Abstract
Characteristics of inhibitors identified by prospective screening may differ from those detected clinically. In a prospective study at 17 hemophilia centers with central inhibitor measurement by Nijmegen-Bethesda assay, 23 (2.8%) of 824 hemophilia A patients had new inhibitors detected: nine high-titer inhibitors (HTI: 7 ≥5.0 NBU plus 2 of 2.6 and 3.4 NBU at immune tolerance induction initiation) and 14 low-titer inhibitors (LTI: 0.5–1.9 NBU). HTI occurred at an earlier age (median 2 years, range 1–18, vs. median 11 years, range 2–61, P = 0.016). Both HTI (22%) and LTI (43%) occurred in non-severe patients. All HTI, but only 64% of LTI, were found to be FVIII-specific by chromogenic Bethesda assay or fluorescence immunoassay (FLI), indicating a high rate of false-positive LTI. Repeat specimens confirmed all HTI, 7/9 LTI, and 7/7 FVIII-specific LTI. FLI results were similar between HTI and FVIII-specific LTI; all included IgG1 and IgG4 subclasses. A comparable prospective study conducted from 1975 to 1979 at 13 U.S. centers found 31 (2.4%) new inhibitors among 1,306 patients. In both studies, one-third of inhibitors occurred in non-severe patients and one-quarter after 150 exposure days (ED). Significant differences were seen in the age at which inhibitors occurred (median 16 years in the older study vs. 5 years currently, P = 0.024) and in ED before inhibitor development, 10% in the older study and 43% currently study occurring within 20 ED, suggesting a temporal change in inhibitor development. Prospective screening detects inhibitors in patients of all severities, ages, and ED. Some LTI, however, are false positives.
Introduction
The development of neutralizing antibodies, referred to as inhibitors, is a significant treatment-associated complication experienced by a subset of hemophilia A (HA) patients following factor VIII (FVIII) infusion therapy. Inhibitors complicate patient management by limiting the effectiveness of FVIII infusions in stopping and/or preventing bleeding episodes. Knowledge of the incidence and prevalence of inhibitors is important to assess the burden of inhibitors on the community and to identify trends in inhibitor occurrence [1]. Few large studies have involved prospective monitoring for inhibitors among previously treated patients of all severities in the U.S. [2]. The Hemophilia Inhibitor Research Study (HIRS) conducted by the Centers for Disease Control and Prevention (CDC) at 17 U.S. hemophilia treatment centers (HTCs) included prospective monitoring for inhibitors through testing in a central laboratory and collection of individual treatment records [3]. The modified Nijmegen-Bethesda assay (NBA) used in the study allowed measurement of FVIII inhibitors in the presence of infused factor VIII [4]. Comparison of the NBA results with results of a chromogenic Bethesda assay (CBA) and a fluorescence immunoassay (FLI) for anti-FVIII antibodies showed that 26% of NBA-positive specimens with Nijmegen-Bethesda units <2.0 failed to react with FVIII in both the CBA and FLI, indicating a high rate of false-positive results among low-titer inhibitors [5]. This report further describes the characteristics of the patients with inhibitors detected by this prospective screening program, compares these results to an earlier U.S. prospective study, and discusses the implications of the findings for surveillance and clinical management.
Materials and Methods
Subjects
People with HA having FVIII activity <50 International Units per deciliter were enrolled from 2006 to 2012 at 17 U.S. Hemophilia Treatment Centers in a study of prospective monitoring for inhibitors, which is described in detail elsewhere [3]. Demographic data and information on number of exposure days (ED) before enrollment and previous inhibitor history were collected from the enrolling site using standardized data collection tools. Treatment product exposure records were collected prospectively from the time of enrollment. Inhibitor measurements were performed centrally at CDC at study entry, annually, before any planned product switch, or for clinical indication of an inhibitor. After detection of an elevated inhibitor titer in a previously negative patient, additional data were collected on outcomes. The protocol was approved by the investigational review boards of CDC and each participating site, and all participants or parents/guardians of minor children gave informed consent. The population studied included 824 patients with HA and no previous history of an inhibitor according to the enrolling sites. Severity was reported by the sites as 498 (60%) severe, 135 (16%) moderate, and 191 (23%) mild. For this report, the clinical characteristics of the 23 HA patients with new inhibitors detected during the study are described.
