Abstract
Background
As CDK-16 has been shown to be upregulated in several transformed cancer lines, we hypothesized that the cyclin-dependent kinase 16 (CDK-16) may be upregulated in serous epithelial ovarian cancer (EOC) cells. Therefore, we comparatively examined the mRNA and protein expression of CDK-16 in samples resected from serous EOC patients and normal controls.
Material/Methods
Tissue samples were collected from 70 serous EOC patients and 40 normal controls. Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) was conducted to assess mRNA expression. CDK-16 protein expression was assessed by semi-quantitative immunohistochemical staining. Differences in mRNA and protein expression between serous EOC cells and normal tissue cells were tested with the Kruskal-Wallis test and analysis of variance (ANOVA).
Results
Both CDK-16 mRNA and protein expression were significantly higher in serous EOC tumor cells as compared to normal control ovarian cells (p<0.01). Although there was no significant correlation between CDK-16 mRNA expression and serous EOC stage (p=0.0794), there was a significant correlation between CDK-16 mRNA expression and serous EOC grade (p<0.0001). Moreover, there were significant correlations between CDK-16 protein expression and serous EOC stage (p<0.0001) and grade (p<0.0001).
Conclusions
CDK-16 upregulation in serous EOC cells may represent a negative feedback loop to promote ovarian cell differentiation in malignantly-transformed serous EOC cells. Further in-depth investigation on CDK-16’s role in serous EOC is needed.
MeSH Keywords: Cyclin-Dependent Kinases, Ovarian Neoplasms, Ovary
Background
Epithelial ovarian cancer (EOC) is a primary cause of mortality among patients with gynecological malignancies [1]. Although several EOC histotypes have been identified (e.g., serous, endometrioid, clear-cell, and mucinous), all EOC patients still receive similar cytotoxic chemotherapeutic regimens regardless of histotype, and their survival outcomes have not significantly improved over the past three decades [2,3]. Moreover, accumulated evidence suggests that EOC histotypes should be considered distinct disease states originating from different ovarian cell types [3,4]. Given this heterogeneity in EOC histotypes, the development of screening and therapeutic strategies based on histotype-specific biomarkers may be critical to improving future clinical outcomes [2].
Based on this paradigm shift in our understanding of EOC, re-analysis of EOC cases based on histotypes has led to the molecular characterization of specific subtypes. For example, the most common EOC histotype – serous EOC – displays an almost 100% association with TP53 gene mutations [3,5]. Moreover, mutations in BRCA1/2 and other DNA repair genes (e.g., PALB2, RAD51, RAD50, BARD1, CHK2, and BRIP1) have been shown to occur more frequently in serous EOC than in other EOC histotypes [3,6]. In addition, an analysis of almost 500 serous EOC cases confirmed that these tumors are characterized by widespread DNA copy number aberrations [3,7]. These findings led Bowtell et al. to postulate that serous EOC cells evolve from initial mutations to DNA repair genes followed by DNA copy number aberrations and subsequent malignant transformation [3,8].
Based on this previous evidence, we hypothesized that the cyclin-dependent kinase 16 (CDK-16, PCTAIRE-1, PCTK1) – which has been shown to be upregulated in several transformed cancer lines [9] – may be upregulated in serous EOC cells. Therefore, here we comparatively examined the mRNA and protein expression of CDK-16 in samples resected from serous EOC patients and normal controls.
Material and Methods
Ethics statement
This study was approved by the Ethics Committee (IRB) of the Gynecology and Obstetrics Hospital of Guizhou Medical University (approval no: 201528). All subjects recruited for this study provided written informed consent prior to participation.
Sample size determination
Patient recruitment
From January 2013 to March 2015, tissue samples were consecutively collected from primary ovarian cancer surgery patients undergoing radical surgical resection of their ovarian tumors at the Gynecology and Obstetrics Hospital of Guizhou Medical University. Independent staff gynecologic pathologists determined the subtypes and grades of all resected tumors, while independent staff gynecologic oncologists scored tumor stage. Inclusion criteria were as follows: (i) primary surgical patient, (ii) serous EOC histopathology subtype, (iii) complete records regarding pre-operative chemotherapy and past medical history, and (iv) signed informed consent for inclusion in this study. Exclusion criteria were as follows: (i) non-primary surgical patients, (ii) non-serous EOC histopathology subtypes, (iii) missing or incomplete records regarding pre-operative chemotherapy or past medical history, (iv) chemotherapy of any kind prior to tumor resection, (v) previous history of any malignancy, or (vi) lack of informed consent.
