Abstract
CAPSULE
Male aging effects on aneuploidy rates in embryos.
OBJECTIVE
Paternal age is associated with decreasing sperm quality; however, it is unknown if it influences chromosomal abnormalities in embryos. The objective of this study is to evaluate if the aneuploidy rates in embryos are affected by advanced paternal age.
METHODS
A total of 286 embryos, obtained from 32 in vitro fertilization/intracytoplasmic sperm injection cycles with donated oocytes in conjunction with preimplantation genetic diagnosis, were allocated according to paternal age in three groups: Group A: ≤39 years (n = 44 embryos); Group B: 40–49 years (n = 154 embryos); and Group C: ≥50 years (n = 88 embryos). Fertilization rates, embryo quality at day 3, blastocyst development, and aneuploidy embryo rates were then compared.
RESULTS
There was no difference in the seminal parameters (volume, concentration, and motility) in the studied groups. Fertilization rate, percentages of zygotes underwent cleavage, and good quality embryos on day 3 were similar between the three evaluated groups. The group of men ≥50 years had significantly more sperm with damaged DNA, low blastocyst development rate, and higher aneuploidy rates in embryos compared to the other two evaluated groups (P < 0.05).
CONCLUSIONS
Our findings suggest that advanced paternal age increases the aneuploidy rates in embryos from donated oocytes, which suggests that genetic screening is necessary in those egg donor cycles with sperm from patients >50 years old.
Keywords: aging, oocyte, aneuploidy, PGD, ART
Introduction
In today’s society, the assisted reproduction technology permits treating the majority of infertile couples, achieving satisfactory pregnancy and implantation rates. In the vast majority of in vitro fertilization (IVF) laboratories around the world, embryo selection is still based on the assessment of morphologic criteria during the preimplantation stage, such as number of pronuclear and polar bodies, cell size and number, evenness of mitotic divisions, multinucleation, amount of cellular fragmentation, extent of blastocoel expansion, and quality of inner cell mass (ICM) and trophectoderm (TE).1–4 However, it is now known that the embryo morphology does not always translate into high implantation rates,5,6 and thus, embryos achieving the best morphologic scores often fail to achieve implantation or do not produce a live birth.7–9 In many cases, the underlying cause of embryo arrest, implantation failure, or miscarriage is the presence of chromosomal abnormalities or aneuploidy, and now it is generally accepted to be the principal genetic factor that affects the human reproduction success.
The frequency of aneuploidies in human preimplantation embryos generated during IVF is estimated to be between 56% and 84%,10 and its occurrence is related to maternal and paternal factors. The principal female factor include aging, which increases the risk of defects in maternal mRNA, disturbing the pool of proteins and mitochondrial function, and finally an incorrect chromosome segregation during cell division process.11 Duncan et al12 showed a reduced cohesion molecule concentration in older women (responsible for binding the sister chromatids together), which causes an unequal separation of chromosomes leading to aneuploidy. Additional factors include accumulation of free radical in the oocytes, exposure to radiation or chemicals, poor vascularization of antral follicle during oocyte maturation, and depletion of critical nutrients during cell divisions leading to chromosome fragmentation and aneuploidy.13–15 In the case of paternal factors leading to embryo aneuploidy, alterations in centrosome, which is composed of two centrioles and paternally inherited, may result in abnormal spindle formation and chromosome malsegregation.16 Besides, the generation of aneuploid gametes during spermatogenesis and patients with oligoasthenoteratospermia or nonobstructive azoospermia (testicular sperm extracted) with severe sperm defects may result in a higher percentage of mitotic abnormalities and chaotic embryos.17 In the past years, male aging has been associated with a decrease in serum steroid levels, testicular volume, progressive motility, daily sperm production, inhibin B/follicle stimulating hormone (FSH) ratio, alteration in testicular histomorphology, risk of chromosomal disorders,18,19 and a significant increase in spermatic DNA fragmentation, particularly in men >50 years.20,21 These changes related to aging are factors that can increase the risk of aneuploidy in embryos causing failure to obtain blastocysts, blockage in embryo development after implantation, increased risk of recurrent miscarriages, reduced chance of successful implantation, and negative effects on the health of the offspring.22–24
Oocyte donation is a successful and well-established treatment where the oocyte and subsequent embryo qualities are optimized by donated oocytes from young women,25 eliminating the effect of maternal age and resulting in high pregnancy rates and good obstetrical outcomes observed in recipients.26,27 It is in this manner that oocyte donation represents an optimal model to study the influence of male aging on reproductive potential.
The aim of the present study is to evaluate the development capacity, embryo quality, and aneuploidy rates in embryos obtained from donated oocytes according to male age.
Materials and Methods
Study design
This is a retrospective nonrandomized study conducted on 286 embryos obtained from 32 IVF/intracytoplasmic sperm injection (ICSI) cycles with oocytes donated in conjunction with preimplantation genetic diagnosis (PGD; IVF: n = 14; ICSI: n = 18). The procedures were done at FERTILAB Laboratory of Assisted Reproduction between January 2012 and August 2015. Written informed consents were obtained from all recipients, and their partners were included in this study to share the outcomes of their cycles for research purposes. This study was approved by the Institutional Review Board and the corresponding Ethics Committee from Clínica Oncogyn.
Thirty-two anonymous oocytes donors (21–28 years old) were subjected to physical, gynecological, and psychological examinations, and there was no family history of hereditary or chromosomal diseases. All participants had a normal karyotype and tested negative in a screening for sexually transmitted diseases. The recruitment of oocyte donors was done based on the recommendations given by other donors, and the donation of their gametes was merely by altruistic reasons.
