Abstract
Purpose
Cediranib is a multi-tyrosine kinase inhibitor targeting vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF) receptors. This phase II study was conducted to assess activity and tolerability of single-agent cediranib in recurrent/persistent endometrial cancer.
Patients and Methods
Eligible patients had recurrent or persistent endometrial cancer after receiving one or two prior cytotoxic regimens, measurable disease, and Gynecologic Oncology Group (GOG) performance status of ≤2 (≤1 if two prior cytotoxic regimens given). Cediranib 30 mg orally daily for a 28 day cycle was administered until disease progression or prohibitive toxicity. Microvessel density (MVD) was measured in tumor tissue from initial hysterectomy specimens and correlated with clinical outcome. Primary endpoints were tumor response and surviving progression-free for six months without subsequent therapy (6-month event-free survival [EFS]).
Results
Of 53 patients enrolled, 48 were evaluable for cediranib efficacy and toxicity. Median age was 65.5 years, 52% of patients had received prior radiation, and 73% of patients received only one prior chemotherapy regimen. A partial response was observed in 12.5%. Fourteen patients (29%) had six-month EFS. Median progression-free survival (PFS) was 3.65 months and median overall survival (OS) 12.5 months. No grade 4 or 5 toxicities were observed. A trend towards improved PFS was found in patients whose tumors expressed high MVD.
Conclusion
Cediranib as a monotherapy treatment for recurrent or persistent endometrial cancer is well tolerated and met protocol set objectives for sufficient activity to warrant further investigation. MVD may be a useful biomarker for activity.
Keywords: targeted therapy, tyrosine kinase inhibitor, angiogenesis, vascular endothelial growth factor receptor, platelet derived growth factor receptor, fibroblast growth factor receptor
INTRODUCTION
The estimated number of new uterine corpus cancer cases diagnosed in the United States last year increased by 5% to 49,560 [1]. Although most cases of endometrial cancer will be cured with surgery alone, approximately 50% with advanced disease will recur [1]. The long-term survival for women diagnosed with metastatic, recurrent endometrial cancer is poor, with limited responses to current therapy. Deaths from endometrial cancer are on the rise, and relative five-year survival has steadily worsened over the past decades, dropping from 88% in 1977 to 84% in 2006 to 81.5% in 2014 [2,3].
Primary treatment for advanced or recurrent metastatic endometrial cancer usually includes platinum-based therapy in combination with paclitaxel and/or doxorubicin (Gynecologic Oncology Group [GOG] 209) [4]. The overall survival (OS) for this population is limited (median OS 32–38 months) and has led to efforts to exploit other targets involved in tumor cell growth [4]. In particular, the epidermal growth factor receptor (EGFR), vascular endothelial growth factor receptor (VEGFR) and mammalian target of rapamycin (mTOR) are involved in signaling cascades which act as primary downstream controllers of proliferation and apoptosis. For patients with recurrent endometrial cancer who failed chemotherapy, clinical activity was observed by targeting growth factors with bevacizumab alone (13.5% clinical response, 40.4% progression-free survival (PFS) for at least six months) and bevacizumab with temsirolimus (24.5% clinical response, 46.9% PFS for at least six months) [5,6]. The impressive six-month PFS associated with these agents led to a trial evaluating these agents in combination with standard cytotoxic chemotherapy, for the primary treatment of advanced metastatic or recurrent disease (GOG-86P, ClinicalTrials.gov Identifier: NCT00977574). GOG 86P recently completed accrual and is now closed with results pending.
Cediranib is an oral agent that inhibits tyrosine kinase activity of all VEGFRs, platelet-derived growth factor (PDGF) receptors alpha and beta, and fibroblast growth factor (FGF) receptor 1. Cediranib monotherapy, studied in phase I trials of solid tumors (colorectal, gastrointestinal, breast, skin/soft tissue, prostate, and renal cell) is generally well-tolerated [7]. Among patients with glioblastoma, a 45 mg daily dose observed a decrease in tumor enhancement in 75% of patients [8]. Patients with epithelial ovarian cancer, primary peritoneal cancer, or fallopian tube cancer demonstrated a 30% clinical benefit (defined as complete response or partial response, stable disease >16 weeks, or CA-125 nonprogression >16 weeks), with cediranib monotherapy [9]. More recently, treatment with cediranib, in combination with chemotherapy (platinum/taxane regimen), for platinum-sensitive recurrent ovarian cancer and recurrent cervical cancer was found to be well-tolerated with significant increases in PFS [10, 11, 12].
GOG 229J was a phase II trial of single-agent cediranib for patients with recurrent or persistent endometrial cancer. The primary objective was to evaluate the efficacy of cediranib in this population defined by the probability of clinical response and PFS without going onto a subsequent therapy for at least six months (six-month event-free survival [EFS]).
Patients and Methods
Eligible patients were required to have recurrent or persistent endometrial cancer and who met the following criteria: Histologic confirmation of the primary tumor completed by central pathology review by the GOG Pathology Committee; measurable disease was present, defined by Response Evaluation Criteria in Solid Tumors (RECIST) (version 1.1); GOG performance status of 0–2 if one prior cytotoxic regimen was given or a GOG performance status of 0–1 if two prior cytotoxic regimens had been administered; any prior therapy directed at the endometrial cancer must be discontinued at least three weeks prior to registration; any hormonal therapy directed at the malignant tumor must be discontinued at least one week prior to registration; free of active infection requiring antibiotics; adequate hematologic parameters (absolute neutrophil count greater than or equal to 1500/mcl, platelets ≥100,000/mcl), creatinine ≤1.5 × the institutional upper limit normal or creatinine clearance ≥60 ml/min, adequate hepatic function (bilirubin ≤1.5 × upper limit normal, SGOT [AST] less ≤2.5 × upper limit normal, alkaline phosphatase ≤2.5 × upper limit normal), neuropathy (sensory and motor) ≤ grade 1 (Common Terminology Criteria for Adverse Events (CTCAE) version 4.0), urine protein/creatinine ratio <1.0 gm, adequate blood coagulation parameters (International Normalized Ratio [INR] is ≤1.5 × upper limit normal, or an in range INR [between 2 and 3] if a patient is on a stable dose of warfarin, and a PTT ≤1.5 × upper limit normal), amylase and lipase ≤ upper limit normal, thyroid stimulating hormone (TSH) level and a free thyroxine (Free T4) level within institutional normal limits; a signed approved informed consent in accordance with federal, state, and local requirements; and authorization permitting release of personal health information. The protocol was approved by institutional review boards.
