Abstract
Studies of influenza-specific immune responses in humans have largely assessed systemic responses involving serum Ab and peripheral blood T cell responses. However, recent evidence indicates that tissue-resident memory T (TRM) cells play an important role in local murine intrapulmonary immunity. Rhesus monkeys were pulmonary exposed to 2009 pandemic H1N1 virus at days 0 and 28 and immune responses in different tissue compartments were measured. All animals were asymptomatic postinfection. Although only minimal memory immune responses were detected in peripheral blood, a high frequency of influenza nucleoprotein–specific memory T cells was detected in the lung at the “contraction phase,” 49–58 d after second virus inoculation. A substantial proportion of lung nucleoprotein-specific memory CD8+ T cells expressed CD103 and CD69, phenotypic markers of TRM cells. Lung CD103+ and CD103- memory CD8+ T cells expressed similar levels of IFN-γ and IL-2. Unlike memory T cells, spontaneous Ab secreting cells and memory B cells specific to influenza hemagglutinin were primarily observed in the mediastinal lymph nodes. Little difference in systemic and local immune responses against influenza was observed between young adult (6–8 y) and old animals (18–28 y). Using a nonhuman primate model, we revealed substantial induction of local T and B cell responses following 2009 pandemic H1N1 infection. Our study identified a subset of influenza-specific lung memory T cells characterized as TRM cells in rhesus monkeys. The rhesus monkey model may be useful to explore the role of TRM cells in local tissue protective immunity after rechallenge and vaccination.
Introduction
Influenza remains a global health problem with high degree of morbidity and mortality in young children and the elderly. Seasonal influenza vaccines, either trivalent inactivated or live attenuated influenza vaccines provide only moderate protection in adults and children with efficacy ranging from 59 to 83% (1). New improved influenza vaccines are needed to further reduce influenza-related morbidity and mortality. Serum hemagglutination-inhibition (HAI) titers against influenza viruses have been commonly used as correlates for protection (2) and serve as markers for designing influenza vaccines to induce strain-matched HAI Ab responses. These Abs are specific to the immunodominant globular domain of hemagglutinin (HA), thereby inhibiting binding of the virus to receptor on host target cells. It is well recognized that seasonal influenza vaccines do not confer protection on all vaccinated individuals. Some individuals with high HAI titers can be infected with influenza virus, whereas in others, clinical protection can be detected in the absence of HAI titers (3, 4) therefore suggest a role of cell-mediated immunity in protection.
Both natural infection and immunization with influenza A vaccines provide complete protection against reinfection with homologous virus. This is termed homotypic immunity. In contrast, heterosubtypic immunity is defined as immunity to an influenza subtype (i.e., heterologous influenza A virus that has a major change in the surface proteins [antigenic shift]). There is strong evidence in animal models that influenza-specific cross-reactive memory T cells are responsible for inducing heterosubtypic immunity (5–7). However, in humans, the role of cross-reactive memory T cells in protecting against influenza is not well elucidated. A recent human influenza challenge study demonstrated that the preexisting CD4+ T cell responses to conserved nucleoprotein (NP) and matrix protein could reduce severe illness in the absence of specific Abs (8). In another study, a higher frequency of preexisting CD8+IFN-γ+IL-2− cross-reactive memory T cells against conserved core proteins (NP, M1, and PB1) in peripheral blood was associated with reduced severity of disease in humans infected with 2009 pH1N1 influenza (9), although this could reflect spillover of the responses initially generated in respiratory tract-draining lymph nodes.
Viral infection and replication take place in the respiratory epithelial cells, yet most studies on influenza-specific memory T and B cells in humans have been conducted on immune cells isolated from peripheral blood, which may not reflect local lung immune responses. The role of local immunity has received more attention lately primarily because of the discovery of a new subset of memory T cells termed TRM cells. These long-lived nonrecirculating TRM cells permanently reside in nonlymphoid tissues including skin, brain, vagina, and lung and provide rapid, effective local protection against reinfection relative to circulating counterpart memory T cells (10, 11). Local immune protection by TRM cells has been consistently documented in murine models of virus and bacterial infections including vaccinia virus, lymphocytic choriomeningitis virus, HSV, influenza, and tuberculosis (12–16). Recent studies have revealed delivery of vaccinia virus in mice via skin scarification generates long-lived skin TRM CD8+ T cells associated with improved long-term immunity (12, 17). In humans, protection against smallpox was first shown in 1796 by Edward Jenner using skin scarification with cowpox, ultimately leading to the eradication of the disease. Intravaginal heterologous prime/boost vaccination with human papillomavirus vectors (human papillomavirus pseudoviruses) expressing HSV Ags in mice preferentially induces cervicovaginal TRM CD8+ T cells and controls genital disease and virus shedding, whereas i.m. injection preferentially induces serum Ab response but fails to control either one (18). Targeting lung dendritic cells (DCs) via intranasal vaccination with Ag coupled to mAbs against endocytic receptors on DCs induces lung TRM CD8+ T cells that protect against lethal influenza challenge (19). Collectively, these data suggest that site of vaccine Ag priming could influence the localization of TRM cells which are crucial for local host defense.
Most information about tissue distribution of memory T and B cells has been generated from a mouse model of microbial infection or vaccination (20–23). Rhesus monkeys are phylogentically close to humans and similarities of T and B cell immune responses in both species have been reported (24–27). Furthermore, TLR expression on Ag presenting DCs is similar in primates and humans (28). Rhesus monkeys have helped predict subsequent human immunogenicity of various formulations of malaria vaccines and such preclinical studies have contributed to development of the soon-to-be licensed RTS,S malaria vaccine (29, 30).The confirmation of comparable distribution pattern of memory T and B cells, resident memory cells in particular, subsets in rhesus monkeys and humans will greatly aid in designing vaccination strategies capable of inducing tissue-specific immunity. Rhesus monkeys have been previously employed to study immune response and immunopathology to 2009 pH1N1 (31‑33). In this paper, we report the tissue distribution of memory T, B, and spontaneous Ab-secreting cells (ASCs) following infection with 2009 pH1N1influenza virus in rhesus monkeys as well as correlations between age and immune responses.
Materials and Methods
Animals and virus inoculations
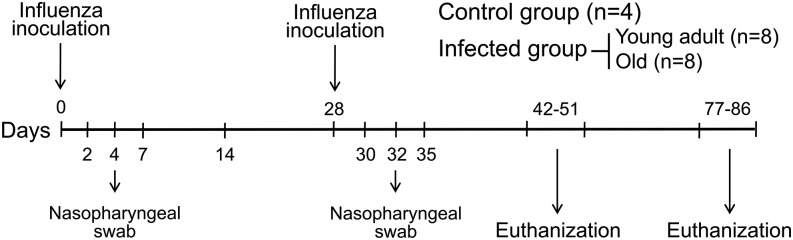
All animal procedures were approved by the institution animal care and use committee in a facility accredited by the American Association of Laboratory Animal Care, incompliance with the Animal Welfare Act and Guide for the Care and Use of Laboratory Animals and in accordance with all applicable U.S. Department of Agriculture, Office of Laboratory Animal Welfare and Department of Defense guidelines. A stock of A/California/04/2009 (H1N1) virus was produced on Madin–Darby canine kidney (MDCK) cells and titrated using 50% tissue culture infective dose (TCID50) assay. A total of 20 healthy Indian rhesus monkeys (Macaca mulatta) with no preexisting immunity to 2009 pH1N1 were selected and divided into two groups: control (n = 4) and infected group (n = 16). In the infected group, animals were subdivided into young adult (6–8 y of age; n = 8) and old monkeys (18–28 y of age; n = 8). Animals were inoculated twice with A/California/04/2009 (H1N1) virus at days 0 and 28, each inoculation consisted of 2 × 106 TCID50 in 2 ml Leibovitz L-15 medium (L-15) with 1 ml administered intranasally (0.5 ml/nostril) and 1 ml intratracheal (Fig. 1). Nasal swabs were collected on days 2, 4, and 7 after each virus inoculation using Copan Flocked swabs and Copan universal transport medium (Copan Diagnostic, Brescia, Italy). Animals in control group were inoculated with L-15 medium. Virus replication in nasal secretions was assessed using a standard plaque assay. Briefly, confluent monolayers of MDCK were inoculated with 10-fold dilutions of samples at 37°C for 1 h. The inoculums were removed, and cells were washed and overlaid with MEM containing 0.6% agarose and 0.2% serum albumin. After 2 d at 37°C, cells were fixed in 37% formaldehyde solution for 1 h before stained with 1.25% crystal violet and plaque numbers (PFU/ml) were evaluated.