Ovarian stimulation and oocyte collection
Donors’ menstrual cycles were stimulated using recombinant FSH (Gonal®) according to the previously established stimulation protocols.28 Medication was started on day 2 of the menstrual cycle until at least three follicles reached ~18 mm in diameter. Oocytes were collected 36 hours after human chorionic gonadotropin administration (Pregnyl®) by transvaginal ultrasound ovum pickup. During the follicular aspiration procedure, oocytes were recovered in Global® HEPES-buffered medium (LifeGlobal) supplemented with 10% volume/volume (vol/vol) Serum Substitute Supplement (SSS; Irvine Scientific). After retrieval, cumulus–oocyte complexes were manually denuded from cumulus cells using sterile needles and cultured in ~200 µL drops of Global® Fertilization medium (LifeGlobal) plus 10% SSS under oil at 37°C and an atmosphere containing 6% of CO2, 5% of O2, and 89% of N2 for five hours before the IVF/ICSI procedure.
Insemination, fertilization, and embryo culture
The recovered oocytes were assessed for their nuclear maturity, and only metaphase II oocytes were submitted to IVF/ICSI. The insemination was made with 50,000–100,000 motile spermatozoa in ~200 µL drops of Global® Fertilization medium + 10% SSS, where one to five oocytes were placed. In the cases of ICSI, all collected oocytes were denuded enzymatically off cumulus cells with hyaluronidase (80 IU/mL; LifeGlobal) and injected following the routine procedures.29
Normal fertilization status, indicated by the presence of two pronuclei, was evaluated for 16–18 hours after IVF/ICSI. The zygotes were individually cultured under mineral oil, in 10-µL droplets of Global® medium (LifeGlobal) supplemented with 10% vol/vol SSS until day 3 when the embryos were moved to fresh 10-µL droplets of Global® medium + 10% SSS and cultured for two more days up to blastocyst stage. On day 3, the embryos were evaluated for cell number, fragmentation, and multinucleation, and on day 5, they were evaluated for blastocyst development and expansion. Good quality day 3 embryos were defined as those with six to eight cells and ≤10% of fragmentation. Good quality blastocysts were defined as having an ICM and TE type A or B.30
Embryo biopsy, fixation, and FISH analysis
On the third day after insemination, one cell per embryo was biopsied following a protocol described elsewhere.31 Individual embryos were placed into calcium/magnesium-free media (PGD Biopsy Medium; LifeGlobal) through a hole of the zona pellucida opened with Tyrode’s acid solution; one nucleated blastomere was removed by aspiration. After biopsies, the embryos were rinsed thoroughly and returned to culture under mineral oil, in 10-µL droplets of Global® medium (LifeGlobal) supplemented with 10% vol/vol SSS.
Blastomeres were fixed individually following the routine protocols to minimize signal overlap and loss of micronuclei.32 PGD analysis was performed by FISH using probes specific for 12 chromosomes 8, 13, 14, 16, 18, 20, 21, 22 (Abbott Laboratories), X, Y, 15, and 17 (Cellay Inc.) following the manufacturer’s instructions.
Sperm collection
Semen samples were collected by masturbation after three to five days of abstinence and on the day of oocyte retrieval for ICSI. Semen analysis was performed according to World Health Organization (WHO) criteria.33 After semen liquefaction, motile spermatozoa were separated from the seminal plasma by centrifugation at 300 × g for 10 minutes through 1.0 mL 95% and 45% Isolate gradients (Irvine Scientific). The pellet was washed once by centrifugation for five minutes and was resuspended in 0.1 mL of Global Fertilization medium + 10% SSS for IVF/ICSI.
Sperm DNA fragmentation assessment
Prior to the hormonal stimulation, sperm DNA fragmentation values were evaluated with the sperm chromatin dispersion test34 using the Halosperm® Kit (Halotech DNA). Briefly, sperm samples from each patient, with a concentration not <5 million and not >10 million spermatozoa per milliliter, were used. The kit contains aliquots of agarose gel in Eppendorf tubes. Each semen sample was processed after the agarose gelled (from immersion in a water bath at 90°C for five minutes). When the Eppendorf tubes reached a temperature of 37°C (five minutes at 37°C in a dry atmosphere), 25 µL of sperm were added and gently mixed. Twenty microliters of this mixture were placed on precoated slides and covered with 22 × 22-mm coverslide. The slides were maintained at 4°C for five minutes to produce a microgel containing embedded spermatozoa. The coverslides were gently removed, and the slides were immersed in a previously prepared acid solution (80 µL of HCl added to 10 mL of distilled water) for seven minutes. After removal from this solution, the slides were incubated for 25 minutes in 10 mL of lysing solution (provided in the Halosperm kit). After rinsing in distilled water, the slides were dehydrated for two minutes in three concentrations of alcohol (70%, 90%, and 100% vol); each and either were stored (storage was possible several months in optimal conditions) or were processed immediately with staining solution for 10 minutes with continuous airflow. Staining was performed with 1:1 (vol/vol) by using Wright’s solution (Merck) and phosphate-buffered saline solution (Merck). The slides were rinsed in tap water, allowed to dry at room temperature, processed for upright or inverted bright-field microscopy at 100×, and covered with 22 × 22 coverslide. Operators scored ≥500 spermatozoa for each patient according to the patterns established by Fernández et al34 Strong staining is preferred to visualize the dispersed DNA loop halos. Removal of sperm nuclear proteins results in nucleoids with a central core and a peripheral halo of dispersed DNA loops. The sperm tails remain intact. The acid treatment produces DNA unwinding that is restricted in those nuclei with high levels of DNA strand breakage. After the subsequent lysis, sperm nuclei with fragmented DNA produce very small or no halos of dispersed DNA. However, nuclei without DNA fragmentation released their DNA loops to form large halos.