Patients were ineligible if they met any of the following criteria: Prior treatment with cediranib (AZD 2171) or other VEGF pathway-targeted therapy; prior therapy with any non-cytotoxic chemotherapy other than hormonal therapy; history of other invasive malignancies (except non-melanomatous skin cancer) evident within three years of prior cancer treatment that contradicts patient eligibility; prior radiotherapy to any portion of the abdominal cavity or pelvis other than for the treatment of endometrial cancer within the last three years; presence of serious, non-healing wound, ulcer, or bone fracture, including abdominal fistula, gastrointestinal perforation or intra-abdominal abscess within 28 days; active bleeding or pathologic conditions that carry high risk of bleeding (bleeding disorder, coagulopathy, tumor involving major vessels); Central nervous system (CNS) disease including primary brain tumor, uncontrolled seizures or any brain metastases; clinically significant cardiovascular disease (uncontrolled hypertension [systolic >150 mmHg, diastolic >100 mmHg]), myocardial infarction or unstable angina within past six months, New York Heart Association Grade II or greater congestive heart failure or serious cardiac arrhythmia requiring medication; prior anthracycline treatment (doxorubicin or liposomal doxorubicin) with an ejection fraction less than institutional lower limit normal; CTCAE grade 2 or greater peripheral vascular disease; history of cerebrovascular accident (CVA), stroke, transient ischemic attack (TIA), or subarachnoid hemorrhage within six months of initiating cediranib therapy; familial history of long QT syndrome or mean QTc > 500 msec; major surgical procedure within 28 days of the initiation of the study.
Treatment
Enrolled patients were to receive cediranib (AZD2171) orally at a dose of 30 mg per day for 28 days (one cycle) with a dose modification based on toxicity assessed by history, physical examination, and laboratory assessment before each treatment cycle with adverse events defined and graded according to CTCAE, version 4.0. A single dose reduction to 20 mg per day for subsequent treatment was allowed. Cediranib was held for peripheral neuropathy ≥ grade 2, renal toxicity ≥ grade 2, or other grade 3 or greater non hematologic toxicities for a maximum of two weeks to allow recovery to ≤ grade 1. If toxicities did not resolve to ≤ grade 1 after two weeks of withholding cediranib, therapy on the trial was stopped. Cediranib was also discontinued for arterial thrombosis ≥ grade 2; a diagnosis of reversible posterior leukoencephalopathy; grade 4 hypertension; grade 3 symptomatic hypertension requiring hospitalization; gastrointestinal perforation, leak or fistula; wound separation or dehiscence requiring intervention; central nervous system (CNS) or pulmonary hemorrhage ≥ grade 2; and grade 4 proteinuria. Specific guidelines were implemented for modifying the treatment in the event of hypertension, proteinuria, and non-CNS, non-pulmonary hemorrhage. There were no dose escalations or re-escalations during the study.
Evaluation Criteria
Activity of cediranib was assessed according to RECIST 1.1. Measurable and non-measurable disease was assessed by radiographic imaging at baseline, before every other cycle for the first six months using the same technique as that which was used at baseline, and then every three months thereafter until disease progression was confirmed.
Microvessel Density Evaluation by Immunohistochemistry
Tissue was submitted from the primary hysterectomy for microvessel density (MVD) immunostaining. After tissue deparafinization and hydration, epitope retrieval in a pressure cooker with 10 mmol/L citrate buffer with pH 6.0 was initiated. Peroxidase quenching was done by incubation at 3% Hydrogen peroxide for 8 minutes. The primary antibody used was monoclonal mouse anti-human CD31 (platelet/endothelial cell adhesion molecule-1, product # M0823, Dako) 1:20 dilution for 15 minutes. The slides were then incubated in mouse DAKO EnVision ™ HRP System for 15 minutes followed by DAB chromagen for 5 minutes and DAB enhancer for 3 minutes. The specimens were counterstained in hematoxylin for 1 minute and mounted. Normal colonic epithelium was used as a positive control as suggested by the manufacturer, while colonic tissue devoid of primary antibody was used as a negative control [13]. The slides were sequentially reviewed by two blinded investigators. Each slide was manually scanned under low magnification (100×) to identify and select three different regions or “hot-spots” with the highest vascularity. The blood vessel density in a 0.75 mm2 area per hotspot was counted under 200× magnification. MVD staining was counted within the tumor and tumor margins. Any endothelial cell or group of cells that stained positive for CD31 and was distinct from neighboring fibroblasts or tumor cells was counted as a microvessel. The average of the values obtained by the two reviewers for each hot-spot was reported as a single numerical value, and the mean count from the three regions was used to determine the MVD score. A score of ≤25 vessels /high power field (HPF) was considered as low and a score of >25 vessels/HPF as high [14]. Final results were then confirmed by a pathologist designated as the GOG liaison for the University of Iowa, but not an investigator on the trial.