FIGURE 1.
Study design for influenza inoculation and tissue collection. Rhesus monkeys were exposed via intranasal and intratracheal inoculation to 2009 pH1N1 virus at days 0 and 28. Peripheral blood, mediastinal lymph nodes, lung, spleen, and bone marrow were collected at days 14–23 and 49–58 after second virus inoculation and assessed for immune responses.
Tissue collection and cell separation
To evaluate memory T and B cell response, half of animals in each group were euthanized between days 14 and 23 and the other half between days 49 and 58 after second virus inoculation. Blood was collected before euthanasia and processed for PBMC by centrifugation using Histopaque-1077 (Sigma-Aldrich, St. Louis, MO). The lung, mediastinal lymph nodes, spleen, and bone marrow were also harvested immediately after euthanasia. Mediastinal lymph nodes, spleen, and bone marrow were homogenized between the frosted ends of two slides, whereas lung tissues were cut into small pieces and homogenized in the presence of 1 μg/ml collagenase type 1 (Life Technologies, Grand Island, NY) using automated tissue dissociation (Miltenyi Biotec, Auburn, CA) and further incubated at 37°C for another 1 h. Digested lung cells and cells derived from mediastinal lymph nodes, spleen, and bone marrow were then passed through 70-μm cell strainer (BD Falcon, Durham, NC). Then, single-cell suspensions were centrifuged using Histopaque-1077 to obtain mononuclear immune cells. They were treated with ammonium chloride–based lysing solution (BD Biosciences, San Jose, CA) if a large amount of RBCs were found to be contaminated in cell preparations. PBMC and mononuclear immune cells derived from different tissues were frozen in liquid nitrogen until use.
ELISA
IgG and IgA specific to influenza HA in nasal secretions were measured by ELISA. Briefly, 96-well ELISA plates (Dynex, Chantilly, VA) were coated with recombinant HA derived from A/California/04/2009 (H1N1) (0.5 μg/well) at 4°C overnight and then washed with PBS containing 0.1% Tween 20 (Sigma-Aldrich). After blocking with 1% BSA in PBS with 0.1% Tween 20, nasal swab fluids (1:4 dilution) were added into coated, blocked plates and incubated for 2 h. Abs specific to HA were detected by anti-monkey IgG (Sigma-Aldrich) or IgA (KPL, Gaithersburg, MD) conjugated with peroxidase and developed with substrate using equal parts of solution A (2,2′-azino-di-(3-ethylbenzthiazoline-6-sulfonate)) and B (H2O2) (KPL). The reaction was stopped with 5% NaDodSO4 stop solution, and the plates were read at 405 nm on a SPECTRAmax plate reader (Molecular Devices).
HAI assay
Monkey serum samples were treated with receptor-destroying enzyme and subsequently heat-inactivated (30 min at 56°C). The HAI assay was performed by World Health Organization standard methods using 8 HA units of influenza virus. Samples were tested in serial 2-fold dilutions by starting at 1:10 dilution. They were mixed with virus and incubated for 15 min at room temperature and then 50 μl of a 0.5% suspension of goose RBCs was added. The Ab titers were defined as the reciprocal of the highest dilution of sera samples that completely inhibited hemagglutination. Titers that were lower than the detection limit were assigned a value of 5 for analysis of geometric mean titer (GMT).
Neutralization assay (NT) for 2009 pH1N1
To detect Abs that could inhibit infection of cells with influenza virus, microneutralization assays were performed using MDCK cells. Samples were heat-inactivated and serial dilutions preincubated with 2009 H1N1 (A/California/04/2009; 100 TCID50) in 96 well plates. After 1- to 2-h incubation at 37°C in a 5% CO2, the mixtures were added to a preformed monolayer of MDCK cells, and the plates were incubated for another 18 h. MDCK monolayers were then washed with PBS and fixed in cold 80% acetone for 1 h. The presence of viral protein was detected by ELISA using a mAb to the influenza A NP. The second Ab conjugated with peroxidase was added and incubated for another 1 h. Plates were washed and specific enzyme substrate added. The reactions were stopped with 1 N sulfuric acid. The absorbance (A) was measured at 450 and 620 nm. The average of difference A450 and A620 was determined for duplicate wells of virus-infected (VC) and -uninfected (CC) control wells, and a neutralizing endpoint was determined by using a 50% specific signal calculation. The endpoint titer was expressed as the reciprocal of the highest dilution of serum with A450 value less than X, where X = [(average [A450-A620] of VC wells) − (average [A450-A620] of CC wells)]/2 + (average [A450-A629] of CC wells). Sera, which tested negative at a dilution of 1:20, were assigned a titer of 10 for analysis of GMT.
ELISPOT assay for spontaneous ASCs
Spontaneous Ab=secreting cells specific to influenza HA were assessed by ELISPOT assay. Briefly, ELISPOT plates (Millipore, Billerica, MA) were coated overnight at 4°C with recombinant influenza HA (0.5 μg/well) and then blocked by incubation with 2% boiled casein in PBS for 1–2 h at 37°C. Plates were washed (three times) and mononuclear immune cells from different tissue compartments (150,000–300,000 cells/200 μl complete medium/well) were added into Ag=coated wells in triplicate. After overnight culture at 37°C, plates were washed (six times), followed by incubation with anti-monkey IgG conjugated with biotin (Alpha Diagnostic International, San Antonio, TX) and incubated for 2 h at 37°C. Plate were washed (six times) and incubated with streptavidin–alkaline phosphatase (Sigma-Aldrich) for 1 h and developed by 5-bromo-4-chloro-3-indolyl phosphate-NBT-blue system (Sigma-Aldrich). The reaction was stopped by washing with tap water and air-dried. Spot-forming cells (SFC) were quantified by automated plate reader (Cellular Technology Ltd.). Keyhole limpet hemocyanin (KLH; Sigma-Aldrich) was used as a negative control Ag. The frequency of HA-specific spontaneous ASCs was defined as the numbers of SFC in HA-coated well subtracted from SFC in KLH-coated well and data are presented as SFC per million input cells.
ELISPOT assay for memory B cells
Mononuclear immune cells from different tissue compartments (1 × 106 cells/1 ml/well) were cultured in 48-well plates for 6 d in the presence of R848 (2.5 μg/ml) (InvivoGen, San Diego, CA) and IL-2 (1000 U/ml) (R&D Systems, Minneapolis, MN). Half of culture medium was replaced with fresh medium containing R848 and IL-2 at day 3. This stimulation method has been found to be optimal for assessment memory B cell response in rhesus monkeys. To enumerate HA-specific ASCs, stimulated cells (150,000–300,000 cells/200 μl complete medium/well) were added into ELISPOT plates and then processed as described for spontaneous Ab secreting cells above. The frequency of HA-specific memory B cells was defined as the numbers of spots in HA-coated well subtracted from spots in KLH-coated well and data are presented as SFC per million input cells. The samples were scored positive for HA-specific memory B cells if the ASCs were greater than the mean of pre-existing ASCs in nonstimulated cells plus 2 SDs.