Laboratory methods
Factor VIII inhibitors were measured using a modified Nijmegen-Bethesda assay (NBA), in which patient plasma was heated to 56°C for 30 minutes and centrifuged before testing, as previously described [4], and expressed in Nijmegen-Bethesda units (NBU). For selected specimens, a CBA, expressed in chromogenic Bethesda units (CBU) and a FLI for FVIII antibodies using combined immunoglobulin G (IgG) and immunoglobulin M (IgM) were also performed as previously described [5]. Immunoglobulin subclasses were determined by FLI [6]. Factor VIII gene sequencing, FVIII inversion testing, and multiple ligand probe amplification were carried out by published methods [7]. Dilute Russell’s viper venom time (DRVVT) was measured using DVVtest and DVVconfirm reagents (American Diagnostica, Stamford, CT). Heparin was quantitated using an anti-factor Xa assay (Liquid Anti-Xa Assay, Diagnostica Stago, Parsippany, NJ).
Statistical methods
Comparisons using Fisher’s exact or Chi-square tests were calculated as appropriate using GraphPad Prism, Version 5 (GraphPad Software Inc., San Diego, CA). Results were considered significant at the 0.05 level.
Results
A prospective monitoring study of 824 HA patients with no previous history of an inhibitor identified an inhibitor in 23 patients (2.8%). Characteristics of these 23 patients are shown in Table I. Nine inhibitors (two high titer) were detected at enrollment in patients reported by the enrolling sites to be negative; 14 developed in patients in whom a negative result was documented in the central laboratory at enrollment. Distributions of severity, age, ethnicity, exposure days to factor products (ED), and mutation type were similar for the two groups of patients, and the groups were combined for further analysis.
TABLE I.
Characteristics of 23 Hemophilia A Patients With New Inhibitors Detected During the Hemophilia Inhibitor Research Study
| Patient | Age | Severity | Mutation | Exposure Days | Peak NBU | Peak CBU | FLI | IgG Subclass | Detection | Repeat | Persistence | Treatment Change |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High titer | ||||||||||||
| 1 | 1.5 | Severe | del exons 7–9 | 0–20 | 688 | 512 | 4.25 | 1, 4 | FU | P | > 3 years | BPA, ITI |
| 2 | 18 | Moderate | Arg1941Gln | 21–100 | 55.6 | 72.8 | 1.84 | 1, 2, 4 | E | P | > 3years | BPA |
| 3 | 3 | Severe | Arg427Stop | 0–20 | 54.4 | 54.7 | ND | ND | E | P | > 10 months | BPA |
| 4 | 2.9 | Severe | 51–52delTTGT | 52 | 36.8 | 14.8 | 3.54 | 1, 2, 3, 4 | FU | P | > 2 years | BPA |
| 5 | 1 | Severe | Inversion 22 | 21–50 | 18.7 | 12.4 | 7.89 | 1, 2, 3, 4 | FU | P | < 7 months | BPA, ITI |
| 6 | 1 | Severe | Met128Ilefs*5 | 9 | 16 | ND | ND | ND | FU | ND | > 1 year | ITI |
| 7 | 4 | Moderate | Inversion 22 | 101–150 | 6.5 | 6.5 | 2.89 | 1, 4 | FU | P | > 1 year | BPA, ITI |
| 8 | 5 | Severe | Inversion 22 | 27 | 3.9 | ND | 1.47 | 1, 4 | FU | ND | Unknown | BPA, ITI |
| 9 | 2 | Severe | 215delAA | 0–20 | 2.6 | 2.9 | 0.54 | ND | FU | ND | < 9 months | ITI |
| Low titer factor VIII-specific | ||||||||||||
| 10 | 12 | Mild | Arg593Cys | 0–20 | 1.8 | 0.9 | 6.17 | 1, 2, 3, 4 | FU | P | > 5 months | BPA |
| 11 | 46 | Mild | Ser535Gly | 0–20 | 1.7 | 3.3 | 6.4 | 1, 2, 3, 4 | FU | P | > 1 month | BPA |
| 12 | 5 | Severe | Inversion 22 | >150 | 1.7 | 0.7 | 0.54 | 1, 4 | FU | P | < 1 year | No change |
| 13 | 61 | Severe | 2229Stop | >150 | 1.5 | 0.8 | 0.58 | 1, 2, 4 | E | P | > 2 years | No change |
| 14 | 2 | Severe | Inversion 22 | 0–20 | 1.3 | 0.9 | 0.5 | 1, 4 | E | P | > 1 year | No change |
| 15 | 2 | Severe | 833ins13 | 21–100 | 0.7 | 0.5 | 3.06 | 1, 3, 4 | E | P | Recurrent | No change |
| 16 | 41 | Moderate | 142delAAGA | >150 | 0.7 | 0.9 | 2.35 | 1, 2, 3, 4 | E | ND | Unknown | BPA, ITI |
| 17 | 5 | Severe | 1615delA | >150 | 0.7 | 0.3 | 0.36a | 1, 4 | E | P | > 1 year | BPA |
| 18 | 2 | Severe | Inversion 22 | 101–150 | 0.5 | 0.7 | ND | ND | E | ND | Unknown | No change |
| Low titer factor VIII non-specific | ||||||||||||
| 19 | 18 | Moderate | Val326Ala | 0 | 1.3 | 0 | ND | ND | FU | N | < 1 year | No prior product |
| 20 | 29 | Mild | Arg593Cys | 0 | 0.9 | 0.3 | 0.19 | ND | E | ND | Unknown | No prior product |
| 21 | 10 | Severe | 1049delGA | >150 | 0.8 | 0 | 0.34 | None | FU | ND | Unknown | No change |
| 22 | 15 | Severe | 1194delA | >150 | 0.7 | 0.1 | 0.03 | ND | FU | N | < 1 month | No change |
| 23 | 6 | Mild | Ala284Glu | 0–20 | 0.6 | 0.1 | ND | ND | FU | ND | Unknown | No change |
NBU = Nijmegen Bethesda units, CBU = chromogenic Bethesda units, FLI = fluorescence immunoassay, N = negative, P = positive, ND = not done, E = at enrollment, FU = during follow-up, BPA = by-passing agent, ITI = immune tolerance induction.
