Abstract
Purpose: To determine whether curcumin offers neuroprotection to minimize the apoptosis of neural cells in the retina of diabetic rats.
Methods: Streptozotocin (STZ)-induced diabetic rats and control rats were used in this study. A subgroup of STZ-induced diabetic rats were treated with curcumin for 12 weeks. Retinal histology, apoptosis of neural cells in the retina, electroretinograms, and retinal glutamate content were evaluated after 12 weeks. Retinal levels of Ca2+/calmodulin-dependent protein kinase II (CaMKII), phospho-CaMKII (p-CaMKII), and cleaved caspase-3 were determined by Western blot analysis.
Results: The amplitudes a-wave, b-wave, and oscillatory potential were reduced by diabetes, but curcumin treatment suppressed this reduction of amplitudes. Curcumin also prevented cell loss from the outer nuclear, inner nuclear, and ganglion cell layers. Apoptosis of retinal neurons was detected in diabetic rats. The concentration of glutamate in the retina was higher in diabetic rats, but was significantly reduced in the curcumin-treated group. Furthermore, p-CaMKII and cleaved caspase-3 expression were upregulated in the diabetic retina, but reduced in curcumin-treated rats.
Conclusions: Curcumin attenuated diabetes-induced apoptosis in retinal neurons by reducing the glutamate level and downregulating CaMKII. Thus, curcumin might be used to prevent neuronal damage in the retina of patients with diabetes mellitus.
Introduction
Diabetic retinopathy is the leading cause of new-onset blindness in the working-age population, even in developed countries.1 Diabetic retinopathy is classically considered to be a disease of the microvasculature. However, recent studies have reported that diabetes causes neuronal damage to the retina in the early stages of the disease.2–5 Apoptosis is probably the mechanism by which neuronal cell death occurs in patients with diabetic retinopathy,6–8 ultimately leading to ophthalmic dysfunction and visual loss. Therefore, it is necessary to identify pharmacological targets that prevent neuronal apoptosis in patients with diabetic retinopathy.
Ca2+/calmodulin-dependent protein kinase II (CaMKII) is an ubiquitous multifunctional serine/threonine protein kinase that regulates the death of neuronal cells, including retinal ganglion cells (RGCs).9–12 Autocamtide-2-related inhibitory peptide, a potent inhibitor of CaMKII, can prevent retinal neuronal cell death both in vivo and in vitro.10,13 Furthermore, when the CaMKII activity is inhibited, the damage to neurons is minimized in mice with focal and global ischemia. Thus, the inhibitors of calmodulin and CaMKII are potently neuroprotective.14,15 Therefore, we postulate that CaMKII is a prospective target for neuron-destroying diseases, such as diabetic retinopathy.
Curcumin (Cur) is the main bioactive component of turmeric (Curcuma longa). It provides diverse health benefits, including protection from metabolic disorders. Therefore, it is used to treat diabetic retinopathy. However, researchers have not yet been able to elucidate the mechanisms by which curcumin prevents the progression of diabetic retinopathy.16–35 Although many studies have reported that curcumin rescues retinal neuronal cell cultures from glutamate toxicity,36,37 researchers have not been able to determine how curcumin treatment ameliorates the diabetes-induced damage to retinal neuronal cells. In this study, we determined whether curcumin has a neuroprotective effect on retinal neural cells from streptozotocin (STZ)-induced diabetic rats. We found that curcumin suppresses glutamate toxicity in the retina, thereby decreasing the action of CaMKII and the expression of cleaved caspase-3. Our results indicate that curcumin is quite effective in therapeutically preventing CaMKII-mediated retinal loss in patients with diabetic retinopathy.
Methods
Ethics statement
This study was carried out according to the recommendations provided by the Guide for the Care and Use of Laboratory Animals, which is a handbook released by the National Institutes of Health, Bethesda, Maryland, USA. The protocol was approved by the Committee on the Ethics of Animal Experiments of Wenzhou Medical University, Zhejiang, China. We performed our experimental research study in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. All surgical procedures were performed after administering sodium pentobarbital anesthesia, and efforts were made to minimize the suffering of the rats.
Materials
Unless stated otherwise, chemicals were reagent-grade quality and were purchased from Sigma Chemicals (St. Louis, MO).
Animals
In this experimental study, we used male Sprague-Dawley rats (8 weeks old) weighing 180–200 g (Shanghai Laboratory Animal Center, Chinese Academy of Sciences). Animals were kept under a 12-h light–12-h dark cycle; the room temperature was maintained in the range of 23°C–25°C, while the humidity was maintained in the range of 55%–60%. Food and water were available ad libitum. The rats were acclimatized for a period of 1 week. Then, they were randomly divided into 2 groups. The first group of rats was administered a 60 mg/kg dose of STZ intraperitoneally, while the second group of rats was administered only a citrate buffer. The first group of rats became diabetic as their blood glucose levels exceeded 16.7 mmol/L, 48 h after STZ administration. The control animals (n=12) were age matched before being administered with an equal volume of sodium citrate. Two weeks after inducing diabetes, rats were divided randomly into 2 subgroups: diabetic rats (DM; n=12) and diabetic rats treated with curcumin at a dose of 100 mg/kg/day (DM+Cur; n=12). Curcumin was suspended in saline containing 0.5% carboxymethylcellulose; the concentration of curcumin in this solution was adjusted to 20 mg/mL and was administered through oral gavage for 12 weeks. Every rat received saline containing 0.5% carboxymethylcellulose. After completing these experiments, the rats were euthanized by anesthetic overdose. Their eyes were removed and fixed for subsequent experiments.