Intracellular cytokine staining
T cell responses were assessed by intracellular cytokine staining. Cryopreserved PBMC and mononuclear immune cells (1 × 106 cells in 200 μl complete medium) from different tissue compartments were stimulated with NP peptide derived from A/California/04/2009 (H1N1) (122 15-mer peptide overlapping by 11 aa) at a final concentration of each peptide of 1μg/ml. All stimulated cell cultures contained 1 μg/ml anti-CD28 (clone L293; BD Biosciences) and 1 μg/ml of anti-CD49 (clone L25; BD Biosciences). Staphylococcal enterotoxin (4 μg/ml) and medium were used as positive and negative controls, respectively. After 2 h of stimulation, GolgiPlug was added to inhibit cytokine secretion, and the cell cultures were further incubated overnight. Then cells were washed and stained with a panel of Abs specific for surface markers, including anti-CD4 (clone L200; BD Biosciences), anti-CD8 (clone SK1; BD Biosciences), anti-CD95 (clone DX2; BioLegend, San Diego CA), anti-CCR7 (clone 150503; R&D Systems), anti-CD103 (clone B-Ly7; eBioscience, San Diego CA), anti–programmed death-1 (PD-1) (clone EH12.2H7; BioLegend), anti-CXCR3 (clone 1C6/CXCR3; BioLegend), and anti-CCR5 (clone 3A9; BioLegend). The stained cells were fixed/permeabilized and intracellular cytokines were stained with mAbs against IFN-γ (clone B 27; BioLegend) and IL-2 (clone MQ1-17H12; BioLegend). Finally, stained cells were analyzed by six-color flow cytometry (BDFACSCanto; BD Biosciences). The samples considered positive were those in which the percentage of cytokine-staining cells was at least twice that for the background or in which there was a distinct population of bright cytokine-positive cells.
To evaluate the role of CD69-positive memory T cells in cytokine responses, lung mononuclear immune cells were stained with anti-CD69 (clone FN50; BD Biosciences) and CD69-positive cells were depleted by fluorescence-activated cell sorter (FACSAria III; BD Biosciences). CD69-depleted lung mononuclear immune cells were stimulated with NP peptide pool and assessed for IFN-γ and IL-2 production compared with the nondepleted population.
Statistical analysis
The data were analyzed using SPSS 12.0 for Windows (SPSS, Chicago, IL). Ab titers were log transformed before testing of differences. Differences in T cell, memory B cell, and plasma cell responses were analyzed by using the Wilcoxon signed rank test and the Mann–Whitney rank sum test. p < 0.05 was considered statistically significant.
Results
2009 pH1N1 infection in rhesus monkeys
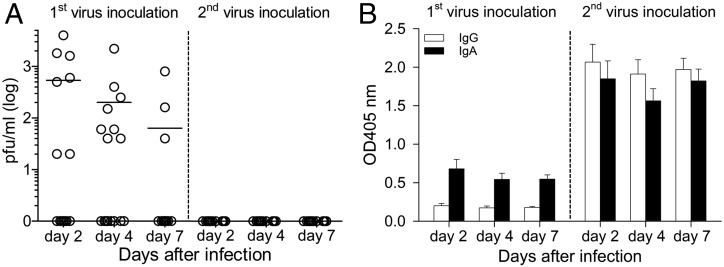
Of 20 monkeys seronegative for serum HAI against 2009 pH1N1, 16 received pulmonary inoculation of 2009 pH1N1 virus (2 × 106 TCID50) at days 0 and 28, whereas 4 monkeys were inoculated with control L-15 medium (Fig. 1). Infection with 2009 pH1N1 in rhesus monkeys induced no clinical signs of disease with no difference in animal activity, body weight loss, or respiratory parameters compared with control animals (data not shown). Viral replication was detected in nasal secretions from 12 of the 16 monkeys (Fig. 2A). Although peak of virus titer was found on day 2, this could be left over inoculum. However, virus titer on days 4 and 7 in nasal wash demonstrated the virus can replicate efficiently in upper respiratory tract of rhesus monkeys. Viral replication in nasal secretions was absent after the second inoculation, wheras concomitant increases in nasal HA-specific IgG and IgA was detected (Fig. 2B), suggesting that these Ab responses may suppress local viral replication. One animal died during the course of the study at 23 d after the second virus inoculation. A diagnostic necropsy was conducted, and the cause of death was determined to be because of tuberculosis.
FIGURE 2.
Replication of 2009 pH1N1 in rhesus monkeys and the presence of HA-specific IgG and IgA in nasal secretions. (A) Virus replication in nasal secretions of 16 infected animals was measured by plaque assay. Each data point represents the value for an individual monkey and horizontal lines are means. (B) ELISA was used to measure HA-specific IgG and IgA in nasal secretions (1:4 dilution) and expressed as OD at 405 nm. Data shown are mean ± SE.
Serum Ab and peripheral blood T cell responses after 2009 pH1N1 infection
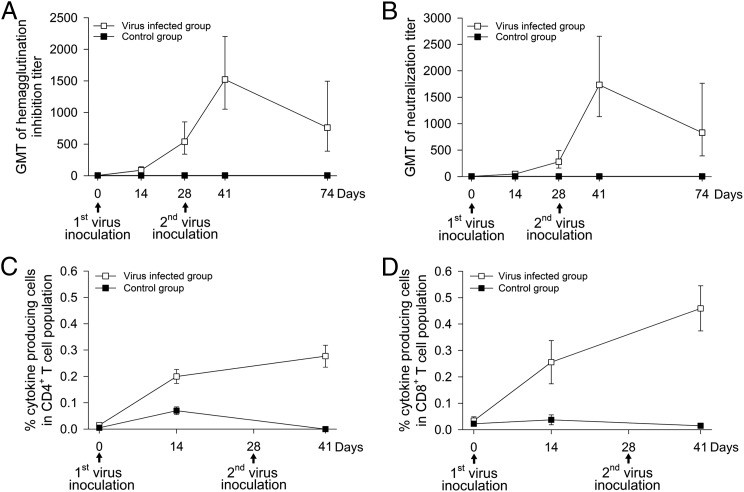
We measured serum Ab and peripheral blood T cell responses specific to 2009 pH1N1 virus before and after virus inoculation. Using HAI and NT assays, we showed that all 16 test animals developed serum Ab responses following infection. HAI titers (GMT = 87; 95% confidence interval [CI] = 54–142) and NT titers (GMT = 50; 95% CI = 31–80) were detected at day 14 after the 1st inoculation, continued to increase and peaked at 13 d after the 2nd inoculation with GMT HAI titers of 1,522; 95% CI = 1052–2202 and GMT NT titers of 1733; 95% CI = 1132–2653 (Fig. 3A, 3B). By day 74, the HAI and NT titers declined ∼50%. Kinetics and magnitude of peripheral blood CD4+ and CD8+ T cell response to NP are shown in Fig. 3C and 3D. The magnitude of the NP-specific CD4+ or CD8+ T cells response was defined as the combined frequency of IFN-γ-, IL-2-, and double-cytokine producing IFN-γ-plus-IL-2 cells within the CD4+ or CD8+ T cell population. Baseline responses were very low; NP-specific cytokine production by CD4+ and CD8+ T cells was observed 14 d after the first virus inoculation and peaked after the second inoculation (mean frequency of cytokine-producing CD4+ T cells ± SE = 0.28 ± 0.04% and CD8+ T cells = 0.46 ± 0.09%). Negligible influenza-specific Ab and T cell responses were detected in the control animal group.
FIGURE 3.
Kinetics of serum Ab and peripheral blood T cell responses. Ab responses were measured by HAI (A) and NT (B) assays and each data point represents the GMT ± SE. Peripheral blood CD4+ (C) and CD8+ (D) T cell responses were assessed by in vitro T cell recall assay against influenza NP. Intracellular cytokine staining was used to assess the frequencies of NP-specific cytokine-secreting T cells. Stained cells were analyzed by multicolor flow cytometry. Data shown are means ± SE of percent cytokine-producing cells (IFN-γ-, IL-2-, and IFN-γ plus IL-2) in CD4+ or CD8+ T cell population.