Statistics
The primary objective was to evaluate the efficacy of cediranib through the frequency of patients who either had objective tumor responses or who achieved six-month event-free survival (EFS). Activity on either dimension is indicative of a regimen worthy of further investigation. The null hypothesis, derived from historical data [5], specified uninteresting probabilities of response and six-month EFS equal to 10% and 15%, respectively. The probability of six-month EFS was approximated with historical data using six-month PFS. EFS is defined as the time from study entry to progression of disease, initiation of another therapy, or death. EFS was chosen as the primary endpoint since it was expected to reduce the rate of incorrectly declaring cediranib an “active” agent. Clinically significant improvements of probabilities under the alternative hypothesis were 25% and 35% for response and six-month EFS. The study accrued patients in two stages using the method of Sill et al [15]. With 27 patients accrued to the first stage, the critical values were 3 and 4 for the number who responded or were six-month EFS. With 48 patients accrued cumulatively, the critical values were 7 and 12 for response and six-month EFS. The study was flexible with actual accrual and had approximately 90% power at the 10% level of significance. The probability of early termination was likely between 46 and 57% under the null, dependent of the true association between response and six-month EFS.
Additonal endpoints of the study included adverse events attributed to the investigational agent as well as the duration of PFS, EFS, and OS. Time at risk was assessed from the date of enrollment. Per the protocol, patients retrospectively not meeting eligibility criteria or who received no investigational therapy were excluded from all analyses. Translational research was carried out in an exploratory fashion to generate hypotheses for future studies. The proposed hypothesis for the translational work was that response to the multi-tyrosine kinase activity of cediranib is related to higher microvessel density, reflective of greater tumor vascularity, in pretreatment specimens. Associations were examined using Spearman’s correlation and Cox regression [16,17]. Associations detected with p-values <0.05 were deemed “suggestive.” Associations with 0.05 < p-values <0.10 were deemed as a “trend.”
RESULTS
From June 2010 to April 2012 GOG member institutions enrolled 53 patients onto this trial. Five patients were deemed ineligible or inevaluable because of wrong primary cancer cell type (n=1), inadequate pathology for central review (n=1), never administered investigational agent (n=1), inadequate data for central review (n=1), and prior treatment making them ineligible (n=1). The remaining 48 patients were assessed for toxicity and efficacy. Patient characteristics are presented in Table 1. Approximately 73% of patients received only one prior chemotherapy regimen and 52% received prior radiation therapy. Patient outcomes are presented in Table 2. Thirty patients have died from disease. A median of two cycles of cediranib were administered (range, 1–15). Forty-eight percent received at least three cycles of study therapy and 31.3% received six or more cycles. Among patients who discontinued therapy, 64.6% stopped for disease progression and 29.2% stopped for toxicity as directed by the protocol.
Table 1.
Patient characteristics
| Characteristic | Category | No. | % |
|---|---|---|---|
| Age | 40–49 | 3 | 6.3 |
| 50–59 | 10 | 20.8 | |
| 60–69 | 20 | 41.7 | |
| 70–79 | 12 | 25.0 | |
| 80–89 | 3 | 6.3 | |
| Race | African-American | 2 | 4.2 |
| White | 46 | 95.8 | |
| Performance Status | 0 | 36 | 75.0 |
| 1 | 10 | 20.8 | |
| 2 | 2 | 4.2 | |
| Cell Type/Grade | Endometrioid, grade 1 | 3 | 6.3 |
| Endometrioid, grade 2 | 14 | 29.2 | |
| Endometrioid, grade 3 | 7 | 14.6 | |
| Serous | 11 | 22.9 | |
| Clear Cell | 3 | 6.3 | |
| Mixed Epithelial | 10 | 20.8 | |
| Prior Chemotherapy | 1 Prior Regimen | 35 | 72.9 |
| 2 Prior Regimens | 13 | 27.1 | |
| Prior Radiation | No | 23 | 47.9 |
| Yes | 25 | 52.1 | |
| Prior Immunotherapy | No | 48 | 100.0 |
| Prior Surgery | No | 1 | 2.1 |
Table 2.
Patient outcomes
| Characteristics | Category | No. | % |
|---|---|---|---|
| Response | Partial response | 6 | 12.5 |
| Stable disease | 18 | 37.5 | |
| Increase disease | 17 | 35.4 | |
| Indeterminate | 7 | 14.6 | |
| PFS > 6 Months | No | 32 | 66.7 |
| Yes | 16 | 33.3 | |
| EFS > 6 Months | No | 34 | 70.8 |
| Yes | 14 | 29.2 | |
| Cycles of Treatment | 1 | 10 | 20.8 |
| 2 | 15 | 31.3 | |
| 3 | 1 | 2.1 | |
| 4 | 6 | 12.5 | |
| 5 | 1 | 2.1 | |
| 6 | 7 | 14.6 | |
| 8+ | 8 | 16.7 | |
| Off Study | Yes | 48 | 100.0 |
| Why Off Study | Disease progression | 31 | 64.6 |
| Refused further treatment | 1 | 2.1 | |
| Toxicity as permitted | 14 | 29.2 | |
| Death | 1 | 2.1 | |
| Other | 1 | 2.1 | |
| Alive | Without progression | 2 | 4.2 |
| With progression | 16 | 33.3 | |
| Dead | From disease | 28 | 58.3 |
| From Rx & disease | 2 | 4.2 |
The 90% confidence interval (CI) for the probability of response is 5.6% ~ 26.8%. The 90% 2-sided CI for event-free survival (EFS) >6 months is 18.6% ~ 41.9%. The 90% 1-sided CI for EFS >6 months is 20.5% ~ 100%.