Electroretinography
Rats were dark adapted for 1 h, and then, they were anesthetized with an intraperitoneal injection of 1% pentobarbital sodium (40 mg/kg). Electroretinography (ERG) was monitored by a procedure described previously.38 The pupils were maximally dilated, and the cornea was topically anesthetized. Light stimuli were generated with a Grass Ps22 photic stimulator (UTAS-E 2000; LKC Technologies, Gaithersburg, MD) at a 2 log-flash intensity (cd.s/m2). Full-field white-light stroboscopic flashes lasting 10 ms were presented at a distance of 30 cm at a rate of 1.0/s. The retinal signals were amplified at a band pass of 75–300 Hz for scotopic responses.
Hematoxylin and eosin staining and observation
After sacrificing rats, their eyes were enucleated and fixed in 4% paraformaldehyde for 24 h at 4°C and then, the anterior segment was removed. The posterior eyecup was dehydrated and embedded in paraffin and cut into 4-μm-thick sections over the entire length of the retina, approximately along the horizontal meridian that passes through the ora serrata and the optic nerve in both the temporal and nasal hemispheres. These sections were stained immediately with hematoxylin and eosin (H&E) using standard techniques. The tissues were scanned microscopically to search for gross disease. The thickness of the following components was measured using morphometric analysis: the total retina, the inner nuclear layer (INL), the inner plexiform layer (IPL), and the ganglion cell layer (GCL). We also measured the number of cells in the outer nuclear layer (ONL), INL, and GCL. The thicknesses were measured in identical regions (between 1 and 2 mm from the center of the optic nerve head). The number of cells in these layers was quantified by counting cells in the same region and the measurements for a region were averaged. In every rat, we measured 3 distinct sections. All measurements were conducted with a light microscope (Leica, Heidelberg, Germany).
Terminal deoxyribonucleotidyl transferase-mediated dUTP nick end labeling staining
Terminal deoxyribonucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining was performed on paraffin-embedded sections using an in situ Cell Death Detection Kit (Roche, Lewes, UK). Staining was performed according to the manufacturer's protocol. Paraffin-embedded sections were deparaffinized, rehydrated, and subjected to TUNEL analysis after incubating them with proteinase K. These slides were mounted in the Vectashield fluorescence medium (Vector Laboratories, Burlingame, CA) and stained with the nuclear stain, 4′6-diamidino-2-phenylindole (DAPI). Then, these DAPI-stained slides were observed under a laser scanning confocal microscope (Zeiss 510, Jena, Germany). Zeiss 4.6 version software was used for collecting images. The apoptotic neurons within the retina emitted green fluorescence.
Western blot analysis
The retinas were removed rapidly and frozen in liquid nitrogen. Then, the retinas were thawed and sonicated in tris-buffered saline (TBS) containing protease inhibitors. The supernatants were collected after centrifugation (12,000 rpm, 15 min, 4°C). Protein concentration was measured using the Bio-Rad DC protein assay kit (Bio-Rad Laboratories, Hercules, CA). We separated 50 μg of protein from each sample on a 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis using a Bio-Rad miniature slab gel apparatus and electrophoretically transferred the separated proteins onto a nitrocellulose sheet. The sheet was blocked overnight at 4°C in TBS containing 0.05% Tween-20 (TBST) and 5% skimmed milk. Then, this sheet was incubated with monoclonal antibodies against rat cleaved caspase-3 (Cell Signaling Technology, Danvers, MA), CaMKII, and phospho-CaMKII (p-CaMKII) (Thr-286) (Abcam, Cambridge, MA) at 1:1,000 dilutions for 2 h. After rinsing in TBST, the membranes were incubated for 2 h with a horseradish-peroxidase-conjugated secondary antibody against rabbit IgG in a 1:1,000 dilution. Finally, SuperSignal West Pico Chemiluminescent Substrates (Pierce, Rockford, IL) were used for detecting the blots, and the band density was determined by Image J software (NIH, Bethesda, MD). The expression of β-actin (1:5,000; monoclonal anti-β-actin) was used as an internal loading control to confirm equivalent total protein loading. These experiments were performed in triplicate.
High-pressure liquid chromatography
Rats were killed and their eyes were enucleated immediately. The retina was detached from the sclera and weighed. Then, this detached retina was placed in 500 μL of 0.1 M perchloric acid and homogenized at 4°C. The mixture was centrifuged at 13,600 g for 20 min at 4°C and 100 μL of the supernatant was removed. This 100 μL liquid was added to 200 μL of ethyl acetate, and the resultant suspension was vortexed for 2 min and centrifuged again under the same conditions. Finally, 10 μL of the supernatant was collected. High-pressure liquid chromatography was used to measure the retinal glutamate level in the 3 groups of rats.38
Statistical analysis
Data are expressed in terms of mean±standard deviation. The differences between the mean values of multiple groups were analyzed by 1-way analysis of variance. Thereafter, Dunnett's post hoc test was performed using SPSS16.0 software (SPSS, Chicago, IL). A P<0.05 was considered to be statistically significant.
Results
Animal data
Within 12 weeks of the onset of diabetes, there was a significant decrease in the body weight of diabetic rats, regardless of whether they were treated with curcumin. Compared to the rats in the control group, diabetic rats also showed a significant increase in blood glucose levels (P<0.05). The long-term (12 weeks) administration of curcumin did not have an impact on the body weight or blood glucose levels of diabetic rats compared with the diabetic rats that were not treated with curcumin (P>0.05) (Table 1).
Table 1.