Prior to patient recruitment, pilot testing was performed to obtain preliminary data on CDK16 expression in serous EOC patients in order to determine a minimum sample size. On this basis, minimum sample sizes of 34 and 37 were calculated based on the CDK16 mRNA and protein expression testing, respectively. After application of the inclusion and exclusion criteria, a total of 70 Han Chinese serous EOC patients were finally recruited into this study. During the same time period, healthy ovaries from 40 patients that had underwent oophorectomy for prolapses or uterine fibromas were also recruited as normal controls.
Ovarian tissue sampling
Within 30 min of surgical resection, tumors were immediately frozen via liquid nitrogen. Each sample was split in two portions: one for histologic assays and one for RNA isolation. Samples were placed in a medium at a suitable temperature for micro-dissection and hematoxylin and eosin (H&E) staining to ascertain the epithelial portion.
Reverse transcription of extracted RNA
From a frozen state, 23 EOC and 12 control samples were carefully dissected out, and then a mechanical rotary homogenizer (Qiagen, Valencia, CA, USA) was used to homogenize the samples. A TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) was applied to extract the total RNA content followed by RNA purification with an RNeasy cleanup kit (Qiagen). Spectrophotometry was applied to assess RNA purity and quantity. An Agilent 2100 Bioanalyser (Santa Clara, CA, USA) was used to assess RNA integrity. Using a SuperScript™ II RT RNaseH-reverse transcriptase kit (Invitrogen Life Technologies), 1 mg RNA was reverse-transcribed in a 20-ml volume.
Gene expression assay
Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) was conducted in triplicate for the CDK-16 gene on an ABI PRISM 7000 Sequence Detection System with TaqMan Universal PCR Master Mix and the anti-human CDK-16 Assay on Demand probe (Hs00178837_m1) (all from Applied Biosystems, Applera, UK). cDNA levels were normalized from with an endogenous anti-human GAPDH control probe (Hs02758991_g1, Applied Biosystems). Separate TaqMan reaction tubes were used for the CDK16 and internal control genes. Reverse transcription volume (5 μl) was conducted in a total PCR reaction volume of 25 μl. The PCR reaction was cycled as follows: 95°C for 10 min, 95°C for 15 sec (40 cycles), and 60°C for 1 min. Fold-changes were calculated using the comparative threshold cycle (Ct) method.
Semi-quantitative immunohistochemistry
To evaluate CDK-16 protein expression, semi-quantitative immunohistochemical staining was conducted on the EOC and normal tissue samples as previously described with minor modifications [10]. First, paraffin-embedded blocks were cut into 6-μm slices. The sections were slide-mounted, dried for 16 h at 37°C, xylene-deparaffinized, rehydrated through an alcohol gradient, and finally lightly washed with faucet water. EOC tumors were classified as “low-grade” or “high-grade” by H&E staining (Sigma-Aldrich, St. Louis, MO, USA).
Then, 0.3% hydrogen peroxide in methyl alcohol was applied to block endogenous peroxidase activity. Thereafter, the sections were lightly washed in distilled water. Microwaving the sections (three cycles at 750 W for 5 min per cycle) in 1.0-mM ethylenediaminetetracetic acid (EDTA) buffer (pH 8.0) was performed for epitope retrieval. Following light washing with phosphate buffer, normal horse serum was applied for 30 min to block non-specific binding. Sections were treated with rabbit anti-human CDK-16 antibody (diluted 1:200 in phosphate buffer with 1% bovine serum albumin (BSA), Sigma-Aldrich) in a humidified chamber for 2 h at 22°C. Antibody detection was performed with an ABC Universal kit. Visualization was conducted with 3, 3-diaminobenzidine (DAB) plus hydrogen peroxide as horseradish peroxidase substrates (staining brown). As a control, the procedure was repeated without the anti-CDK-16 antibody. Sections were lightly washed in faucet water, hematoxylin-counterstained, and lightly washed again in running faucet water for 15 min. An alcohol gradient and xylene were applied to dehydrate the sections, which were then Permount-mounted (Fisher Scientific, Pittsburgh, PA, USA).
Microscopy was used to assess CDK-16 protein localization and expression as previously described with minor modifications [10]. Three sections were used for triplicate evaluation. First, sections were scanned at a ×12.5 magnification. Then, section thickness was assessed in three unique regions at a ×600 magnification in order to calculate the mean section thickness therefrom. Then, a ×600 magnification under oil immersion was used for cell counts, which were calculated with a grid size of 800×800 μm and a frame size of 60×60 μm within a 7-μm dissector height. Therefrom, we calculated the mean ± standard deviation (SD) of CDK-16+ cells.
