The Organ Procurement and Transplantation Network. Guidance for reporting potential deceased and living donor-derived disease transmission events (PDTE) [Accessed February 6, 2014];2012 Nov 11; Retrieved from: http://optn.transplant.hrsa.gov/SharedContentDocuments/PDDTEExhibitBGuidance.pdf.