Body Weight and Blood Glucose Level in the Experimental Groups at Week 12
| Control | DM | DM+Cur | |
|---|---|---|---|
| Body weight (g) | 537±36 | 298±27a | 319±32a,b |
| Blood glucose (mmol/l) | 5.5±0.4 | 28.1±4.2a | 23.8±4.6a,b |
P<0.05 for the difference between the diabetic and control groups or between the control and DM+Cur groups.
P>0.05 for the difference between the DM and DM+Cur groups.
Cur, curcumin; DM, diabetic rats.
Electroretinogram
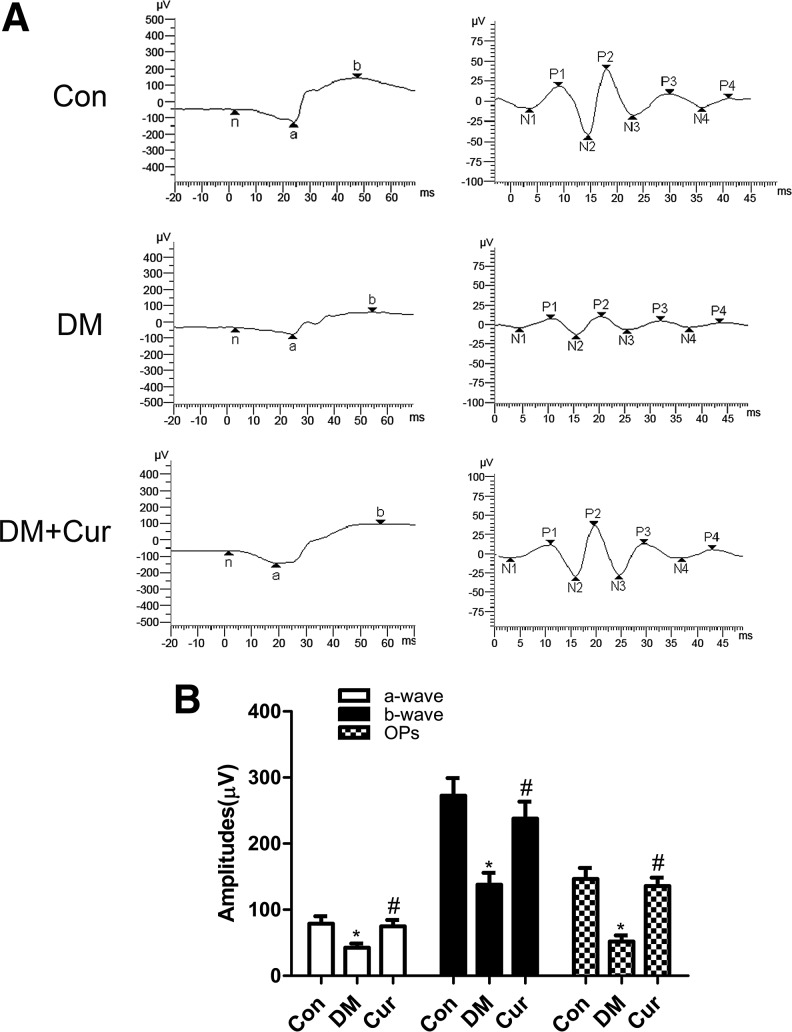
ERG was used to evaluate the functional status of the inner and outer retina. To evaluate the effect of constant curcumin intake on the visual function of diabetic rats, we used full-field flash ERG. As shown in Figure 1, compared with those in the age-matched controls, the amplitudes of a-waves, b-waves, and oscillatory potential (Ops) in STZ-induced diabetic rats were significantly decreased at the end of the experiment. However, these decreases of amplitudes and Ops were successfully prevented by treating diabetic rats with curcumin.
FIG. 1.
(A) Representative waves of electroretinography responses from control rats (Con), diabetic rats (DM), and diabetic rats treated with curcumin (DM+Cur). (B) There were significant reductions in amplitudes of a-waves, b-waves, and oscillatory potential (Ops) in diabetic rats compared with control rats. The reductions were partially reversed in the retinas of curcumin-treated diabetic rats. *P<0.01 versus Con, #P<0.01 versus DM. Cur, curcumin.
H&E staining and observation
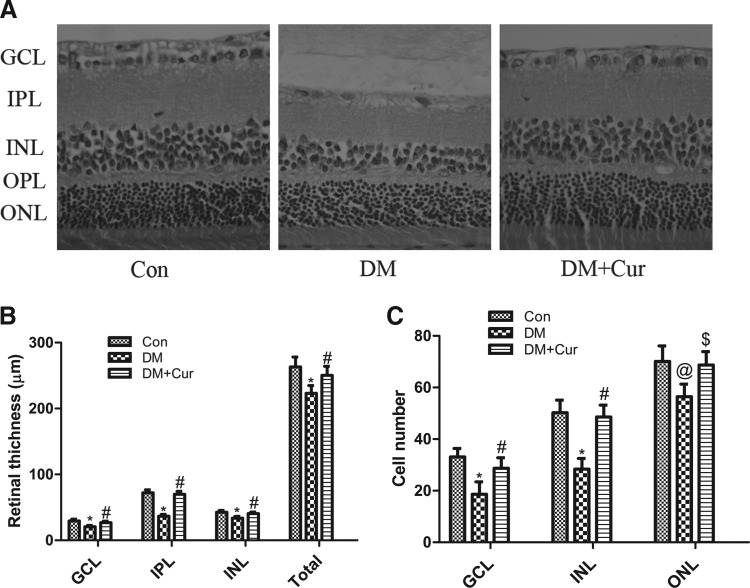
The retinas of all the rats were observed after staining them with H&E. The thickness of the total retina (223±12 μm vs. 263±15 μm, P<0.01) was reduced by 15.2% in diabetic rats compared with the control rats. Furthermore, retinal thinning was significantly prevented in diabetic rats, which were continuously treated with curcumin (DM+Cur 250±13 μm vs. DM, P<0.01, Fig. 2A, B). Compared with the healthy rats of the control group, the retinal thickness of GCL, IPL, and INL was significantly reduced in diabetic rats (GCL thickness: DM 21±2 μm vs. control 29±3 μm, P<0.01; IPL thickness: DM 37±3 μm vs. control 72±4 μm, P<0.01; INL thickness: DM 34±3 μm vs. control 42±3 μm, P<0.01, Fig. 2A, B). The reductions in the thickness of GCL, IPL, and INL of diabetic retinas were significantly minimized by treating some diabetic rats with curcumin for 12 weeks (GCL: DM+Cur 27±2 μm vs. DM, P<0.01; IPL: DM+Cur 70±5 μm vs. DM, P<0.01; INL: DM+Cur 41±2 μm vs. DM, P<0.01, Fig. 2A, B).