Positive for IgG1 and IgG4 subclasses.
Peak inhibitor titers before initiation of immune tolerance induction therapy (ITI) were used for classification. Seven patients (30%) had inhibitors ≥5.0 NBU. Two (9%) had inhibitors of 2.6 and 3.9 NBU and were placed on ITI immediately following their inhibitor detection. Fourteen (61%) had inhibitors of 0.5–1.9 NBU. For purposes of analysis, the 2.6 and 3.9 NBU patients were included in the high-titer group. Table II shows characteristics of the patients with high-titer inhibitors (HTI) and low-titer inhibitors (LTI), as well as those LTI which were identified as being FVIII-specific and non-specific by reaction in the CBA and/or FLI.
TABLE II.
Clinical Characteristics of Patients With New Inhibitors Detected, Including High Titer, Low Titer, and Factor VIII (FVIII)-Specific Low Titer (Positive in Chromogenic Bethesda Assay and/or Fluorescence Immunoassay), n (%)
| High | All Low | FVIII-Specific Low | FVIII Non-Specific Low | |
|---|---|---|---|---|
| Number of patients | 9 | 14 | 9 | 5 |
| Age | ||||
| Median | 2 | 11a | 5 | 15a |
| Range | 1–18 | 2–61 | 2–61 | 6–29 |
| Severity | ||||
| Severe | 7 (78) | 8 (57) | 6 (67) | 2 (40) |
| Moderate | 2 (22) | 2 (14) | 1 (12) | 1 (20) |
| Mild | 0 | 4 (29) | 2 (25) | 2 (40) |
| Exposure days | ||||
| 0–20 | 4 (44) | 6 (43) | 3 (38) | 3 (60) |
| 21–100 | 4 (44) | 1 (7) | 1 (12) | 0 |
| 101–150 | 1 (11) | 1 (7) | 1 (12) | 0 |
| >150 | 0 | 6 (43) | 4 (44) | 2 (40) |
| Race | ||||
| White | 5 (56) | 11 (79) | 6 (67) | 5 (100) |
| Black | 1 (11) | 0 | 0 | 0 |
| Hispanic | 0 | 3 (21) | 3 (33) | 0 |
| Other | 3 (33) | 0 | 0 | 0 |
| High-risk mutation | 8 (89) | 10 (71) | 7 (88) | 3 (60) |
| Detected clinically | 6 (67) | 3 (21) | 3 (33) | 0 |
| Treatment change | ||||
| No change | 0 | 8 (57)a | 5 (56)a | 3 (60)a |
| Desmopressin to factor | 0 | 2 (14) | 0 | 2 (40) |
| Bypassing agent only | 3 (33) | 3 (21) | 3 (33) | 0 |
| ITI only | 2 (22) | 0 | 0 | 0 |
| Bypassing agent and ITI | 4 (44) | 1 (7) | 1 (11) | 0 |
Significantly different from high-titer patients at P <0.05.
High-titer inhibitors
HTI were identified in nine patients, including seven with inhibitors detected during study monitoring and two detected at enrollment. Eight patients were between 1 and 5 years old at the time of initial inhibitor detection; one was 18 years old. Among the younger patients, four had fewer than 20 ED, three had 21–50 ED, and one had 52 ED; the 18-year-old was reported to have between 21 and 100 ED. Four inhibitors were first detected locally, and five were detected through study screening at annual visits or before a planned product switch. Six were positive on multiple specimens with peak titers ranging from 6.5 to 688.2 NBU. The two patients placed on ITI immediately (Patients 8 and 9) were not retested in the central laboratory.