Adverse Events
The safety of cediranib in all 48 patients was analyzed descriptively (Table 3). No fatal events occurred as a result of the study drug and no grade 4 or 5 toxicities were attributed to the investigational agent. Vascular disorders accounted for the most common grade 3 toxicity which included hypertension (n=16) and pulmonary embolus (n=3). Two of the patients with hypertension experienced non-life-threatening hemorrhage, leading to cessation of the study. Seven patients experienced grade 3 diarrhea and 10 patients reported grade 3 fatigue. During study treatment, one patient sustained a colonic perforation; another developed ischemic bowel in the presence of a hernia, and a third developed a rectal fistula where tumor progression in the rectovaginal septum was noted. Cediranib was possibly related to the colonic perforation, but deemed unrelated and unlikely related to the rectal fistula and the ischemic bowel, respectively. Among the three patients with significant bowel complications, radiation (vaginal brachytherapy) had been previously administered only to the individual who sustained a colonic perforation. Reversible posterior leukoencephalopathy was diagnosed in a single patient.
Table 3.
Adverse Effects of Cediranib using Common Toxicity Criteria (CTC) Version 4.0
| AE Category | 0 | 1 | 2 | 3 | 4 | 5 | Total |
|---|---|---|---|---|---|---|---|
| Leukopenia | 40 | 7 | 1 | 0 | 0 | 0 | 48 |
| Thrombocytopenia | 38 | 9 | 1 | 0 | 0 | 0 | 48 |
| Neutropenia | 44 | 3 | 1 | 0 | 0 | 0 | 48 |
| Anemia | 37 | 7 | 4 | 0 | 0 | 0 | 48 |
| Other Investigations | 21 | 17 | 8 | 2 | 0 | 0 | 48 |
| Ear and labyrinth | 45 | 2 | 1 | 0 | 0 | 0 | 48 |
| Endocrine | 35 | 5 | 8 | 0 | 0 | 0 | 48 |
| Eye | 46 | 0 | 2 | 0 | 0 | 0 | 48 |
| Nausea | 28 | 10 | 10 | 0 | 0 | 0 | 48 |
| Vomiting | 35 | 6 | 5 | 2 | 0 | 0 | 48 |
| Other Gastrointestinal | 9 | 20 | 10 | 9 | 0 | 0 | 48 |
| General and administration site | 7 | 15 | 16 | 10 | 0 | 0 | 48 |
| Infections/Infestations | 46 | 0 | 2 | 0 | 0 | 0 | 48 |
| Injury/poisoning | 47 | 1 | 0 | 0 | 0 | 0 | 48 |
| Metabolism/nutrition | 24 | 12 | 8 | 4 | 0 | 0 | 48 |
| Musculoskeletal/connective tissue | 40 | 4 | 2 | 2 | 0 | 0 | 48 |
| Peripheral sensory neuropathy | 42 | 5 | 1 | 0 | 0 | 0 | 48 |
| Nervous system | 33 | 11 | 4 | 0 | 0 | 0 | 48 |
| Psychiatric | 47 | 1 | 0 | 0 | 0 | 0 | 48 |
| Renal/urinary | 38 | 4 | 6 | 0 | 0 | 0 | 48 |
| Respiratory/thoracic/mediastinal | 37 | 7 | 3 | 1 | 0 | 0 | 48 |
| Skin/subcutaneous | 41 | 6 | 1 | 0 | 0 | 0 | 48 |
| Vascular disorders | 15 | 2 | 13 | 18 | 0 | 0 | 48 |
Activity of Cediranib
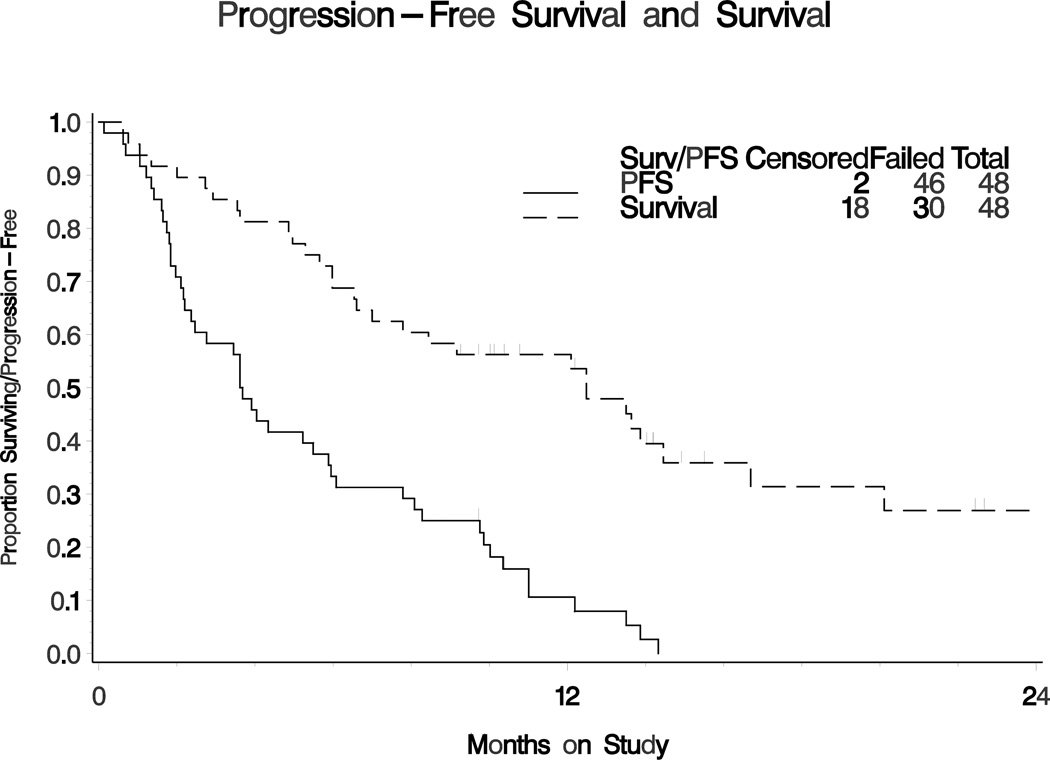
The activity of cediranib was analyzed in 48 patients (Table 2). Six patients had a partial response for an overall response rate (ORR) of 12.5% (90% 2-sided CI for the probability of response 5.6% – 26.8%). The drug was not sufficiently active by its ORR to declare it interesting. Stable disease was observed in 18 (37.5%) patients. Fourteen patients had six-month EFS (29.2%; 90% 2-sided confidence interval (CI) is 18.6 ~ 41.9%). Sixteen patients (33.3%, 90% two-sided CI 22 ~ 46%) had six-month PFS. The frequency of patients who had six-month EFS met criteria for declaring this regimen active. The median EFS was 3.61 months. The median PFS was 3.65 months, and the median OS was 12.5 months (Figure 1).