FIG. 2.
(A) Representative images of retinal sections from control rats (Con), diabetic rats (DM), and diabetic rats treated with curcumin (DM+Cur). (B) The diabetic retina showed significant thinning in the ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), and the total retina. Twelve weeks of curcumin treatment significantly prevented the reduction in GCL, IPL, INL, and total retinal thickness. (C) The cell numbers in the GCL (per 500 μm), INL (per 100 μm), and outer nuclear layer (ONL) (per 50 μm) significantly decreased after 12 weeks in diabetic rats compared to controls, and curcumin treatment significantly prevented diabetes-induced cell loss in the GCL, INL, and ONL. *P<0.01 versus Con, #P<0.01 versus DM, @P<0.05 versus Con, $P<0.05 versus DM.
The cell numbers in the GCL, INL, and ONL were significantly lower in diabetic rats compared to the healthy rats of the control group, but were higher after treating the diabetic rats with curcumin for 12 weeks (Fig. 2A, C).
TUNEL staining
To further determine whether curcumin actually prevented neuronal loss in the retina of diabetic rats, we performed TUNEL staining to detect DNA fragmentation of cells undergoing apoptosis. In the control group, the retinal nuclei were essentially negative with TUNEL staining. Abundant green fluorescent retinal nuclei were found in the inner nuclear, outer nuclear, and ganglion cells of diabetic rats, but were sparsely found in diabetic rats treated with curcumin (Fig. 3A). Compared with the DM group, cell apoptosis in GCL, INL, and ONL (P<0.05 in GCL, P<0.01 in INL and ONL) was significantly inhibited in diabetic rats treated with curcumin (Fig. 3B).
FIG. 3.
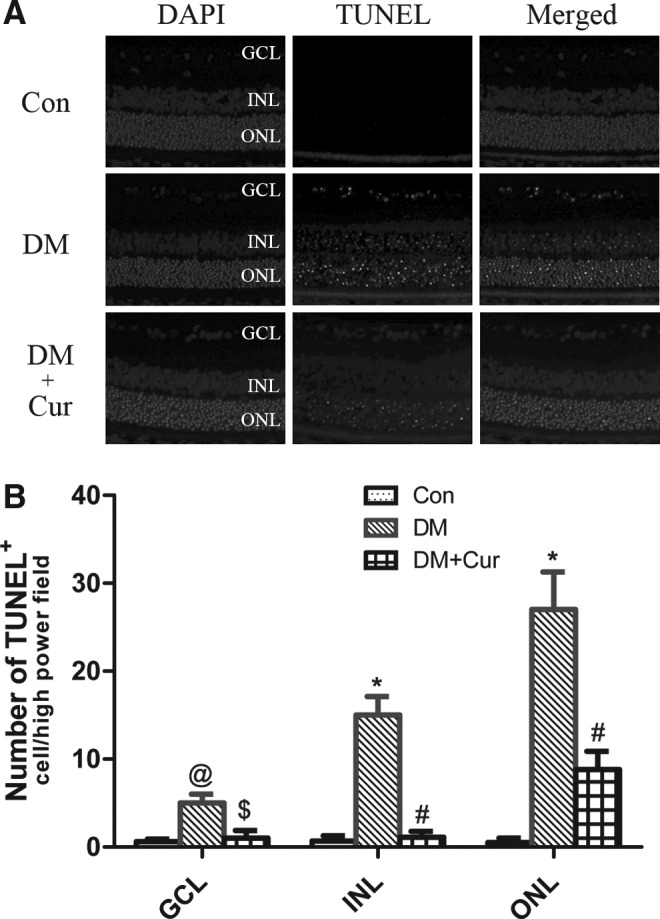
(A) Fluorescence detection of neuronal apoptosis. No terminal deoxyribonucleotidyl transferase-mediated dUTP nick end labeling (TUNEL)-positive cells were seen in the control group (Con); abundant fluorescent nuclei were observed in the diabetic rats (DM); and few fluorescent nuclei were observed in diabetic rats treated with curcumin (DM+Cur). (B) Quantitative results of TUNEL-positive cells in the GCL, INL, and ONL (n=6 different samples in each group). *P<0.01 versus Con, #P<0.01 versus DM, @P<0.05 versus Con, $P<0.05 versus DM.