Of the two patients with HTI detected at enrollment, one was a 3-year-old with fewer than 20 EDs who had an inhibitor titer of 54.4 NBU (Patient 3). The results of previous local inhibitor testing were not reported by the site. The other patient was an 18-year-old with moderate hemophilia whose initial study result was 10.2 NBU (Patient 2). A test performed three years before enrollment by the patient’s HTC was reported as negative. Six months after the positive study test result reported to the HTC, a local test was said to be negative. The patient presented for an emergency appendectomy one year later and was treated with several FVIII products, with hemostasis achieved only with recombinant factor VIIa. The peak inhibitor titer detected by the CDC laboratory post-surgery was 55.6 NBU. These two patients and Patient 4 were not noted to have clinical indication of an inhibitor at the time that their positive study specimens were submitted.
Low-titer inhibitors
LTI were detected in 14 patients ranging from 2 to 61 years of age. Seven were detected during study monitoring and seven at enrollment. Of the nine LTI patients with repeat specimens available, seven (78%) were consistently positive, while two were negative on repeat specimens. One LTI patient became negative within three months, but his inhibitor reappeared two years later (Patient 15). One LTI was detected locally (Patient 12), and two other specimens were submitted due to poor response to treatment (Patients 10 and 11). Eleven were not noted to have clinical indication of an inhibitor. Three patients with LTI were over age 40. Patient 11 had mild disease and fewer than 20 ED. His inhibitor appeared at age 46, three weeks after he underwent surgery for hernia repair and liver biopsy. His mutation, Ser535Gly, is one of the missense mutations previously seen with inhibitors [8]. Patients 13 and 16, who were severe and moderate, respectively, had >150 ED. Their inhibitors were detected at enrollment, and the events surrounding their occurrence are not known. Patient 13 was 61 years old and reported to have history of intracranial hemorrhage and drug allergies; he had no recent procedures. Additional data on Patient 16, age 41, were not available.
Comparison of HTI and LTI
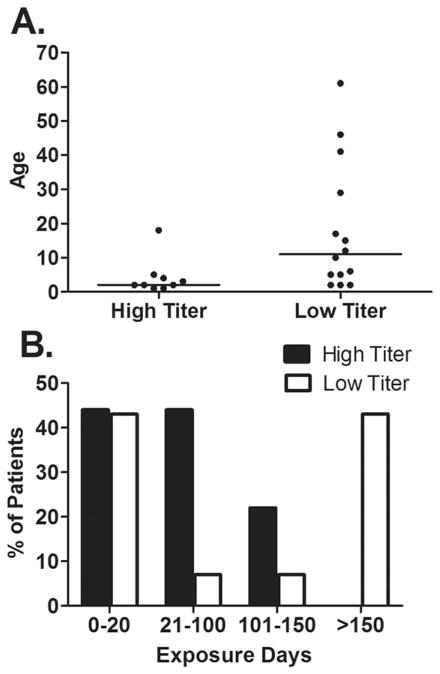
Subjects with HTI were younger at inhibitor detection than those with LTI (median age 2 years vs. 11 years, P = 0.016). All HTI were seen in patients age 18 or younger, while LTI occurred in patients with a wide age distribution, including three patients over 40 years of age (Fig. 1A). Despite the difference in the age distributions, the proportions of HTI and LTI patients with fewer than 20 EDs were similar, 44% and 43%, respectively, due to the presence of more mild and moderate patients in the LTI group. All HTI developed within the first 150 ED, while 43% of LTI developed after 150 ED (Fig. 1B). Race and mutation type frequencies were similar between HTI and LTI groups (Table II). All HTI required change in treatment to a bypassing agent, ITI, or both; whereas, only 43% of those with LTI underwent a treatment change (P = 0.029).
Figure 1.
Comparison of high titer and low titer inhibitors for age at inhibitor detection (A) and exposure days before inhibitor detection (B). Bar represents the median.