Statistical analysis
Differences in mRNA and protein expression between serous EOC cells and normal tissue cells were analyzed by Kruskal-Wallis and analysis of variance (ANOVA) testing. A p-value was considered significant if less than 0.05.
Results
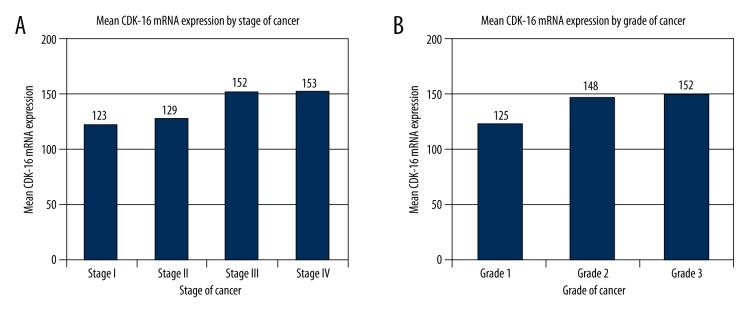
CDK-16 mRNA expression was significantly higher in serous EOC tumor cells as compared to normal control ovarian cells (p<0.01; Table 1, Figure 1). The target amplified gene was confirmed as human CDK-16 (Hs00178837_m1, Applied Biosystems) (data not shown). Although there was no significant correlation between CDK-16 mRNA expression and serous EOC stage (p=0.0794), there was a significant correlation between CDK-16 mRNA expression and serous EOC grade (p<0.0001) (Table 1, Figure 1).
Table 1.
Demographic and clinical characteristics of included patients.
| Group | N | Mean age ±SD | Mean CDK mRNA expression | Mean CDK protein expression |
|---|---|---|---|---|
| Serous EOC patients | 70 | 49.53±11.79 | 146.73±11.02* | 185.61±23.08* |
| By stage | ||||
| Stage I | 15 | 123.20±9.42 | 163.31±21.37 | |
| Stage II | 3 | 129.40±8.79 | 167.96±18.21 | |
| Stage III | 38 | 152.27±11.70 | 201.43±21.31 | |
| Stage IV | 8 | 153.51±6.59 | 217.20±9.72 | |
| Not collected | 6 | – | – | |
| By grade | ||||
| G1 | 25 | 125.93±7.30 | 129.73±14.91 | |
| G2 | 22 | 148.29±9.50 | 197.49±18.30 | |
| G3 | 12 | 152.03±6.13 | 212.03±21.30 | |
| Not collected | 11 | – | – | |
| Controls | 40 | 52.95±8.04 | 46.08±10.05* | 43.66±8.01* |
Statistically significant differences between serous EOC and controls, p<0.01.
Figure 1.
CDK16 mRNA expression in serous EOC tissues by stage and grade. (A) mRNA expression by stage and (B) mRNA expression by grade.
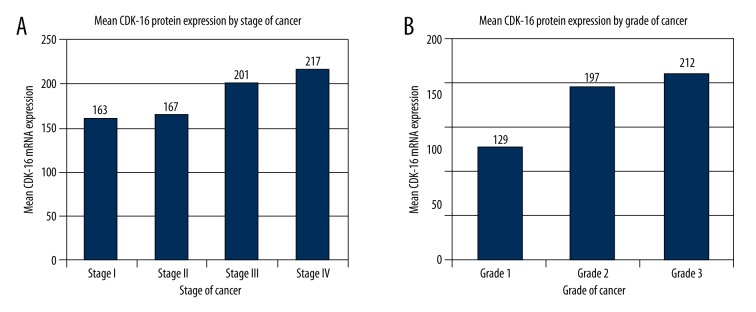
Similarly, CDK-16 protein expression was significantly higher in serous EOC tumor cells as compared to normal control ovarian cells (p<0.01; Table 1; Figures 2, 3). Moreover, there were significant correlations between CDK-16 protein expression and serous EOC stage (p<0.0001) and grade (p<0.0001) (Table 1, Figure 3).