Tissue distribution of influenza-specific memory T cells in rhesus monkeys
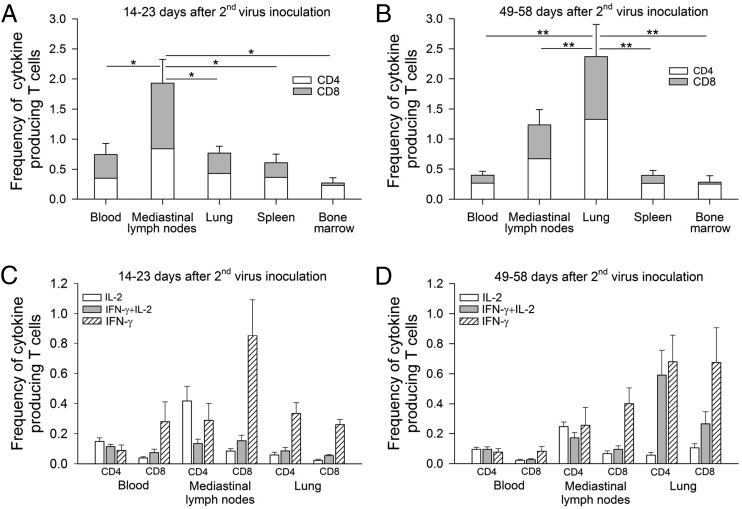
We next measured and compared the magnitude of memory T cell responses in different tissue compartments. The frequency of NP-specific memory T cells in peripheral blood, lung-draining mediastinal lymph nodes, lung, spleen, and bone marrow were evaluated at two time points. Half of the animals (n = 8) were euthanized between days 14 and 23 after the second virus inoculation when peak immune response was expected (34). This time period was termed “expansion phase.” The other half were euthanized between days 49 and 58 after the second virus inoculation, when a stable distribution of memory immune cells was expected (34), termed the “contraction phase.” Fig. 4A shows that during the expansion phase (days 14–23), a significantly higher frequency of NP-specific memory T cells were observed in the mediastinal lymph nodes (total frequency of 1.9%; CD4+ T cells = 0.8%, CD8+ T cells = 1.1%) compared with other tissues (p < 0.05): lung (total frequency of 0.7%; CD4+ T cells = 0.4%, CD8+ T cells = 0.3%), blood (total frequency of 0.7%; CD4+ T cells = 0.3%, CD8+ T cells = 0.4%), spleen (total frequency of 0.6%; CD4+ T cells = 0.4%, CD8+ T cells = 0.2%), and bone marrow (total frequency of 0.29%; CD4+ T cells = 0.25%, CD8+ T cells = 0.04%).
FIGURE 4.
Generation of NP-specific memory CD4+ plus CD8+ T cells in different tissue compartments (peripheral blood, mediastinal lymph nodes, lung, spleen, and bone marrow) at the expansion phase (14–23 d after the second virus inoculation) (A) and the contraction phase (49–58 d after the second virus inoculation) (B). Data shown are means ± SE (*p < 0.05, **p < 0.01, Wilcoxon test). Cytokine profiles of NP-specific memory CD4+ and CD8+ T cells at the expansion phase (C) and contraction phase (D) were also evaluated by separating cells into three distinct populations based on the production of IL-2 alone, IFN-γ alone, and IFN-γ plus IL-2. Data shown are means ± SE.
Although the T cell response during the contraction phase was still evident, a 0.4- to 2-fold decline in NP-specific memory T cell frequency was observed in the mediastinal lymph nodes, blood, and spleen compared with the expansion phase. In contrast, a 3.3-fold increase in memory T cell frequency was detected in the lung (Fig. 4B). During the contraction phase, the frequency of NP-specific memory T cells in the lung was significantly higher than that in the mediastinal lymph nodes (p < 0.01, total frequency of 2.4 versus 1.2%). Only a minimal number of NP-specific memory T cells (range 0.2–0.4%) were detected in blood, spleen, and bone marrow. There were not significant differences in the ratio of NP-specific memory CD4+ to CD8+ T cells in each tissue sample at the time points studied.
We then next evaluated the cytokine profiles of influenza-specific memory T cell responses in the lung, mediastinal lymph nodes, and peripheral blood at both time points; the expansion phase (Fig. 4C) and contraction phase (Fig. 4D). In the lung, a large proportion of NP-specific memory CD4+ and CD8+ T cells produced IFN-γ alone (70–77%) during the expansion phase, whereas at the contraction phase, NP-specific memory CD4+ and CD8+ T cells produced either IFN-γ alone (51–65%) or IFN-γ plus IL-2 (25–45%). In the mediastinal lymph nodes, we detected, at both time points, NP-specific memory CD4+ T cells producing either IL-2 alone (37–50%) or IFN-γ alone (34–38%), whereas most NP-specific memory CD8+ T cells produced IFN-γ alone (71–78%). In peripheral blood, about equal proportions of NP-specific memory CD4+ T cells producing either IFN-γ alone (28–28%), IL-2 alone (35–42%), or IFN-γ plus IL-2 (33–35%) were observed at both time points, whereas most NP-specific memory CD8+ T cells produced IFN-γ alone (63–71%).
Rhesus lung NP-specific memory T cells expressed phenotypic markers of TRM cells
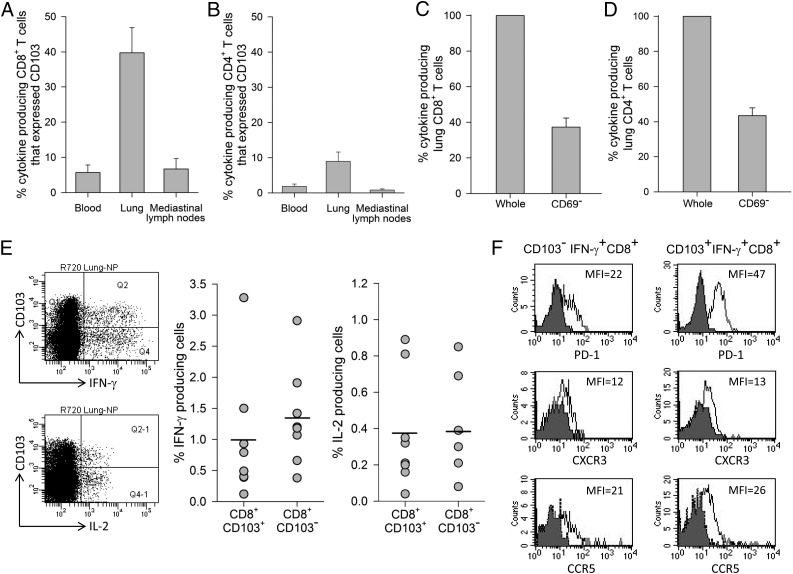
To investigate NP-specific memory T cells found in the lung at the contraction phase characterized as TRM cells, we measured the expression of CD103 (αEβ7 integrin) and CD69 (C-type lectin), both of which are cell surface signature markers of TRM cells involved in cell adhesion and tissue retention (10). Flow cytometry analysis (Fig. 5A) showed that ∼40% of lung NP-specific memory CD8+ T cells that produced cytokines (IFN-γ and IL-2) expressed CD103. Negligible numbers of CD103 positive NP-specific memory CD8+ T cells were observed in the mediastinal lymph nodes and peripheral blood, suggesting that NP-specific memory CD8+ T cells established residency only in the lung. In contrast, only a minimal proportion of lung NP-specific memory CD4+ T cells (<10%) expressed CD103 (Fig. 5B).
FIGURE 5.
Expression of CD103 and CD69, phenotypic markers of TRM on NP-specific lung memory T cells at the contraction phase. Percentage of cytokine producing CD8+ (A) or CD4+ (B) memory T cells that expressed CD103 in peripheral blood, lung, and mediastinal lymph nodes. Depletion of CD69-positive cells from lung mononuclear immune cells and cytokine response in NP-specific CD8+ (C) or CD4+ (D) memory T cells. Means ± SE of n = 8 are shown. Representative flow cytometry plot of lung CD103+ and CD103−CD8+ T cells expressing IFN-γ and IL-2 and frequencies of IFN-γ and IL-2 production from lung CD103+ and CD103−CD8+ T cells from eight animals (E). Each data point represents the value for an individual monkey and horizontal lines are means. Phenotype of IFN-γ–producing CD8+ T cells in the lung (F). Shaded histograms represent isotype control and solid lines represent lung CD103− or CD103+IFNγ+CD8+ T cells expressing PD-1, CXCR3, and CCR5. Data are representative of four monkeys.