FVIII specificity
CBA and anti-FVIII FLI were performed on all samples that tested positive by the NBA to rule out non-FVIII-specific inhibition of the clot-based assay. All HTI tested gave positive reactions in the CBA and FLI (Table III). For eight of the 14 LTI, FVIII specificity was indicated by a positive reaction in the CBA. Seven of the eight were also positive by FLI; one was not tested. Patient 17 with 0.7 NBU was CBA-negative at 0.3 CBU and negative by FLI using combined IgG/IgM reagents at 0.36 (negative <0.47). He remained NBA-positive at 0.5 NBU and CBA-negative 16 months later, when testing for immunoglobulin subclass determination [6] revealed positivity for IgG1 and IgG4. Among the 5 LTI not shown to be FVIII-specific were those from Patients 19 and 20, who at ages 18 and 29 had titers of 1.3 and 0.9 NBU despite having received no treatment other than desmopressin, clearly illustrating false positive test results; Patient 22, whose inhibitor titer decreased from 0.7 NBU to 0.3 NBU within 1 month, representing either a false positive or a transient inhibitor; and Patients 21 and 23, whose inhibitors of 0.8 and 0.6 NBU detected late in the study were not repeated, but as both were negative in the CBA, which is more sensitive than the NBA [5], are also likely to be false positives. Four of the five non-FVIII-specific specimens had negative DRVVT; Patient 23 was not tested. None of the specimens had heparin contamination. Overall, 36% of LTI, with titers of 0.6–1.3 NBU, failed to react in the CBA or FLI. FVIII-specific LTI had similar titers of 0.5–1.8 NBU but remained positive over time and more often resulted in treatment change than LTI that were not FVIII-specific (Table II).
TABLE III.
Characteristics of New Inhibitors Detected, Including High Titer, Low Titer, and Factor VIII (FVIII)-Specific Low Titer (Positive in Chromogenic Bethesda Assay and/or Fluorescence Immunoassay)
| High | Low | FVIII-Specific Low | |
|---|---|---|---|
| Inhibitor confirmation, n (%) | |||
| FVIII-specific | 8/8 (100) | 9/14 (64) | – |
| Repeat specimen positive | 7/7 (100) | 7/9 (78) | 7/7 (100) |
| Peak titer, median (range) | |||
| Nijmegen-Bethesda assay | 18.7 (2.6–688) | 0.9 (0.5–1.8) | 1.4 (0.5–1.8) |
| Chromogenic Bethesda assay | 14.8 (2.9–512) | 0.8 (0–3.3) | 0.9 (0.5–3.3) |
| Fluorescence Immunoassay | 2.89 (0.54–7.89) | 0.50 (0.03–6.17) | 2.71 (0.50–6.17) |
Levels of antibodies binding to FVIII in the FLI (Table III) were similar among patients with HTI (median 2.89, range 0.54–7.89) and FVIII-specific LTI (median 2.71, range 0.50–6.17). Immunoglobulin subclass types also were similar between HTI and LTI, with all FVIII-specific inhibitors tested having IgG1 and IgG4.
Study comparison
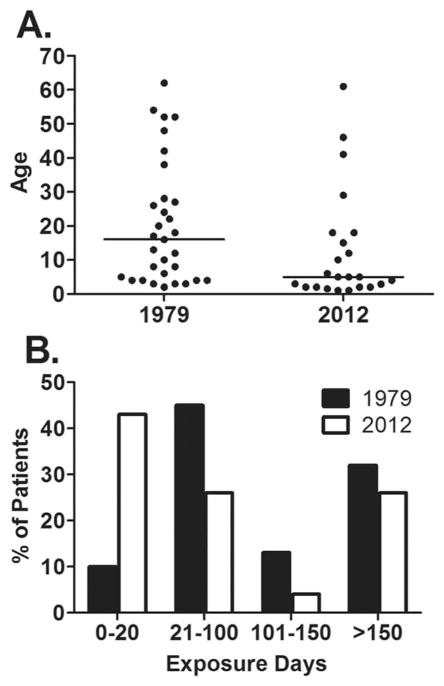
Table IV compares data from the current study with the only similar prospective study of inhibitors in the U.S., which was conducted by the National Heart, Lung, and Blood Institute (NHLBI) between 1975 and 1979 [2]. Among 1,306 enrolled HA patients, 31 new inhibitors (2.4%) were detected. Of patients developing inhibitors, 32% and 35% were non-severe in the NHLBI study and the current study, respectively. Clinical indications prompted testing for an inhibitor before study screening in 35% and 39% of patients who developed new inhibitors in the NHLBI and the current study, respectively, and repeat specimens were positive in 77% of the NHLBI patients and 88% of the patients in the current study. The age ranges at time of inhibitor development were similar, but median age was significantly higher in the earlier study (16 years vs. 5 years, P = 0.024) (Fig. 2A). Later occurrence of inhibitors is also suggested by a significant difference in the distribution of EDs (Chi-square = 31.0, P = 0.032), with 10% of inhibitors in the early study and 43% in the current study developing before 20 ED (Fig. 2B). Age distributions for the enrolled subjects in the two studies were not significantly different (data not shown); distribution of EDs for the entire NHBLI population was not provided [9]
TABLE IV.