Figure 1. Progression-free survival (PFS) and overall survival (OS) of endometrial cancer patients receiving single agent cediranib.
Kaplan-Meier plot of progression-free survival (solid line) and overall survival (dashed line). The median PFS was 3.65 (90% CI 2.37 ~ 5.49). The median OS was 12.5 (90% CI 7.0 ~ 14.5).
Microvessel Density (MVD) as a predictor of progression-free survival
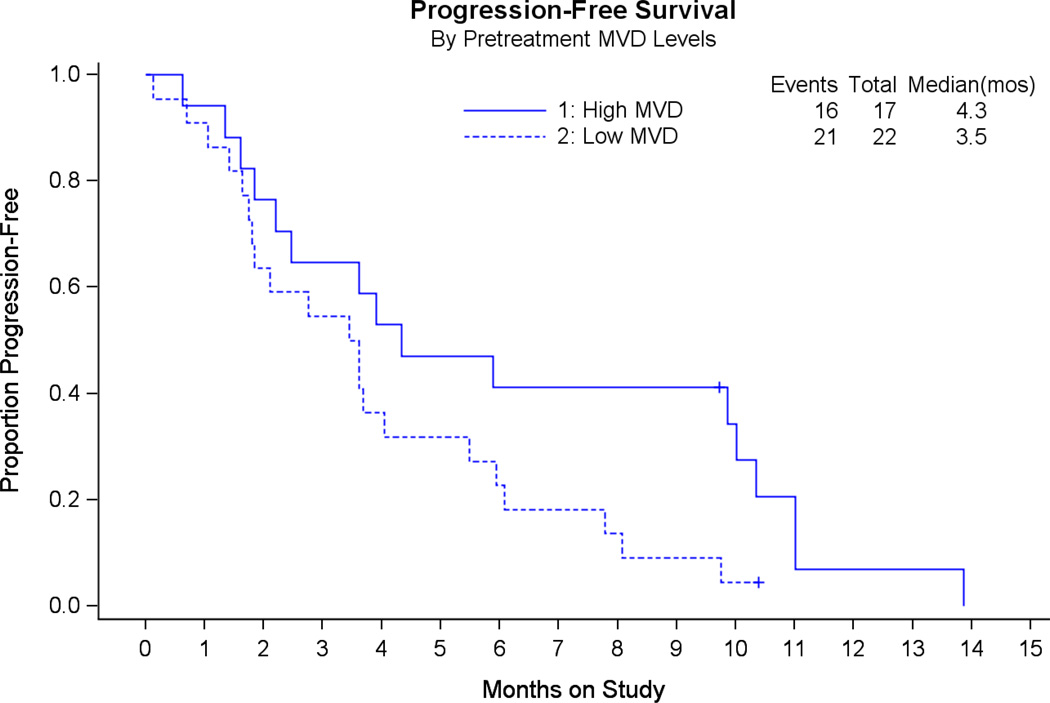
Among the 48 evaluable patients in this trial, histologic slides were available for 42 pretreatment hysterectomy specimens. Three cases had insufficient tissue on the slides to complete the MVD assays. Forty-two cases had formalin-fixed, paraffin-embedded (FFPE) tissue submitted from the primary hysterectomy for MVD immunostaining. Data were obtained on 39 patients with primary tumor tissue. Of the 39 evaluable cases stained for MVD, 17 had high MVD and 22 had low MVD (Figure 2). Median PFS in cases with high MVD was 4.3 months vs. 3.5 months for patients with low MVD (Figure 3). The estimated hazard ratio was 0.51 (95% 2-sided CI 0.25 ~ 1.04). This indicated a trend towards prolonged PFS in patients with high MVD.
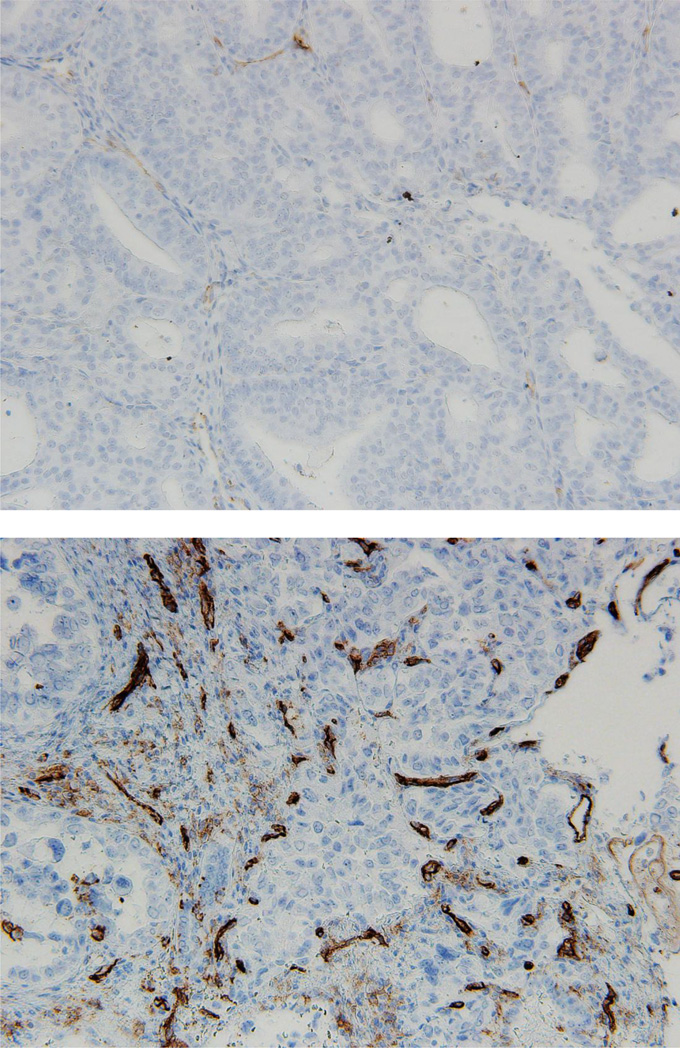
Figure 2. Microvessel density (MVD) as determined by immunostaining for cluster of differentiation 31 (CD31).