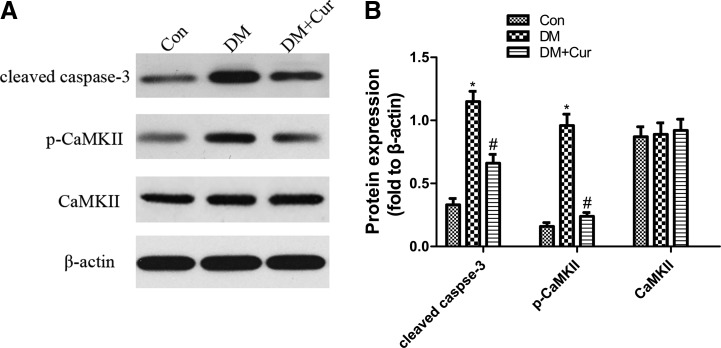
Western blot analysis of cleaved caspase-3, p-CaMKII, and CaMKII expression
The expression of cleaved caspase-3 and p-CaMKII increased significantly, while there was no significant difference in CaMKII of the 2 groups. Although curcumin could decrease the expression of cleaved caspase-3 and p-CaMKII, it did not have a significant effect on the expression of CaMKII (Fig. 4).
FIG. 4.
(A) Western blotting analysis showing expression of cleaved caspase-3, phospho-CaMKII (p-CaMKII), and calmodulin-dependent protein kinase II (CaMKII) protein in the whole retina of control rats (Con), diabetic rats (DM), and diabetic rats treated with curcumin (DM+Cur). β-Actin served as the loading control. (B) Bars represent mean±SD from at least 3 independent experiments. *P<0.01 versus Con; #P<0.01 versus DM.
Retinal glutamate content
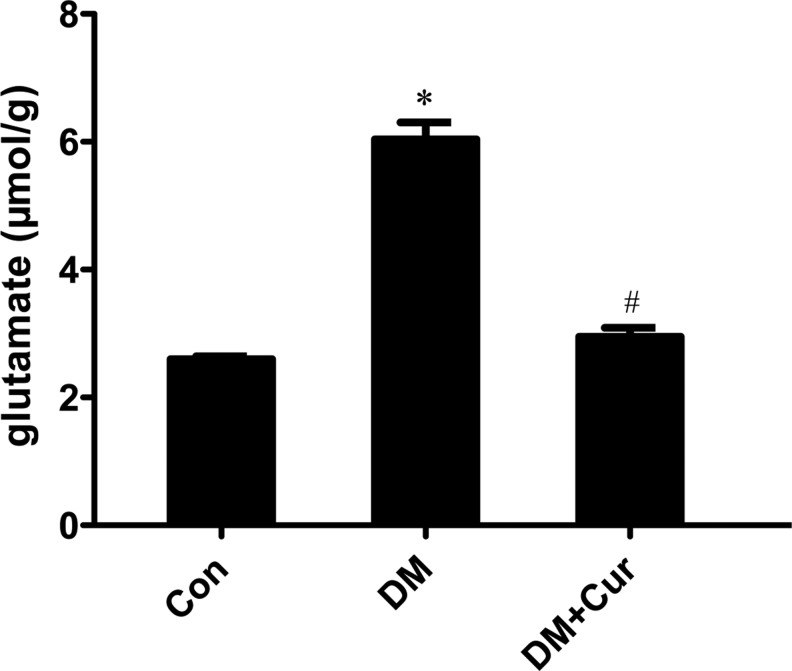
The glutamate content in diabetic rats was significantly greater than that in age-matched control rats (Fig. 5, 6.0±0.3 μmol/g vs. 2.60±0.05 μmol/g, P<0.05). The increase in glutamate levels was suppressed when a subgroup of diabetic rats were continuously treated with curcumin for 12 weeks (2.9±0.1 μmol/g vs. 6.0±0.3 μmol/g, P<0.05).
FIG. 5.
Glutamate content in the retinas of control rats (Con), diabetic rats (DM), and diabetic rats treated with curcumin (DM+Cur) was determined by high-pressure liquid chromatography and quantification of retinal glutamate in the 3 groups of rats. The results shown are mean±SD from triplicate experiments (*P<0.05 vs. Con; #P<0.05 vs. DM).
Discussion
Curcumin has been used to treat diabetic retinopathy; however, the underlying mechanisms by which curcumin cures diabetic retinopathy is unclear.16–30,33,34,39 In this study, we partially elucidated the molecular mechanisms of cell apoptosis in diabetes-induced rats and demonstrated that curcumin was excellent for treatment. We found that neuronal loss occurred in the retinas of diabetic rats. Moreover, we found that cell apoptosis was prevented in diabetic rats treated with curcumin. Curcumin suppressed cell apoptosis in diabetic rats by Ca2+/CaMKII -dependent pathways. We found that curcumin effectively prevented neuronal loss in the retinas of diabetic rats. Curcumin also upregulated glutamate levels in the retina of STZ-induced diabetic rats. Moreover, it inhibited CaMKII and caspase-3 activity, thereby preventing the apoptosis of retinal neurons in diabetic rats.