Comparison of the Results of the Current Study and a Previous Large U.S. Prospective Study Conducted by the NHLBI [2], n (%)
| NHLBI study | Current study | |
|---|---|---|
| Study Period | 1975–1979 | 2006–2012 |
| Number of Patients Enrolled | 1306 | 824 |
| Number of New Inhibitors | 31 (2.4%) | 23 (2.8%) |
| Severity | ||
| Severe | 21 (68) | 15 (65) |
| Moderate or mild | 10 (32) | 8 (35) |
| Age | ||
| Median | 16 | 5a |
| Range | 2–62 | 1–61 |
| Exposure days | ||
| 0–20 | 3 (10) | 10 (43)a |
| 21–100 | 14 (45) | 6 (26) |
| 101–150 | 4 (13) | 1 (4) |
| >150 | 10 (32) | 6 (26) |
| Clinical indicationb | 11 (35) | 9 (39) |
| Repeat specimen positivec | 24/31 (77) | 14/16 (88) |
Significantly different at P <0.05.
Detected when tested due to clinical suspicion of an inhibitor rather than at study screening.
Of those repeated.
Figure 2.
Comparison of 1975–1979 study [2] and current study for age at inhibitor detection (A) and exposure days before inhibitor detection (B). Bar represents the median.
Discussion
The findings from prospective studies have important implications for both clinical management and population surveillance. For both purposes, it is crucial that the appropriate population is monitored and that tests are used that minimize the number of false results, both false positive and false negative. In this prospective study on inhibitor development in HA patients, HTI occurred in non-severe as well as severe patients. Although almost one-half of all inhibitors occurred within the first 20 EDs in both LTI and HTI groups, there was a second peak of inhibitor development in the LTI group that occurred after 150 EDs. This difference accounted for the significant median age difference between HTI and LTI groups. The LTI group included three patients over age 40, although one was mild with fewer than 20 ED at the time of inhibitor development. These findings are similar to the report from the United Kingdom of a second peak of inhibitors later in life [10].
At the time of initial detection, it is not possible to determine whether a LTI will progress to a high-responding inhibitor, remain low-responding, or be transient [11]. In the current study, 39% of new inhibitors detected had not yet come to clinical attention. Early recognition of inhibitors may improve outcomes of ITI, which is reported to be more effective at lower titers [12]. Although the modified NBA used in this study facilitates patient screening, because it allows for testing patients with exogenous FVIII present [4], traditional inhibitor assays may miss LTIs if a wash-out period is not employed, because the presence of residual infused FVIII can act as a reservoir for anti-FVIII antibodies, thereby decreasing the observed inhibitor titer and potentially producing a false-negative test result. This study also demonstrates that false-positive results can occur but illustrates that they can be distinguished from true positives by performing follow-up tests and utilizing alternative testing methods to confirm FVIII specificity.
The validity of a single LTI result is often questioned due to the known high rate of false-positive tests, which has been documented by proficiency testing programs in North America and attributed to differences in the test methods used [13]. Indeed, 70% of North American coagulation laboratories report using a hybrid of Bethesda and Nijmegen methods and not strictly following either [14]. Such differences make it difficult to compare results among laboratories and to collect national data on inhibitor occurrence. Using carefully standardized methods in a single laboratory, we have shown it is possible to achieve a high rate of precision for known positive and negative specimens [4]. We also found, however, that one-quarter of all inhibitors <2.0 NBU in the NBA failed to react with FVIII in more sensitive and specific assays [5]. These discrepancies may be due to assay variability, lupus anticoagulants, or non-specific inhibition of clot-based assays. Use of alternative assays, particularly the CBA, which can be performed in many clinical laboratories with automated analyzers, allows rapid identification of true positive inhibitors that our current data suggest may be more likely to persist and require treatment change. These data, and the fact that CBA and NBA results correlate well for inhibitors greater than 2.0 NBU [5], suggest that the adoption of the CBA for all inhibitor testing might be advantageous.
The clinical significance of persistent LTIs is not well documented; however, our limited data show that almost one-half of those with FVIII-specific LTI were placed on by-passing agents. It is also of interest that levels of antibodies directed against FVIII, as measured by FLI, were often as high in those with LTI as in those with HTI, suggesting that in some patients the NBA titer may not accurately reflect the antibody load. In addition, FVIII-specific LTI contained both IgG1 and IgG4 subclasses, a pattern similar to that seen in HTI [6,15]. Clinically, response to therapy is the most useful determinant of the significance of a LTI [11], but data on clinical response were not collected in this study. Further studies on the natural history and clinical significance of LTI are warranted.