Unlike CD103 expression, direct assessment of CD69 on NP-specific memory T cells from lung tissue was not feasible since in vitro stimulation with NP peptide pools led to CD69 expression on all NP-specific memory T cells (data not shown). We therefore first depleted CD69-positive T cells and then CD69-depleted lung mononuclear immune cells were stimulated with the NP peptide pool. In this case, the percent reduction of cytokine producing cells in a recall T cell assay of a depleted population compared with a nondepleted population would correspond to the proportion of lung NP-specific memory T cells expressing CD69. As depicted in Fig. 5C and 5D, depletion of CD69 cells resulted in a reduction of cytokine-producing cells to 37% for CD8+ T cells and 43% for CD4+ T cells. These data imply that 63 and 57% of lung NP-specific memory CD8+ and CD4+ T cells, respectively, expressed CD69. Taken together, these data suggest that following influenza infection, a substantial proportion of NP-specific memory T cells in the lung of rhesus monkeys expressed phenotypic markers of TRM cells, CD103 and CD69.
Because CD103 was mainly expressed on lung memory CD8+ T cells, we then compared cytokine response between lung NP-specific CD103+CD8+ and CD103−CD8+ T cells. After in vitro stimulation with the NP peptide pool, we observed no significant difference in the frequency of IFN-γ or IL-2 produced by CD103+CD8+ T cells versus CD103−CD8+ T cells (p range 0.46 – 0.74) (Fig. 5E). As PD-1, a cell surface marker of activated T cells is known to express on TRM cells (16, 35), we showed that high expression of PD-1 was consistently detected on lung CD103+ compared with CD103−IFN-γ+CD8+ T cells. In addition, the expression levels of chemokine receptors involved in T cell migration into the lung (36, 37), CXCR3 and CCR5, were minimal with no significant difference between either subset (Fig. 5F).
Tissue distribution of spontaneous ASCs and memory B cells
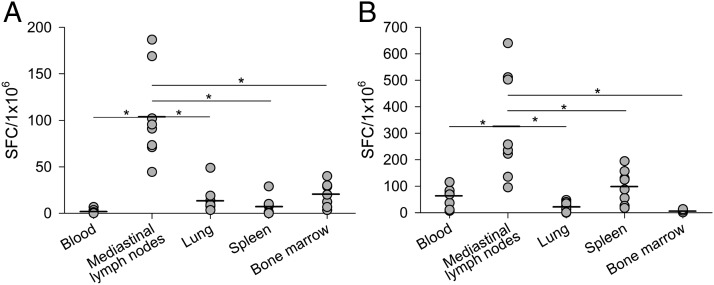
We also assessed for the presence of spontaneous ASCs and memory B cells at the contraction phase. The frequency of spontaneous ASCs and memory B cells specific to HA was different in each studied tissue sample. As shown in Fig. 6A, unlike NP-specific memory T cells, a significantly higher frequency of HA-specific spontaneous ASCs was detected in the mediastinal lymph nodes (104 SFC/106 mononuclear immune cells) compared with other tissue compartments (p < 0.01). Fewer numbers of spontaneous ASCs were detected in the bone marrow (20 SFC/106 mononuclear immune cells), lung (13 SFC/106 mononuclear immune cells), and spleen (7 SFC/106 mononuclear immune cells). HA-specific spontaneous ASCs could not be detected in peripheral blood (below the established limit of detection for the assay).
FIGURE 6.
Generation of HA-specific spontaneous ASCs and memory B cells at the contraction phase. Mononuclear immune cells isolated from different tissue compartments were assayed for the presence of spontaneous ASCs (A) and memory B cells (B) specific to influenza HA using ELISPOT assay. Each data point represents value for an individual monkey and horizontal lines are means (*p < 0.01, Wilcoxon test).
For memory B cell response, a polyclonal stimulation with R848 (TLR7/8 ligand) and IL-2 were used. This type of stimulation was found to be optimal for differentiation rhesus monkey memory B cells into ASCs, without losing cell viability (Supplemental Fig. 1A, 1B). After 6 d of in vitro activation in cell culture medium, significantly higher frequency of HA-specific IgG memory B cells was detected in the mediastinal lymph nodes (325 SFC/106 mononuclear immune cells) compared with other tissue compartments (p < 0.01) (Fig. 6B). The frequency of HA-specific IgG memory B cells was low in spleen (99 SFC/106 mononuclear immune cells), peripheral blood (64 SFC/106 mononuclear immune cells), lung (20 SFC/106 mononuclear immune cells), and negligible in bone marrow.
KLH was used as a control Ag for coating plates. We consistently observed a negligible number of spots in Ag control KLH coated wells, suggesting the assay was specific for detecting HA. Due to a limited number of cells, we could not perform the assay to determine the number of spontaneous Ab secreting cells and memory B cells that secrete HA-specific IgA.
Influenza-specific systemic and local immune responses in young adult and old monkeys
To study age-related changes, we compared serum Ab, T cell responses in peripheral blood and local memory T, B, and spontaneous ASCs in the lung, and mediastinal lymph nodes between young adult and old monkeys. Following 2009 pH1N1 infection, all animals, regardless of age, developed immune responses against influenza. No significant difference in the magnitude and kinetics of serum Ab (HAI and NT) responses in young adult monkeys (n = 8; mean age ± SE = 6.5 ± 0.2 y) versus old monkeys (n = 8; mean age ± SE = 20.4 + 1.2 y) (Supplemental Fig. 2A). Comparable peripheral blood T cell responses between young adult and old monkeys were observed with the exception at 14 d after first virus inoculation at which the cytokine response was higher in young adult animals.
There were not significant differences in the magnitude of cytokine-producing lung memory T cells, CD103 expression on cytokine-producing lung memory CD8+ T cells, mediastinal lymph node spontaneous ASCs, and memory B cells in young adult monkeys (n = 4; mean age ± SE = 6.5 ± 0.3) and old monkeys (n = 4; mean age ± SE = 20.5 ± 2.5) at the contraction phase (Supplemental Fig. 2B).
Discussion
One of the key challenges for vaccinologists is to develop vaccines that induce site-specific protective immunity to attempt to mimic the immune response to natural infection. The generation of TRM cells and memory B cells as part of the local lung immune response against influenza infection has recently been demonstrated in a mouse model of influenza infection (15, 22, 23, 35). To further explore the role of local immunity, we used a nonhuman primate model of influenza infection to analyze the tissue distribution of memory T and B cells. Our findings are consistent with previous reports that pulmonary infection of rhesus monkeys with 2009 pH1N1 induces no clinical disease associated with limited viral replication (32, 33) and that rhesus monkeys pulmonary infected with 2009 pH1N1 developed both systemic and local immune responses. Influenza-specific adaptive immune cells including memory T, B, and spontaneous ASCs were detected in several tissue compartments but differed in tissue tropisms.
During the expansion phase 14–23 d after second virus inoculation, the proportion of NP-specific memory T cells in the lung-draining mediastinal lymph nodes was significantly higher than in the lung. These finding may not be surprising because priming of naive T cells by dendritic APCs occurs in the mediastinal lymph nodes (38, 39). Preferential localization of memory T cells to the lungs occurred during the contraction phase, 49–58 d after second virus inoculation. These results agree with previous mouse data, which suggest that the tissue tropism of the natural infection influences the localization of memory T cells (40). The markedly increased lung NP-specific memory T cells and the decrease of mediastinal lymph node memory T cells at the later contraction phase likely reflect the migration of memory T cells from the lymph nodes to the lung. Despite similarity in the magnitude of NP-specific memory T cell responses in peripheral blood and in the lung at the expansion phase, the frequency of NP-specific memory T cells in the lung at the contraction phase was 6-fold greater than that in peripheral blood. In addition, the number of polyfunctional memory CD4+ and CD8+ T cells producing double cytokines, IFN-γ plus IL-2, in the lung was 6- to 10-fold greater in frequency than that in peripheral blood, suggesting a possible functional difference between memory T cells from the two compartments. Increase in the number of polyfunctional T cells that produce multiple cytokines is known to associate with protective immunity in many infection models (41–44).