Diabetic retinopathy causes neuronal damage in the retina. Diabetic retinopathy is typically characterized by reduced ERG responses40–42 and progressive loss of retinal structure.5,43 The electrophysiological changes in diabetic retina reportedly occur before the appearance of visible lesions. In this study, we observed that the amplitudes of a-wave, b-wave, and OPs of diabetic rats were lower than those of the control rats after 12 weeks, indicating that photoreceptors, bipolar cells, and inner retina (including microcirculation) were adversely affected in diabetic rats within 12 weeks. The retinal thickness was lower in diabetic rats compared with controls. Diabetes mellitus is an energy wasting disease, resulting in subcutaneous fat consumption and weight loss in rats. The animal model used here has been widely used and our results agree well with a previous study.44 Therefore, the change in the thickness of retina does not seem to be related to body weight or the size of the eyes, since the body weight and size of eyes did not recover in the DM+Cur group, yet the thickness of the retina was restored in this group. We also found that diabetes not only induces a decrease in the retinal thickness but also causes a loss of neural cells in the retina. However, curcumin significantly improved the reduced ERG responses and morphological changes induced by diabetes. This indicates that curcumin effectively treats the neuronal loss that is caused by diabetic retinopathy. The results of our study are in good agreement with those of previous studies, which showed that curcumin regulates CaMKII action and effectively cures hippocampal neuronal loss induced by chronic stress.45
CaMKII is an ubiquitous, multifunctional serine–threonine protein kinase that regulates a variety of neuronal functions.9,46,47 An overexpression of CaMKII can trigger caspase-3-dependent cell death in damaged retinas.9,48,49 Previous studies, which have investigated the relationship between diabetes and CaMKII, have reported that diabetes stimulates the action of CaMKII, which plays an important role in the pathogenesis of diabetic complications, such as diabetic retinopathy.49–52 In this study, we found that compared with the healthy rats of the control group, active CaMKII (p-CaMKII) and cleaved caspase-3 levels were significantly increased in STZ-induced diabetic rats. However, these increases in the levels of active CaMKII (p-CaMKII) and cleaved caspase-3 levels were minimized by treating the diabetic rats with curcumin. Based on these results, we conclude that curcumin inhibits neuronal loss in the retina. The results of our experiments indicate that the oral administration of curcumin effectively blocks the activation of CaMKII and neuronal death in retinas damaged by diabetes. Our results agree well with the results of a study that showed resveratrol, another polyphenol whose physiological properties are similar to those of curcumin, suppresses CaMKII action and CaMKII-mediated RGC loss induced by diabetes.50
We have not been able to clearly determine the precise mechanism by which curcumin inactivates CaMKII action and prevents neuronal death. However, we believe that this mechanism is also involved in the regulation of glutamate levels.35–37 Glutamate is the main excitatory neurotransmitter in the retina, but it is neurotoxic when present in excessive amounts.53,54 Many research studies have reported that when glutamate is released in excess amounts, it over stimulates the N-methyl-D-aspartate receptor, promoting autophosphorylation and activation of CaMKII.12,14,55 Based on these facts, we hypothesize that CaMKII-mediated cell death in diabetic rats may be prevented by blocking glutamate. It has been reported that curcumin protects neurons, including RGCs, from glutamate excitotoxicity.35–37,56 In this study, we have found that curcumin reduces in vivo glutamate to nearly normal levels in the retina. Previous studies demonstrated that the release of glutamate could be directly inhibited by curcumin, possibly by suppressing the activity of mitogen-activated protein kinase (MAPK).16,39 Accordingly, we hypothesized that curcumin probably normalizes the level of glutamate in the retina of diabetic rats by inactivating MAPK.
In summary, we found that curcumin can protect retinal neurons in rats afflicted with diabetic retinopathy. Owing to the neuroprotective effect of curcumin, the apoptosis of neuronal cells was inhibited in the retina. Curcumin reduced the diabetes-induced overproduction of glutamate in the retina and also repressed the activity of CaMKII. These results indicate that, because curcumin is neuroprotective, it could potentially be used to the treat diabetic retinopathy.
Acknowledgment
This work was supported by Traditional Chinese Medicine of Zhejiang province, Zhejiang, China (Grant No. 2012ZA137).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ferris F.L., 3rd, Davis M.D., and Aiello L.M. Treatment of diabetic retinopathy. N. Engl. J. Med. 341:667–678, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Barber A.J. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog. Neuropsychopharmacol. Biol. Psychiatry. 27:283–290, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Villarroel M., Ciudin A., Hernandez C., and Simo R. Neurodegeneration: an early event of diabetic retinopathy. World J. Diabetes. 1:57–64, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa G., Pereira T., Neto A.M., Cristovao A.J., Ambrosio A.F., and Santos P.F. High glucose changes extracellular adenosine triphosphate levels in rat retinal cultures. J. Neurosci. Res. 87:1375–1380, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Martin P.M., Roon P., Van Ells T.K., Ganapathy V., and Smith S.B. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest. Ophthalmol. Vis. Sci. 45:3330–3336, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Barber A.J., Lieth E., Khin S.A., Antonetti D.A., Buchanan A.G., and Gardner T.W. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J. Clin. Invest. 