A national cooperative study conducted by the NHLBI from 1975 to 1979 in 1,306 HA patients at 13 hemophilia treatment centers [2] is the only prospective U.S. study comparable in size to HIRS involving a patient population including all hemophilia severities. In spite of the changes seen in treatment over the past 35 years, characteristics of patients identified with inhibitors in the two studies were similar. Both studies found close to one-third of new inhibitors occurring in non-severe patients and more than one-quarter in those with >150 ED. Only 35% and 39% of inhibitors were detected due to clinical suspicion of an inhibitor and the rest by study screening. The proportion of detected inhibitors negative on repeat specimens was 24% in the NHLBI study, but only 12% in HIRS, perhaps reflecting improved specificity of the Nijmegen method over the Bethesda assay for inhibitor detection, although the difference was not statistically significant. There were two striking differences between the groups of patients with new inhibitors identified. In HIRS, the inhibitors occurred at a significantly earlier age with a median age of 5 years compared to 16 years in the NHLBI study. There was also a shift in the distribution of EDs: 43% of HIRS inhibitors occurred before 20 EDs, compared to only 10% of those in the NHLBI study, in which 45% of new inhibitors occurred between 21 and 100 ED. During the NHLBI study, only plasma-derived factor VIII concentrates were available, and their introduction was relatively recent after prior dependence on cryoprecipitate and plasma treatment. This temporal shift in inhibitor occurrence could reflect the change from plasma-derived to recombinant factor. The appearance of inhibitors in younger patients may explain the perception that more inhibitors appeared with introduction of recombinant products, although a difference often could not be documented in studies conducted at the time [16]. Other changes in treatment practices, such as introduction of factor concentrates earlier in life and prophylaxis, may also have contributed. Neither the NHLBI study nor the current study was restricted to previously untreated patients and thus cannot address the important question of immunogenicity in that population. These two prospective studies, with strikingly similar results although decades apart, show that the population at risk for inhibitors includes patients of all severities, ages, and EDs and that more than one-third of HTIs had not been recognized clinically at the time of laboratory detection.
Acknowledgments
Contract grant sponsor: CDC Foundation through grants from Pfizer Inc. and Baxter Healthcare.
Footnotes
Conflict of interest: Nothing to report.
Addendum
The Hemophilia Inhibitor Research Study Investigators include authors from the following study sites: Thomas C. Abshire, Amy L Dunn, and Christine L. Kempton, Emory University, Atlanta GA; Paula L. Bockenstedt, University of Michigan Hemophilia and Coagulation Disorders, Ann Arbor, MI; Doreen B. Brettler, New England Hemophilia Center, Worcester, MA; Jorge A. Di Paola, Mohamed Radhi and Steven R. Lentz, University of Iowa Carver College of Medicine, Iowa City, IA; Gita Massey and John C. Barrett, Virginia Commonwealth University, Richmond, VA; Anne T. Neff, Vanderbilt University Medical Center, Nashville, TN; Amy D. Shapiro, Indiana Hemophilia and Thrombosis Center, Indianapolis, IN; Michael Tarantino, Comprehensive Bleeding Disorders Center, Peoria, IL; Brian M. Wicklund, Kansas City Regional Hemophilia Center, Kansas City, MO; Marilyn J. Manco-Johnson, Mountain States Regional Hemophilia and Thrombosis Center, University of Colorado and The Children’s Hospital, Aurora, CO; Christine Knoll, Phoenix Children’s Hospital Hemophilia Center, Phoenix, AZ; Miguel A. Escobar, Gulf States Hemophilia and Thrombophilia Center, Houston, TX; M. Elaine Eyster, Hemophilia Center of Central Pennsylvania, Hershey, PA; Joan C. Gill, Comprehensive Center for Bleeding Disorders, Milwaukee, WI; Cindy Leissinger, Louisiana Center for Bleeding and Clotting Disorders, New Orleans, LA; Hassan Yaish, Primary Children’s Medical Center, Salt Lake City, UT.