Top image (A) is a photomicrograph of endometrial cancer with strong staining for CD31 and MVD score = 81.3. Bottom image (B) is a photomicrograph of a tumor with low staining for CD31 and MVD score = 8.3.
Figure 3. Progression-free survival (PFS) as a function of microvessel density.
A trend towards improved PFS in patients with high MVD (solid line) is suggested.
DISCUSSION
Endometrial cancer is the most common gynecologic malignancy in the US, and the prognosis is poor for patients with advanced disease. This trial, GOG 229J, tested the hypothesis that the oral multi-tyrosine kinase inhibitor cediranib (AZD 2171) is a tolerable oral therapy and would demonstrate a clinically significant six-month event-free survival in patients with recurrent or advanced disease who had previously failed chemotherapy. The results of this trial identify cediranib as among one of the first tyrosine kinase inhibitors, studied by the GOG, with sufficient activity to warrant further investigation in advanced endometrial cancer.
Several phase II trials have examined the efficacy of targeted molecular inhibitors in patients with advanced or recurrent endometrial cancer [5,6,18–20]. Bevacizumab (anti-VEGF antibody) and temsirolimus (anti-mTOR small molecule) were the first targeted agents to demonstrate clinical activity [5,21]. Cediranib (AZD 2171) is an oral agent that inhibits tyrosine kinase activity in all VEGF receptors and PDGF receptors alpha and beta. Anti-tumor activity has been demonstrated both in tumor xenograft models of various types of cancer as well as in clinical trials of cediranib monotherapy [7,8,22]. Cediranib was well tolerated in each monotherapy trial. The tolerability, ease of administration, and clinical observations with cediranib therapy made this drug a compelling choice for investigation in patients with recurrent or persistent endometrial cancer.
Tumor xenograft mouse models for colon, lung, prostate, breast, and ovarian cancer have demonstrated a response to once daily dosing of cediranib as evidenced by a reduction in tumor growth, tumor vessel density, and vascular regression [22]. Similar anti-tumor activity was observed in initial clinical trials where a single daily oral dose of cediranib was used in patients with advanced solid tumors [11]. Further investigation of cediranib activity in ovarian, fallopian tube, and peritoneal cancers identified clinical benefit which ultimately led to trials using this agent in combination with cytotoxic chemotherapy for platinum-sensitive ovarian cancer. The International Collaboration for Ovarian Neoplasia 6 (ICON 6) trial showed cediranib to be sufficiently well tolerated on initial toxicity assessment such that it progressed to stage 2 [10]. Four-hundred-fifty-six patients were recruited into this trial and initial reports indicated that the trial met its primary endpoint. Patients receiving cediranib with chemotherapy plus maintenance cediranib had significantly improved PFS (medians 9.4 to 12.5 months; HR 0.57; log rank test p=0.00001) compared to those who received chemotherapy alone [11]. Efficacy was also observed in the Cediranib In Recurrent Cervical Cancer (CIRCCa) phase II trial of carboplatin and paclitaxel in combination with cediranib or placebo in patients with relapsed or metastatic cervical cancer [12]. Specifically, response rates were 66% for the 34 patients who received cediranib vs. 42% for the 35 patients who received placebo, with a modest but significant increase in PFS from 30 to 35 weeks. Clinical benefit has also been observed for cediranib combined with chemotherapy in the treatment of chemotherapy-naïve patients with advanced non-small cell lung cancer; 40% demonstrated a partial response to cediranib in combination with carboplatin and paclitaxel, while 53% of the 15 patients had stable disease [23].
Data from this trial, GOG-229J, are comparable to the findings of GOG-229E, where bevacizumab treatment resulted in a response rate of 13.5%, and 40% of patients had PFS greater than six months. While no direct comparison of efficacy has been studied between cediranib and bevacizumab, the multi-targeted anti-angiogenic TKI was felt to potentially have greater theoretical benefit due to the additional blockade of PDGF and FGF receptors. PDGF receptors are highly expressed in uterine cancers and multiple FGF receptors and their ligands have been identified in endometrial cells and their respective tumors [24, 25]. Cediranib, like bevacizumab, demonstrated a clinically significant 6-month PFS (33.3%) and should also be considered an agent of clinical interest in this disease. Although cediranib was well tolerated with no grade 4 or 5 toxicities, 14 (29.2%) patients discontinued therapy due to toxicity as permitted by the protocol. The rate of discontinuing therapy for toxicity-related reasons for this tyrosine kinase inhibitor is considerably higher than the 5.8% rate which was reported for the pure VEGF antagonist, bevacizumab, in GOG-229E. Perhaps the additional blocking activity of FGF and PDGF receptors led to higher rates of grade 3 diarrhea (15%) and grade 3 fatigue (21%), neither of which were reported at grade 3 levels with bevacizumab. In short, patients who are treated with cediranib should be carefully followed for not only the toxicities commonly reported among agents blocking VEGF activity, but also for diarrhea and fatigue as seen in this trial.