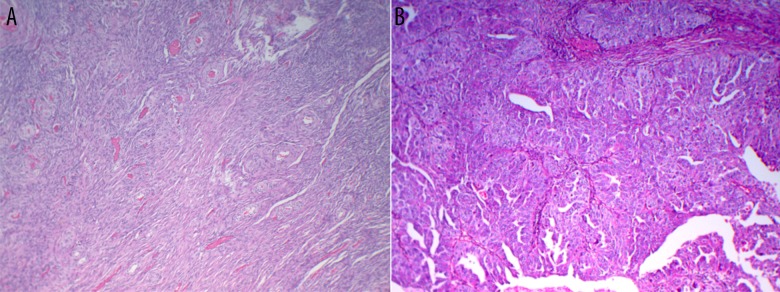
Figure 2.
Immunohistochemical staining of ovarian tissues. (A) Normal tissue and (B) serous EOC tissue.
Figure 3.
CDK16 protein expression in serous EOC tissues by stage and grade. (A) mRNA expression by stage and (B) mRNA expression by grade.
Discussion
The human CDK-16 gene maps to the X chromosome (Xp11.3–p11.23) and belongs to the CMGC protein kinase family that includes all cyclin-dependent kinases (CDKs), mitogen-activated protein kinases (MAPKs), glycogen synthase kinase, and CDK-like kinases [11–13]. CDK-16 is one of three members of the PCTAIRE family that contain a highly-conserved PSTAIRE motif which plays a key role in cyclin binding [11,14]. Despite this well-conserved cyclin-binding motif and CDK’s widely-acknowledged role in cell cycle regulation, CDK-16 has not been conclusively shown to be involved in cell cycle regulation [11]. Rather, CDK-16 is expressed in post-mitotic cells and has been associated with vesicle trafficking, neurite outgrowth, and spermatogenesis [11].
Of specific relevance to the current study is CDK-16’s vital role in spermatogenesis, as ovarian cells share some homology with testicular cells and are capable of reorganizing into testicular structures under the proper conditions [15]. In the testis, CDK-16 expression is upregulated during spermatid differentiation but is remarkably absent in mature spermatozoa [16,17]. Specifically, CDK-16 is sequestered within the cytoplasmic residual body, which is shed after spermatogenesis is completed [16,17]. In vivo, CDK-16-knockout mice develop normally, but male mice are infertile with spermatozoa displaying a bent morphology, malformed heads, thinning and elongation of the annulus, and impaired motility. In contrast, targeting of other CDKs or their associated cyclins led to arrest in spermatogonium proliferation or disruption of meiosis [16]. These findings suggest that CDK-16 deficiency does not affect meiosis but adversely affects terminal differentiation of sperm cells [16].
Here, we found that CDK-16 mRNA and protein expression was significantly higher in serous EOC tumor cells compared to normal control ovarian cells. Moreover, CDK-16 mRNA and protein expression were significantly higher in high-grade serous EOC tumor cells as compared to low-grade serous EOC tumor cells. Based on the previous findings in the testis in which CDK-16 is required for proper spermatid differentiation, we speculate that CDK-16 upregulation in serous EOC cells may represent a negative feedback loop to promote ovarian cell differentiation in malignantly-transformed serous EOC cells. This hypothesis is supported by abundant evidence of negative feedback loops involving CDKs and cyclins that play regulatory roles in human cancers [18–21]. Since no other study has yet examined CDK-16 expression in ovarian cancer cells, further research is necessary to support this hypothesis.
There are several limitations to this study. First, the sample size of participants in this study was limited. Second, the population under investigation was rather homogenous. Third, serous EOC was only compared to normal control tissue, not benign ovarian tumors. Therefore, larger, multi-center studies that include women of different ethnicities with benign tumors would provide more robust conclusions. Third, due to the short duration of this study, we did not correlate CDK-16 expression with specific histopathological grades nor with clinical outcomes. Fourth, we did not examine EOC morphology (beyond the low-grade versus high grade classification), the levels of other CDK’s or cyclins, or the potential mechanism(s) underlying CDK-16’s effect on EOC cells.
Conclusions
CDK-16 mRNA and protein expression was significantly higher in serous EOC tumor cells compared to normal control ovarian cells. Moreover, CDK-16 mRNA and protein expression were significantly higher in high-grade serous EOC tumor cells as compared to low-grade serous EOC tumor cells. CDK-16 upregulation in serous EOC cells may represent a negative feedback loop to promote ovarian cell differentiation in malignantly-transformed serous EOC cells. Further in-depth investigation on CDK-16’s role in serous EOC is needed.