Statistical analysis
Statistical analysis was carried out using the statistic package Stata 10 (StataCorp). Data were represented as mean ± SD. Group comparisons were made using the χ2 test and Student’s t-test. It was considered a statistical significant difference when P < 0.05.
Results
Results of chromosomal status from 286 biopsied embryos were allocated to three groups according to paternal age as follows:
≤39 years (range 34–39 years; n = 5)
40–49 years (range 40–47 years; n = 17)
≥50 years (range 50–72 years; n = 10)
There was no difference in the age of oocyte donors (22.7 ± 1.53, 24.2 ± 1.74, and 24.1 ± 1.85 years), days of stimulation (8.4 ± 1.14, 8.9 ± 1.11, and 8.3 ± 1.06), and mean of recombinant FSH treatment (1345 ± 329.96, 1363.2 ± 288.61, and 1385 ± 290.64 IU/mL) between the three evaluated groups (data not shown).
The results of the basic semen parameters and sperm DNA fragmentation according to male age are presented in Table 1. Our data showed that men ≥50 years old had significantly high percentages of sperm with fragmented DNA (37.1 ± 17.61% versus 17.4 ± 10.79% and 21.3 ± 13.48%; P < 0.05). Values of semen volume, sperm concentration, and progressive motility were similar in the three evaluated groups (P = not significant). According to WHO (2010) criteria, men ≥50 years old had significantly less sperm with normal morphology (4.7 ± 3.51% versus 11.3 ± 4.88% and 7.9 ± 3.99%) compared to men from groups ≤39 years old and 40–49 years old, respectively (P < 0.05).
Table 1.
Comparison of seminal characteristics between three evaluated groups.
| ≤39 | 40–49 | ≥50 | |
|---|---|---|---|
| Semen volume (mL) (Mean ± SD) | 3.2 ± 1.39 | 2.1 ± 1.38 | 2 ± 1.15 |
| Sperm concentration (×106/mL) | 90.1 ± 41.83 | 101.5 ± 40.12 | 57.8 ± 51.62 |
| Progressive motility (%) | 29.6 ± 11.55 | 29.3 ± 7.05 | 21 ± 9.07 |
| Sperm morphology (%) | 11.3 ± 4.88 | 7.9 ± 3.99 | 4.7 ± 3.51* |
| Sperm DNA fragmentation (%) | 17.4 ± 10.79 | 21.3 ± 13.48 | 37.1 ± 17.61* |
Note:
P < 0.05 in relation to the groups of ≤39 years and 40–49 years.
Results of laboratory and preimplantation genetic screening (PGS) from evaluated groups are summarized in Table 2. A total of 58, 207, and 131 oocytes were inseminated from the groups of ≤39 years, 40–49 years, and ≥50 years, respectively. There was no difference in the normal fertilization (2PN) between the studied groups (≤39 years: 82.8%; 40–49 years: 75.8%; and ≥50 years: 82.4%). Percentages of zygotes that underwent cleavage (100%, 92.2%, and 95.4%), mean cell number (7.3 ± 1.01, 7.3 ± 0.93, and 6.5 ± 1.22), and good quality embryos on day 3 (83.3%, 85.2%, and 70.4%) were similar from the groups of ≤39 years, 40–49 years, and ≥50 years, respectively. Blastocyst formation rate was significantly lower in the group of men ≥50 years compared to the other two evaluated groups (P < 0.05), but the percentages of good quality blastocysts were not associated with advancing paternal age (P = no significant). According to the chromosomal status of embryos, the advanced paternal age was significantly associated with high aneuploidy rates in embryos; thus, 73.9% embryos from the group of ≥50 years were aneuploidies compared to 59.1% in the group of ≤39 years and 61.1% in the group of 40–49 years (P < 0.05) (Fig. 1).
Table 2.
Results of fertilization, embryo cleavage, blastocyst development, and aneuploidy rates according male age.
| ≤39 | 40–49 | ≥50 | |
|---|---|---|---|
| No. total oocytes | 61 | 237 | 152 |
| No. total inseminated oocytes | 58 | 207 | 131 |
| No. total fertilized oocytes (2PN) (%) | 48 (82.8) | 169 (75.8) | 108 (82.4) |
| No. total cleaved embryo at day 3 (%) | 48 (100) | 166 (92.2) | 103 (95.4) |
| No. cell/embryo at day 3 (Mean ± SD) | 7.3 ± 1.01 | 7.3 ± 0.93 | 6.5 ± 1.22 |
| Good-quality embryos at day 3 (%) | 83.3 | 85.2 | 70.4 |
| Blastocyst formation/2PN (%) | 54.2 | 49.1 | 26.9* |
| Good-quality blastocysts (%) | 95.2 | 82.1 | 79.3 |
| No. total embryos biopsied/2PN (%) | 44 (91.7) | 154 (91.1) | 88 (81.5) |
| Aneuploidy rate (%) | 59.1 | 61.1 | 73.9* |
Note:
P < 0.05 in relation to the groups of ≤39 years and 40–49 years.
Figure 1.