102:783–791, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oshitari T., Yamamoto S., Hata N., and Roy S. Mitochondria- and caspase-dependent cell death pathway involved in neuronal degeneration in diabetic retinopathy. Br. J. Ophthalmol. 92:552–556, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Abu-El-Asrar A.M., Dralands L., Missotten L., Al-Jadaan I.A., and Geboes K. Expression of apoptosis markers in the retinas of human subjects with diabetes. Invest. Ophthalmol. Vis. Sci. 45:2760–2766, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Goebel D.J. Selective blockade of CaMKII-alpha inhibits NMDA-induced caspase-3-dependent cell death but does not arrest PARP-1 activation or loss of plasma membrane selectivity in rat retinal neurons. Brain Res. 1256:190–204, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Fan W., Agarwal N., Kumar M.D., and Cooper N.G. Retinal ganglion cell death and neuroprotection: involvement of the CaMKIIalpha gene. Brain Res. Mol. Brain Res. 139:306–316, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Fan W., Agarwal N., and Cooper N.G. The role of CaMKII in BDNF-mediated neuroprotection of retinal ganglion cells (RGC-5). Brain Res. 1067:48–57, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Fan W., and Cooper N.G. Glutamate-induced NFkappaB activation in the retina. Invest. Ophthalmol. Vis. Sci. 50:917–925, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laabich A., and Cooper N.G. Neuroprotective effect of AIP on N-methyl-D-aspartate-induced cell death in retinal neurons. Brain Res. Mol. Brain Res. 85:32–40, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Waxham M.N., Grotta J.C., Silva A.J., Strong R., and Aronowski J. Ischemia-induced neuronal damage: a role for calcium/calmodulin-dependent protein kinase II. J. Cereb. Blood Flow Metab. 16:1–6, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Takano H., Fukushi H., Morishima Y., and Shirasaki Y. Calmodulin and calmodulin-dependent kinase II mediate neuronal cell death induced by depolarization. Brain Res. 962:41–47, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Suh H.W., Kang S., and Kwon K.S. Curcumin attenuates glutamate-induced HT22 cell death by suppressing MAP kinase signaling. Mol. Cell. Biochem. 298:187–194, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Jeenger M.K., Shrivastava S., Yerra V.G., Naidu V.G., Ramakrishna S., and Kumar A. Curcumin: a pleiotropic phytonutrient in diabetic complications. Nutrition. 31:276–282, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Sulaiman R.S., Basavarajappa H.D., and Corson T.W. Natural product inhibitors of ocular angiogenesis. Exp. Eye Res. 129:161–171, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lou J., Hu W., Tian R., Zhang H., Jia Y., Zhang J., and Zhang L. Optimization and evaluation of a thermoresponsive ophthalmic in situ gel containing curcumin-loaded albumin nanoparticles. Int. J. Nanomed. 9:2517–2525, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aldebasi Y.H., Aly S.M., and Rahmani A.H. Therapeutic implications of curcumin in the prevention of diabetic retinopathy via modulation of anti-oxidant activity and genetic pathways. Int. J. Physiol. Pathophysiol. Pharmacol. 5:194–202, 2013 [PMC free article] [PubMed] [Google Scholar]
- 21.Pescosolido N., Giannotti R., Plateroti A.M., Pascarella A., and Nebbioso M. Curcumin: therapeutical potential in ophthalmology. Planta Med. 80:249–254, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Zuo Z.F., Zhang Q., and Liu X.Z. Protective effects of curcumin on retinal muller cell in early diabetic rats. Int. J. Ophthalmol. 6:422–424, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huynh T.P., Mann S.N., and Mandal N.A. Botanical compounds: effects on major eye diseases. Evid. Based Complemen. Alternat. Med. 2013:549174, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L.L., Sun Y., Huang K., and Zheng L. Curcumin, a potential therapeutic candidate for retinal diseases. Mol. Nutr. Food Res. 57:1557–1568, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Steigerwalt R., Nebbioso M., Appendino G., Belcaro G., Ciammaichella G., Cornelli U., Luzzi R., Togni S., Dugall M., Cesarone M.R., Ippolito E., Errichi B.M., Ledda A., Hosoi M., and Corsi M. Meriva®, a lecithinized curcumin delivery system, in diabetic microangiopathy and retinopathy. Panminerva Med. 54:11–16, 2012 [PubMed] [Google Scholar]
- 26.Wang C., George B., Chen S., Feng B., Li X., and Chakrabarti S. Genotoxic stress and activation of novel DNA repair enzymes in human endothelial cells and in the retinas and kidneys of streptozotocin diabetic rats. Diabetes Metab. Res. Rev. 28:329–337, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Schrier S.A., and Falk M.J. Mitochondrial disorders and the eye. Curr. Opin. Ophthalmol. 22:325–331, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta S.K., Kumar B., Nag T.C., Agrawal S.S., Agrawal R., Agrawal P., Saxena R., and Srivastava S. Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J. Ocul. Pharmacol. Ther. 27:123–130, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Allegri P., Mastromarino A., and Neri P. Management of chronic anterior uveitis relapses: efficacy of oral phospholipidic curcumin treatment. Long-term follow-up. Clin. Ophthalmol. 4:1201–1206, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mrudula T., Suryanarayana P., Srinivas P.N., and Reddy G.B. Effect of curcumin on hyperglycemia-induced vascular endothelial growth factor expression in streptozotocin-induced diabetic rat retina. Biochem. Biophys. Res. Commun. 361:528–532, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Rema M., and Pradeepa R. Diabetic retinopathy: an Indian perspective. Indian J. Med. Res. 125:297–310, 2007 [PubMed] [Google Scholar]