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Andersen CL, Sikora MJ, Luthra S, et al. Utilizing ER target genes as biomarkers of endocrine response in serous ovarian carcinoma. Cancer Research. 2014;74(19 Suppl):2103. [Google Scholar]
- 3.Berns EM, Bowtell DD. The changing view of high-grade serous ovarian cancer. Cancer Res. 2012;72(11):2701–4. doi: 10.1158/0008-5472.CAN-11-3911. [DOI] [PubMed] [Google Scholar]
- 4.Vaughan S, Coward JI, Bast RC, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;11(10):719–25. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed AA, Etemadmoghadam D, Temple J, et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J Pathol. 2010;221(1):49–56. doi: 10.1002/path.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh T, Casadei S, Lee MK, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci USA. 2011;108(44):18032–37. doi: 10.1073/pnas.1115052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Network CGAR. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowtell DD. The genesis and evolution of high-grade serous ovarian cancer. Nat Rev Cancer. 2010;10(11):803–8. doi: 10.1038/nrc2946. [DOI] [PubMed] [Google Scholar]
- 9.Endicott JA, Noble M. Structural characterization of the cyclin-dependent protein kinase family. Biochem Soc Trans. 2013;41:1008–16. doi: 10.1042/BST20130097. [DOI] [PubMed] [Google Scholar]
- 10.Penumatsa K, Edassery SL, Barua A, et al. Differential expression of aldehyde dehydrogenase 1a1 (ALDH1) in normal ovary and serous ovarian tumors. J Ovarian Res. 2010;3(1):28. doi: 10.1186/1757-2215-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shehata SN, Hunter RW, Ohta E, et al. Analysis of substrate specificity and cyclin Y binding of PCTAIRE-1 kinase. Cell Signal. 2012;24(11):2085–94. doi: 10.1016/j.cellsig.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thiselton DL, McDowall J, Brandau O, et al. An integrated, functionally annotated gene map of the DXS8026-ELK1 Interval on Human Xp11. 3-Xp11. 23: Potential hotspot for neurogenetic disorders. Genomics. 2002;79(4):560–72. doi: 10.1006/geno.2002.6733. [DOI] [PubMed] [Google Scholar]
- 13.Manning G, Whyte DB, Martinez R, et al. The protein kinase complement of the human genome. Science. 2002;298(5600):1912–34. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 14.Jeffrey PD, Russo AA, Polyak K, et al. Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex. Nature. 1995;376(6538):313–20. doi: 10.1038/376313a0. [DOI] [PubMed] [Google Scholar]
- 15.Zenzes MT, Wolf U, Engel W. Organization in vitro of ovarian cells into testicular structures. Hum Genet. 1978;44(3):333–38. doi: 10.1007/BF00394298. [DOI] [PubMed] [Google Scholar]
- 16.Mikolcevic P, Sigl R, Rauch V, et al. Cyclin-dependent kinase 16/PCTAIRE kinase 1 is activated by cyclin Y and is essential for spermatogenesis. Mol Cell Biol. 2012;32(4):868–79. doi: 10.1128/MCB.06261-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Besset V, Rhee K, Wolgemuth DJ. The cellular distribution and kinase activity of the Cdk family member Pctaire1 in the adult mouse brain and testis suggest functions in differentiation. Cell Growth Differ. 1999;10(3):173–81. [PubMed] [Google Scholar]
- 18.Ladam F, Damour I, Dumont P, et al. Loss of a negative feedback loop involving Pea3 and Cyclin D2 is required for Pea3-induced migration in transformed mammary epithelial cells. Mol Cancer Res. 2013;11(11):1412–24. doi: 10.1158/1541-7786.MCR-13-0229. [DOI] [PubMed] [Google Scholar]
- 19.Sheppard KE, McArthur GA. The cell-cycle regulator CDK4: an emerging therapeutic target in melanoma. Clin Cancer Res. 2013;19(19):5320–28. doi: 10.1158/1078-0432.CCR-13-0259. [DOI] [PubMed] [Google Scholar]
- 20.Yu Z, Wang C, Wang M, et al. A cyclin D1/microRNA 17/20 regulatory feedback loop in control of breast cancer cell proliferation. J cell Biol. 2008;182(3):509–17. doi: 10.1083/jcb.200801079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X, Feng M, Jiang X, et al. miR-449a and miR-449b are direct transcriptional targets of E2F1 and negatively regulate pRb–E2F1 activity through a feedback loop by targeting CDK6 and CDC25A. Genes Dev. 2009;23(20):2388–93. doi: 10.1101/gad.1819009. [DOI] [PMC free article] [PubMed] [Google Scholar]