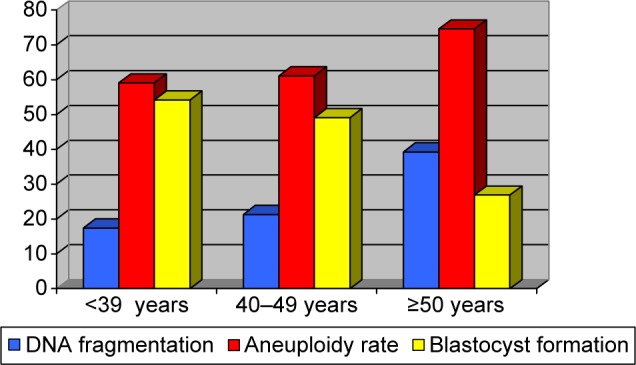
Sperm DNA fragmentation, aneuploidy rate, and blastocyst formation according to male age.
Discussion
In the present study, we have evaluated the effect of male age on embryo quality/development and aneuploidy rates, including only IVF/ICSI cycles using donor oocyte for controlling female age. The data obtained demonstrate a significant negative effect of paternal age beginning from ≥50 years on the aneuploidy and blastocyst formation rates. These results are very important because they directly show the paternal aging effect on chromosomal status in embryos when the effect of the female age is controlled. Other studies that evaluated the paternal contribution to aneuploidy have shown an increase in the proportion of chromosomally abnormal embryos according to the severity of the male factor condition17 but have no relationship to paternal aging in the first trimester pregnancy loss.35
Nowadays, it is known that most human aneuploidies found in embryos originate from the egg and not the sperm,36–39 likely due to one critical difference in the meiotic process between males and females. It is now well established that aneuploidy is present in embryos from infertile patients and dramatically increases with maternal age40–42 from 73% for patients younger than 35 years and 87% for patients 41 and older.10 Nevertheless, controversy exists whether aneuploidy rates in embryos are influenced by the advanced paternal age.
Deficiencies in sperm nuclear genome can be detected as early as the one-cell human zygote (early paternal effect) or throughout preimplantation development after eight-cell stage (late paternal effect).43 During early paternal effect, centrosome dysfunction, disturbance in the number and spatial distribution of the nucleolus precursor body at pronuclear zygote stage, delayed cleavage divisions, and increases in the degree of cleaving embryo fragmentation can occur. The late paternal effect causes failure in the blastocyst formation and low clinical outcomes, and this effect has been associated with an increased incidence of sperm DNA fragmentation.43,44 The present study has not shown difference in fertilization rates, mean cell/embryo, and good quality at day 3 in the three age groups, suggesting a minimal or absence of early paternal effect on preimplantation development. Similar results were reported for fertilization rates45–47 and embryo quality45,47,48 when the effect of paternal age on assisted reproduction outcome was tested.
Evaluating the late paternal effect, a statistically significant decrease in the number of blastocysts on day 5 directly related to an increase in the sperm DNA damage was observed in the group of aging men. In patients ≥50 years old, only 26.9% of the embryos reached the blastocyst stage by the fifth day of development compared to 54.2% and 49.1% in the other two groups of younger men. These data confirm that when the male genomic activation occurs between the four- and eight-cell stage of human preimplantation development, factors such as age and sperm DNA quality induce chromosomal alterations that affect directly the embryo’s capacity to reach the blastocyst stage, and then these strong paternal influences reduce the pregnancy and implantation rates in aging men inclusive of those cases using donated oocytes. Similar results were shown by Frattarelli et al47 and Luna et al.49
Sperm DNA fragmentation might be the most frequent cause of paternal DNA anomaly transmission to progeny and is found in a high percentage of spermatozoa from subfertile and infertile men. Apoptosis, abnormal chromatin packaging, and reactive oxygen species are the principal molecular mechanisms leading to sperm DNA fragmentation.50 Studies by Lopes et al51 and Gandini et al52 have shown that spermatozoa with DNA fragmentation are able to fertilize an oocyte but are related to poor quality embryos, blockage of blastocyst development, and lower pregnancy rates through either natural or using intrauterine insemination, IVF, or ICSI procedures.53–56 Our study, similar to that of García-Ferreyra et al,20 Plastira et al,57 and Vagnini et al,58 showed an age-dependent increase in sperm DNA fragmentation, but additionally we observed that this event was significant and directly related to embryonic aneuploidy when the male was ≥50 years old. These embryos with chromosomal abnormalities could be the more important cause to miscarriages in subfertile couples, older patients, and those cases from donated oocyte, including older men. This finding is important because it shows that advanced paternal age actively contributes to the generation of chromosomal abnormalities in the resulting embryos, and when the type and extent of DNA damage cannot be balanced by the reparative ability of the oocyte (including cases of egg donor), then the genetic screening in embryos should be considered to improve the clinical outcomes.
Male germ cells divide continuously. Approximately 30 spermatogonial stem cell divisions take place before puberty, while undergoing meiotic divisions. From then on, 23 mitotic divisions per year occur, resulting in 150 replications by the age of 20 years and 840 replications by the age of 50 years.59 In older men, these numerous divisions in the stem cells joined to possible attacks from endogenous and exogenous factors can induce a wide range of DNA lesions, affecting normal cellular processes such as transcriptions, recombination and replication.60 One of the main theories of aging states that aging results from the accumulation of unrepaired DNA lesions over life in many tissues, including the brain, the liver, and the testis.61,62 Paul et al62 showed that there is an age-related accumulation of DNA damage in the testis, particularly caused by oxidative stress in the form of 8-oxodG lesions, and a lower capacity of germ cells to repair such DNA damage that leads to a decline in genome integrity that may produce aneuploidy embryos and/or be passed on to future generations, specifically the offspring of older males.