TRM cells coexpressing CD103 and CD69 have been identified in mice and recently in humans (11, 45, 46). The αEβ7 integrin CD103 promotes cell adherence to E-cadherin expressed on tissue epithelial cells (47), whereas C-type lectin CD69 inhibits sphingosine-1-phosphate receptor 1, leading to tissue retention (48, 49). In this study, we identified influenza-specific lung memory T cells expressing CD103 and CD69 in rhesus monkeys. Consistent with mouse data, CD103 expression was detected mainly on CD8+ memory T cells but negligible on CD4+ memory T cells, whereas CD69 expression was detected on both memory CD8+ and CD4+ T cells (11, 45). Recent data show a differentiation pathway from CD69+CD103− to CD69+CD103+ TRM cells and suggest a delay of CD103 expression compared with CD69 expression (50). These findings may explain why the proportion of lung CD103-positive NP-specific CD8+ T cells observed in this study was consistently less than CD69-positive NP-specific CD8+ T cells (40 versus 63%). A limitation of our study is that we did not sample far out enough in time to assess whether CD103 would increase in number. Similar to influenza-infected mice (51), influenza infection in rhesus monkeys generates two populations of lung memory T cells, ones expressing TRM cell phenotypic markers CD103 and CD69, which are involved in cell adhesion and tissue retention and likely localize in lung parenchyma, whereas the others lack CD69 and CD103 expression and thus likely localize in lung vasculature. Unlike mice in which the anatomical location of vascular and lung tissue memory T cells can be differentiated by intravascular Ab staining combined with tetramer labeling (51, 52), there are significant technical restrictions to using such a technique in rhesus monkeys.
It has been suggested that the ability of memory T cells to home to the lung parenchyma as TRM is critical to control lung pathogens such as influenza and Mycobacterium tuberculosis (15, 16). Strategically well-positioned in the lung tissue, TRM cells could rapidly recognize, mediate cytotoxicity and secrete cytokines to control local microbe-infected cells. Our study on cytokine profiles of NP-specific lung memory T cells at the contraction phase indicates that a large proportion of both lung CD8+ and CD4+ memory T cells produced IFN-γ alone or IFN-γ plus IL-2. Further detailed analysis suggests that there is no different in the levels of IFN-γ and IL-2 responses between lung CD103+CD8+ versus CD103−CD8+ memory T cells. Recent observations suggest that airway CD8+ TRM cells produce IFN-γ faster than systemic memory CD8+ T cells and are responsible for rapid protection against respiratory infection (53). In this study, we did not compare the kinetics of cytokine production between lung CD103+CD8+ versus CD103-CD8+ memory T cells. The ability to secrete IFN-γ and IL-2 is crucial for local lung immunity. TRM cell–derived IFN-γ is known to be critical for recruitment of circulating memory CD8+ T and B cells to the site of infection via an IFN-γ–induced VCAM-1 pathway (54) and is responsible for the induction of broad antiviral protein; the IFN-induced transmembrane protein 3 in local tissue (55). Mouse lung TRM cells that lack IFN-induced transmembrane protein 3 are more susceptible to influenza infection than their normal counterparts (56). IL-2 produced by TRM cells could rapidly upregulate granzyme B on innate NK cells involved in direct killing of infected cells (54). The ability of rhesus lung-resident memory T cells to recognize a conserved influenza NP suggests that they may play a role in heterosubtypic immunity against emerging influenza virus variants.
Our findings that lung CD103+IFN-γ+CD8+ T cells express higher levels of PD-1 than CD103−IFN-γ+CD8+ T cells seems to support previous observations in mice that influenza-specific lung TRM express higher PD-1 than non-TRM cells (35). Alternatively, the observed expression of PD-1 may result from an in vitro stimulation with NP peptide pool (57), and this may suggest a high activation status of CD103+IFN-γ+CD8+ T cells. In any event, more work is needed to determine regulatory role of PD-1 expression of TRM cells. In addition, we observed only minimal expression of CXCR3 and CCR5, on both CD103+ and CD103−IFN-γ+CD8+ T cell subsets. These findings differ from recent observations in M. tuberculosis–infected mice, which show high expression of CXCR3 on lung TRM cells (16). More study of chemokine receptors on influenza-specific lung TRM cells is required to elucidate to importance of chemokine receptors in establishing tissue residence.
Plasma cells and memory B cells are components of humoral immunity crucial for protection against influenza infection. We observed a high frequency of long-lived HA-specific spontaneous ASCs and memory B cells in the mediastinal lymph nodes and only low frequencies in the lung of rhesus monkeys. The findings differ from recent mouse studies in which large numbers of influenza-specific ASCs and memory B cells are detected in the lung after influenza infection (22, 23, 58). These mouse ASCs and memory B cell are generated during germinal center activation within ectopic lymphoid-like structure in the lung known as induced BALT (iBALT) (59, 60). Development of iBALT in murine lung is commonly observed and persists for a long time after influenza infection (61). We did not investigate the formation of iBALT in the lung of influenza-infected monkeys. However, infection of rhesus monkeys with 2009 pH1N1in our study was self-limited and asymptomatic with no clinical signs of intense lung inflammation, conditions that do not favor iBALT formation (62, 63).
Accumulating data suggest that humoral and cellular immune responses are impaired in aged individuals, leading to the increased susceptibility to influenza infection and decreased immune response to influenza vaccine (64). Our findings show that the serum Ab (HAI and NT titers) and peripheral blood T cell responses were mostly comparable between young adult and old monkeys following 2009 pH1N1infection. These findings differ from recent observations indicating that influenza vaccine-induced Ab responses are significantly reduced in old rhesus monkeys compared with young adult animals (65). We did not detect any significant difference in young adult versus old monkeys with regard to the frequency of NP-specific memory T in the lung and the proportion of lung NP-specific memory T cells expressing CD103. In addition, the frequency of spontaneous Ab secreting cells and memory B cells in the lung mediastinal lymph nodes did not correlate with the age of animals. This study was conducted using a small sample size, and the average age in the older group was 20.4 y (equivalent to a 63-y-old humans), limiting conclusions to some degree.
Our findings from a rhesus monkey model of 2009 pH1N1 infection support the current concept that the site of pathogen infection determines the localization of immune memory cells. Following influenza infection, we detected preferential localization of memory T and B cells in the lung and lung-draining mediastinal lymph nodes, respectively. We confirm recent observations that immune responses in the lung differ from those in peripheral blood, which are commonly used as an indicator of memory response following infection or vaccination. Analysis of immune responses in specific tissue compartment where protection is needed against localized infection such as influenza, tuberculosis, and liver-stage malaria provides important insights into protective immunity and will help guide vaccine development.
Supplementary Material
Acknowledgments
We thank Dr. R. Burke, Dr. K. Chumpolkulwong, and A. Hanrujirakomjorn for assistance in animal experiments. We also thank Dr. Evelina Angov for CelTOS Ag.
This work was supported by National Institutes of Health, National Institute of Allergy and Infectious Diseases Grant Y1-AI-5026-01, Thailand Research Fund Grant BRG5880003, and a Ratchadapisek endowment.
The views of the authors do not purport to reflect official policy of the U.S. Department of the Army or the Department of Defense.
The online version of this article contains supplemental material.