References
- 1.Soucie JM, Miller CH, Kelly FM, Aschman D, DiMichele D, Konkle BA, Kulkarni R Monahan PE the CDC. Inhibitor Surveillance Working Group. National surveillance for hemophilia inhibitors in the United States: summary report of an expert meeting. Am J Hematol. 2014;89:621–625. doi: 10.1002/ajh.23704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMillan CW, Shapiro SS, Whitehurst D, Hoyer LW, Rao AV, Lazerson J. The natural history of factor VIII:C inhibitors in patients with hemophilia A: A national cooperative study. II. Observations on the initial development of factor VIII:C inhibitors. Blood. 1988;71:344–348. [PubMed] [Google Scholar]
- 3.Soucie JM, Miller CH, Kelly FM, Payne AB, Creary M, Bockenstedt PL, Kempton CL, Manco-Johnson MJ, Neff AT the Haemophilia Inhibitor Research Study Investigators. A study of prospective surveillance for inhibitors among persons with haemophilia in the United States. Haemophilia. 2014;20:230–237. doi: 10.1111/hae.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller CH, Platt SJ, Rice AS, Kelly F, Soucie JM the Hemophilia Inhibitor Research Study Investigators. Validation of Nijmegen–Bethesda assay modifications to allow inhibitor measurement during replacement therapy and facilitate inhibitor surveillance. J Thromb Haemost. 2012;10:1055–1061. doi: 10.1111/j.1538-7836.2012.04705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller CH, Rice AS, Boylan B, Shapiro AD, Lentz SR, Wicklund BM, Kelly FM, Soucie JM the Hemophilia Inhibitor Research Study Investigators. Comparison of clot-based, chromogenic and fluorescence assays for measurement of factor VIII inhibitors in the U.S. Hemophilia Inhibitor Research Study. J Thromb Haemost. 2013;11:1300–1309. doi: 10.1111/jth.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boylan B, Rice AS, Dunn AL, Tartantino MD, Brettler DB, Barrett JC, Miller CH the Hemophilia Inhibitor Research Study Investigators. Characterization of the anti-factor VIII immunoglobulin profile in patients with hemophilia using a fluorescence-based immunoassay. J Thromb Haemost. 2015;13:47–53. doi: 10.1111/jth.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller CH, Benson J, Ellingsen D, Driggers J, Payne A, Kelly FM, Soucie JM, Hooper WC the Hemophilia Inhibitor Research Study Investigators. F8 and F9 mutations in U.S. hemophilia patients: correlation with history of inhibitor and race/ethnicity. Haemophilia. 2012;18:375–382. doi: 10.1111/j.1365-2516.2011.02700.x. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. [Accessed February 5, 2015];CDC Hemophilia A Mutation Project (CHAMP) Available at: http://www.cdc.gov/hemophiliamutations.
- 9.Gill FM. The natural history of factor VIII inhibitors in patients with hemophilia A. In: Hoyer LW, editor. Factor VIII Inhibitors. New York: Liss; 1984. pp. 19–29. [PubMed] [Google Scholar]
- 10.Hay CRM, Palmer B, Chalmers E, Liesner R, Maclean R, Rangarajan S, Williams M, Collins PW United Kingdom Haemophilia Centre Doctors’ Organisation (UKHCDO) Incidence of factor VIII inhibitors throughout life in severe hemophilia A in the United Kingdom. Blood. 2011;117:6367–6370. doi: 10.1182/blood-2010-09-308668. [DOI] [PubMed] [Google Scholar]
- 11.Blanchette VS, Key NS, Ljung LR, Manco-Johnson MJ, van den Berg HM, Srivastava A. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12:1935–1939. doi: 10.1111/jth.12672. [DOI] [PubMed] [Google Scholar]
- 12.DiMichele DM, Kroner BL. The North American immune tolerance registry: practices, outcomes, outcome predictors. Thromb Haemost. 2002;87:52–57. [PubMed] [Google Scholar]
- 13.Peerschke EIB, Castellone DD, Ledford-Kraemer M, Van Cott EM, Meijer P NASCOLA Proficiency Testing Committee. Laboratory assessment of factor VIII inhibitor titer. Am J Clin Pathol. 2009;131:552–558. doi: 10.1309/AJCPMKP94CODILWS. [DOI] [PubMed] [Google Scholar]
- 14.Pruthi RK, Plumhoff EA, Nichols WL, Meijer P, van Cott EM. Quality of factor VIII inhibitor testing in North American specialized coagulation laboratories. Am J Hematol. 2014;89:E27. doi: 10.1111/ijlh.12359. [DOI] [PubMed] [Google Scholar]
- 15.Whelan SF, Hofbauer CJ, Horling FM, Allacher P, Wolfsegger MJ, Oldenburg J, Male C, Windyga J, Tiede A, Schwarz HP, Scheiflinger F, Reipert BM. Distinct characteristics of antibody responses against factor VIII in healthy individuals and in different cohorts of hemophilia A patients. Blood. 2013;121:1039–1048. doi: 10.1182/blood-2012-07-444877. [DOI] [PubMed] [Google Scholar]
- 16.Scharrer I, Bray GL, Neutzling O. Incidence of inhibitors in haemophilia A patients: a review of recent studies of recombinant and plasma-derived factor VIII concentrates. Haemophilia. 1999;5:145–154. doi: 10.1046/j.1365-2516.1999.00300.x. [DOI] [PubMed] [Google Scholar]