Chromosomal mosaicism is defined as the presence of two or more karyotypically distinct cell lines within an individual, occurs in ~15–90% of all cleavage human embryos,63,64 and may represent a major source of misdiagnosis in PGS because of both false-positive and false-negative results.65 The genetic analysis on one cell or two cells reduces the chance of misdiagnosis because of mosaicism; however, based on the low incidence (5%) of mosaicism encountered in spontaneous abortions and vital pregnancies (2%), it is likely that most mosaic embryos are eliminated before the first trimester of pregnancy.66 Developmental potential in mosaic embryos is related to the proportion and type of aneuploid cells involved, and when multiple chromosome anomalies on different cells are present, high rates of developmental arrest are observed. On the other hand, studies of Johnson et al67 and Fragouli et al68 have showed lower level of mosaicism at the blastocyst stage (16–33%), suggesting that preimplantation genetic screening via TE biopsy may reduce misdiagnosis by mosaicism. We suggest that future studies should be carried out by TE genetic analysis to avoid mosaic embryos misdiagnosed.
Programs of IVF with egg donor are believed to be so successful largely because oocyte quality is greatly improved when the donor’s age is low, thus yielding better pregnancy rates and reduced miscarriage risk. One would hope that these good clinical outcomes could be due to absent or minimal chromosomal errors present in oocytes from healthy young donor; however, a previous study showed that 17% of human egg collected from healthy women at the age of 22–25 during natural cycle had spindle abnormalities,69 which cause aneuploidy in embryos derived from donated oocytes. Our study showed a global aneuploidy rate of 65.1%, which is similar to the previously observed rates by Sills et al70 and Haddad et al,71 but unlike these studies, we have also showed that the advanced paternal age significantly increases the aneuploidy embryo rate since ≥50 years old, and this event is related to high values of sperm DNA fragmentation.
Conclusion
This study shows that male aging actively contributes to the generation of chromosomal abnormalities in the resulting embryos from oocytes donated and the effect on aneuploidy and blastocyst development rates significantly begin from ≥50 years old. Embryonic genetic screening should be performed in cycles of egg donor if paternal age is >50 years to improve the clinical outcomes and reduce the miscarriage risk in this group.
Footnotes
ACADEMIC EDITOR: Zeev Blumenfeld, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report.
Reviewers’ reports totaled 778 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: JGF. Analyzed the data: JGF and DL. Made critical revisions and approved final version: JGF, DL, LV, RR, PZ, RH, and JDC. All the authors reviewed and approved the final manuscript.
REFERENCES
- 1.Cummins JM, Breen TM, Harrison KL, Shaw JM, Wilson LM, Hennessey JF. A formula for scoring human embryo growth rates in in vitro fertilization: its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J In Vitro Fert Embryo Transf. 1986;3:284–295. doi: 10.1007/BF01133388. [DOI] [PubMed] [Google Scholar]
- 2.Puissant F, Van Rysselberge M, Barlow P, Dewese J, Leroy F. Embryo scoring as a prognostic tool in IVF treatment. Hum Reprod. 1987;2:705–708. doi: 10.1093/oxfordjournals.humrep.a136618. [DOI] [PubMed] [Google Scholar]
- 3.Van Royen E, Mangelschots K, De Neubourg D, et al. Characterization of a top quality embryo, a step toward single-embryo transfer. Hum Reprod. 1999;14:2345–2349. doi: 10.1093/humrep/14.9.2345. [DOI] [PubMed] [Google Scholar]
- 4.Gianaroli L, Magli MC, Ferraretti AP, Lappi M, Borghi E, Ermini B. Oocyte euploidy, pronuclear zygote morphology and embryo chromosomal complement. Hum Reprod. 2007;22:241–249. doi: 10.1093/humrep/del334. [DOI] [PubMed] [Google Scholar]
- 5.Moyaeri SE, Allen RB, Brewster WR, Kim MH, Porto M, Werlin LB. Day-3 embryo morphology predicts euploidy among older subjects. Fertil Steril. 2008;89:118–123. doi: 10.1016/j.fertnstert.2007.01.169. [DOI] [PubMed] [Google Scholar]
- 6.Alfarawati S, Fragouli E, Colls P, et al. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertil Steril. 2011;95:520–524. doi: 10.1016/j.fertnstert.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Magli MC, Gianaroli L, Munné S, Ferraretti AP. Incidence of chromosomal abnormalities from a morphologically normal cohort of embryos in poor-prognosis patients. J Assist Reprod Genet. 1998;15:297–301. doi: 10.1023/A:1022596528036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardarson T, Caisander G, Sjogren A, Hanson C, Hamberger L, Lundin K. A morphological and chromosomal study of blastocyst developing from morphologically suboptimal pre-embryos compared with control blastocyst. Hum Reprod. 2003;18:399–407. doi: 10.1093/humrep/deg092. [DOI] [PubMed] [Google Scholar]