- ASC
- Ab-secreting cell
- CI
- confidence interval
- DC
- dendritic cell
- GMT
- geometric mean titer
- HA
- hemagglutinin
- HAI
- hemagglutination-inhibition
- iBALT
- induced BALT
- KLH
- keyhole limpet hemocyanin
- MDCK
- Madin–Darby canine kidney
- NP
- nucleoprotein
- PD-1
- programmed death-1
- SFC
- spot-forming cell
- TCID50
- 50% tissue culture infective dose
- TRM
- tissue-resident memory.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Osterholm M. T., Kelley N. S., Sommer A., Belongia E. A. 2012. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect. Dis. 12: 36–44. [DOI] [PubMed] [Google Scholar]
- 2.Hobson D., Curry R. L., Beare A. S., Ward-Gardner A. 1972. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J. Hyg. (Lond.) 70: 767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black S., Nicolay U., Vesikari T., Knuf M., Del Giudice G., Della Cioppa G., Tsai T., Clemens R., Rappuoli R. 2011. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr. Infect. Dis. J. 30: 1081–1085. [DOI] [PubMed] [Google Scholar]
- 4.Gravenstein S., Drinka P., Duthie E. H., Miller B. A., Brown C. S., Hensley M., Circo R., Langer E., Ershler W. B. 1994. Efficacy of an influenza hemagglutinin-diphtheria toxoid conjugate vaccine in elderly nursing home subjects during an influenza outbreak. J. Am. Geriatr. Soc. 42: 245–251. [DOI] [PubMed] [Google Scholar]
- 5.Yap K. L., Ada G. L. 1978. Cytotoxic T cells in the lungs of mice infected with an influenza A virus. Scand. J. Immunol. 7: 73–80. [DOI] [PubMed] [Google Scholar]
- 6.Webster R. G., Askonas B. A. 1980. Cross-protection and cross-reactive cytotoxic T cells induced by influenza virus vaccines in mice. Eur. J. Immunol. 10: 396–401. [DOI] [PubMed] [Google Scholar]
- 7.Guo H., Santiago F., Lambert K., Takimoto T., Topham D. J. 2011. T cell-mediated protection against lethal 2009 pandemic H1N1 influenza virus infection in a mouse model. J. Virol. 85: 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkinson T. M., Li C. K., Chui C. S., Huang A. K., Perkins M., Liebner J. C., Lambkin-Williams R., Gilbert A., Oxford J., Nicholas B., et al. 2012. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 18: 274–280. [DOI] [PubMed] [Google Scholar]
- 9.Sridhar S., Begom S., Bermingham A., Hoschler K., Adamson W., Carman W., Bean T., Barclay W., Deeks J. J., Lalvani A. 2013. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med. 19: 1305–1312. [DOI] [PubMed] [Google Scholar]
- 10.Shin H., Iwasaki A. 2013. Tissue-resident memory T cells. Immunol. Rev. 255: 165–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schenkel J. M., Masopust D. 2014. Tissue-resident memory T cells. Immunity 41: 886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang X., Clark R. A., Liu L., Wagers A. J., Fuhlbrigge R. C., Kupper T. S. 2012. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature 483: 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofmann M., Pircher H. 2011. E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc. Natl. Acad. Sci. USA 108: 16741–16746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin H., Iwasaki A. 2012. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491: 463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teijaro J. R., Turner D., Pham Q., Wherry E. J., Lefrançois L., Farber D. L. 2011. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 187: 5510–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakai S., Kauffman K. D., Schenkel J. M., McBerry C. C., Mayer-Barber K. D., Masopust D., Barber D. L. 2014. Cutting edge: control of Mycobacterium tuberculosis infection by a subset of lung parenchyma-homing CD4 T cells. J. Immunol. 192: 2965–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L., Zhong Q., Tian T., Dubin K., Athale S. K., Kupper T. S. 2010. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat. Med. 16: 224–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Çuburu N., Wang K., Goodman K. N., Pang Y. Y., Thompson C. D., Lowy D. R., Cohen J. I., Schiller J. T. 2015. Topical herpes simplex virus 2 (HSV-2) vaccination with human papillomavirus vectors expressing gB/gD ectodomains induces genital-tissue-resident memory CD8+ T cells and reduces genital disease and viral shedding after HSV-2 challenge. J. Virol. 89: 83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wakim L. M., Smith J., Caminschi I., Lahoud M. H., Villadangos J. A. 2015. Antibody-targeted vaccination to lung dendritic cells generates tissue-resident memory CD8 T cells that are highly protective against influenza virus infection. Mucosal Immunol. 8: 1060–1071. [DOI] [PubMed] [Google Scholar]
- 20.Masopust D., Vezys V., Marzo A. L., Lefrançois L. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291: 2413–2417. [DOI] [PubMed] [Google Scholar]
- 21.Çuburu N., Graham B. S., Buck C. B., Kines R. C., Pang Y. Y., Day P. M., Lowy D. R., Schiller J. T. 2012. Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. J. Clin. Invest. 122: 4606–4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joo H. M., He Y., Sangster M. Y. 2008. Broad dispersion and lung localization of virus-specific memory B cells induced by influenza pneumonia. Proc. Natl. Acad. Sci. USA 105: 3485–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onodera T., Takahashi Y., Yokoi Y., Ato M., Kodama Y., Hachimura S., Kurosaki T., Kobayashi K. 2012. Memory B cells in the lung participate in protective humoral immune responses to pulmonary influenza virus reinfection. Proc. Natl. Acad. Sci. USA 109: 2485–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pitcher C. J., Hagen S. I., Walker J. M., Lum R., Mitchell B. L., Maino V. C., Axthelm M. K., Picker L. J. 2002. Development and homeostasis of T cell memory in rhesus macaque. J. Immunol. 168: 29–43. [DOI] [PubMed] [Google Scholar]
- 25.Mooij P., Balla-Jhagjhoorsingh S. S., Beenhakker N., van Haaften P., Baak I., Nieuwenhuis I. G., Heidari S., Wolf H., Frachette M. J., Bieler K., et al. 2009. Comparison of human and rhesus macaque T-cell responses elicited by boosting with NYVAC encoding human immunodeficiency virus type 1 clade C immunogens. J. Virol. 83: 5881–5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zahorsky-Reeves J. L., Gregory C. R., Cramer D. V., Patanwala I. Y., Kyles A. E., Borie D. C., Kearns-Jonker M. K. 2006. Similarities in the immunoglobulin response and VH gene usage in rhesus monkeys and humans exposed to porcine hepatocytes. BMC Immunol. 7: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gujer C., Sundling C., Seder R. A., Karlsson Hedestam G. B., Loré K. 2011. Human and rhesus plasmacytoid dendritic cell and B-cell responses to Toll-like receptor stimulation. Immunology 134: 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ketloy C., Engering A., Srichairatanakul U., Limsalakpetch A., Yongvanitchit K., Pichyangkul S., Ruxrungtham K. 2008. Expression and function of Toll-like receptors on dendritic cells and other antigen presenting cells from non-human primates. Vet. Immunol. Immunopathol. 125: 18–30. [DOI] [PubMed] [Google Scholar]
- 29.Stewart V. A., McGrath S. M., Walsh D. S., Davis S., Hess A. S., Ware L. A., Kester K. E., Cummings J. F., Burge J. R., Voss G., et al. 2006. Pre-clinical evaluation of new adjuvant formulations to improve the immunogenicity of the malaria vaccine RTS,S/AS02A. Vaccine 24: 6483–6492. [DOI] [PubMed] [Google Scholar]
- 30.Garçon N., Heppner D. G., Cohen J. 2003. Development of RTS,S/AS02: a purified subunit-based malaria vaccine candidate formulated with a novel adjuvant. Expert Rev. Vaccines 2: 231–238. [DOI] [PubMed] [Google Scholar]