- 32.Kowluru R.A., and Kanwar M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr. Metab. 4:8, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balasubramanyam M., Koteswari A.A., Kumar R.S., Monickaraj S.F., Maheswari J.U., and Mohan V. Curcumin-induced inhibition of cellular reactive oxygen species generation: novel therapeutic implications. J. Biosci. 28:715–721, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Okamoto T., Yamagishi S., Inagaki Y., Amano S., Koga K., Abe R., Takeuchi M., Ohno S., Yoshimura A., and Makita Z. Angiogenesis induced by advanced glycation end products and its prevention by cerivastatin. FASEB J. 16:1928–1930, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Lin T.Y., Lu C.W., Wang C.C., Wang Y.C., and Wang S.J. Curcumin inhibits glutamate release in nerve terminals from rat prefrontal cortex: possible relevance to its antidepressant mechanism. Prog. Neuropsychopharmacol. Biol. Psychiatry. 35:1785–1793, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Matteucci A., Cammarota R., Paradisi S., Varano M., Balduzzi M., Leo L., Bellenchi G.C., De Nuccio C., Carnovale-Scalzo G., Scorcia G., Frank C., Mallozzi C., Di Stasi A.M., Visentin S., and Malchiodi-Albedi F. Curcumin protects against NMDA-induced toxicity: a possible role for NR2A subunit. Invest. Ophthalmol. Vis. Sci. 52:1070–1077, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Matteucci A., Frank C., Domenici M.R., Balduzzi M., Paradisi S., Carnovale-Scalzo G., Scorcia G., and Malchiodi-Albedi F. Curcumin treatment protects rat retinal neurons against excitotoxicity: effect on N-methyl-D: -aspartate-induced intracellular Ca(2+) increase. Exp. Brain Res. 167:641–648, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Zhu B., Wang W., Gu Q., and Xu X. Erythropoietin protects retinal neurons and glial cells in early-stage streptozotocin-induced diabetic rats. Exp. Eye Res. 86:375–382, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Lin T.Y., Lu C.W., Huang S.K., and Wang S.J. Curcumin inhibits glutamate release from rat prefrontal nerve endings by affecting vesicle mobilization. Int. J. Mol. Sci. 13:9097–9109, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shinoda K., Rejdak R., Schuettauf F., Blatsios G., Volker M., Tanimoto N., Olcay T., Gekeler F., Lehaci C., Naskar R., Zagorski Z., and Zrenner E. Early electroretinographic features of streptozotocin-induced diabetic retinopathy. Clin. Exp. Ophthalmol. 35:847–854, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Arden G.B., Hamilton A.M., Wilson-Holt J., Ryan S., Yudkin J.S., and Kurtz A. Pattern electroretinograms become abnormal when background diabetic retinopathy deteriorates to a preproliferative stage: possible use as a screening test. Br. J. Ophthalmol. 70:330–335, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karadeniz S., Kir N., Yilmaz M.T., Ongor E., Dinccag N., Basar D., Akarcay K., Satman I., and Devrim A.S. Alteration of visual function in impaired glucose tolerance. Eur. J. Ophthalmol. 6:59–62, 1996 [DOI] [PubMed] [Google Scholar]
- 43.van Dijk H.W., Kok P.H., Garvin M., Sonka M., Devries J.H., Michels R.P., van Velthoven M.E., Schlingemann R.O., Verbraak F.D., and Abramoff M.D. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 50:3404–3409, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Q., Gorbey S., Pfister F., Hoger S., Dorn-Beineke A., Krugel K., Berrone E., Wu L., Korff T., Lin J., Busch S., Reichenbach A., Feng Y., and Hammes H.P. Long-term treatment with suberythropoietic Epo is vaso- and neuroprotective in experimental diabetic retinopathy. Cell. Physiol. Biochem. 27:769–782, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Xu Y., Lin D., Li S., Li G., Shyamala S.G., Barish P.A., Vernon M.M., Pan J., and Ogle W.O. Curcumin reverses impaired cognition and neuronal plasticity induced by chronic stress. Neuropharmacology. 57:463–471, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Gao J., Duan B., Wang D.G., Deng X.H., Zhang G.Y., Xu L., and Xu T.L. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron. 48:635–646, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Gu Z., Liu W., and Yan Z. {beta}-Amyloid impairs AMPA receptor trafficking and function by reducing Ca2+/calmodulin-dependent protein kinase II synaptic distribution. J. Biol. Chem. 284:10639–10649, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cooper N.G., Laabich A., Fan W., and Wang X. The relationship between neurotrophic factors and CaMKII in the death and survival of retinal ganglion cells. Prog. Brain Res. 173:521–540, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Kim Y.H., Kim Y.S., Park S.Y., Park C.H., Choi W.S., and Cho G.J. CaMKII regulates pericyte loss in the retina of early diabetic mouse. Mol. Cells. 31:289–293, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim Y.H., Kim Y.S., Kang S.S., Cho G.J., and Choi W.S. Resveratrol inhibits neuronal apoptosis and elevated Ca2+/calmodulin-dependent protein kinase II activity in diabetic mouse retina. Diabetes. 59:1825–1835, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yousif M.H., Akhtar S., Walther T., and Benter I.F. Role of Ca2+/calmodulin-dependent protein kinase II in development of vascular dysfunction in diabetic rats with hypertension. Cell. Biochem. Funct. 26:256–263, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Yousif M.H., Benter I.F., and Akhtar S. Inhibition of calcium/calmodulin-dependent protein kinase II normalizes diabetes-induced abnormal vascular reactivity in the rat perfused mesenteric vascular bed. Auton. Autacoid Pharmacol. 23:27–33, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Vorwerk C.K., Naskar R., Schuettauf F., Quinto K., Zurakowski D., Gochenauer G., Robinson M.B., Mackler S.A., and Dreyer E.B. Depression of retinal glutamate transporter function leads to elevated intravitreal glutamate levels and ganglion cell death. Invest. Ophthalmol. Vis. Sci. 41:3615–3621, 2000 [PubMed] [Google Scholar]
- 54.Sisk D.R., and Kuwabara T. Histologic changes in the inner retina of albino rats following intravitreal injection of monosodium L-glutamate. Graefes Arch. Clin. Exp. Ophthalmol. 223:250–258, 1985 [DOI] [PubMed] [Google Scholar]
- 55.Tang K., Liu C., Kuluz J., and Hu B. Alterations of CaMKII after hypoxia-ischemia during brain development. J. Neurochem. 91:429–437, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang R., Li Y.B., Li Y.H., Xu Y., Wu H.L., and Li X.J. Curcumin protects against glutamate excitotoxicity in rat cerebral cortical neurons by increasing brain-derived neurotrophic factor level and activating TrkB. Brain Res. 1210:84–91, 2008 [DOI] [PubMed] [Google Scholar]