- 9.Eaton JL, Hacker MR, Harris D, Thornton KL, Penzias AS. Assessment of day-3 morphology and euploidy for individual chromosomes in embryos that develop to the blastocyst stage. Fertil Steril. 2009;91:2432–2436. doi: 10.1016/j.fertnstert.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Fragouli E, Alfarawati S, Spath K, Wells D. Morphological and cytogenetic assessment of cleavage and blastocyst stage embryos. Mol Hum Reprod. 2014;20:117–126. doi: 10.1093/molehr/gat073. [DOI] [PubMed] [Google Scholar]
- 11.Braude P, Bolton V, Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature. 1988;332:459–461. doi: 10.1038/332459a0. [DOI] [PubMed] [Google Scholar]
- 12.Duncan FE, Hornick JE, Lampson MA, Schultz RM, Shea LD, Woodruff TK. Chromosome cohesion decreases in human eggs with advanced maternal age. Aging Cell. 2012;11:1121–1124. doi: 10.1111/j.1474-9726.2012.00866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilding M, De Placido G, De Matteo L, Marino M, Alviggi C, Dale B. Chaotic mosaicism in human preimplantation embryos is correlated with a low mitochondrial membrane potential. Fertil Steril. 2003;79:340–346. doi: 10.1016/s0015-0282(02)04678-2. [DOI] [PubMed] [Google Scholar]
- 14.Wells D, Bermudez MG, Steuerwald N, et al. Expression of genes regulating chromosome segregation, the cell cycle and apoptosis during human preimplantation development. Hum Reprod. 2005;20:1339–1348. doi: 10.1093/humrep/deh778. [DOI] [PubMed] [Google Scholar]
- 15.Jaroudi S, Kakourou G, Cawood S, et al. Expression profiling of DNA repair genes in human oocytes and blastocysts using microarrays. Hum Reprod. 2009;24:2649–2655. doi: 10.1093/humrep/dep224. [DOI] [PubMed] [Google Scholar]
- 16.Palermo G, Munné S, Cohen J. The human zygote inherits its mitotic potential from the male gamete. Hum Reprod. 1994;9:1220–1225. doi: 10.1093/oxfordjournals.humrep.a138682. [DOI] [PubMed] [Google Scholar]
- 17.Magli MC, Gianaroli L, Ferraretti AP, Gordts S, Fredericks V, Crippa A. Paternal contribution to aneuploidy in preimplantation embryos. Reprod Biomed Online. 2009;18:536–542. doi: 10.1016/s1472-6483(10)60131-9. [DOI] [PubMed] [Google Scholar]
- 18.Handelsman DJ, Staraj S. Testicular size: the effect of aging, malnutrition, and illness. J Androl. 1985;6:144–151. doi: 10.1002/j.1939-4640.1985.tb00830.x. [DOI] [PubMed] [Google Scholar]
- 19.Mahmoud AM, Goemaere S, El-Garem Y, Van Potterlberrgh I, Comhaire FH, Kaufman JM. Testicular volume in relation to hormonal indices of gonadal function in community-dwelling elderly men. J Clin Endocrinol Metab. 2003;88:179–184. doi: 10.1210/jc.2002-020408. [DOI] [PubMed] [Google Scholar]
- 20.García-Ferreyra J, Romero R, Hilario R, Dueñas-Chacón J. High levels of DNA fragmentation observed in an infertile population attending a fertility center are related to advanced paternal age. J Fertili In Vitro. 2012;2:1–5. [Google Scholar]
- 21.García-Ferreyra J, Villegas L, Romero R, et al. Sperm DNA fragmentation is significantly increased in those men with morphologically abnormal spermatozoa. J Fertili In Vitro. 2014;2:1–5. [Google Scholar]
- 22.Seli E, Gardner DK, Schoolcraft WB, Moffalt O, Sakkas D. Extend of nuclear DNA damage in ejaculated spermatozoa impacts on blastocyst development after in vitro fertilization. Fertil Steril. 2004;82:378–383. doi: 10.1016/j.fertnstert.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 23.Borini A, Tarozzi N, Bizzaro D, et al. Sperm DNA fragmentation: paternal effect on early post-implantation embryo development in ART. Hum Reprod. 2006;21:2876–2881. doi: 10.1093/humrep/del251. [DOI] [PubMed] [Google Scholar]
- 24.Bungum M, Humaidan P, Axmon A, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007;22:174–179. doi: 10.1093/humrep/del326. [DOI] [PubMed] [Google Scholar]
- 25.Wong IL, Legro RS, Lindheim SR, Paulson RJ, Sauer MV. Efficacy of oocytes donated by older women in an oocyte donation programme. Hum Reprod. 1996;11:820–823. doi: 10.1093/oxfordjournals.humrep.a019260. [DOI] [PubMed] [Google Scholar]
- 26.Sauer MV, Kavic SM. Oocyte and embryo donation 2006: reviewing two decades of innovation and controversy. Reprod Biomed Online. 2006;12:153–162. doi: 10.1016/s1472-6483(10)60855-3. [DOI] [PubMed] [Google Scholar]
- 27.Budak E, Garrido N, Soares SR, et al. Improvements achieved in an oocyte donation program over a 10-year period: sequential increase in implantation and pregnancy rates and decrease in high-order multiple pregnancies. Fertil Steril. 2007;88:342–349. doi: 10.1016/j.fertnstert.2006.11.118. [DOI] [PubMed] [Google Scholar]
- 28.Tavmergen E, Goker E, Sendag F, Sendag H, Levi R. Comparison of short and long ovulation induction protocols used in ART applications according to the ovarian response and outcome of pregnancy. Arch Gynecol Obstet. 2002;266:5–11. doi: 10.1007/pl00007494. [DOI] [PubMed] [Google Scholar]