In order to refine the patient population most likely to respond to cediranib, we performed MVD analyses of patient tumors using FFPE slides from the original hysterectomy blocks. We reasoned that cediranib, as an inhibitor of angiogenesis, may be particularly effective against tumors with high MVD. Our clinical data show a prognostic relationship between MVD and PFS and may be among the first to confirm pre-clinical observations that tumors with high MVD respond best to anti-angiogenic therapy [26]. Our finding is particularly interesting, first, because the studies were performed on the original tumor, not on the recurrent lesions. Hence, these data speculate that high MVD may be a consistent tumor characteristic which is predictable at the outset of therapy. Second, it is expected that tumors with high MVD may be the most aggressive lesions in the absence of anti-angiogenic treatment and would otherwise portend a poor prognosis. The fact that high MVD may be associated with longer PFS in patients on anti-angiogenic treatment such as cediranib underscores the potential benefit of these agents. The limitations of our study include the relatively small sample size, a lack of concurrent control, insufficient tissue or histology slides available for nine pretreatment tumor specimens, and the fact that these are recurrent cases. The missing data were assumed to be missing completely at random, a hypothesis difficult to verify. In fact, with 20% of the population missing, the analysis could be biased to a considerable degree, so caution should be exercised when interpreting it. The original tumor phenotype at hysterectomy (the source for the MVD analysis in this study) may not fully represent the phenotype of the recurrent cancer. Also, the absence of a control group in this study prevents the determination of MVD as a predictive biomarker, and should be addressed in future clinical trials where chemotherapy is used with and without cediranib. Nevertheless, these data, when considered in aggregate with the reports from others [27, 28], indicate that MVD may be a useful discriminator of tumors most likely to respond to anti-angiogenic agents.
In conclusion, the tyrosine kinase inhibitor, cediranib, was shown in GOG-229J to have sufficient activity against endometrial cancer, warranting further treatment strategies with this agent. Multiple studies have supported its use as a single agent as well as in combination with cytotoxic chemotherapy for a variety of gynecologic and non-gynecologic malignancies. Therefore, given the need to address the evidence of increasing incidence and decreasing survival in women with advanced endometrial cancer, we propose similar combinations of cediranib with cytotoxic agents be considered for this population.
RESEARCH HIGHLIGHTS.
Cediranib is an active multi-tyrosine kinase inhibitor in uterine cancer
Cediranib for recurrent uterine cancer had a 33% six-month progression free survival
Cediranib is a safe and well-tolerated oral treatment for recurrent uterine cancer
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group (GOG) Administrative Office, the GOG Core Laboratory for Receptors and Targets and the GOG Tissue Bank (U24 CA114793), the GOG Statistical and Data Center (CA 37517), NRG Oncologay Grant # 1 U10 CA180822, NRG Operations Grant # U10CA180868 and K. Leslie (R01-CA099908). We also thank and acknowledge the Barbara Beach Fund to support endometrial cancer research (to K. Leslie).
The following GOG member institutions participated in this protocol: Duke University Medical Center, Abington Memorial Hospital, Fred Hutchinson Cancer Research Center, University of Cincinnati, Indiana University Hospital, University of California Medical Center at Irvine, Rush University Medical Center, Washington University School of Medicine, University of Oklahoma, Women and Infants Hospital, Central Connecticut, Georgia Core, Carolinas Medical Center and Community Clinical Oncology Program.
Dr. Henry Reyes received a WRHR grant from NICHD (K12-HD063117). Dr. Heidi Gray is a contributor for the “UpToDate” online educational publication. Dr. Robert Mannel is a consultant for Amgen, Advaxix, MedImmune, AstraZeneca, Oxigene, and Endocyte – All advisory boards for clinical trial design. Dr. Jeanne Schilder receives funds from the NRG/GOG grants. She is an Associate Professor at Indiana University and also receives money from GOG/NRG for other studies. Dr. Kimberly Leslie receives funds from NIH Grant #CA99908.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
All other co-authors have no conflicts of interest to declare.
Contributor Information
David Bender, Email: david-bender@uiowa.edu.
Michael W. Sill, Email: msill@gogstats.org.
Heather A. Lankes, Email: hlankes@gogstats.org.
Henry D. Reyes, Email: henry-reyes@uiowa.edu.
Christopher J. Darus, Email: cdarus@gmail.com.
James E. Delmore, Email: james.delmore@awhobgyn.com.
Jacob Rotmensch, Email: jacob_rotmensch@rush.edu.
Heidi J. Gray, Email: hgray@u.washington.edu.
Robert S. Mannel, Email: robert-mannel@ouhsc.edu.
Jeanne M. Schilder, Email: jschilde@iupui.edu.
Mark I. Hunter, Email: hunterm@health.missouri.edu.
Carolyn K. McCourt, Email: McCourtc@wudosis.wustl.edu.