- 31.Weinfurter J. T., Brunner K., Capuano S. V., III, Li C., Broman K. W., Kawaoka Y., Friedrich T. C. 2011. Cross-reactive T cells are involved in rapid clearance of 2009 pandemic H1N1 influenza virus in nonhuman primates. PLoS Pathog. 7: e1002381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boonnak K., Paskel M., Matsuoka Y., Vogel L., Subbarao K. 2012. Evaluation of replication, immunogenicity and protective efficacy of a live attenuated cold-adapted pandemic H1N1 influenza virus vaccine in non-human primates. Vaccine 30: 5603–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Josset L., Engelmann F., Haberthur K., Kelly S., Park B., Kawoaka Y., García-Sastre A., Katze M. G., Messaoudi I. 2012. Increased viral loads and exacerbated innate host responses in aged macaques infected with the 2009 pandemic H1N1 influenza A virus. J. Virol. 86: 11115–11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richards K. A., Chaves F. A., Sant A. J. 2011. The memory phase of the CD4 T-cell response to influenza virus infection maintains its diverse antigen specificity. Immunology 133: 246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu T., Hu Y., Lee Y. T., Bouchard K. R., Benechet A., Khanna K., Cauley L. S. 2014. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J. Leukoc. Biol. 95: 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wareing M. D., Lyon A. B., Lu B., Gerard C., Sarawar S. R. 2004. Chemokine expression during the development and resolution of a pulmonary leukocyte response to influenza A virus infection in mice. J. Leukoc. Biol. 76: 886–895. [DOI] [PubMed] [Google Scholar]
- 37.Kohlmeier J. E., Miller S. C., Smith J., Lu B., Gerard C., Cookenham T., Roberts A. D., Woodland D. L. 2008. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity 29: 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.GeurtsvanKessel C. H., Willart M. A., van Rijt L. S., Muskens F., Kool M., Baas C., Thielemans K., Bennett C., Clausen B. E., Hoogsteden H. C., et al. 2008. Clearance of influenza virus from the lung depends on migratory langerin+CD11b‑ but not plasmacytoid dendritic cells. J. Exp. Med. 205: 1621–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matheu M. P., Teijaro J. R., Walsh K. B., Greenberg M. L., Marsolais D., Parker I., Rosen H., Oldstone M. B., Cahalan M. D. 2013. Three phases of CD8 T cell response in the lung following H1N1 influenza infection and sphingosine 1 phosphate agonist therapy. PLoS One 8: e58033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knudson C. J., Weiss K. A., Hartwig S. M., Varga S. M. 2014. The pulmonary localization of virus-specific T lymphocytes is governed by the tissue tropism of infection. J. Virol. 88: 9010–9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darrah P. A., Patel D. T., De Luca P. M., Lindsay R. W., Davey D. F., Flynn B. J., Hoff S. T., Andersen P., Reed S. G., Morris S. L., et al. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13: 843–850. [DOI] [PubMed] [Google Scholar]
- 42.Abdel-Hakeem M. S., Bedard N., Murphy D., Bruneau J., Shoukry N. H. 2014. Signatures of protective memory immune responses during hepatitis C virus reinfection. Gastroenterology 147: 870‑881 e878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Derrick S. C., Yabe I. M., Yang A., Morris S. L. 2011. Vaccine-induced anti-tuberculosis protective immunity in mice correlates with the magnitude and quality of multifunctional CD4 T cells. Vaccine 29: 2902–2909. [DOI] [PubMed] [Google Scholar]
- 44.Boaz M. J., Waters A., Murad S., Easterbrook P. J., Vyakarnam A. 2002. Presence of HIV-1 Gag-specific IFN-γ+IL-2+ and CD28+IL-2+ CD4 T cell responses is associated with nonprogression in HIV-1 infection. J. Immunol. 169: 6376–6385. [DOI] [PubMed] [Google Scholar]
- 45.Farber D. L., Yudanin N. A., Restifo N. P. 2014. Human memory T cells: generation, compartmentalization and homeostasis. Nat. Rev. Immunol. 14: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sathaliyawala T., Kubota M., Yudanin N., Turner D., Camp P., Thome J. J., Bickham K. L., Lerner H., Goldstein M., Sykes M., et al. 2013. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 38: 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cepek K. L., Shaw S. K., Parker C. M., Russell G. J., Morrow J. S., Rimm D. L., Brenner M. B. 1994. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the αEβ7 integrin. Nature 372: 190–193. [DOI] [PubMed] [Google Scholar]
- 48.Skon C. N., Lee J. Y., Anderson K. G., Masopust D., Hogquist K. A., Jameson S. C. 2013. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat. Immunol. 14: 1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mackay L. K., Braun A., Macleod B. L., Collins N., Tebartz C., Bedoui S., Carbone F. R., Gebhardt T. 2015. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J. Immunol. 194: 2059–2063. [DOI] [PubMed] [Google Scholar]
- 50.Hofmann M., Oschowitzer A., Kurzhals S. R., Krüger C. C., Pircher H. 2013. Thymus-resident memory CD8+ T cells mediate local immunity. Eur. J. Immunol. 43: 2295–2304. [DOI] [PubMed] [Google Scholar]
- 51.Turner D. L., Bickham K. L., Thome J. J., Kim C. Y., D’Ovidio F., Wherry E. J., Farber D. L. 2014. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 7: 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson K. G., Mayer-Barber K., Sung H., Beura L., James B. R., Taylor J. J., Qunaj L., Griffith T. S., Vezys V., Barber D. L., Masopust D. 2014. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat. Protoc. 9: 209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McMaster S. R., Wilson J. J., Wang H., Kohlmeier J. E. 2015. Airway-resident memory CD8 T cells provide antigen-specific protection against respiratory virus challenge through rapid IFN-γ production. J. Immunol. 195: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schenkel J. M., Fraser K. A., Beura L. K., Pauken K. E., Vezys V., Masopust D. 2014. T cell memory: resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 346: 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ariotti S., Hogenbirk M. A., Dijkgraaf F. E., Visser L. L., Hoekstra M. E., Song J. Y., Jacobs H., Haanen J. B., Schumacher T. N. 2014. T cell memory: skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science 346: 101–105. [DOI] [PubMed] [Google Scholar]
- 56.Wakim L. M., Gupta N., Mintern J. D., Villadangos J. A. 2013. Enhanced survival of lung tissue-resident memory CD8+ T cells during infection with influenza virus due to selective expression of IFITM3. Nat. Immunol. 14: 238–245. [DOI] [PubMed] [Google Scholar]
- 57.Agata Y., Kawasaki A., Nishimura H., Ishida Y., Tsubata T., Yagita H., Honjo T. 1996. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 8: 765–772. [DOI] [PubMed] [Google Scholar]
- 58.Jones P. D., Ada G. L. 1986. Influenza virus-specific antibody-secreting cells in the murine lung during primary influenza virus infection. J. Virol. 60: 614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moyron-Quiroz J. E., Rangel-Moreno J., Kusser K., Hartson L., Sprague F., Goodrich S., Woodland D. L., Lund F. E., Randall T. D. 2004. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat. Med. 10: 927–934. [DOI] [PubMed] [Google Scholar]
- 60.Boyden A. W., Legge K. L., Waldschmidt T. J. 2012. Pulmonary infection with influenza A virus induces site-specific germinal center and T follicular helper cell responses. PLoS One 7: e40733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moyron-Quiroz J. E., Rangel-Moreno J., Hartson L., Kusser K., Tighe M. P., Klonowski K. D., Lefrançois L., Cauley L. S., Harmsen A. G., Lund F. E., Randall T. D. 2006. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity 25: 643–654. [DOI] [PubMed] [Google Scholar]
- 62.Pitzalis C., Jones G. W., Bombardieri M., Jones S. A. 2014. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat. Rev. Immunol. 14: 447–462. [DOI] [PubMed] [Google Scholar]
- 63.Aloisi F., Pujol-Borrell R. 2006. Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol. 6: 205–217. [DOI] [PubMed] [Google Scholar]
- 64.Liu W. M., van der Zeijst B. A., Boog C. J., Soethout E. C. 2011. Aging and impaired immunity to influenza viruses: implications for vaccine development. Hum. Vaccin. 7(Suppl.): 94–98. [DOI] [PubMed] [Google Scholar]
- 65.Coe C. L., Lubach G. R., Kinnard J. 2012. Immune senescence in old and very old rhesus monkeys: reduced antibody response to influenza vaccination. Age (Dordr.) 34: 1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.