- 29.García J Noriega Hoces L, GF Gonzales. Sperm chromatin stability and its relationship with fertilization rate after intracytoplasmic sperm injection (ICSI) in an assisted reproduction program. J Assist Reprod Genet. 2007;24:587–593. doi: 10.1007/s10815-007-9174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardner DK, Schoolcraft WB. In vitro culture of human blastocysts. In: Jansen R, Mortimer D, editors. Towards Reproductive Certainly: Infertility and Genetics Beyond; The Plenary Proceedings of the 11th World Congress on In Vitro Fertilization and Human Reproductive Genetics. Pearl River: Parthenon Press; 1999. pp. 378–388. [Google Scholar]
- 31.Munné S, Sandalinas M, Escudero T, Ary B. Improved implantation after pre-implantation genetic of aneuploidy. Reprod Biomed Online. 2003;7:91–97. doi: 10.1016/s1472-6483(10)61735-x. [DOI] [PubMed] [Google Scholar]
- 32.Velilla E, Escudero T, Munné S. Blastomere fixation technique and risk of misdiagnosis for PGD of aneuploidy. Reprod Biomed Online. 2002;4:210–217. doi: 10.1016/s1472-6483(10)61808-1. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization . WHO Laboratory Manual for Examination and Processing. 5th ed. World Health Organization; Geneva, Switzerland: 2010. [Google Scholar]
- 34.Fernández JL, Muriel L, Rivero MT, Goyanes V, Vasquez R, Alvarez JG. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl. 2003;24:59–66. [PubMed] [Google Scholar]
- 35.Kushnir VA, Scott RT, Frattarelli JL. Effect of paternal age on aneuploidy rates in first trimester pregnancy loss. J Med Genet Genomics. 2010;2:38–43. [Google Scholar]
- 36.Hassold T, Jacobs PA, Leppert M, Sheldon M. Cytogenetic and molecular studies of trisomy 13. Med Genet. 1987;24:725–732. doi: 10.1136/jmg.24.12.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.May KM, Jacobs PA, Lee M, et al. The paternal origin of the extra chromosome in 47, XXX females. Am J Hum Genet. 1990;46:754–761. [PMC free article] [PubMed] [Google Scholar]
- 38.Takaesu N, Jacobs PA, Cockwell A, et al. Nondisjunction of chromosome 21. Am J Med Genet Suppl. 1990;7:175–181. doi: 10.1002/ajmg.1320370735. [DOI] [PubMed] [Google Scholar]
- 39.Munné S, Chen S, Colls P, et al. Maternal age, morphology, development and chromosome abnormalities in over 6000 cleavage-stage embryos. Reprod Biomed Online. 2007;14:628–634. doi: 10.1016/s1472-6483(10)61057-7. [DOI] [PubMed] [Google Scholar]
- 40.Spandorfer SD, Chung PH, Kligman I, Liu HC, David OK, Rosenwaks Z. An analysis of the effect of age on implantation rates. J Assist Reprod Genet. 2000;17:303–306. doi: 10.1023/A:1009422725434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Platteau P, Staessen C, Michiels A, Van Steirteghem A, Liebaers I, Devroey P. Preimplantation genetic diagnosis for aneuploidy screening in women older than 37 years. Fertil Steril. 2005;84:319–324. doi: 10.1016/j.fertnstert.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Liu J, Wang W, Sun X, et al. DNA microarray reveals that high proportions of human blastocysts from women of advanced maternal age are aneuploidy and mosaic. Biol Reprod. 2012;87:1–9. doi: 10.1095/biolreprod.112.103192. [DOI] [PubMed] [Google Scholar]
- 43.Tesarik J. Paternal effects on cell division in the human preimplantation embryo. Reprod Biomed Online. 2005;10:370–375. doi: 10.1016/s1472-6483(10)61798-1. [DOI] [PubMed] [Google Scholar]
- 44.Tesarik J, Greco E, Mendoza C. Late, but not early, paternal effect on human embryo development is related to sperm DNA fragmentation. Hum Reprod. 2004;19:611–615. doi: 10.1093/humrep/deh127. [DOI] [PubMed] [Google Scholar]
- 45.Gallardo E, Simón C, Levy M, Guanes PP, Remohí J, Pellicier A. Effect of age on sperm fertility potential: oocyte donation as a model. Fertil Steril. 1996;66:260–264. doi: 10.1016/s0015-0282(16)58450-7. [DOI] [PubMed] [Google Scholar]
- 46.Paulson RJ, Milligan RC, Sokol RZ. The lack of influence of age on male infertility. Am J Obstet Gynecol. 2001;184:818–822. doi: 10.1067/mob.2001.113852. [DOI] [PubMed] [Google Scholar]
- 47.Frattarelli JL, Miller KA, Miller BT, Elkind-Hirsch K, Scott RT., Jr Male age negatively impacts embryo development and reproductive outcome in donor oocyte ART cycles. Fertil Steril. 2008;90:97–103. doi: 10.1016/j.fertnstert.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 48.Ferreira RC, Braga DP, Bonetti TC, Pasqualotto FF, Iaconelli A, Jr, Borges E., Jr Negative influence of paternal age on clinical intracytoplasmic sperm injection cycle outcomes in oligozoospermic patients. Fertil Steril. 2010;93:1870–1874. doi: 10.1016/j.fertnstert.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 49.Luna M, Finkler E, Barritt J, et al. Paternal age and assisted reproductive technology outcome in ovum recipients. Fertil Steril. 2009;92:1772–1775. doi: 10.1016/j.fertnstert.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 50.Sakkas D, Mariethoz E, Manicardi G, Bizzaro PG, Bianchi U. Origin of DNA damage in ejaculated human spermatozoa. Rev Reprod. 1999;4:431–437. doi: 10.1530/ror.0.0040031. [DOI] [PubMed] [Google Scholar]