Kimberly K. Leslie, Email: Kimberly-leslie@uiowa.edu.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Facts and Figures American Cancer Society, 2011 [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 2013. 1975–2010 [Google Scholar]
- 4.Miller DS, Filiaci V, Fleming G, Mannel R, Cohn D, Matsumoto T, et al. Abstract: Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:771–773. [Google Scholar]
- 5.Aghajanian C, Sill MW, Darcy KM, Greer B, McMeekin DS, Rose PG, et al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2011;29:2259–2265. doi: 10.1200/JCO.2010.32.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvarez EA, Brady WE, Walker JL, Rotmensch J, Zhou XC, Kendrick JE, et al. Phase II trial of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2013;129:22–27. doi: 10.1016/j.ygyno.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Drevs J, Siegert P, Medinger M, Mross K, Strecker R, Zirrgiebel U, et al. Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2007;25:3045–3054. doi: 10.1200/JCO.2006.07.2066. [DOI] [PubMed] [Google Scholar]
- 8.Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matulonis UA, Berlin S, Ivy P, Tyburski K, Krasner C, Zarwan C, et al. Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J Clin Oncol. 2009;27:5601–5606. doi: 10.1200/JCO.2009.23.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raja FA, Griffin CL, Qian W, Hirte H, Parmar MK, Swart AM, et al. Initial toxicity assessment of ICON6: a randomised trial of cediranib plus chemotherapy in platinum-sensitive relapsed ovarian cancer. Br J Cancer. 2011;105:884–889. doi: 10.1038/bjc.2011.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ledermann JA, Perren T, Raja FA, et al. Abstract: Randomised double-blind phase III trial of cediranib (AZD 2171) in relapsed platinum sensitive ovarian cancer: Results of the ICON6 trial. Presented at the 2013 NCRI Cancer Conference; 2013; Liverpool, UK. [Google Scholar]
- 12.Symonds P, Gourley C, Davidson S, et al. CIRCCa: A randomised double blind phase II trial of carboplatin-paclitaxel plus cediranib versus carboplatin-paclitaxel plus placebo in metastatic/recurrent cervical cancer. Presented at the ESMO 2014 Congress; 2014; Madrid, Spain. [Google Scholar]
- 13.DAKO. [accessed 6 July 2015];Atlas of Stains: A Better Path for Cancer Diagnostics. (4th ed.). 2012 http://www.dako.com/us/00230_atlas_of_stains.pdf. [Google Scholar]
- 14.Nadkarni NJ, Geest KD, Neff T, Young BD, Bender DP, Ahmed A, et al. Microvessel density and p53 mutations in advanced-stage epithelial ovarian cancer. Cancer Lett. 2013;331:99–104. doi: 10.1016/j.canlet.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Sill MW, Rubinstein L, Litwin S, Yothers G. A method for utilizing co-primary efficacy outcome measures to screen regimens for activity in two-stage Phase II clinical trials. Clin Trials. 2012;9:385–395. doi: 10.1177/1740774512450101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spearman C. The proof and measurement of association between two things. Amer J Psychol. 1904;15:72–101. [PubMed] [Google Scholar]
- 17.Cox DR. Regression models and life tabels. J R Stat Soc Series B. 1972;34:187–122. [Google Scholar]
- 18.Leslie KK, Sill MW, Lankes HA, et al. Lapatinib and potential prognostic value of EGFR mutations in a Gynecologic Oncology Group phase II trial of persistent or recurrent endometrial cancer. Gynecol Oncol. 2012;127:345–350. doi: 10.1016/j.ygyno.2012.07.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leslie KK, Sill MW, Fischer E, Darcy KM, Mannel RS, Tewari KS, et al. A phase II evaluation of gefitinib in the treatment of persistent or recurrent endometrial cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2013;129:486–494. doi: 10.1016/j.ygyno.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coleman RL, Sill MW, Lankes HA, Fader AN, Finkler NJ, Hoffman JS, et al. A phase II evaluation of aflibercept in the treatment of recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;127:538–543. doi: 10.1016/j.ygyno.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oza AM, Elit L, Tsao MS, Kamel-Reid S, Giagi J, Provencher DM, et al. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. J Clin Oncol. 2011;29:3278–3285. doi: 10.1200/JCO.2010.34.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wedge SR, Kendrew J, Hennequin LF, Valentine PJ, Barry ST, Brave SR, et al. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65:4389–4400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- 23.Laurie SA, Gauthier I, Arnold A, Shepherd FA, Ellis PM, Chen E, et al. Phase I and pharmacokinetic study of daily oral AZD2171, an inhibitor of vascular endothelial growth factor tyrosine kinases, in combination with carboplatin and paclitaxel in patients with advanced non-small-cell lung cancer: the National Cancer Institute of Canada clinical trials group. J Clin Oncol. 2008;26:1871–1878. doi: 10.1200/JCO.2007.14.4741. [DOI] [PubMed] [Google Scholar]
- 24.Roh JW, Huang J, Hu W, Yang X, Jennings NB, Sehgal V, et al. Biologic effects of platelet-derived growth factor receptor α blockade in uterine cancer. Clin Cancer Res. 2014;15:2740–2750. doi: 10.1158/1078-0432.CCR-13-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee PS, Secord AA. Targeting molecular pathways in endometrial cancer: a focus on the FGFR pathway. Cancer Treat Rev. 2014;40:507–512. doi: 10.1016/j.ctrv.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Zhang K, Waxman DJ. Impact of tumor vascularity on responsiveness to antiangiogenesis in a prostate cancer stem cell-derived tumor model. Mol Cancer Ther. 2013;12:787–798. doi: 10.1158/1535-7163.MCT-12-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han ES, Burger RA, Darcy KM, Sill MW, Randall LM, Chase D, et al. Predictive and prognostic angiogenic markers in gynecologic oncology group phase II trial of bevacizumab in recurrent and persistent ovarian or peritoneal cancer. Gynecol Oncol. 2010;119:484–490. doi: 10.1016/j.ygyno.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Randall LM, Monk BJ, Darcy KM, Tian C, Burger RA, Liao SY, et al. Markers of angiogenesis in high-risk, early-stage cervical cancer: A Gynecologic Oncology Group study. Gynecol Oncol. 2009;112:583–589. doi: 10.1016/j.ygyno.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]