- 51.Lopes S, Jurisicova A, Sun JG, Casper RF. Reactive oxygen species: potential cause for DNA fragmentation in human spermatozoa. Hum Reprod. 1998;13:896–900. doi: 10.1093/humrep/13.4.896. [DOI] [PubMed] [Google Scholar]
- 52.Gandini L, Lombardo F, Paoli D, et al. Full-term pregnancies achieved with ICSI despite high levels of sperm chromatin damage. Hum Reprod. 2004;19:1409–1417. doi: 10.1093/humrep/deh233. [DOI] [PubMed] [Google Scholar]
- 53.Larson KL, DeJonge CJ, Barnes AM, Jost LK, Evenson DP. Sperm chromatin structure assay parameters as predictors of failed pregnancy following assisted reproduction techniques. Hum Reprod. 2000;15:1717–1722. doi: 10.1093/humrep/15.8.1717. [DOI] [PubMed] [Google Scholar]
- 54.Duran EH, Morshedi M, Taylor S, Oehninger S. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod. 2002;17:3122–3128. doi: 10.1093/humrep/17.12.3122. [DOI] [PubMed] [Google Scholar]
- 55.Henkel R, Hajimohammad M, Stalf T, et al. Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertil Steril. 2004;81:965–972. doi: 10.1016/j.fertnstert.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 56.Muriel L, Garrido N, Fernandez JL, et al. Value of sperm deoxyribonucleic acid fragmentation level, as measured by the sperm chromatin dispersion test, in the outcome of in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 2006;85:371–383. doi: 10.1016/j.fertnstert.2005.07.1327. [DOI] [PubMed] [Google Scholar]
- 57.Plastira K, Msaouel P, Angelopoulou R, et al. The effects of age on DNA fragmentation, chromatin packaging and conventional semen parameters in spermatozoa of oligoasthenoteratozoospermic patients. J Assist Reprod Genet. 2007;24:437–443. doi: 10.1007/s10815-007-9162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vagnini L, Baruffi RL, Mauri AL, et al. The effects of male age on sperm DNA damage in an infertile population. Reprod Biomed Online. 2007;15:514–519. doi: 10.1016/s1472-6483(10)60382-3. [DOI] [PubMed] [Google Scholar]
- 59.Crow JF. The origins, patterns and implications of human spontaneous mutation. Nat Rev Genet. 2000;1:40–47. doi: 10.1038/35049558. [DOI] [PubMed] [Google Scholar]
- 60.Baarends VW, Van der Laan R, Grootegoed JA. DNA repair mechanisms and gametogenesis. Reproduction. 2001;121:31–39. doi: 10.1530/rep.0.1210031. [DOI] [PubMed] [Google Scholar]
- 61.Moller P, Lohr M, Folkmann JK, Mikkelsen L, Loft S. Aging and oxidatively damaged nuclear DNA in animal organs. Free Radic Biol Med. 2010;48:1275–1285. doi: 10.1016/j.freeradbiomed.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 62.Paul C, Nagano M, Robaire B. Aging results in differential regulation of DNA repair pathways in pachytene spermatocytes in the Brown Norway Rat. Biol Reprod. 2011;85:1269–1278. doi: 10.1095/biolreprod.111.094219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daphnis DD, Delhanty JD, Jerkovic S, Geyer J, Craft I, Harper JC. Detailed FISH analysis of day 5 human embryos reveals the mechanisms leading to mosaic aneuploidy. Hum Reprod. 2005;20:129–137. doi: 10.1093/humrep/deh554. [DOI] [PubMed] [Google Scholar]
- 64.Rubio C, Rodrigo L, Mercader A, et al. Impact of chromosomal abnormalities on preimplantation embryo development. Prenat Diagn. 2007;27:748–756. doi: 10.1002/pd.1773. [DOI] [PubMed] [Google Scholar]
- 65.Munné S, Sandalinas M, Escudero T, Marquez C, Cohen J. Chromosome mosaicism in cleavage-stage human embryos: evidence of a maternal age effect. Reprod Biomed Online. 2002;4:223–232. doi: 10.1016/s1472-6483(10)61810-x. [DOI] [PubMed] [Google Scholar]
- 66.Los F, Van Opstal D, van de Berg C. The development of cytogenetically normal, abnormal and mosaic embryos: a theoretical model. Hum Reprod Update. 2004;10:79–94. doi: 10.1093/humupd/dmh005. [DOI] [PubMed] [Google Scholar]
- 67.Johnson DS, Cinnioglu C, Ross R, et al. Comprehensive analysis of karyotypic mosaicism between trophectoderm and inner cell mass. Mol Hum Reprod. 2010;16:944–949. doi: 10.1093/molehr/gaq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fragouli E, Alfarawati S, Daphnis DD, et al. Cytogenetic analysis of human blastocysts with the use of FISH, CGH and aCGH: scientific data and technical evaluation. Hum Reprod. 2011;26:480–490. doi: 10.1093/humrep/deq344. [DOI] [PubMed] [Google Scholar]
- 69.Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod. 1996;11:2217–2222. doi: 10.1093/oxfordjournals.humrep.a019080. [DOI] [PubMed] [Google Scholar]
- 70.Sills ES, Li X, Frederick JL, Khoury CD, Potter DA. Determining parental origin of embryo aneuploidy: analysis of genetic error observed in 305 embryos derived from anonymous donor oocyte IVF cycles. Mol Cytogenet. 2014;7:1–8. doi: 10.1186/s13039-014-0068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haddad G, Deng M, Wang CT, et al. Assessment of aneuploidy formation in human blastocysts resulting from donated eggs and the necessity of the embryos for aneuploidy screening. J Assist Reprod Genet. 2015;32:999–1006. doi: 10.1007/s10815-015-0492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


